Advanced Biomaterials for Lacrimal Tissue Engineering: A Review
Abstract
1. Introduction
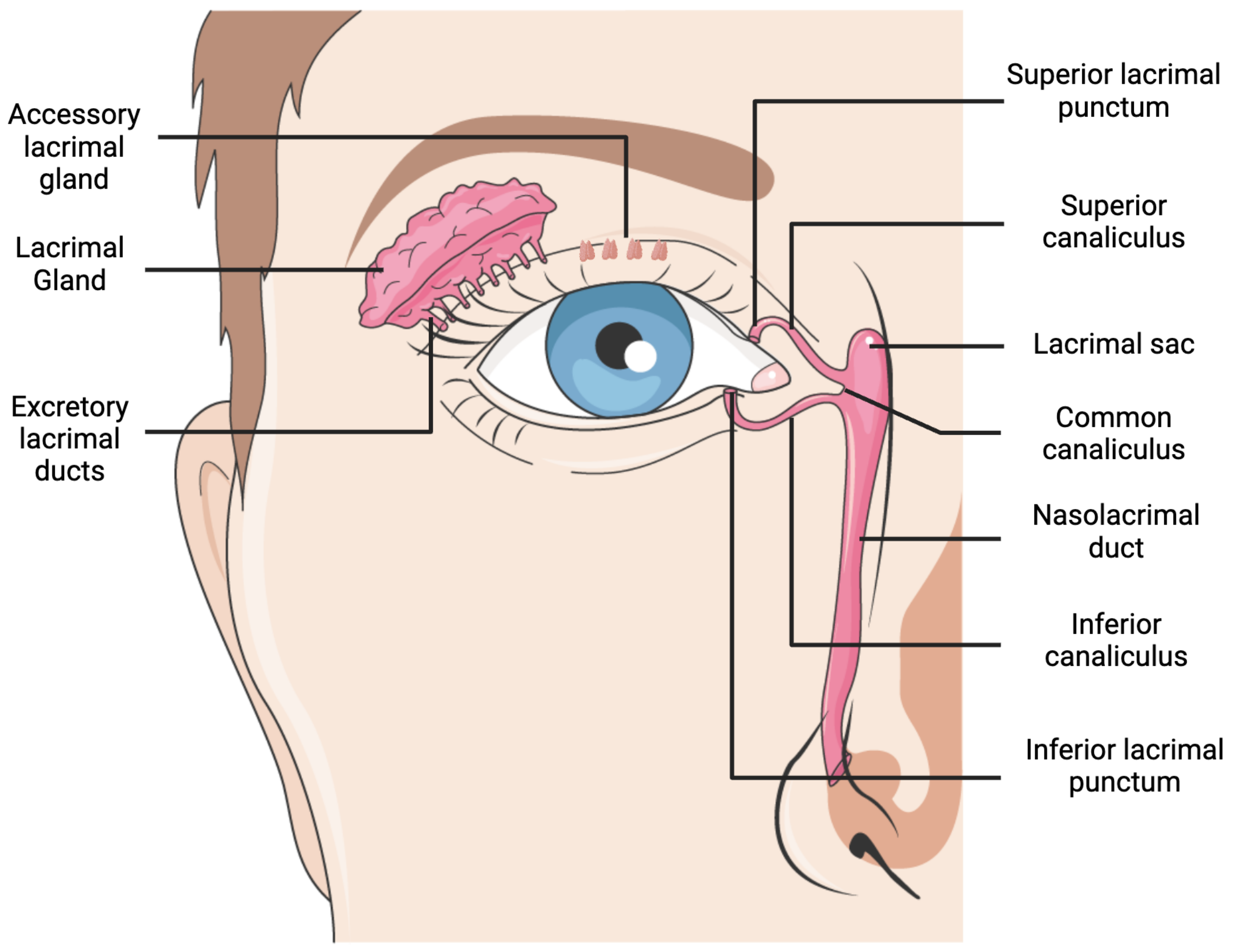
2. Lacrimal Gland Anatomy and Disorders
3. Biomaterials for Lacrimal Tissue Engineering
3.1. Natural Biomaterials
| Biomaterial | Derivation | Features | Disadvantages | References |
|---|---|---|---|---|
| Matrigel | ECM proteins of EHS mouse sarcoma | Supports cell proliferation, acinar differentiation, mimics natural basement membrane, and promotes gland-like structure formation | Variability between batches and animal-derived, hence limiting clinical applications; decreased expression of proteins after some time, hence limiting long-term use; indeterminate degradation rate; and may be immunogenic | [28,29,31,34,35,36,37] |
| Decellularized Lacrimal Gland Hydrogel | Porcine decellularized lacrimal gland, most commonly | Maintains the native biochemical composition of the lacrimal gland ECM | Rapid degradation limits long-term use; requires genipin crosslinking for enhanced mechanical stability; limited availability; and incomplete decellularization can lead to immune response | [37,38,39,40] |
| Chitosan | Polysaccharide from chitin | Promotes branching morphogenesis; interacts with endogenous growth factors; biocompatible; nontoxic; and biodegradable | Limited mechanical strength | [43] |
| Silk Fibroin Hydrogel | Silk fibroin from silk-producing arthropods | Customizable mechanical properties; photo-crosslinkable for controlled solidification in situ; and excellent biocompatibility | Long-term in vivo outcomes unknown and complex preparation process | [44] |
| Human Amniotic Membrane | Innermost layer of placenta (amnion) | Supports cell viability and differentiation and reduces inflammation and fibrosis | Lacks the ability to support vascularization and only grows on surfaces, hence not suitable for true 3D scaffolds | [51,52,53] |
| Collagen | Multiple, typically derived from animal sources | Promotes 3D structure formation and facilitates branching morphogenesis | Rapid degradation | [55,56,57,58] |
| Fibrin | Fibrinogen, usually from human plasma | Promotes cell adhesion, migration, vascularization, and nerve formation; supports epithelial tissue organization | Low mechanical strength and fast degradation rates, hindering long-term tissue support | [60] |
3.2. Synthetic Biomaterials
| Biomaterial | Features | Disadvantages | References |
|---|---|---|---|
| Polyethersulfone (PES) | Excellent mechanical stability; semipermeable and supports nutrient transfer while blocking immunoglobulins; and promotes acinar cell attachment | Hydrophobic surface; lower acinar cell proliferation compared to other endothelial cells; limited immunogenicity testing; and lack of vivo studies | [62] |
| Polyester | Supports cell polarity, tight junctions, protein secretion, and acinar cell proliferation | Lack of in vivo studies to assess the long-term biocompatibility and stability | [66] |
| Poly-I-lactic acid (PLLA) | Biodegradable; good mechanical properties; and supports acinar morphology, secretory granules, and junctional complexes | Very slow degradation, acidic byproducts may affect lacrimal gland cells; and a hydrophobic nature | [67,68] |
| Poly-D,L-lactide-co-glycolide (PLGA) | Biodegradable scaffold with good biocompatibility | Degrades into acidic byproducts and some loss in the mechanical integrity over time, with lower acinar secretory functions compared to PLLA | [67,68] |
| Silicon | Biocompatible and remains stable over an extended period of time | Non-biodegradable and requires further testing for long-term stability and potential immune responses in vivo | [68] |
4. Cell Sources and Growth Factors
5. Advances in Tissue-Engineering Techniques
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, H.; McCann, P.; Lien, T.; Xiao, M.; Abraham, A.G.; Gregory, D.G.; Hauswirth, S.G.; Qureshi, R.; Liu, S.-H.; Saldanha, I.J.; et al. Prevalence of Dry Eye and Meibomian Gland Dysfunction in Central and South America: A Systematic Review and Meta-Analysis. BMC Ophthalmol. 2024, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Messmer, E.M. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch. Ärztebl. Int. 2015, 112, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Shen Lee, B.; Kabat, A.G.; Bacharach, J.; Karpecki, P.; Luchs, J. Managing Dry Eye Disease and Facilitating Realistic Patient Expectations: A Review and Appraisal of Current Therapies. Clin. Ophthalmol. Auckl. NZ 2020, 14, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Saldanha, I.J.; Abraham, A.G.; Rittiphairoj, T.; Hauswirth, S.; Gregory, D.; Ifantides, C.; Li, T. Topical Corticosteroids for Dry Eye. Cochrane Database Syst. Rev. 2022, 2022, CD015070. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Machiele, R.; Lopez, M.J.; Czyz, C.N. Anatomy, Head and Neck: Eye Lacrimal Gland. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chang, A.Y.; Purt, B. Biochemistry, Tear Film. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Dartt, D.A. Neural Regulation of Lacrimal Gland Secretory Processes: Relevance in Dry Eye Diseases. Prog. Retin. Eye Res. 2009, 28, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Wei, S.; Cao, Q.; Guo, C.; Wu, X.; Di, G. Corneal Epithelium–Derived Netrin-1 Alleviates Dry Eye Disease via Regulating Dendritic Cell Activation. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease: An Immune-Mediated Ocular Surface Disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Papas, E.B. The Global Prevalence of Dry Eye Disease: A Bayesian View. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. Optom. 2021, 41, 1254–1266. [Google Scholar] [CrossRef]
- Conrady, C.D.; Joos, Z.P.; Patel, B.C.K. Review: The Lacrimal Gland and Its Role in Dry Eye. J. Ophthalmol. 2016, 2016, 7542929. [Google Scholar] [CrossRef] [PubMed]
- Carsons, S.E.; Patel, B.C. Sjogren Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ebright, B.; Yu, Z.; Dave, P.; Dikeman, D.; Hamm-Alvarez, S.; de Paiva, C.S.; Louie, S. Effects of Age on Lacrimal Gland Bioactive Lipids. Ocul. Surf. 2024, 33, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous Serum Eye Drops in Dry Eye Disease: Preferred Practice Pattern Guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Qi, Q.; Ma, X. Effect of Moisture Chamber Spectacles on Tear Functions in Dry Eye Disease. Optom. Vis. Sci. 2016, 93, 158. [Google Scholar] [CrossRef]
- Baxter, S.A.; Laibson, P.R. Punctal Plugs in the Management of Dry Eyes. Ocul. Surf. 2004, 2, 255–265. [Google Scholar] [CrossRef]
- Schrader, S.; Kremling, C.; Klinger, M.; Laqua, H.; Geerling, G. Cultivation of Lacrimal Gland Acinar Cells in a Microgravity Environment. Br. J. Ophthalmol. 2009, 93, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, P.; Hetz, S.; Dieckow, J.; Schicht, M.; Richter, A.; Kruse, C.; Schroeder, I.S.; Jung, M.; Paulsen, F.P. Isolation and Investigation of Presumptive Murine Lacrimal Gland Stem Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4350–4363. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; He, H.; Botsford, B.; Yiu, S. Lacrimal Gland Repair after Short-Term Obstruction of Excretory Duct in Rabbits. Sci. Rep. 2017, 7, 8290. [Google Scholar] [CrossRef]
- Tiwari, S.; Nair, R.M.; Vamadevan, P.; Ali, M.J.; Naik, M.N.; Honavar, S.G.; Vemuganti, G.K. Establishing and Characterizing Lacrispheres from Human Lacrimal Gland for Potential Clinical Application. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 717–727. [Google Scholar] [CrossRef]
- Henker, R.; Scholz, M.; Gaffling, S.; Asano, N.; Hampel, U.; Garreis, F.; Hornegger, J.; Paulsen, F. Morphological Features of the Porcine Lacrimal Gland and Its Compatibility for Human Lacrimal Gland Xenografting. PLoS ONE 2013, 8, e74046. [Google Scholar] [CrossRef]
- Schechter, J.E.; Warren, D.W.; Mircheff, A.K. A Lacrimal Gland Is a Lacrimal Gland, but Rodent’s and Rabbit’s Are Not Human. Ocul. Surf. 2010, 8, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Passaniti, A.; Kleinman, H.K.; Martin, G.R. Matrigel: History/Background, Uses, and Future Applications. J. Cell Commun. Signal. 2022, 16, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of Multiple Active Growth Factors in Basement Membrane Matrigel Suggests Caution in Interpretation of Cellular Activity Related to Extracellular Matrix Components. Exp. Cell Res. 1992, 202, 1–8. [Google Scholar] [CrossRef]
- Arnaoutova, I.; George, J.; Kleinman, H.K.; Benton, G. Basement Membrane Matrix (BME) Has Multiple Uses with Stem Cells. Stem Cell Rev. Rep. 2012, 8, 163–169. [Google Scholar] [CrossRef]
- Schechter, J.; Stevenson, D.; Chang, D.; Chang, N.; Pidgeon, M.; Nakamura, T.; Okamoto, C.T.; Trousdale, M.D.; Mircheff, A.K. Growth of Purified Lacrimal Acinar Cells in Matrigel Raft Cultures. Exp. Eye Res. 2002, 74, 349–360. [Google Scholar] [CrossRef]
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The Use of Mixed Collagen-Matrigel Matrices of Increasing Complexity Recapitulates the Biphasic Role of Cell Adhesion in Cancer Cell Migration: ECM Sensing, Remodeling and Forces at the Leading Edge of Cancer Invasion. PLoS ONE 2020, 15, e0220019. [Google Scholar] [CrossRef]
- Stanton, A.E.; Tong, X.; Yang, F. Extracellular Matrix Type Modulates Mechanotransduction of Stem Cells. Acta Biomater. 2019, 96, 310. [Google Scholar] [CrossRef]
- Tiwari, S.; Ali, M.J.; Balla, M.M.S.; Naik, M.N.; Honavar, S.G.; Reddy, V.A.P.; Vemuganti, G.K. Establishing Human Lacrimal Gland Cultures with Secretory Function. PLoS ONE 2012, 7, e29458. [Google Scholar] [CrossRef]
- Yoshino, K.; Tseng, S.C.; Pflugfelder, S.C. Substrate Modulation of Morphology, Growth, and Tear Protein Production by Cultured Human Lacrimal Gland Epithelial Cells. Exp. Cell Res. 1995, 220, 138–151. [Google Scholar] [CrossRef]
- Yoshino, K. Establishment of a Human Lacrimal Gland Epithelial Culture System with in Vivo Mimicry and Its Substrate Modulation. Cornea 2000, 19, S26. [Google Scholar] [CrossRef]
- Asal, M.; Koçak, G.; Sarı, V.; Reçber, T.; Nemutlu, E.; Utine, C.A.; Güven, S. Development of Lacrimal Gland Organoids from iPSC Derived Multizonal Ocular Cells. Front. Cell Dev. Biol. 2023, 10, 1058846. [Google Scholar] [CrossRef]
- Gleixner, S.; Zahn, I.; Dietrich, J.; Singh, S.; Drobny, A.; Schneider, Y.; Schwendner, R.; Socher, E.; Blavet, N.; Bräuer, L.; et al. A New Immortalized Human Lacrimal Gland Cell Line. Cells 2024, 13, 622. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Xu, L.; Wang, G.; Shi, R.; Wang, K.; Wang, S.; Li, C. Distinctive Small Molecules Blend: Promotes Lacrimal Gland Epithelial Cell Proliferation in Vitro and Accelerates Lacrimal Gland Injury Repair in Vivo. Ocul. Surf. 2024, 34, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Zakour, K.E.W.-B.; Kaya, S.; Matros, J.C.; Hacker, M.C.; Cheikh-Rouhou, A.; Spaniol, K.; Geerling, G.; Witt, J. Enhancement of Lacrimal Gland Cell Function by Decellularized Lacrimal Gland Derived Hydrogel. Biofabrication 2024, 16, 025008. [Google Scholar] [CrossRef]
- Lin, H.; Sun, G.; He, H.; Botsford, B.; Li, M.; Elisseeff, J.H.; Yiu, S.C. Three-Dimensional Culture of Functional Adult Rabbit Lacrimal Gland Epithelial Cells on Decellularized Scaffold. Tissue Eng. Part A 2016, 22, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Massie, I.; Spaniol, K.; Barbian, A.; Poschmann, G.; Stühler, K.; Geerling, G.; Metzger, M.; Mertsch, S.; Schrader, S. Evaluation of Decellularized Porcine Jejunum as a Matrix for Lacrimal Gland Reconstruction In Vitro for Treatment of Dry Eye Syndrome. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5564–5574. [Google Scholar] [CrossRef] [PubMed]
- Spaniol, K.; Kunze, A.; Metzger, M.; Geerling, G.; Schrader, S. Decellularization of Porcine Lacrimal Gland Tissue for Development of a Lacrimal Gland Scaffold. Investig. Ophthalmol. Vis. Sci. 2013, 54, 914. [Google Scholar]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and Its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Yang, T.-L. Regulating Temporospatial Dynamics of Morphogen for Structure Formation of the Lacrimal Gland by Chitosan Biomaterials. Biomaterials 2017, 113, 42–55. [Google Scholar] [CrossRef]
- Dai, M.; Xu, K.; Xiao, D.; Zheng, Y.; Zheng, Q.; Shen, J.; Qian, Y.; Chen, W. In Situ Forming Hydrogel as a Tracer and Degradable Lacrimal Plug for Dry Eye Treatment. Adv. Healthc. Mater. 2022, 11, e2200678. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, Y.; Xu, J.; Song, G.; Ding, M.; Zhao, H.; Wang, J. An RGD-Containing Peptide Derived from Wild Silkworm Silk Fibroin Promotes Cell Adhesion and Spreading. Polymers 2018, 10, 1193. [Google Scholar] [CrossRef]
- Mu, X.; Sahoo, J.K.; Cebe, P.; Kaplan, D.L. Photo-Crosslinked Silk Fibroin for 3D Printing. Polymers 2020, 12, 2936. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, J.H.; Nollert, M.U. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Barski, D.; Gerullis, H.; Ecke, T.; Varga, G.; Boros, M.; Pintelon, I.; Timmermans, J.-P.; Otto, T. Human Amniotic Membrane Dressing for the Treatment of an Infected Wound Due to an Entero-Cutaneous Fistula: Case Report. Int. J. Surg. Case Rep. 2018, 51, 11–13. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Schrader, S.; Wedel, T.; Kremling, C.; Laqua, H.; Geerling, G. Amniotic Membrane as a Carrier for Lacrimal Gland Acinar Cells. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1699–1704. [Google Scholar] [CrossRef]
- Singh, V.K.; Sharma, P.; Vaksh, U.K.S.; Chandra, R. Current Approaches for the Regeneration and Reconstruction of Ocular Surface in Dry Eye. Front. Med. 2022, 9, 885780. [Google Scholar] [CrossRef]
- Ogawa, Y.; He, H.; Mukai, S.; Imada, T.; Nakamura, S.; Su, C.-W.; Mahabole, M.; Tseng, S.C.G.; Tsubota, K. Heavy Chain-Hyaluronan/Pentraxin 3 from Amniotic Membrane Suppresses Inflammation and Scarring in Murine Lacrimal Gland and Conjunctiva of Chronic Graft-versus-Host Disease. Sci. Rep. 2017, 7, 42195. [Google Scholar] [CrossRef]
- Chen, L.; Gong, B.; Wu, Z.; Jetton, J.; Chen, R.; Qu, C. A New Method Using Xenogeneicacellular Dermal Matrix in the Reconstruction of Lacrimal Drainage. Br. J. Ophthalmol. 2014, 98, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Ogawa, M.; Oshima, M.; Sekine, Y.; Ishida, K.; Yamashita, K.; Ikeda, K.; Shimmura, S.; Kawakita, T.; Tsubota, K.; et al. Functional Lacrimal Gland Regeneration by Transplantation of a Bioengineered Organ Germ. Nat. Commun. 2013, 4, 2497. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Endo, K.; Nakata, K.; Shirasawa, E.; Okahara, A.; Danjo, Y.; Kiritoshi, A.; Tano, Y. Cultured Rabbit Lacrimal Epithelial Cells Form Branching Processes in a Collagen Matrix. In Vitro Cell. Dev. Biol. Anim. 1996, 32, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Rusch, R.M.; Ogawa, Y.; Sato, S.; Morikawa, S.; Inagaki, E.; Shimizu, E.; Tsubota, K.; Shimmura, S. MSCs Become Collagen-Type I Producing Cells with Different Phenotype in Allogeneic and Syngeneic Bone Marrow Transplantation. Int. J. Mol. Sci. 2021, 22, 4895. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, C.E.; Bang, S.Y.; Yang, J. Anti-Inflammatory Effects of Collagen Type II 1α Based Novel Peptide in a Dry Eye Mouse Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 409. [Google Scholar]
- Nam, K.; dos Santos, H.T.; Maslow, F.; Small, T.; Samuel, R.Z.; Lei, P.; Andreadis, S.T.; Baker, O.J. Fibrin Hydrogels Fortified with FGF-7/10 and Laminin-1 Peptides Promote Regeneration of Irradiated Salivary Glands. Acta Biomater. 2023, 172, 147–158. [Google Scholar] [CrossRef]
- Li, S.; Dan, X.; Chen, H.; Li, T.; Liu, B.; Ju, Y.; Li, Y.; Lei, L.; Fan, X. Developing Fibrin-Based Biomaterials/Scaffolds in Tissue Engineering. Bioact. Mater. 2024, 40, 597–623. [Google Scholar] [CrossRef]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-Mediated Cellular Traction Directs Stem Cell Fate in Covalently Crosslinked Three-Dimensional Hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef]
- Long, L.; Liu, Z.; Wang, T.; Deng, X.; Yang, K.; Li, L.; Zhao, C. Polyethersulfone Dead-End Tube as a Scaffold for Artificial Lacrimal Glands in Vitro. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 409–416. [Google Scholar] [CrossRef]
- Wang See, C.; Kim, T.; Zhu, D. Hernia Mesh and Hernia Repair: A Review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Râpă, M.; Frąckowiak, S. Special Features of Polyester-Based Materials for Medical Applications. Polymers 2022, 14, 951. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA Nanospheres Optimized by Design of Experiments for Ocular Administration of Dexibuprofen-in Vitro, Ex Vivo and in Vivo Characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef]
- Selvam, S.; Thomas, P.B.; Gukasyan, H.J.; Yu, A.S.; Stevenson, D.; Trousdale, M.D.; Mircheff, A.K.; Schechter, J.E.; Smith, R.E.; Yiu, S.C. Transepithelial Bioelectrical Properties of Rabbit Acinar Cell Monolayers on Polyester Membrane Scaffolds. Am. J. Physiol. Cell Physiol. 2007, 293, C1412–C1419. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.; Liu, L.; Kasper, K.; Geerling, G. Generation of Two-and Three-Dimensional Lacrimal Gland Constructs. Dev. Ophthalmol. 2010, 45, 49–56. [Google Scholar] [CrossRef]
- Selvam, S.; Thomas, P.B.; Trousdale, M.D.; Stevenson, D.; Schechter, J.E.; Mircheff, A.K.; Jacob, J.T.; Smith, R.E.; Yiu, S.C. Tissue-Engineered Tear Secretory System: Functional Lacrimal Gland Acinar Cells Cultured on Matrix Protein-Coated Substrata. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80B, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Gromova, A.; Voronov, D.A.; Yoshida, M.; Thotakura, S.; Meech, R.; Dartt, D.A.; Makarenkova, H.P. Lacrimal Gland Repair Using Progenitor Cells. Stem Cells Transl. Med. 2017, 6, 88–98. [Google Scholar] [CrossRef]
- Delcroix, V.; Mauduit, O.; Lee, H.S.; Ivanova, A.; Umazume, T.; Knox, S.M.; de Paiva, C.S.; Dartt, D.A.; Makarenkova, H.P. The First Transcriptomic Atlas of the Adult Lacrimal Gland Reveals Epithelial Complexity and Identifies Novel Progenitor Cells in Mice. Cells 2023, 12, 1435. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Dartt, D.A. Myoepithelial Cells: Their Origin and Function in Lacrimal Gland Morphogenesis, Homeostasis, and Repair. Curr. Mol. Biol. Rep. 2015, 1, 115–123. [Google Scholar] [CrossRef]
- Dc, D.; Wc, S.; Sz, L. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Jaffet, J.; Mohanty, A.; Veernala, I.; Singh, S.; Ali, M.J.; Basu, S.; Vemuganti, G.K.; Singh, V. Human Lacrimal Gland Derived Mesenchymal Stem Cells—Isolation, Propagation, and Characterization. Investig. Ophthalmol. Vis. Sci. 2023, 64, 12. [Google Scholar] [CrossRef] [PubMed]
- Veernala, I.; Jaffet, J.; Fried, J.; Mertsch, S.; Schrader, S.; Basu, S.; Vemuganti, G.K.; Singh, V. Lacrimal Gland Regeneration: The Unmet Challenges and Promise for Dry Eye Therapy. Ocul. Surf. 2022, 25, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Wang, Y.-X.; Hua, H. Characteristics of Labial Gland Mesenchymal Stem Cells of Healthy Individuals and Patients with Sjögren’s Syndrome: A Preliminary Study. Stem Cells Dev. 2017, 26, 1171–1185. [Google Scholar] [CrossRef]
- Hayashi, R.; Okubo, T.; Kudo, Y.; Ishikawa, Y.; Imaizumi, T.; Suzuki, K.; Shibata, S.; Katayama, T.; Park, S.-J.; Young, R.D.; et al. Generation of 3D Lacrimal Gland Organoids from Human Pluripotent Stem Cells. Nature 2022, 605, 126–131. [Google Scholar] [CrossRef]
- Lin, H.; Liu, Y.; Yiu, S. Three Dimensional Culture of Potential Epithelial Progenitor Cells in Human Lacrimal Gland. Transl. Vis. Sci. Technol. 2019, 8, 32. [Google Scholar] [CrossRef]
- Basova, L.V.; Tang, X.; Umazume, T.; Gromova, A.; Zyrianova, T.; Shmushkovich, T.; Wolfson, A.; Hawley, D.; Zoukhri, D.; Shestopalov, V.I.; et al. Manipulation of Panx1 Activity Increases the Engraftment of Transplanted Lacrimal Gland Epithelial Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5654–5665. [Google Scholar] [CrossRef]
- Voronov, D.; Gromova, A.; Liu, D.; Zoukhri, D.; Medvinsky, A.; Meech, R.; Makarenkova, H.P. Transcription Factors Runx1 to 3 Are Expressed in the Lacrimal Gland Epithelium and Are Involved in Regulation of Gland Morphogenesis and Regeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3115–3125. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finburgh, E.N.; Mauduit, O.; Noguchi, T.; Bu, J.J.; Abbas, A.A.; Hakim, D.F.; Bellusci, S.; Meech, R.; Makarenkova, H.P.; Afshari, N.A. Role of FGF10/FGFR2b Signaling in Homeostasis and Regeneration of Adult Lacrimal Gland and Corneal Epithelium Proliferation. Investig. Ophthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef]
- Ueda, Y.; Karasawa, Y.; Satoh, Y.; Nishikawa, S.; Imaki, J.; Ito, M. Purification and Characterization of Mouse Lacrimal Gland Epithelial Cells and Reconstruction of an Acinarlike Structure in Three-Dimensional Culture. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1978–1987. [Google Scholar] [CrossRef]
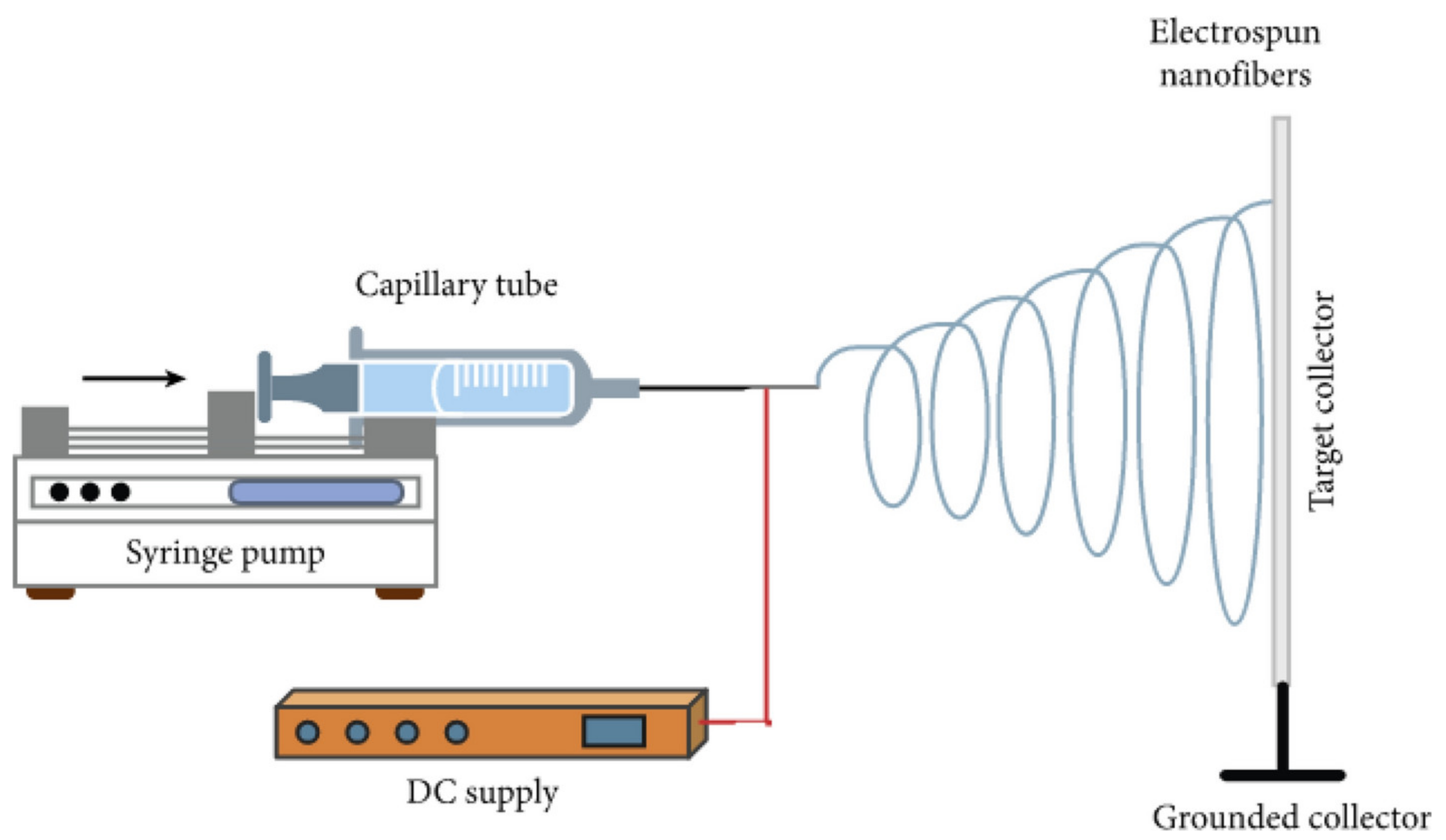
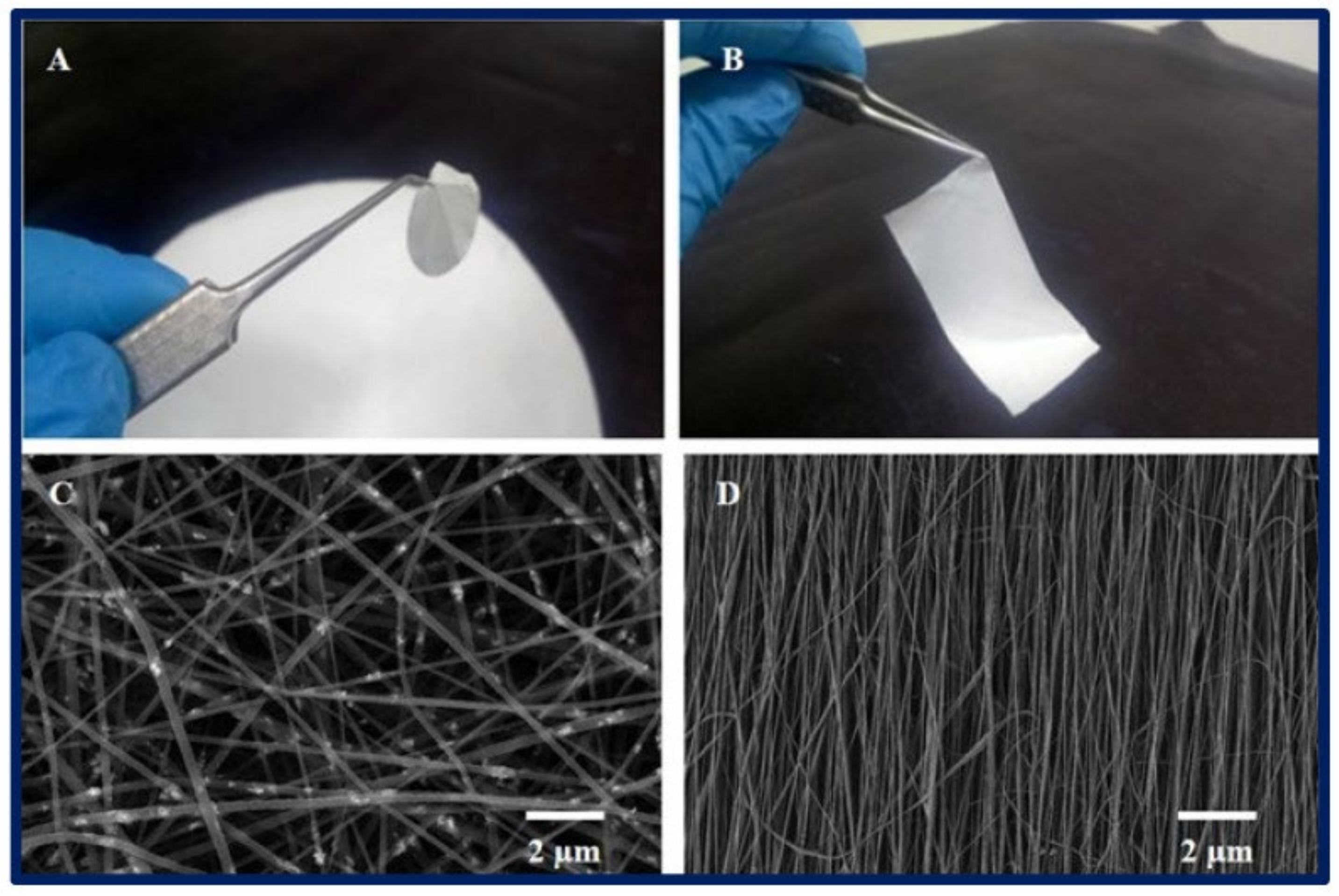
- Maurmann, N.; Sperling, L.-E.; Pranke, P. Electrospun and Electrosprayed Scaffolds for Tissue Engineering. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Chun, H.J., Park, C.H., Kwon, I.K., Khang, G., Eds.; Springer: Singapore, 2018; pp. 79–100. ISBN 9789811309502. [Google Scholar]
- Barud, H.S.; Sousa, F.B.D. Electrospun Materials for Biomedical Applications. Pharmaceutics 2022, 14, 1556. [Google Scholar] [CrossRef]
- Aoki, H.; Miyoshi, H.; Yamagata, Y. Electrospinning of Gelatin Nanofiber Scaffolds with Mild Neutral Cosolvents for Use in Tissue Engineering. Polym. J. 2015, 47, 267–277. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Wang, Y. Recent Progress in Cellulose-Based Electrospun Nanofibers as Multifunctional Materials. Nanoscale Adv. 2021, 3, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-Throughput Production of Silk Fibroin-Based Electrospun Fibers as Biomaterial for Skin Tissue Engineering Applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Doherty, K.G.; Hsuan, J.D.; Cray, S.P.; D’Sa, R.A.; Pineda Molina, C.; Badylak, S.F.; Williams, R.L. Material Characterisation and Stratification of Conjunctival Epithelial Cells on Electrospun Poly(ε-Caprolactone) Fibres Loaded with Decellularised Tissue Matrices. Pharmaceutics 2021, 13, 318. [Google Scholar] [CrossRef]
- Soscia, D.A.; Sequeira, S.J.; Schramm, R.A.; Jayarathanam, K.; Cantara, S.I.; Larsen, M.; Castracane, J. Salivary Gland Cell Differentiation and Organization on Micropatterned PLGA Nanofiber Craters. Biomaterials 2013, 34, 6773–6784. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, Y.; Gong, W.; Zhou, J.; Yu, D.-G.; Xie, Y. Electrospun Chitosan//Ethylcellulose-Vitamin E//Ethylcellulose-Curcumin Tri-Chamber Eccentric Janus Nanofibers for a Joint Antibacterial and Antioxidant Performance. Int. J. Biol. Macromol. 2024, 2024, 135753. [Google Scholar] [CrossRef]
- Dong, R.; Gong, W.; Guo, Q.; Liu, H.; Yu, D.-G. Synergistic Effects of Radical Distributions of Soluble and Insoluble Polymers within Electrospun Nanofibers for an Extending Release of Ferulic Acid. Polymers 2024, 16, 2614. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Socci, M.C.; Rodríguez, G.; Oliva, E.; Fushimi, S.; Takabatake, K.; Nagatsuka, H.; Felice, C.J.; Rodríguez, A.P. Polymeric Materials, Advances and Applications in Tissue Engineering: A Review. Bioengineering 2023, 10, 218. [Google Scholar] [CrossRef]
- Grumm, L.; Wiebe-Ben Zakour, K.E.; Kaya, S.; Groeber-Becker, F.; Geerling, G.; Witt, J. Designing a Hybrid Hydrogel of Lacrimal Gland Extracellular Matrix and Alginate for 3D Bioprinting. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3291. [Google Scholar]
- Rodboon, T.; Yodmuang, S.; Chaisuparat, R.; Ferreira, J.N. Development of High-Throughput Lacrimal Gland Organoid Platforms for Drug Discovery in Dry Eye Disease. SLAS Discov. 2022, 27, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.N.; Bhummaphan, N.; Chaisuparat, R.; Phan, T.V.; Oo, Y.; Jaru-ampornpan, P.; Matangkasombut, O.; Mutirangura, A. Unveiling Senescence-Associated Ocular Pathogenesis via Lacrimal Gland Organoid Magnetic Bioassembly Platform and HMGB1-Box A Gene Therapy. Sci. Rep. 2024, 14, 21784. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Vázquez-Rosado, E.J.; Wu, D.; Viswananthan, V.; Farach, A.; Farach-Carson, M.C.; Harrington, D.A. Microfluidic Coaxial 3D Bioprinting of Cell-Laden Microfibers and Microtubes for Salivary Gland Tissue Engineering. Biomater. Adv. 2023, 154, 213588. [Google Scholar] [CrossRef]
- Wiebe-Ben Zakour, K.E.; Kaya, S.; Grumm, L.; Matros, J.; Hacker, M.C.; Geerling, G.; Witt, J. Modulation of Decellularized Lacrimal Gland Hydrogel Biodegradation by Genipin Crosslinking. Investig. Ophthalmol. Vis. Sci. 2024, 65, 24. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhao, H.; Liu, H.; Zhao, P.; Yu, D.-G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels 2023, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Prasain, N.; Lee, M.R.; Vemula, S.; Meador, J.L.; Yoshimoto, M.; Ferkowicz, M.J.; Fett, A.; Gupta, M.; Rapp, B.M.; Saadatzadeh, M.R.; et al. Differentiation of Human Pluripotent Stem Cells to Cells Similar to Cord-Blood Endothelial Colony–Forming Cells. Nat. Biotechnol. 2014, 32, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Keshavarz, M.; Barhouse, P.; Smith, Q. Strategies for Regenerative Vascular Tissue Engineering. Adv. Biol. 2023, 7, 2200050. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Holtmann, C.; Roth, M.; Filler, T.; Bergmann, A.K.; Hänggi, D.; Muhammad, S.; Borrelli, M.; Geerling, G. Microvascular Anastomosis of the Human Lacrimal Gland: A Concept Study towards Transplantation of the Human Lacrimal Gland. Graefes Arch. Clin. Exp. Ophthalmol. 2023, 261, 1443–1450. [Google Scholar] [CrossRef]
- Boneva, R.; Folks, T. Xenotransplantation and Risks of Zoonotic Infections. Ann. Med. 2004, 36, 504–517. [Google Scholar] [CrossRef]
- Trousdale, M.D.; Zhu, Z.; Stevenson, D.; Schechter, J.E.; Ritter, T.; Mircheff, A.K. Expression of TNF Inhibitor Gene in the Lacrimal Gland Promotes Recovery of Tear Production and Tear Stability and Reduced Immunopathology in Rabbits with Induced Autoimmune Dacryoadenitis. J. Autoimmune Dis. 2005, 2, 6. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Dave, A.; Daigle, P.; Tran, S.D. Advanced Biomaterials for Lacrimal Tissue Engineering: A Review. Materials 2024, 17, 5425. https://doi.org/10.3390/ma17225425
Wu KY, Dave A, Daigle P, Tran SD. Advanced Biomaterials for Lacrimal Tissue Engineering: A Review. Materials. 2024; 17(22):5425. https://doi.org/10.3390/ma17225425
Chicago/Turabian StyleWu, Kevin Y., Archan Dave, Patrick Daigle, and Simon D. Tran. 2024. "Advanced Biomaterials for Lacrimal Tissue Engineering: A Review" Materials 17, no. 22: 5425. https://doi.org/10.3390/ma17225425
APA StyleWu, K. Y., Dave, A., Daigle, P., & Tran, S. D. (2024). Advanced Biomaterials for Lacrimal Tissue Engineering: A Review. Materials, 17(22), 5425. https://doi.org/10.3390/ma17225425







