Strength Development and Environmental Impact of Waste-Glass-Based Cements Activated with Portland Cement, NaOH, Na-Silicate or Na-Carbonates at Ambient Temperature
Abstract
:1. Introduction
- -
- Strong bases (e.g., alkali hydroxides and alkali silicates) are effective at dissolving glass particles, but they are corrosive, which can make them difficult to use in certain practical applications. Sodium hydroxide, however, has the advantage of being less expensive and more widely available, making it one of the most commonly used alkali hydroxides in the synthesis of alkali-activated materials. The literature highlights the importance of controlling the amount of activator used to activate glass powder [22,23]. Studies show that excessive amounts of NaOH can be detrimental to the system; a study by Idir et al. [31] suggests that the optimal concentration is 3 mol/L.
- -
- Liquid sodium silicate is known to be highly effective for activating blast furnace slags [32] and low calcium precursors such as fly ashes [25] and metakaolin [33]. Sodium silicates are generally defined by their SiO2/Na2O modulus, and the literature suggests that, for metakaolin and fly ash geopolymers, the preferred modulus range is 1.7–2.2 [34,35,36]. In the case of slags, lower moduli are sometimes used [37,38], referred to as metasilicates which, unlike higher modulus silicates, are in dry powder form.
- -
- Alkali carbonates, which are less corrosive (though still potentially irritant), have not been as thoroughly studied. Due to their lower pH, strength development at early ages is slower [39,40]. However, several studies on slags [41,42] have shown that long-term performance with alkali carbonates is comparable to or even better than with alkali silicates. Furthermore, Duran et al. [43], demonstrated that using carbonate to activate slag results in shrinkage that is comparable to or less than other activators in cement-based mortars. As a result, the use of carbonates in alkali-activated materials has increased over the years. The reaction mechanism, particularly with blast furnace slag, has been explained by Bernal et al. [44]. In addition, the use of carbonate in powder form has enabled the development of a one-part, ready-to-use binder composed of dry material (precursor and activator), requiring only water to prepare the fresh mixture, similar to Portland cement-based materials.
- -
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Activated Glass Pastes
2.2.2. Compositions of Activated Glass Pastes
2.2.3. Testing Methods
3. Results
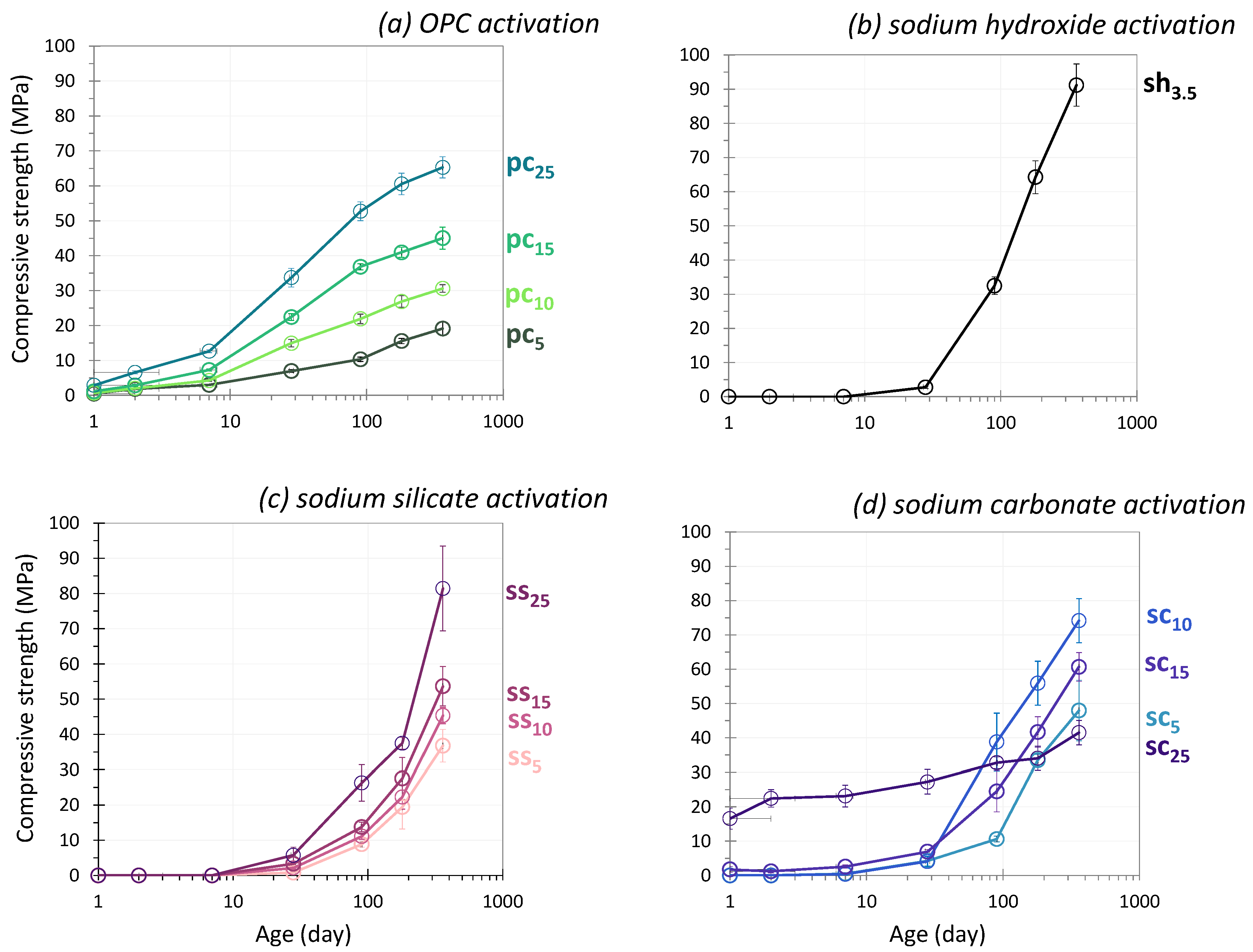
3.1. Compressive Strength
3.1.1. OPC Activation
3.1.2. NaOH Activation
3.1.3. Sodium Silicate Activation
3.1.4. Sodium Carbonate Activation
- Early age (0–7 days)
- Medium term (7–28 days)
- Long term (28–360 days)
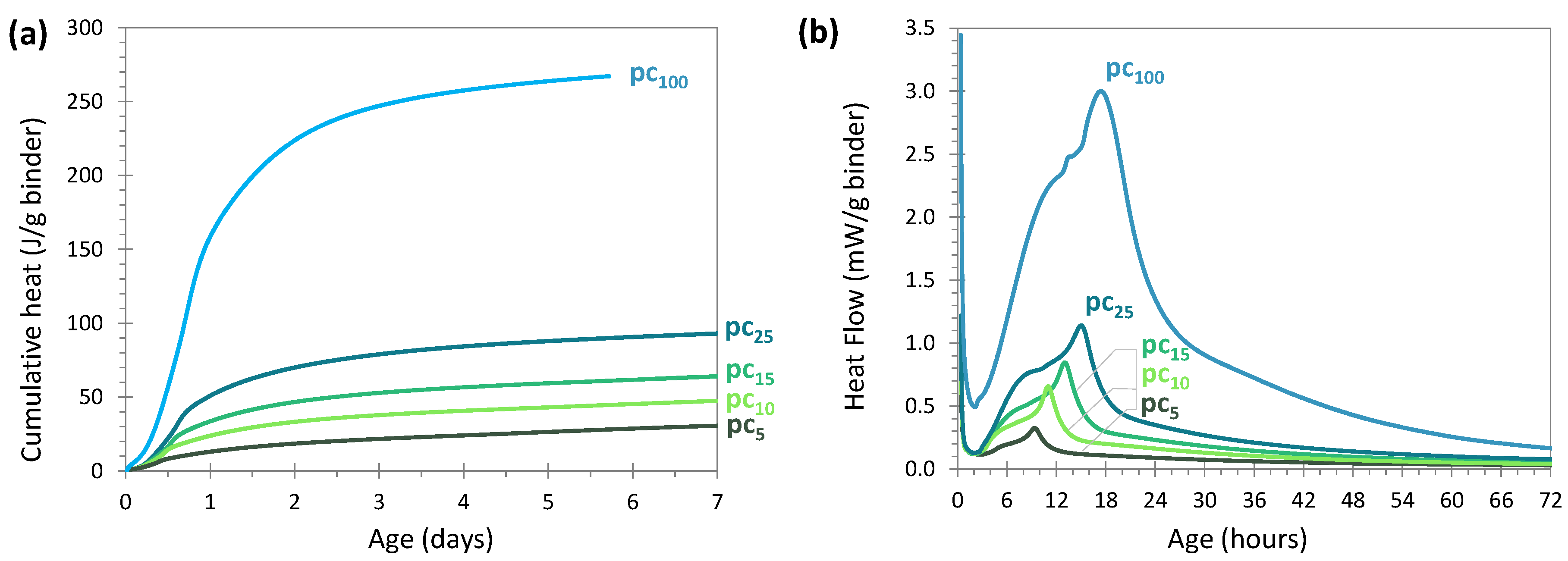
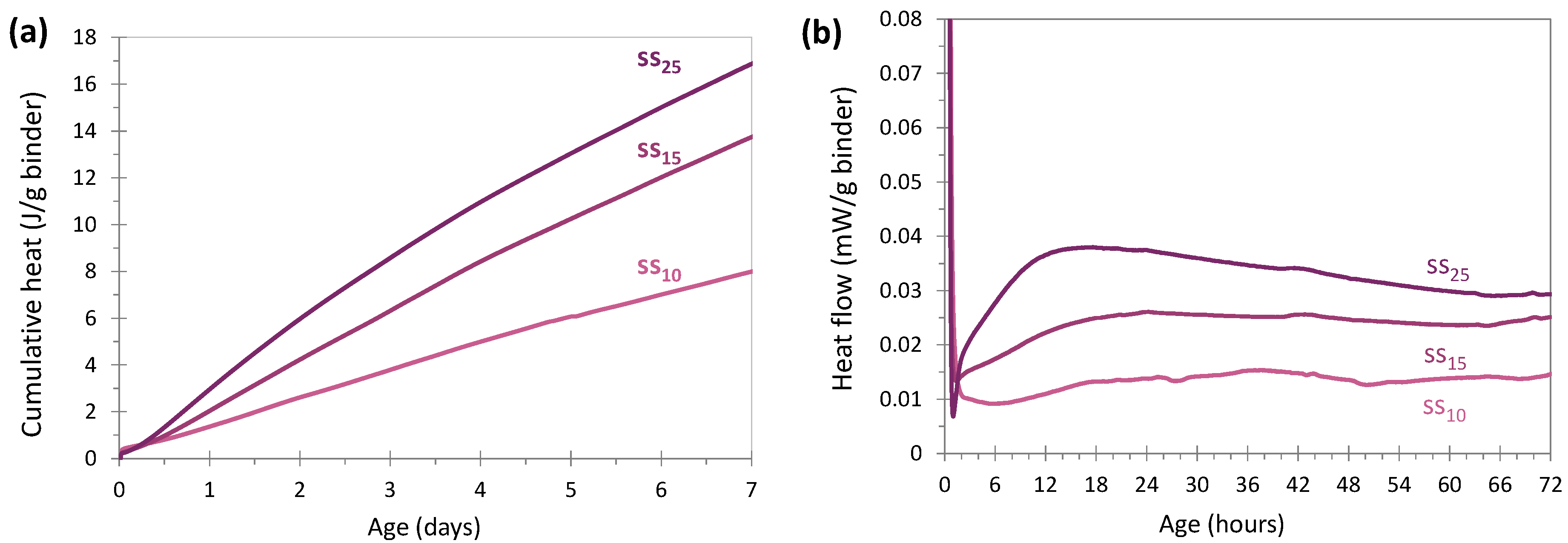
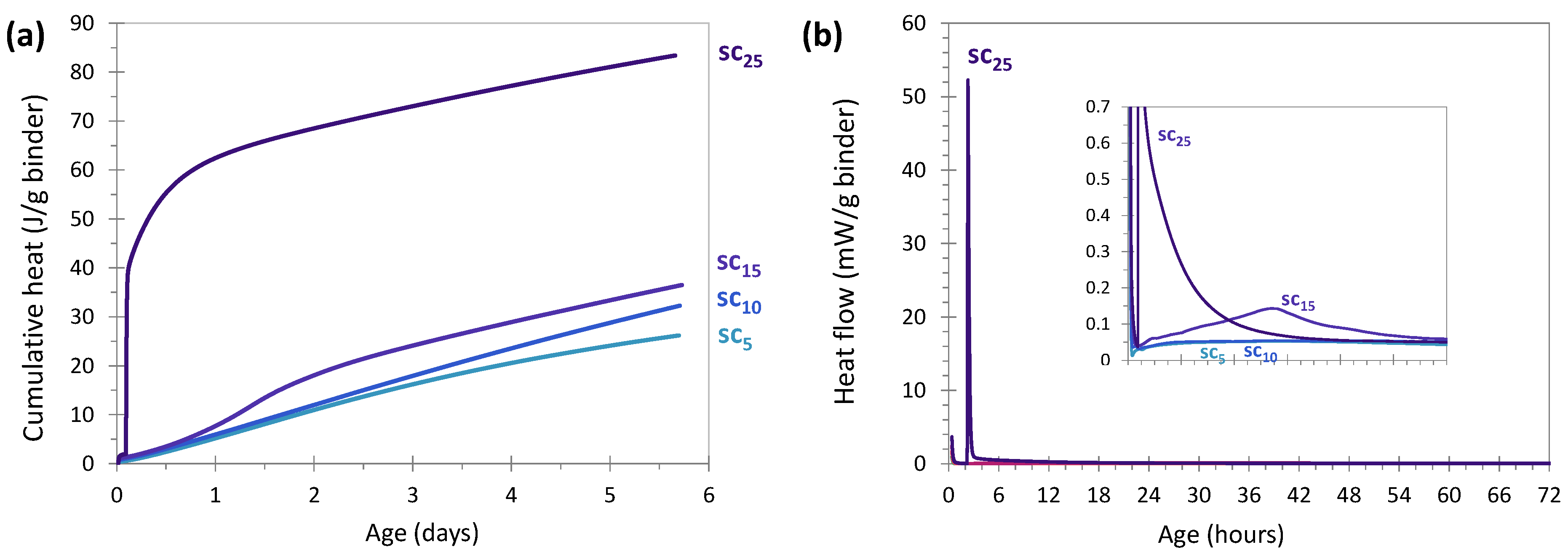
3.2. Hydration Kinetics
3.2.1. OPC Activation
Behavior of the Systems With and Without Glass
3.2.2. Sodium Hydroxide Activation
3.2.3. Sodium Silicate Activation
Behavior of the Glass and Sodium Silicate Systems in Comparison with Cement Activation
Effect of the Amount of Sodium Silicate on the Heat Flow
3.2.4. Sodium Carbonate Activation
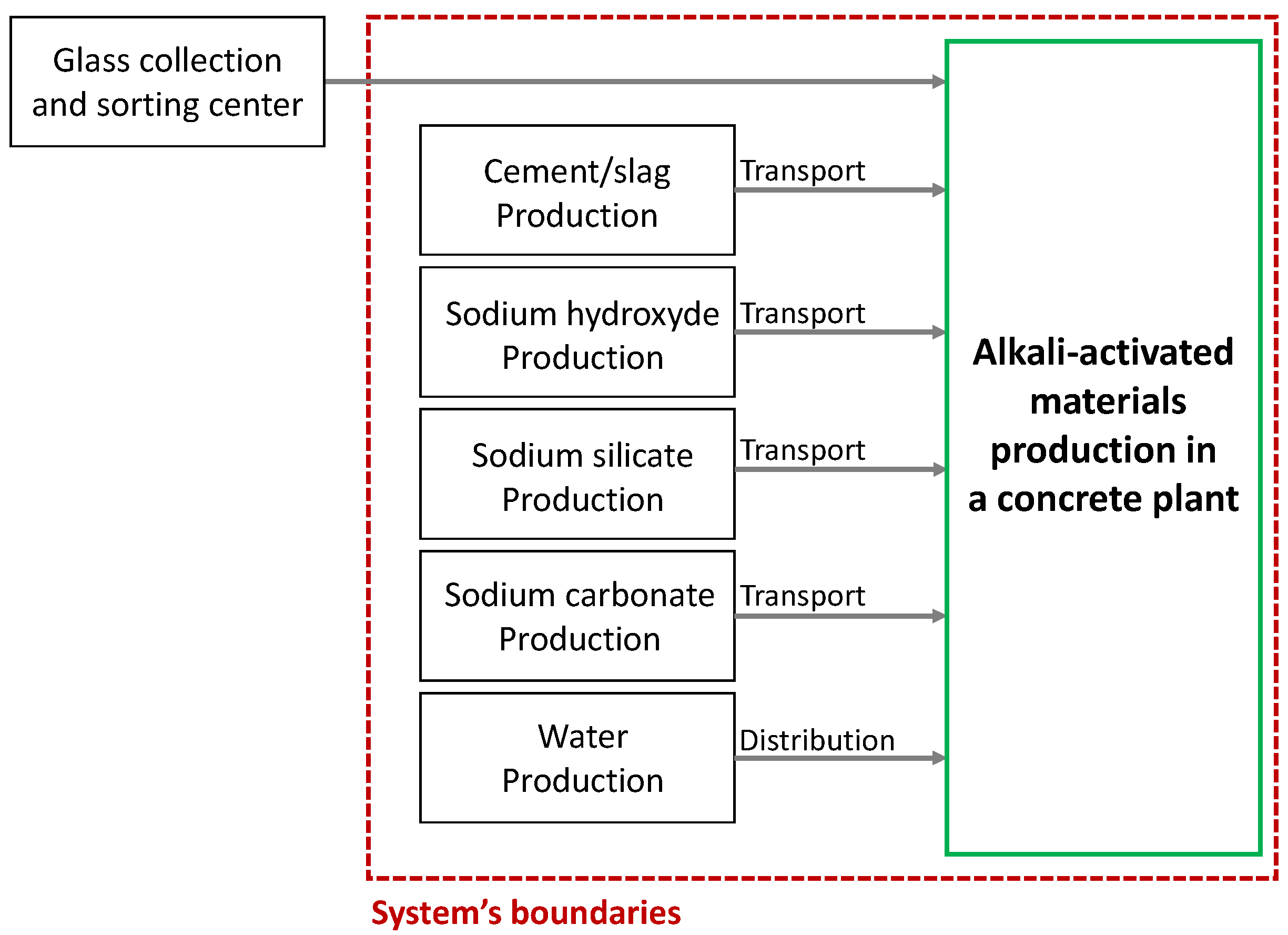
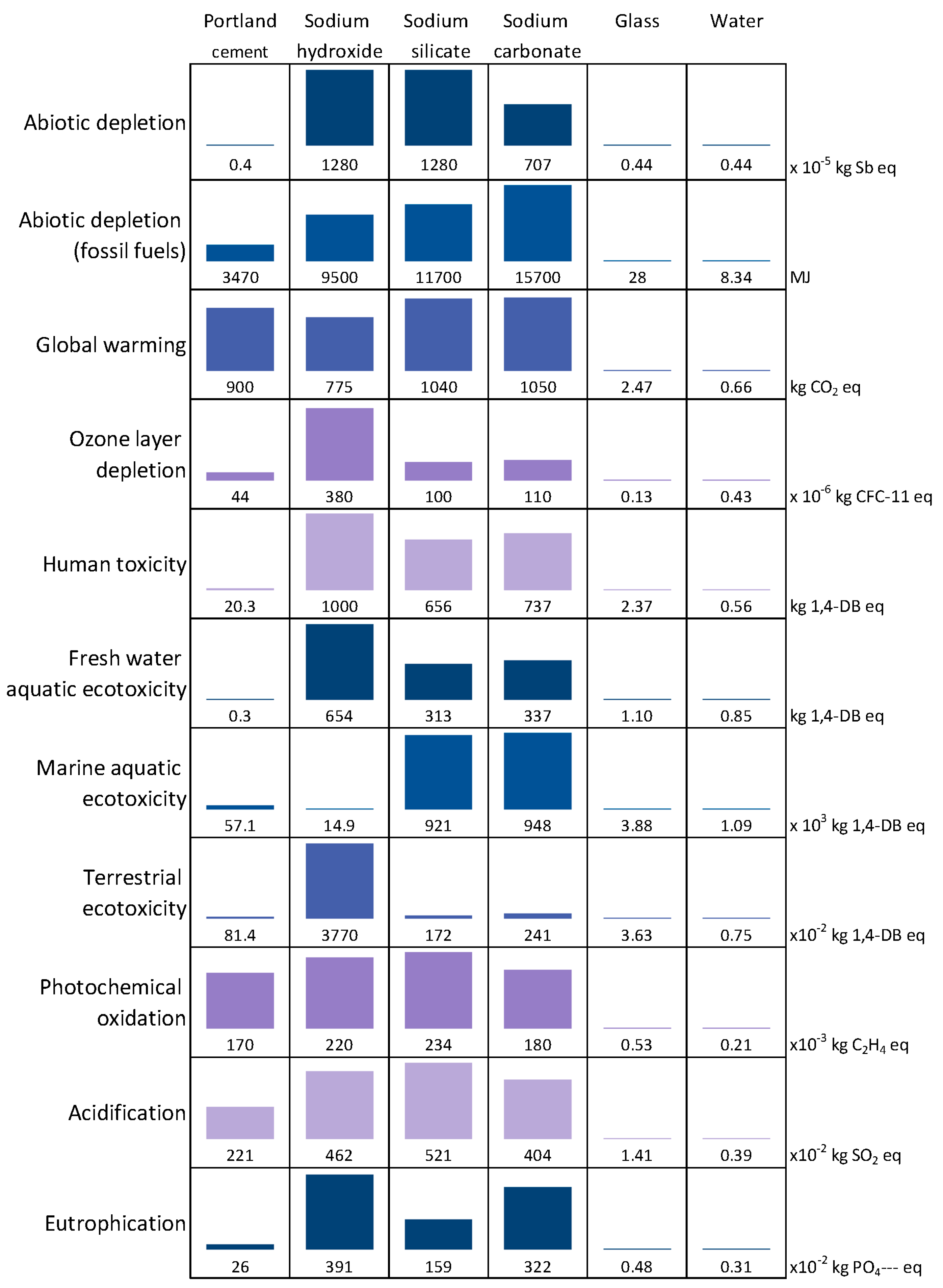
4. Environmental Impact
4.1. LCA for 1 m3 of Activated Glass Cullet Pastes
4.1.1. Contribution of the Different Constituents
4.1.2. Comparison of the Impacts of the Different Systems
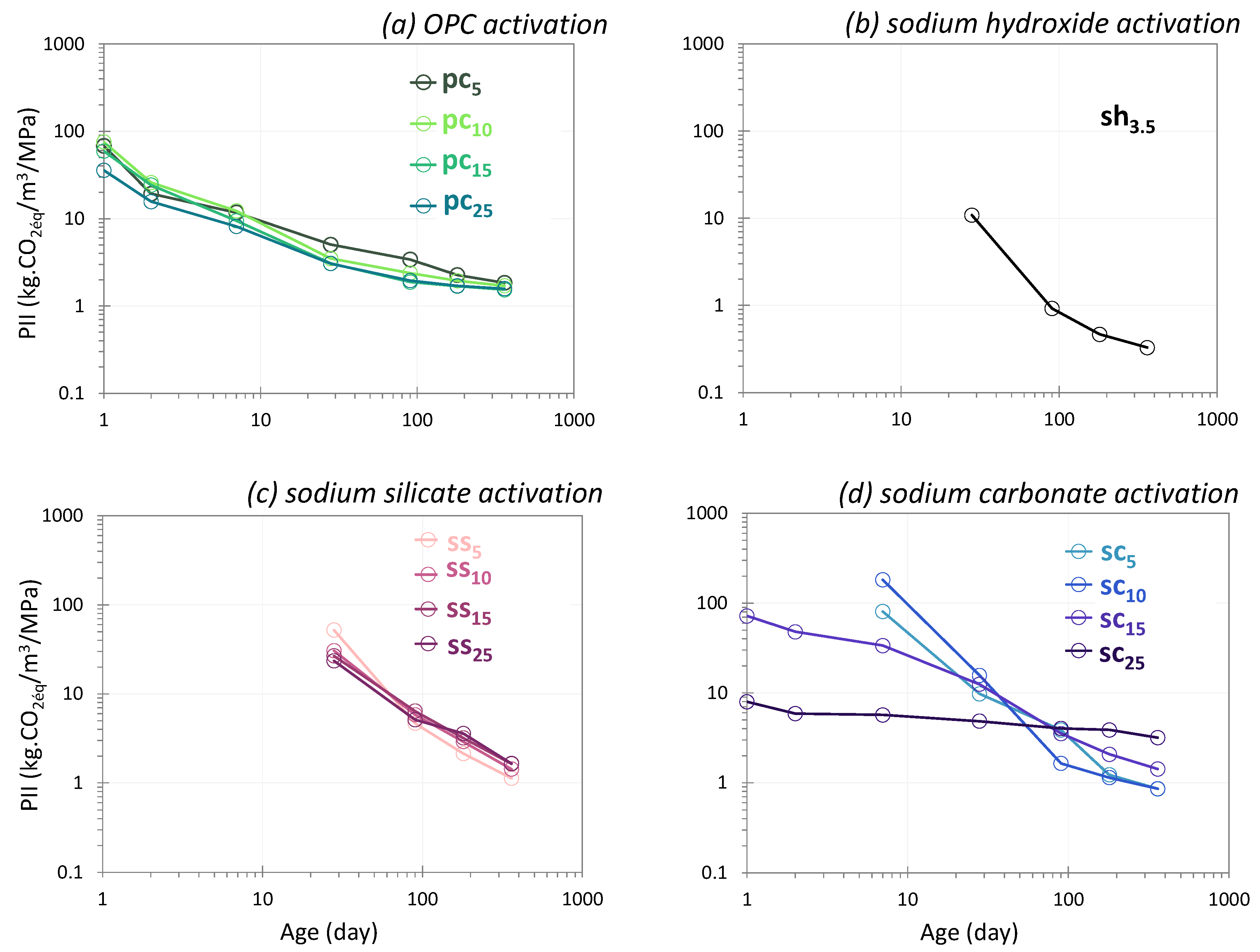
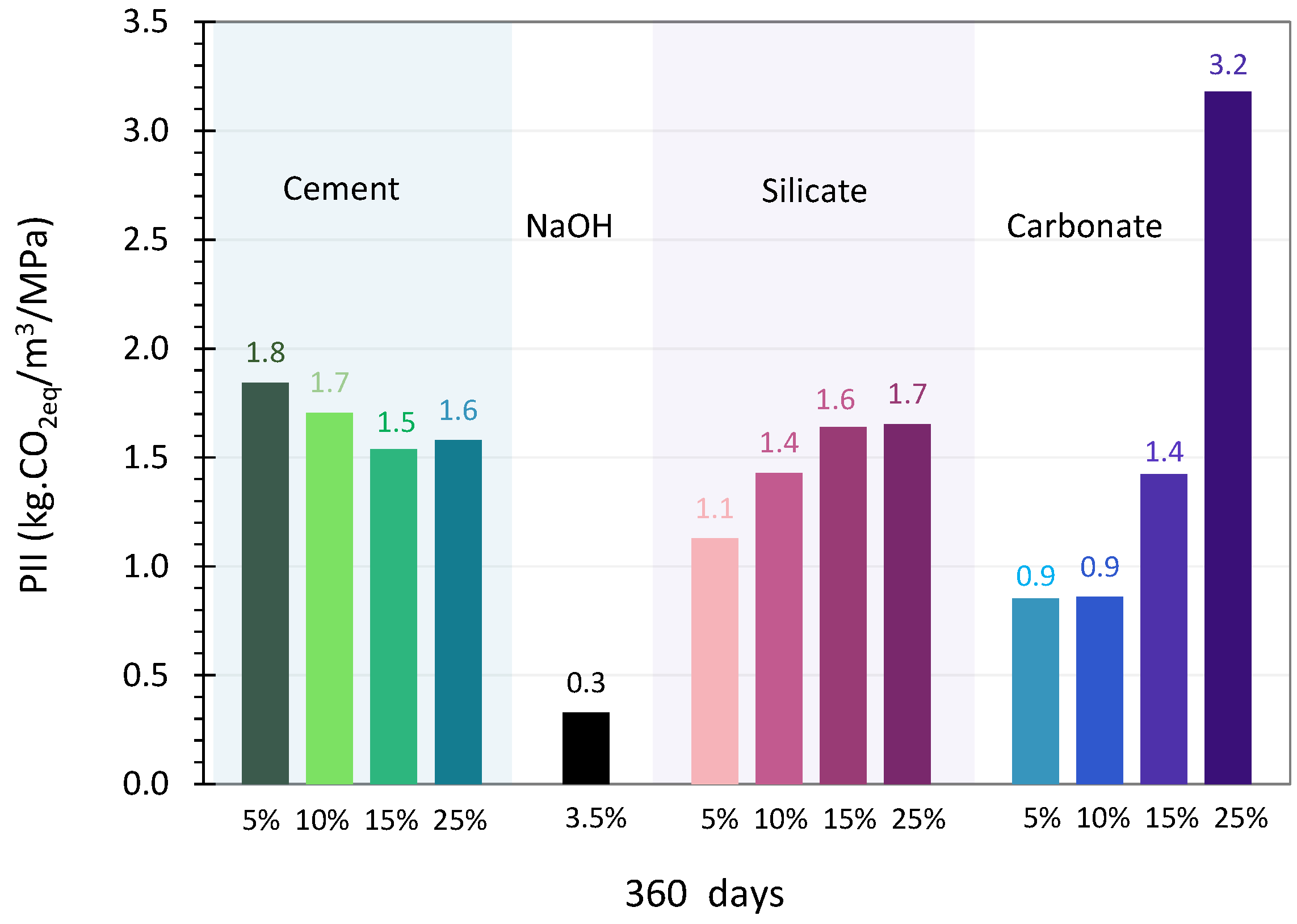
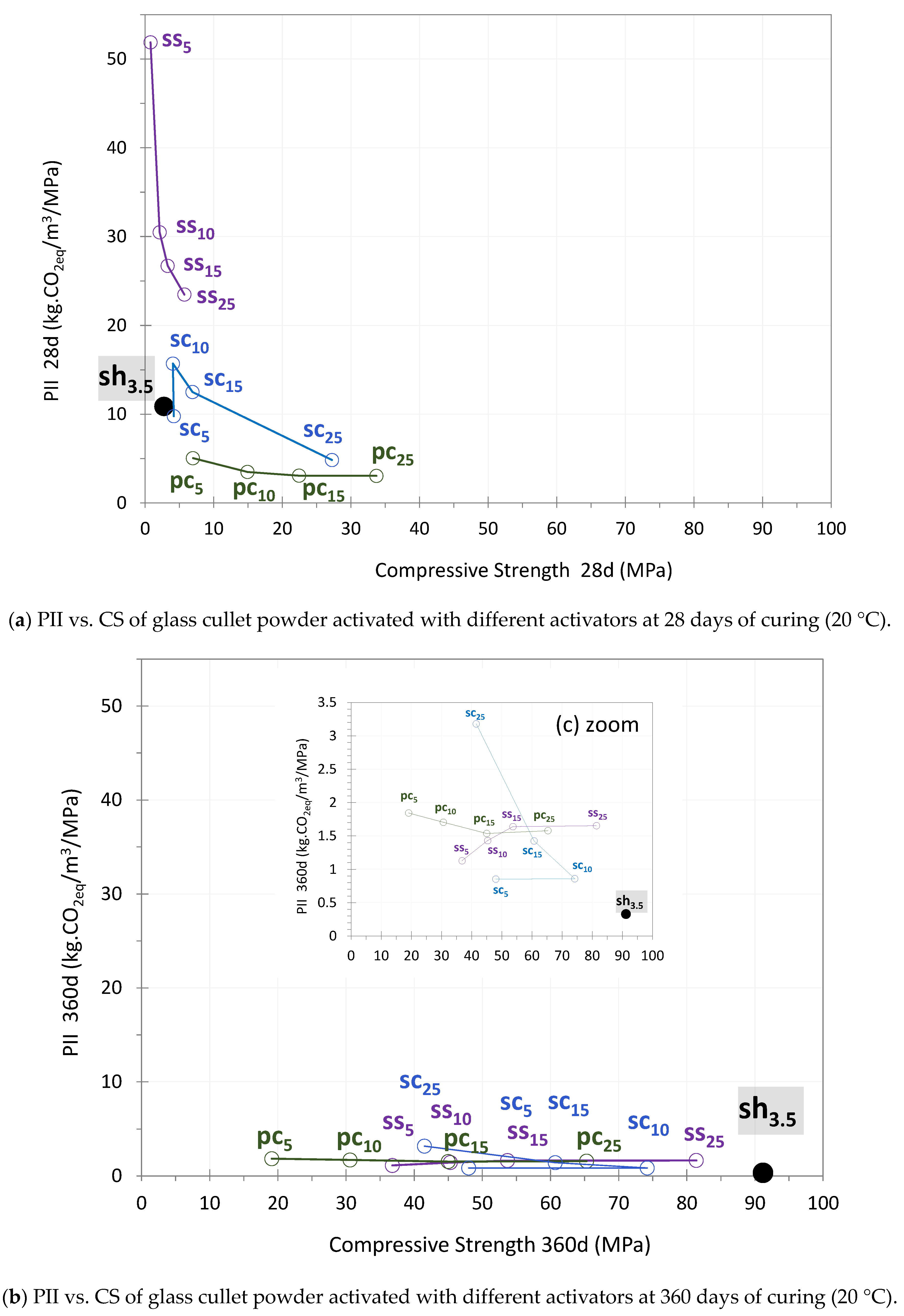
4.2. The Performance Impact Indicator
4.2.1. OPC Activation
4.2.2. Sodium Hydroxide Activation
4.2.3. Sodium Silicate Activation
4.2.4. Sodium Carbonate Activation
5. Discussion
6. Conclusions
- Glass cullet powder could be used as a single precursor at room temperature and with different types of activator to reach a strength compatible with engineering properties (more than 80 MPa), although it presents slow reaction kinetics due to its high stability in alkaline media.
- The short-term strength of activated glass is usually low, except when there is a high content of an activator such as cement or sodium carbonate. These activators are thus preferable to obtain strength at an early age (0–7 days), but have a significantly higher environmental impact.
- Long-term strength could reach very high values, especially with activators that are slow in the first few days (sodium hydroxide, sodium silicate and sodium carbonate). These systems achieved high performance while limiting the use of activator, which is of environmental and economic interest.
- In the long-term, sodium hydroxide and sodium carbonate offer the best compromise between mechanical performance and environmental impact.
- Depending on the performances needed (strength at an early age or longer term, environmentally friendly), the choice of the activator could be adjusted, allowing for a wide range of compositions for this material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Idir, R. Mécanismes D’action des Fines et des Granulats de Verre sur la réaction Alcali-Silice et la réaction Pouzzolanique. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2009. [Google Scholar]
- Rakshvir, M.; Barai, S.V. Studies on recycled aggregates-based concrete. Waste Manag. Res. 2006, 24, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Lee, B.-C. Studies on expansion properties in mortar containing waste glass and fibers. Cem. Concr. Res. 2004, 34, 1145–1152. [Google Scholar] [CrossRef]
- Topçu, İ.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Pike, R.G.; Kubbard, D.; Newman, E.S. Binary silicate glasses in the study of alkali-aggregate reaction. Highw. Res. Board Bull. 1960, 275, 39–44. [Google Scholar]
- Arulrajah, A.; Kua, T.-A.; Horpibulsuk, S.; Phetchuay, C.; Suksiripattanapong, C.; Du, Y.-J. Strength and microstructure evaluation of recycled glass-fly ash geopolymer as low-carbon masonry units. Constr. Build. Mater. 2016, 114, 400–406. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Pugazhmani, G.; Sripragadeesh, R.; Muthu, D.; Venkatasubramanian, C. Experimental study on the mechanical and durability properties of concrete with waste glass powder and ground granulated blast furnace slag as supplementary cementitious materials. Constr. Build. Mater. 2017, 156, 739–749. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Performance of glass powder as a pozzolanic material in concrete: A field trial on concrete slabs. Cem. Concr. Res. 2006, 36, 457–468. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Role of the nature of reaction products in the differing behaviours of fine glass powders and coarse glass aggregates used in concrete. Mater. Struct. 2013, 46, 233–243. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Dhir, R.K. The influence of the use of recycled aggregates on the compressive strength of concrete: A review. Eur. J. Environ. Civ. Eng. 2015, 19, 825–849. [Google Scholar] [CrossRef]
- González-Fonteboa, B.; Seara-Paz, S.; De Brito, J.; González-Taboada, I.; Martínez-Abella, F.; Vasco-Silva, R. Recycled concrete with coarse recycled aggregate. An overview and analysis. Mater. Constr. 2018, 68, 151. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V.; Petrović, R. Physical–mechanical and microstructural properties of alkali-activated fly ash–blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Puertas, F.; Martínez-Ramírez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; Abd Ela-ziz, H. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Komljenović, M.M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. Decalcification resistance of alkali-activated slag. J. Hazard. Mater. 2012, 233–234, 112–121. [Google Scholar] [CrossRef]
- Martinez-Lopez, R.; Ivan Escalante-Garcia, J. Alkali activated composite binders of waste silica soda lime glass and blast furnace slag: Strength as a function of the composition. Constr. Build. Mater. 2016, 119, 119–129. [Google Scholar] [CrossRef]
- Redden, R.; Neithalath, N. Microstructure, strength, and moisture stability of alkali activated glass powder-based binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Pascual, M.T.T.; Tognonvi, M.T.; Tagnit-Hamou, A. Waste glass powder-based alkali-activated mortar. Int. J. Res. Eng. Technol. 2014, 3, 15–19. [Google Scholar]
- Cyr, M.; Idir, R.; Poinot, T. Properties of inorganic polymer (geopolymer) mortars made of glass cullet. J. Mater. Sci. 2012, 47, 2782–2797. [Google Scholar] [CrossRef]
- Marchand, B.; Lanier, S.; Davy, C.A.; Albert-Mercier, C.; Tricot, G. Are calcium silicate hydrates (C-S-H) present in alkali-activated glass cullet cement? Mater. Lett. 2018, 219, 104–108. [Google Scholar] [CrossRef]
- Tho-In, T.; Sata, V.; Boonserm, K.; Chindaprasirt, P. Compressive strength and microstructure analysis of geopolymer paste using waste glass powder and fly ash. J. Clean. Prod. 2018, 172, 2892–2898. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass as a precursor in alkaline activation: Chemical process and hydration products. Constr. Build. Mater. 2017, 139, 342–354. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, R.; Jiang, X.; Li, W.; Zhu, X.; Huang, B. Effect of particle size and curing temperature on mechanical and microstructural properties of waste glass-slag-based and waste glass-fly ash-based geopolymers. J. Clean. Prod. 2020, 273, 122970. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Rivera, J.F.; Cuarán-Cuarán, Z.I.; Vanegas-Bonilla, N.; Mejía de Gutiérrez, R. Novel use of waste glass powder: Production of geopolymeric tiles. Adv. Powder Technol. 2018, 29, 3448–3454. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Pavoine, A. Investigations on the durability of alkali-activated recycled glass. Constr. Build. Mater. 2020, 236, 117477. [Google Scholar] [CrossRef]
- Rovnaník, P.; Bayer, P.; Rovnaníková, P. Characterization of alkali activated slag paste after exposure to high temperatures. Constr. Build. Mater. 2013, 47, 1479–1487. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Cheng, H.; Lin, K.L.; Cui, R.; Hwang, C.L.; Chang, Y.M.; Cheng, T.W. The effects of SiO2/Na2O molar ratio on the characteristics of alkali-activated waste catalyst–metakaolin based geopolymers. Constr. Build. Mater. 2015, 95, 710–720. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van Deventer, J.S. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Provis, J.L.; Yong, C.Z.; Duxson, P.; van Deventer, J.S.J. Correlating mechanical and thermal properties of sodium silicate-fly ash geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2009, 336, 57–63. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, T.; Zhou, S.; Wei, H.; Zhang, Z. Calcium carbide residue as auxiliary activator for one-part sodium carbonate-activated slag cements: Compressive strength, phase assemblage and environmental benefits. Constr. Build. Mater. 2021, 308, 125015. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, Z.; Zhang, F.; Gao, Y.; Wu, Q. Chloride and heavy metal binding capacities of hydrotalcite-like phases formed in greener one-part sodium carbonate-activated slag cements. J. Clean. Prod. 2020, 253, 120047. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-activated slag mortars. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of carbonates in the chemical evolution of sodium carbonate-activated slag binders. Mater. Struct. 2015, 48, 517–529. [Google Scholar] [CrossRef]
- Xiao, R.; Ma, Y.; Jiang, X.; Zhang, M.; Zhang, Y.; Wang, Y.; Huang, B.; He, Q. Strength, microstructure, efflorescence behavior and environmental impacts of waste glass geopolymers cured at ambient temperature. J. Clean. Prod. 2020, 252, 119610. [Google Scholar] [CrossRef]
- NF EN 196-6; Methods of Testing Cement—Determination of Fineness. Association Francaise de Normalisation: Saint-Denis, France, 2018.
- EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- Bruijn, H.; Duin, R.; Huijbregts, M.A.J.; Guinee, J.B.; Gorree, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; Koning, A.; Oers, L.; et al. Handbook on Life Cycle Assessment. Operational Guide to the ISO Standard; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for the production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Khatib, J.M.; Hibbert, J.J. Selected engineering properties of concrete incorporating slag and metakaolin. Constr. Build. Mater. 2005, 19, 460–472. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. High strength geopolymer binder based on waste-glass powder. Adv. Powder Technol. 2017, 28, 215–222. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Chemical Reactions of Glass Cullet Used as Cement Component. J. Mater. Civ. Eng. 2001, 13, 412–417. [Google Scholar] [CrossRef]
- Sagoe-Crentsil, K.; Weng, L. Dissolution processes, hydrolysis and condensation reactions during geopolymer synthesis: Part II. High Si/Al ratio systems. J. Mater. Sci. 2007, 42, 3007–3014. [Google Scholar] [CrossRef]
- Ben Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Özkan, Ö.; Yüksel, İ. Studies on mortars containing waste bottle glass and industrial by-products. Constr. Build. Mater. 2008, 22, 1288–1298. [Google Scholar] [CrossRef]
- Thieme, C. Sodium Carbonates. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publ. Limited: Oxford, UK, 2009. [Google Scholar]
- Chlorine and Sodium Hydroxide Life Cycle Inventories of Chemicals Data v2.0; Ecoinvent: Zürich, Switzerland, 2007.
- Breton, C. Carbonate de sodium. Procédé Solvay à l’ammoniac. Techniques de l’Ingénieur 2002. Available online: https://www.techniques-ingenieur.fr/base-documentaire/procedes-chimie-bio-agro-th2/fabrication-des-grands-produits-industriels-en-chimie-et-petrochimie-42319210/carbonate-de-sodium-j6195/procede-de-fabrication-j6195niv10001.html (accessed on 1 June 2021).
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the eco-efficiency of cement use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Puertas, F. Waste glass in the geopolymer preparation. Mechanical and microstructural characterisation. J. Clean. Prod. 2015, 90, 397–408. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]



| Blaine | D10 (µm) | D50 (µm) | D90 (µm) | |
|---|---|---|---|---|
| Glass powder | 3000 cm2/g | 2.6 | 15.6 | 59.8 |
| CEM I 52.5N | 3850 cm2/g | - | - | - |
| % Mass | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | SO3 | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|
| Glass powder | 71.5 | 2.3 | 0.9 | 10.4 | 1.5 | 12.3 | 0.5 | 0.1 | - | 0.5 |
| CEM I 52.5N | 19.3 | 5.3 | 2.6 | 63.2 | 2.0 | 0.1 | 0.9 | - | 3.5 | 2.9 |
| Portland cement activation | pc5 | pc10 | pc15 | pc25 |
| Cement (%) | 5 | 10 | 15 | 25 |
| Cement (g) | 20 | 40 | 60 | 100 |
| Glass (g) | 400 | |||
| Water (g) | 126 | 132 | 138 | 150 |
| Water/solid * | 0.30 | |||
| Water/cement | 6.3 | 3.3 | 2.3 | 1.5 |
| Sodium hydroxide activation | sh3.5 | |||
| NaOH (%) | 3.5 (equivalent to 3 mol/L) | |||
| NaOH (g) | 14 | |||
| Glass (g) | 400 | |||
| Water (g) | 120 | |||
| Water/solid ** | 0.30 | |||
| Sodium silicate activation | ss5 | ss10 | ss15 | ss25 |
| Sodium silicate solution (%) | 5 | 10 | 15 | 25 |
| Sodium silicate solution (g) | 20 | 40 | 60 | 100 |
| Glass (g) | 400 | |||
| Total water (g) | 122 | 124 | 126 | 131 |
| Water/solid *** | 0.30 | |||
| Sodium carbonate activation | sc5 | sc10 | sc15 | sc25 |
| Sodium carbonate (%) | 5 | 10 | 15 | 25 |
| Sodium carbonate (g) | 20 | 40 | 60 | 100 |
| Glass (g) | 400 | |||
| Water (g) | 126 | 132 | 138 | 150 |
| Water/solid **** | 0.30 | |||
| Process | Modulus Used | Data Sources | Assumptions |
|---|---|---|---|
| Production of glass | / | Laboratory estimation | Average over the power grinders |
| Production of cement | Portland cement (CEM I), production mix, at plant, EN 197-1 (location: RER) | Ecoinvent© Zürich, Switzerland | - |
| Production of sodium silicate | / | [49] | - |
| Production of sodium carbonate | Sodium carbonate from ammonium chloride production, at plant/GLO U | Ecoinvent© [50] | - |
| Production of sodium hydroxide | Sodium hydroxide, 50% in H2O, production mix, at plant RER | Ecoinvent© [50] | Consideration of the French electricity energy mix |
| Production of slag | / | Industrial data | - |
| Transport | Transport, lorry 16–32 t, EURO5/RER | Ecoinvent© [50] | Estimation on distances |
| Production of water | Tap water, at user/CH S | Ecoinvent© [50] | - |
| Time | pc5 | pc10 | pc15 | pc25 | sh3.5 | ss5 | ss10 | ss15 | ss25 | sc5 | sc10 | sc15 | sc25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | ND | ND | ND | ND | ND | ND | ND | ||||||
| 2 d | ND | ND | ND | ND | ND | ND | ND | ||||||
| 7 d | ND | ND | ND | ND | ND | ||||||||
| 28 d | |||||||||||||
| 90 d | |||||||||||||
| 180 d | |||||||||||||
| 360 d | |||||||||||||
| ND | Not detected | <3 MPa | 3–15 MPa | 15–50 MPa | 50–80 MPa | >80 MPa | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemesre, L.; Idir, R.; Cyr, M. Strength Development and Environmental Impact of Waste-Glass-Based Cements Activated with Portland Cement, NaOH, Na-Silicate or Na-Carbonates at Ambient Temperature. Materials 2024, 17, 5097. https://doi.org/10.3390/ma17205097
Lemesre L, Idir R, Cyr M. Strength Development and Environmental Impact of Waste-Glass-Based Cements Activated with Portland Cement, NaOH, Na-Silicate or Na-Carbonates at Ambient Temperature. Materials. 2024; 17(20):5097. https://doi.org/10.3390/ma17205097
Chicago/Turabian StyleLemesre, Louise, Rachida Idir, and Martin Cyr. 2024. "Strength Development and Environmental Impact of Waste-Glass-Based Cements Activated with Portland Cement, NaOH, Na-Silicate or Na-Carbonates at Ambient Temperature" Materials 17, no. 20: 5097. https://doi.org/10.3390/ma17205097
APA StyleLemesre, L., Idir, R., & Cyr, M. (2024). Strength Development and Environmental Impact of Waste-Glass-Based Cements Activated with Portland Cement, NaOH, Na-Silicate or Na-Carbonates at Ambient Temperature. Materials, 17(20), 5097. https://doi.org/10.3390/ma17205097








