Synthesis and Electrochemical Properties of Oleylamine as a Sour Saline Corrosion Inhibitor Under Laminar Flow at 40 °C
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the Sour Corrosion Inhibitor
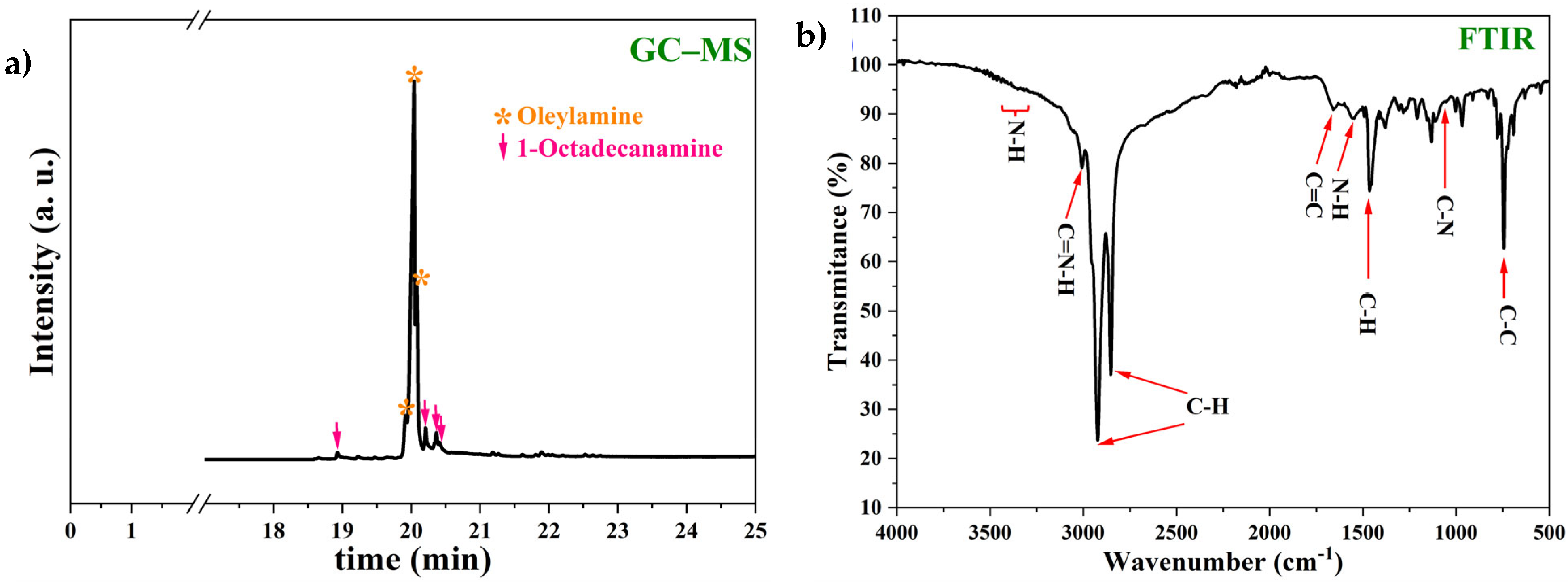
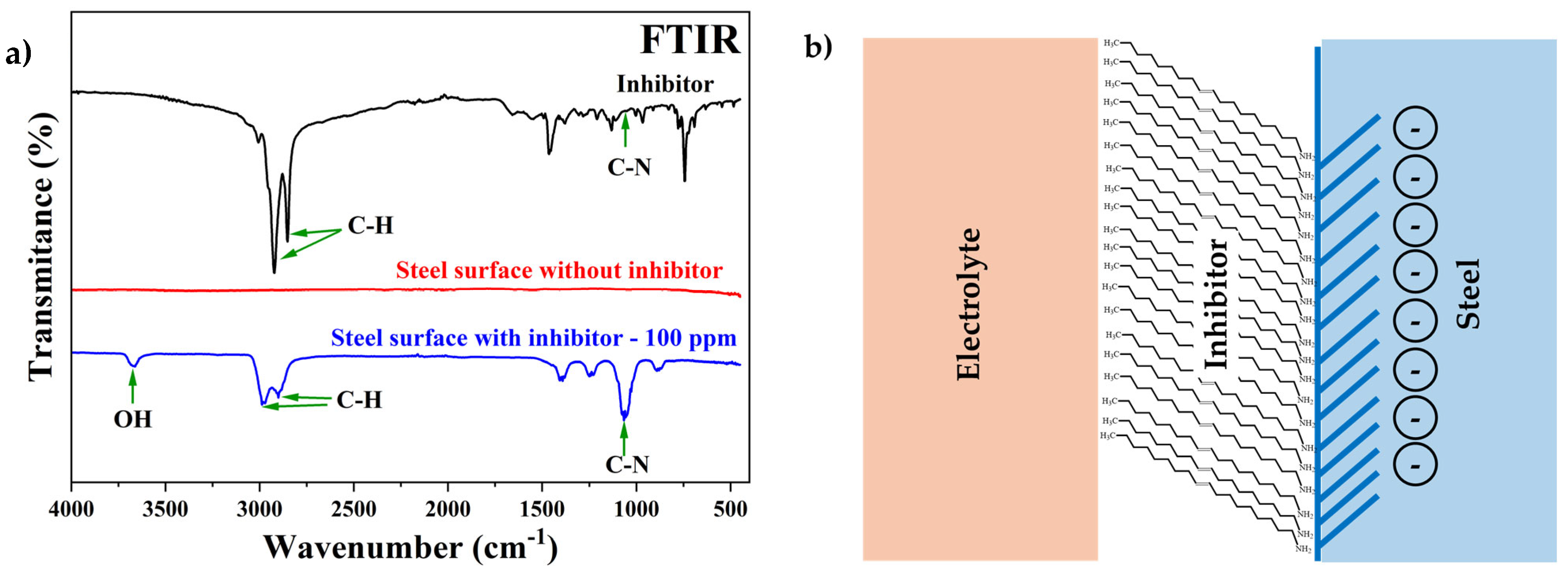
2.1.1. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflection (FTIR-ATR)
2.1.2. Gas Chromatography–Mass Spectroscopy (GC-MS)
2.2. Preparation of Steel Cylinders as Working Electrodes
2.3. Preparation of the Sour Saline Electrolyte
2.4. Setup of the Electrochemical Cell
2.5. Electrochemical Measurement
2.6. Characterization of the Steel Surface After Electrochemical Tests Using Scanning Electron Microscopy (SEM)
3. Results
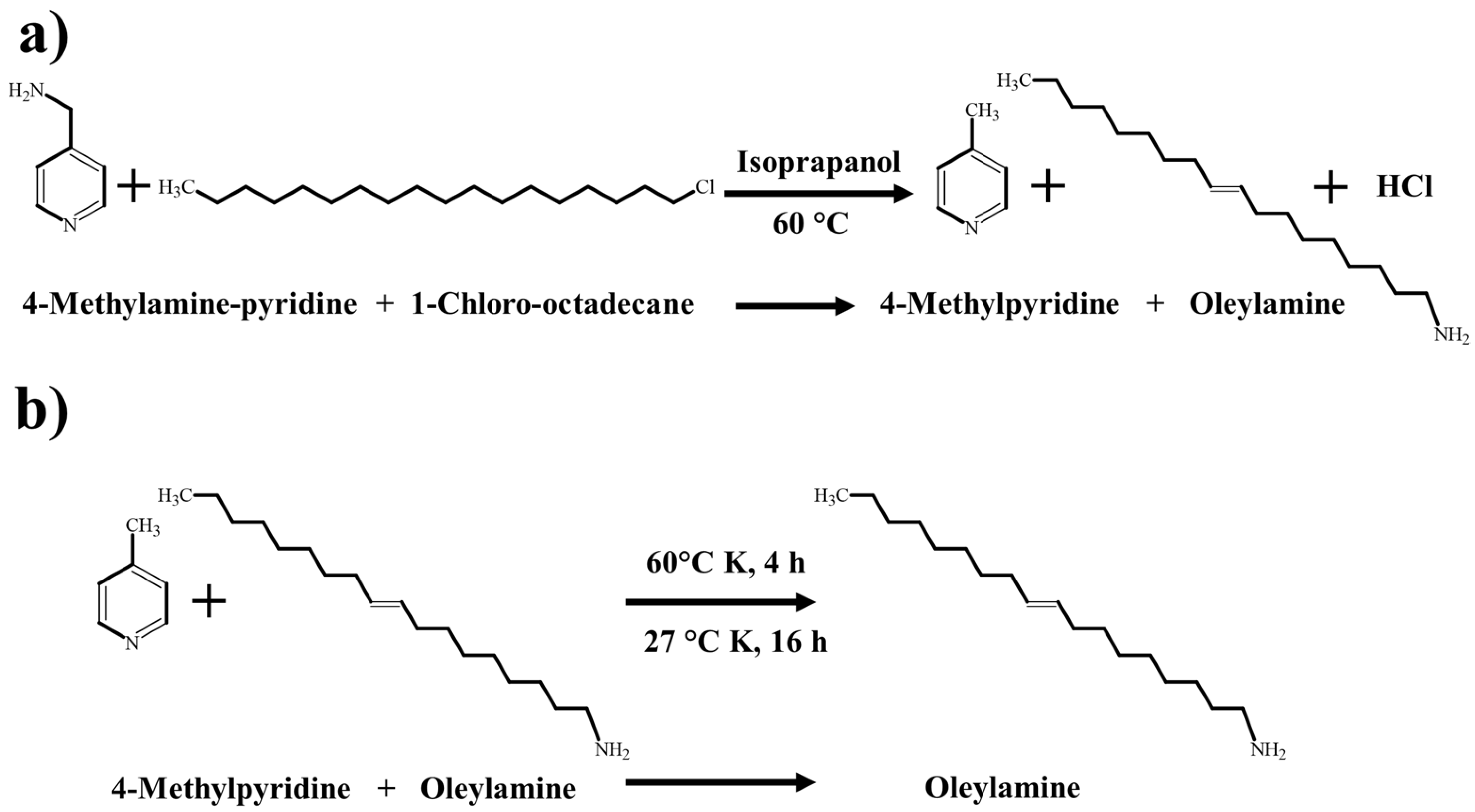
3.1. Corrosion Inhibitor Synthesis
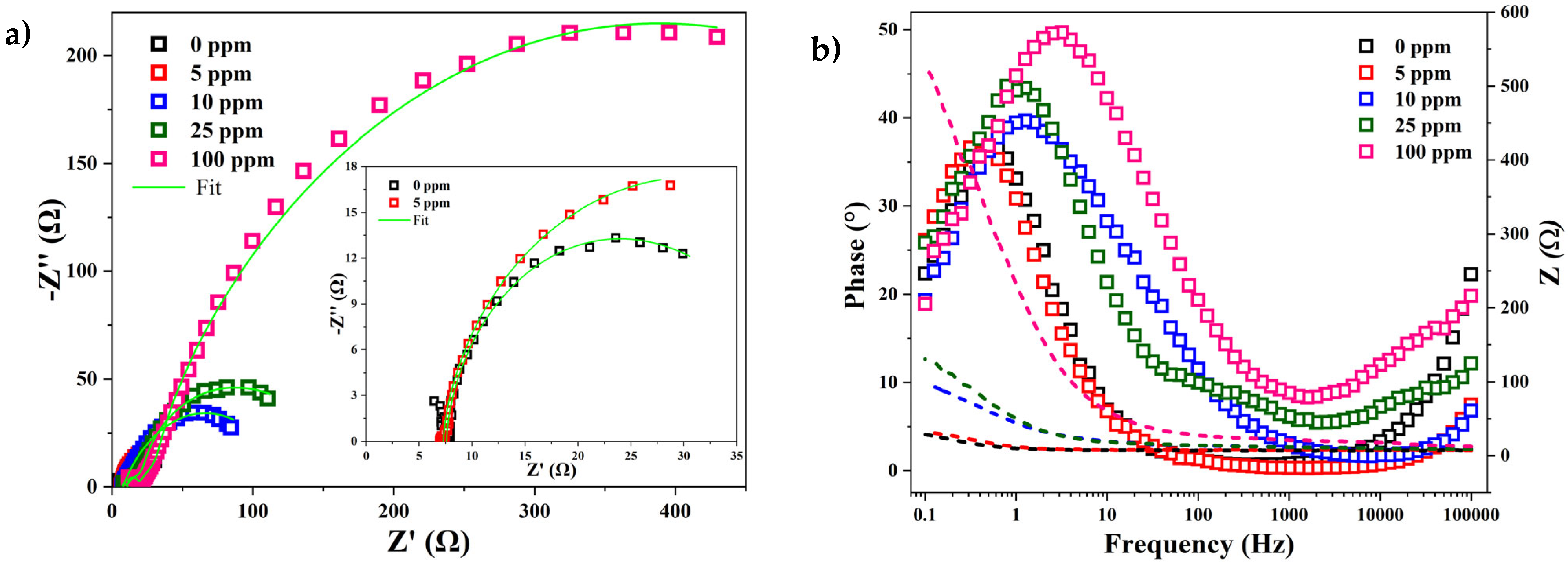
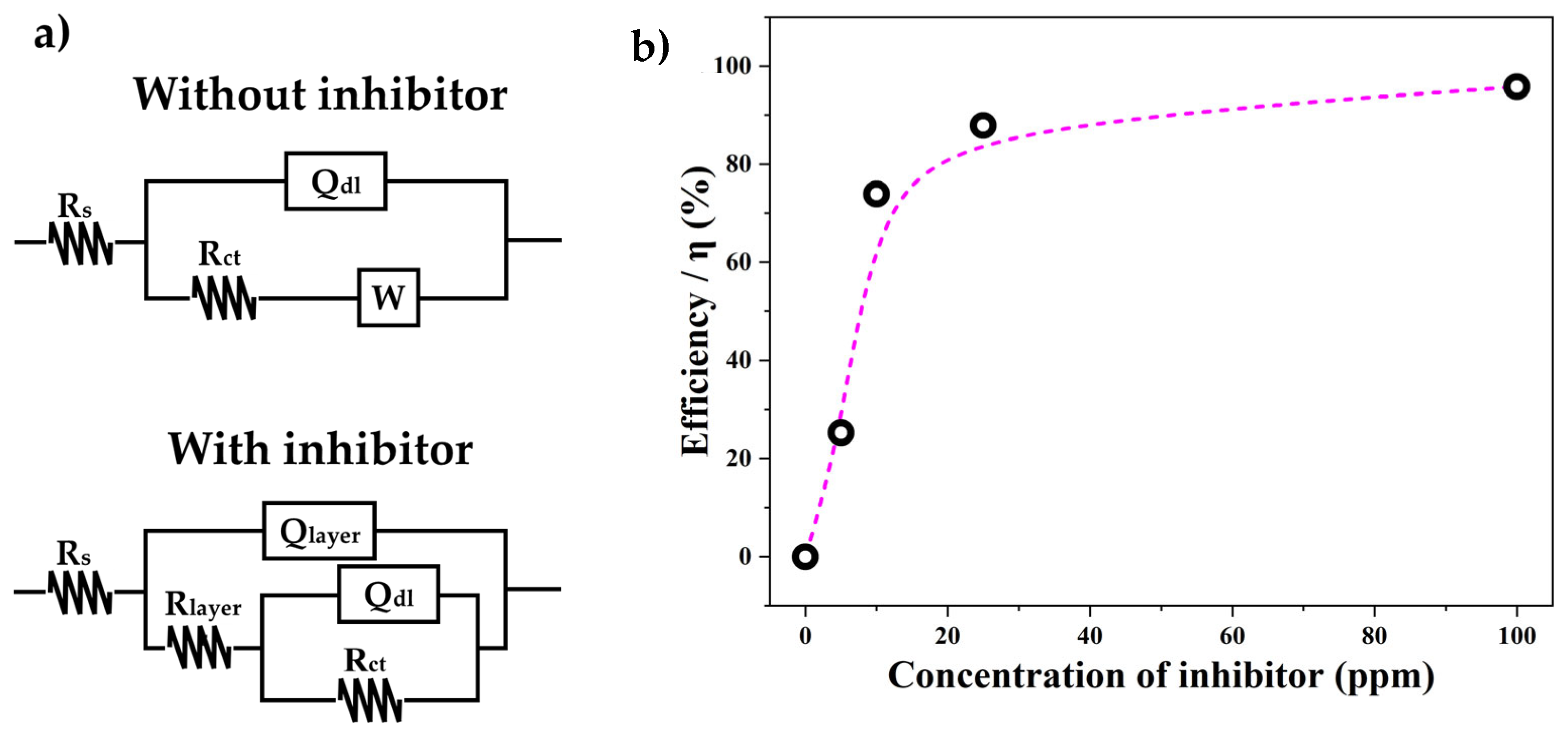
3.2. Electrochemical Properties of the Inhibitor
3.3. Characterization of Cylindrical Mild Steel Surface After Electrochemical Tests
4. Conclusions
- (1)
- Through the reaction route proposed in this report, the oleylamine synthesis reached an excellent reaction yield of 91.23%.
- (2)
- On the other hand, the electrochemical tests revealed that oleylamine possesses anticorrosive properties as an inhibitor when used on low-carbon mild steel (AISI 1018) surfaces exposed to sour saline electrolytes. According to the Icorr and Rct parameters for the inhibitor concentration in the electrolyte, the efficiency of oleylamine as a corrosion inhibitor has exponential behavior.
- (3)
- The application of 20 ppm of the inhibitor in the electrolyte reached an inhibition efficiency of more than 85%, and a maximum efficiency higher than 95% was reached at 100 ppm.
- (4)
- Furthermore, SEM analysis confirmed the anticorrosive properties of oleylamine in steels exposed to sour electrolytes. In addition, oleylamine may be an excellent candidate as a corrosion inhibitor for application in sour saline environments at temperatures ≥40 °C due to its high boiling point (364 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sotoodeh, K. Subsea Valves and Actuators for the Oil and Gas Industry; Gulf Professional Publishing: Houston, TX, USA, 2021. [Google Scholar]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Obot, I.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Liu, L.; Case, R. The influence of H2S on hydrogen absorption and sulfide stress cracking resistance of high strength low alloy carbon steel c110. J. Nat. Gas Sci. Eng. 2022, 99, 104418. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Saji, V.S. Corrosion inhibitors for sour oilfield environment (H2S corrosion). In Corrosion Inhibitors in the Oil and Gas Industry; Wiley: Hoboken, NJ, USA, 2020; pp. 229–254. [Google Scholar]
- Pessu, F.; Barker, R.; Chang, F.; Chen, T.; Neville, A. Iron sulphide formation and interaction with corrosion inhibitor in h2s-containing environments. J. Pet. Sci. Eng. 2021, 207, 109152. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Amiery, A.; Alazawi, R.; Al-Ghezi, M.; Abass, R. Corrosion inhibitors. a review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar]
- Vakili, M.; Koutn, P.; Kohout, J. Addressing hydrogen sulfide corrosion in oil and gas industries: A sustainable perspective. Sustainability 2024, 16, 1661. [Google Scholar] [CrossRef]
- Zhao, F.; Cui, C.; Dong, S.; Xu, X.; Liu, H. An overview on the corrosion mechanisms and inhibition techniques for amine-based post-combustion carbon capture process. Sep. Purif. Technol. 2023, 304, 122091. [Google Scholar] [CrossRef]
- Mok, W.Y.; Jenkins, A.; Keenan, S.R.; Gamble, C.G. Control of localized corrosion using green corrosion inhibitors. In Proceedings of the NACE CORROSION, NACE, Houston, TX, USA, 3–7 April 2005; p. NACE-05289. [Google Scholar]
- Kashkovskiy, R.; Kuznetsov, Y.I.; Kazansky, L. Inhibition of hydrogen sulfide corrosion of steel in gas phase by tributylamine. Corros. Sci. 2012, 64, 126–136. [Google Scholar] [CrossRef]
- Rashid, K.H.; Khadom, A.A. 3-methoxypropyl-amine as corrosion inhibitor for x80 steel in simulated saline water. J. Mol. Liq. 2020, 319, 114326. [Google Scholar] [CrossRef]
- Ge, H.-H.; Zhou, G.-D.; Liao, Q.-Q.; Lee, Y.; Loo, B. A study of anti-corrosion behavior of octadecylamine-treated iron samples. Appl. Surf. Sci. 2000, 156, 39–46. [Google Scholar] [CrossRef]
- Yoshioka, H.; Yoshida, K.; Noguchi, N.; Ueki, T.; Murai, K.-I.; Watanabe, K.; Nakahara, M. Microscopic structure and binding mechanism of the corrosionprotective film of oleylpropanediamine on copper in hot water. J. Phys. Chem. C 2022, 126, 6436–6447. [Google Scholar] [CrossRef]
- Kuilya, S.; Subramanian, V.; Bera, S.; Rangarajan, S. Evaluation of octadecylamine for the corrosion inhibition of incoloy 800. Corros. Eng. Sci. Technol. 2021, 56, 119–128. [Google Scholar] [CrossRef]
- Jäppinen, E.; Ikäläinen, T.; Järvimäki, S.; Saario, T.; Sipilä, K.; Bojinov, M. Corrosion behavior of carbon steel coated with octadecylamine in the secondary circuit of a pressurized water reactor. J. Mater. Eng. Perform. 2017, 26, 6037–6046. [Google Scholar] [CrossRef]
- Lin, S.-K.; March, J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; McGraw-Hill: New York, NY, USA, 1977. [Google Scholar]
- Blandamer, M.J.; Scott, J.M.; Robertson, R.E. Solvolysis revisited. Prog. Phys. Org. Chem. 1985, 149–196. [Google Scholar]
- ASTM G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- Davis, B., Jr. Wheel test method used for evaluation of film persistent inhibitors for oilfield applications. Mater. Perform. 1982, 21, 45–47. [Google Scholar]
- ASTM G5-94; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004.
- Inghram, M.G. Modern mass spectroscopy. In Advances in Electronics and Electron Physics; Elsevier: Amsterdam, The Netherlands, 1948; Volume 1, pp. 219–268. [Google Scholar]
- Fereshteh, Z.; Salavati-Niasari, M.; Saberyan, K.; Hosseinpour-Mashkani, S.M.; Tavakoli, F. Synthesis of nickel oxide nanoparticles from thermal decomposition of a new precursor. J. Clust. Sci. 2012, 23, 577–583. [Google Scholar] [CrossRef]
- Ge, S.; Wong, K.W.; Tam, S.K.; Mak, C.H.; Ng, K.M. Facile synthesis of wo 3x nanorods with controlled dimensions and tunable near-infrared absorption. J. Nanoparticle Res. 2018, 20, 288. [Google Scholar] [CrossRef]
- Shukla, N.; Liu, C.; Jones, P.M.; Weller, D. Ftir study of surfactant bonding to fept nanoparticles. J. Magn. Magn. Mater. 2003, 266, 178–184. [Google Scholar] [CrossRef]
- Lan, F.; Bai, J.; Wang, H. The preparation of oleylamine modified micro-size sphere silver particles and its application in crystalline silicon solar cells. RSC Adv. 2018, 8, 16866–16872. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G. Sustainable chemistry metrics. ChemSusChem Chem. Sustain. Energy Mater. 2009, 2, 905–919. [Google Scholar] [CrossRef]
- Azghandi, M.V.; Davoodi, A.; Farzi, G.A.; Kosari, A. Water-base acrylic terpolymer as a corrosion inhibitor for sae1018 in simulated sour petroleum solution in stagnant and hydrodynamic conditions. Corros. Sci. 2012, 64, 44–54. [Google Scholar] [CrossRef]
- Rivera-Grau, L.; Casales, M.; Regla, I.; Ortega-Toledo, D.; Gutierrez, J.A.; Gonzalez-Rodriguez, J.; Martinez-Gomez, L. H2s corrosion inhibition of carbon steel by a coconut-modified imidazoline. Int. J. Electrochem. Sci. 2012, 7, 12391–12403. [Google Scholar] [CrossRef]
- Ayagou, M.D.D.; Tran, T.T.M.; Tribollet, B.; Kittel, J.; Sutter, E.; Ferrando, N.; Mendibide, C.; Duret-Thual, C. Electrochemical impedance spectroscopy of iron corrosion in H2S solutions. Electrochim. Acta 2018, 282, 775–783. [Google Scholar] [CrossRef]
- Sosa, E.; Cabrera-Sierra, R.; Garcıa, I.; Oropeza, M.; González, I. The role of different surface damages in corrosion process in alkaline sour media. Corros. Sci. 2002, 44, 1515–1528. [Google Scholar] [CrossRef]
- Galicia, P.; González, I. Modification of 1018 carbon steel corrosion process in alkaline sour medium with a formulation of chemical corrosion inhibitors. Electrochim. Acta 2005, 50, 4451–4460. [Google Scholar] [CrossRef]
- Noor, E.A. Evaluation of inhibitive action of some quaternary n-heterocyclic compounds on the corrosion of al–cu alloy in hydrochloric acid. Mater. Chem. Phys. 2009, 114, 533–541. [Google Scholar] [CrossRef]
- Wen, X.; Bai, P.; Luo, B.; Zheng, S.; Chen, C. Review of recent progress in the study of corrosion products of steels in a hydrogen sulphide environment. Corros. Sci. 2018, 139, 124–140. [Google Scholar] [CrossRef]



| Inhibitor Concentration | Corrosion Potential (Ecorr vs. Ag/AgCl) | Corrosion Current (Icorr) | Corrosion Rate | Efficiency η |
|---|---|---|---|---|
| ppm | V | µA/cm2 | mm/year | % |
| 0 | −1.059 | 352.0 | 4.08 | 0 |
| 5 | −1.028 | 341.8 | 3.96 | 2.94 |
| 10 | −0.816 | 43.8 | 0.51 | 87.50 |
| 25 | −0.800 | 39.8 | 0.46 | 88.72 |
| 100 | −0.452 | 0.04 | 4.64 × 10−4 | 99.98 |
| Sample | Rs | Rlayer | Rct | Efficiency/η |
|---|---|---|---|---|
| Units | Ω | Ω | Ω | % |
| Blank | 7.6 | --- | 31.2 | 0 |
| 5 ppm | 7.2 | 0.98 | 39.8 | 25.26 |
| 10 ppm | 7.8 | 1.95 | 118.4 | 73.86 |
| 25 ppm | 6.7 | 3.67 | 253.3 | 87.85 |
| 100 ppm | 7.8 | 14.6 | 724.0 | 95.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Malpica, J.; Carrera-Gutiérrez, K.; Chinchillas-Chinchillas, M.; Herrera Zaldivar, M.; Martinez-Garcia, A.; Orozco-Carmona, V.M. Synthesis and Electrochemical Properties of Oleylamine as a Sour Saline Corrosion Inhibitor Under Laminar Flow at 40 °C. Materials 2024, 17, 5284. https://doi.org/10.3390/ma17215284
Alvarez-Malpica J, Carrera-Gutiérrez K, Chinchillas-Chinchillas M, Herrera Zaldivar M, Martinez-Garcia A, Orozco-Carmona VM. Synthesis and Electrochemical Properties of Oleylamine as a Sour Saline Corrosion Inhibitor Under Laminar Flow at 40 °C. Materials. 2024; 17(21):5284. https://doi.org/10.3390/ma17215284
Chicago/Turabian StyleAlvarez-Malpica, Jorge, Karime Carrera-Gutiérrez, Manuel Chinchillas-Chinchillas, Manuel Herrera Zaldivar, Alfredo Martinez-Garcia, and Victor M. Orozco-Carmona. 2024. "Synthesis and Electrochemical Properties of Oleylamine as a Sour Saline Corrosion Inhibitor Under Laminar Flow at 40 °C" Materials 17, no. 21: 5284. https://doi.org/10.3390/ma17215284
APA StyleAlvarez-Malpica, J., Carrera-Gutiérrez, K., Chinchillas-Chinchillas, M., Herrera Zaldivar, M., Martinez-Garcia, A., & Orozco-Carmona, V. M. (2024). Synthesis and Electrochemical Properties of Oleylamine as a Sour Saline Corrosion Inhibitor Under Laminar Flow at 40 °C. Materials, 17(21), 5284. https://doi.org/10.3390/ma17215284









