Dissolution Behavior of M5 Cladding in Hydrofluoric–Nitric Mixed Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dissolution Testing
2.3. Characterization
3. Results
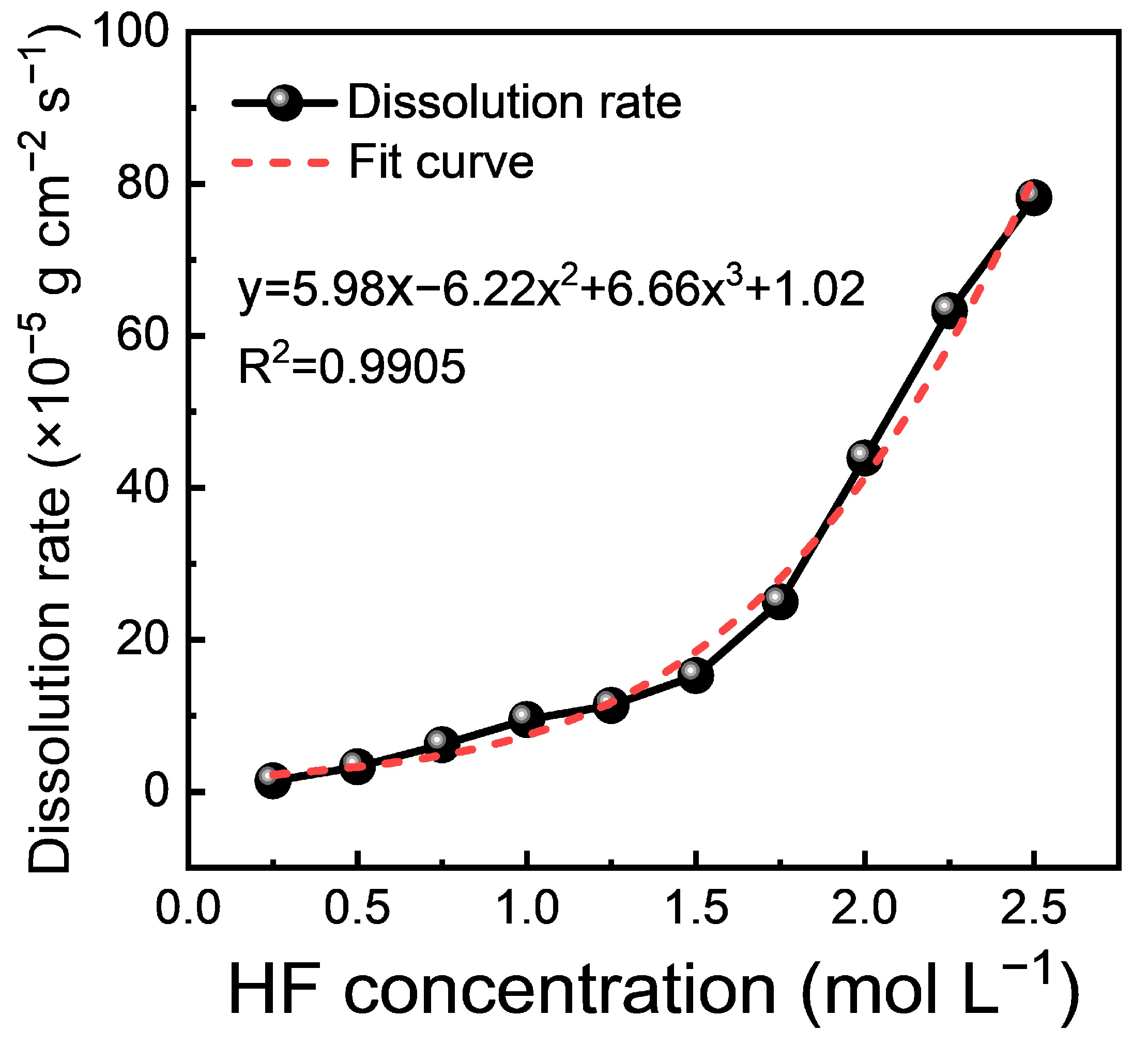
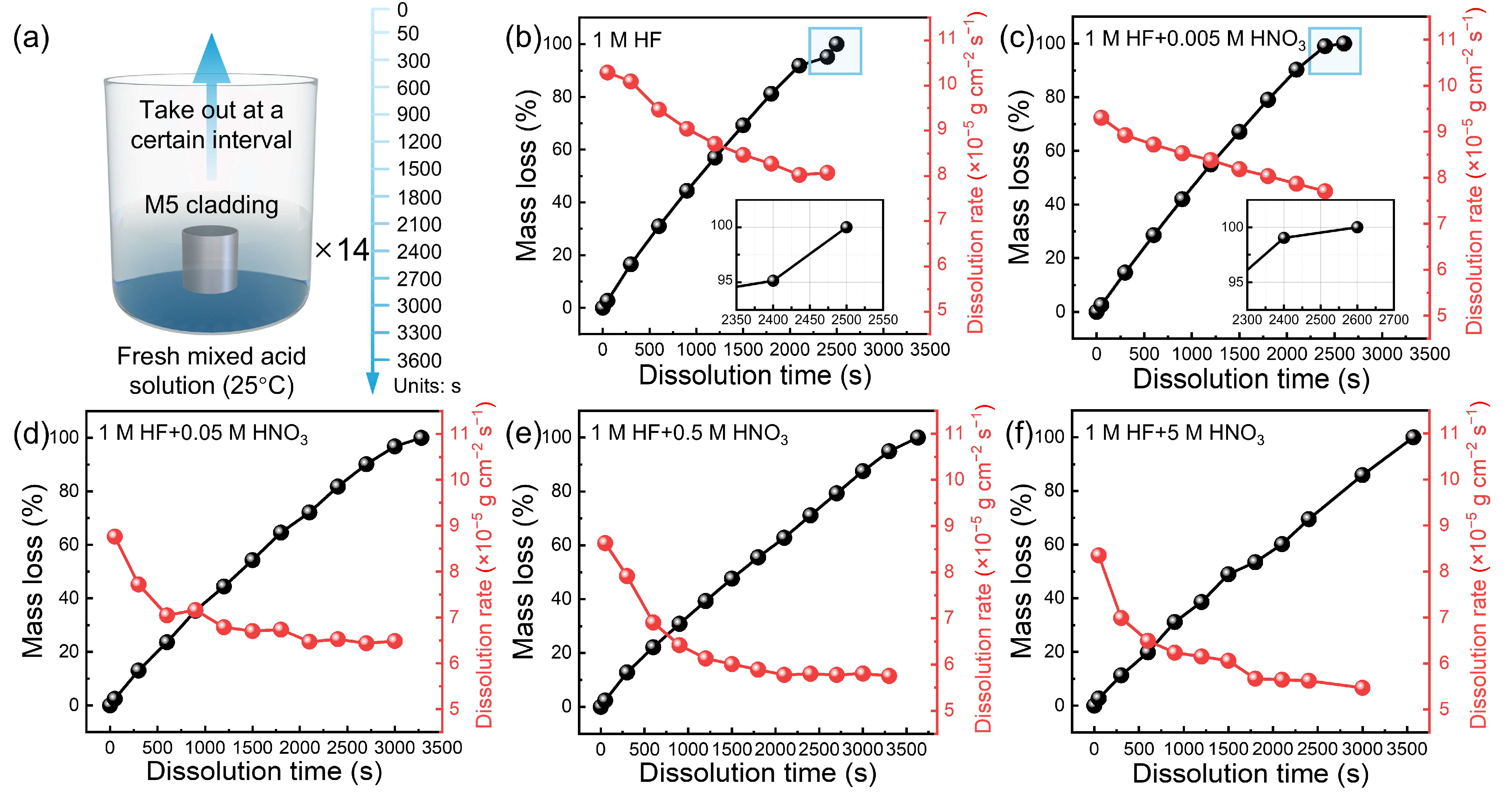
3.1. Dissolution Rate
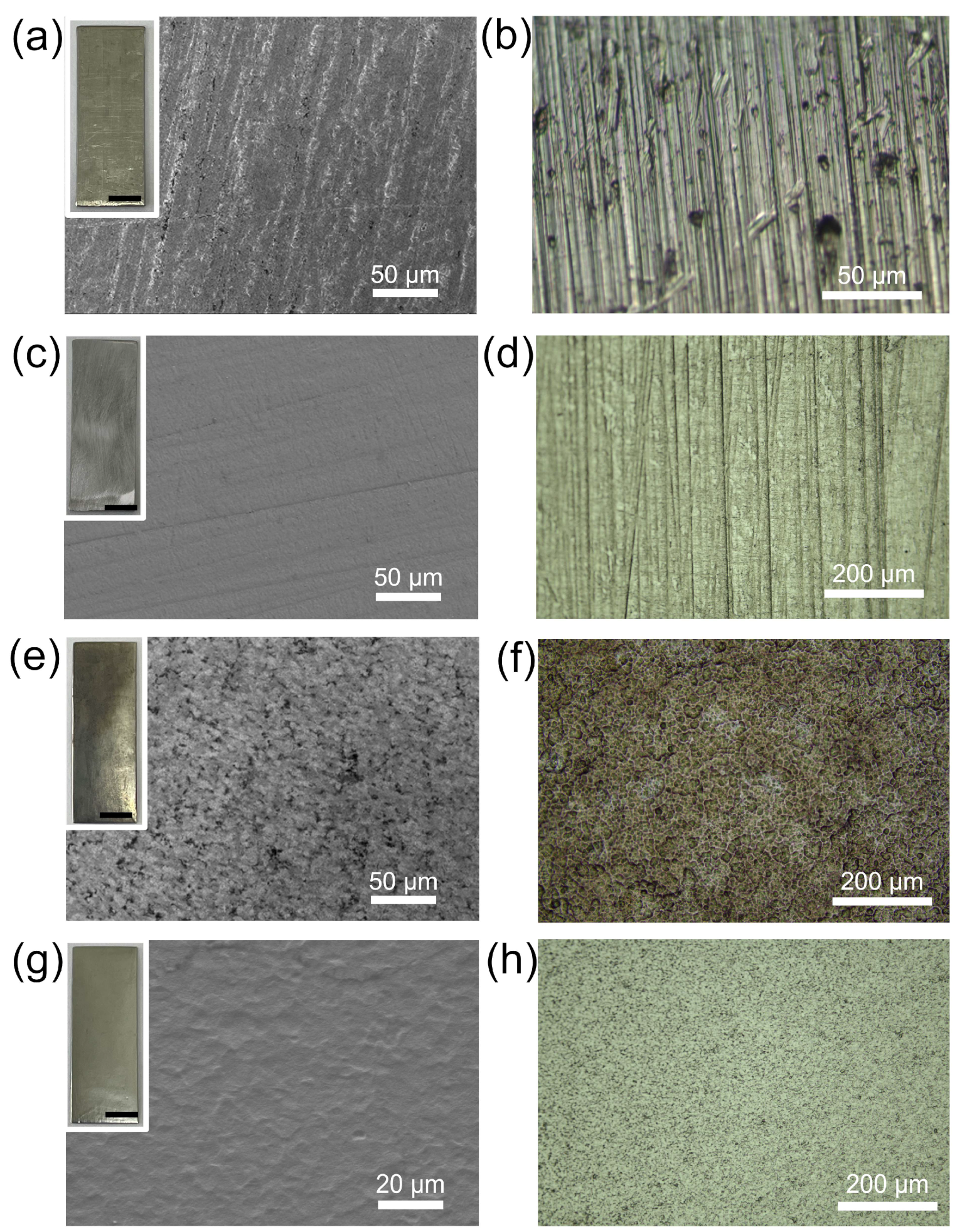
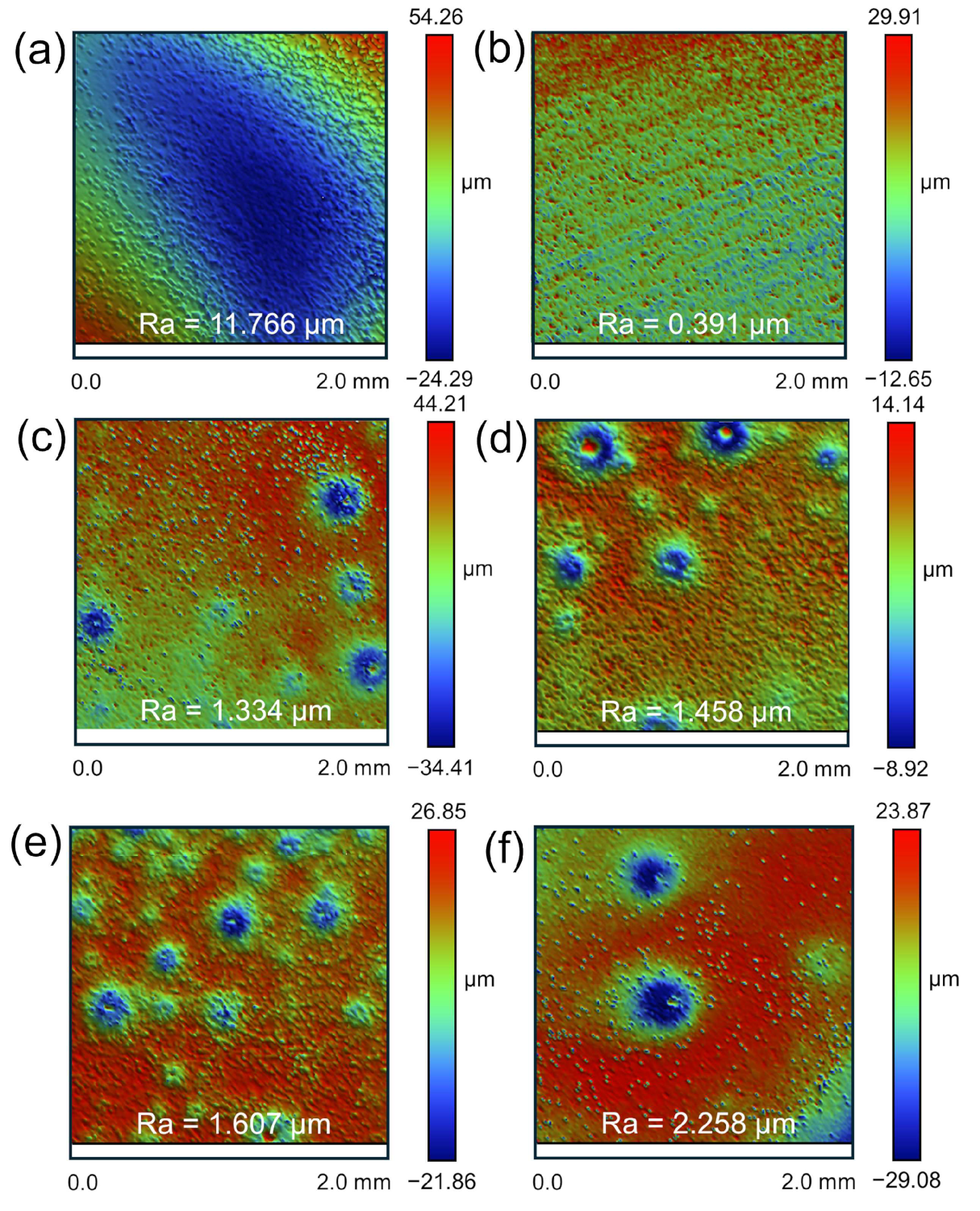
3.2. Surface Morphology and Uniformity
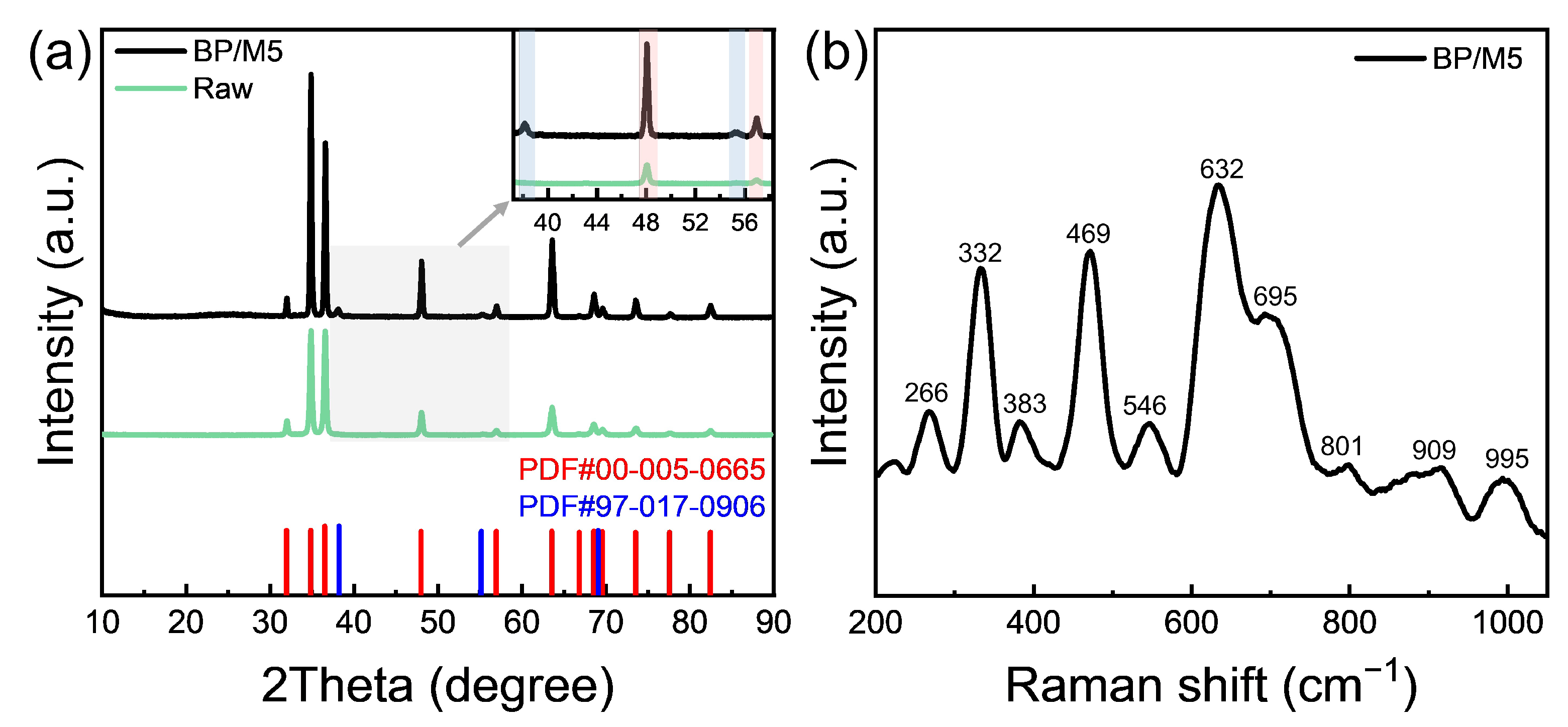
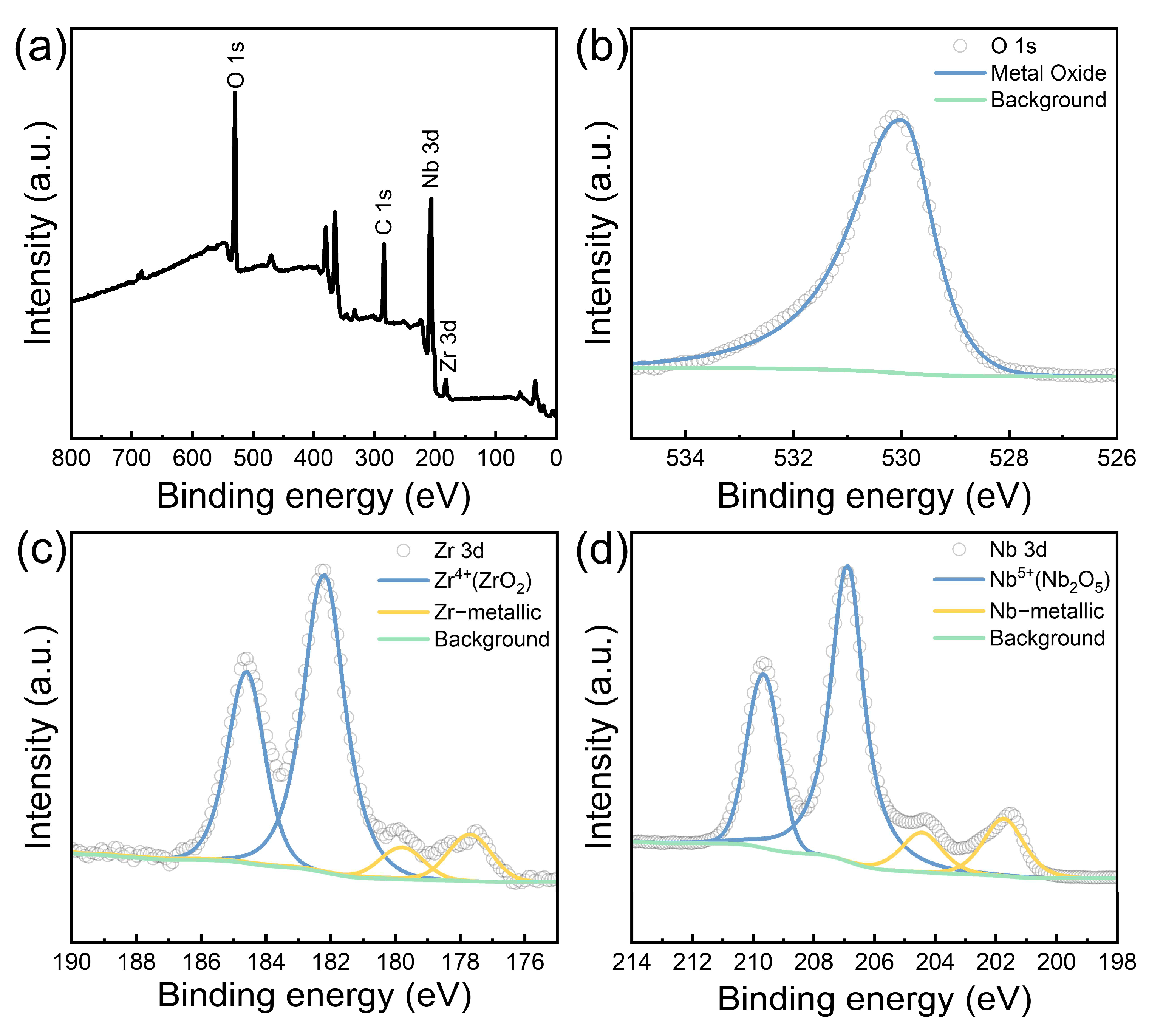
3.3. Analysis of the Passivation Layer
4. Discussion
5. Conclusions
- (1)
- The dissolution kinetics of M5 cladding indicate that the dissolution rate is positively correlated with the HF concentration in HF-HNO3 mixed acid. However, when the HF concentration exceeded 0.5 mol/L, the dissolution rate was significantly reduced due to the strong oxidizing effect of HNO3.
- (2)
- Increasing the HNO3 concentration in the mixed acid mitigates the aggressive erosive action of HF, reduces pitting corrosion, and enhances dissolution uniformity. Under optimized acid conditions (1:5), the M5 cladding achieves uniform dissolution, with surface roughness reduced to 0.812 μm.
- (3)
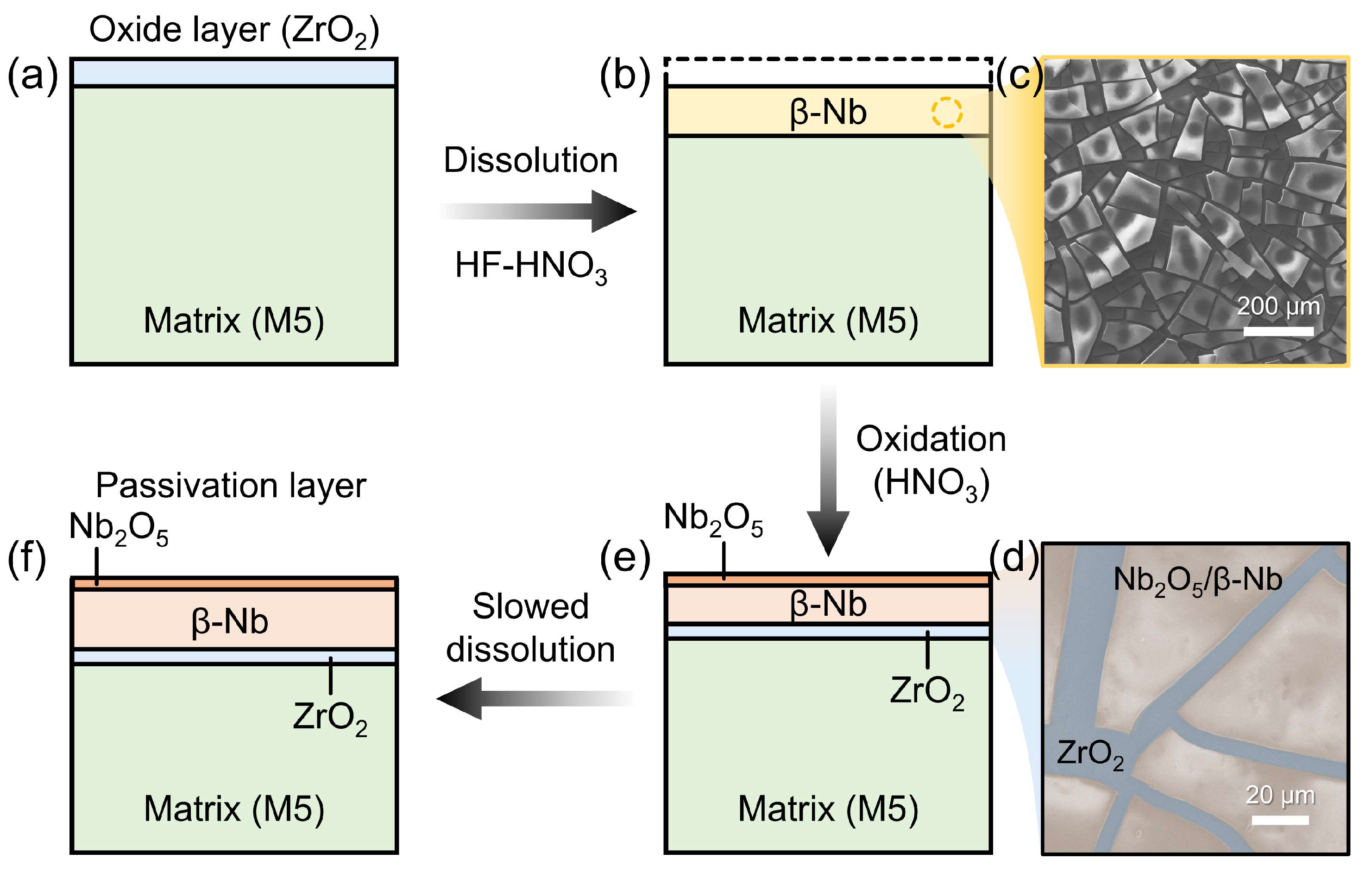
- During dissolution, SPPs (β-Nb) precipitate as a black powder. The outer layer of the precipitate is partially oxidized to stable Nb2O5 by a high concentration of HNO3, while the underlying M5 matrix continues to oxidation to ZrO2. As a result, the surface of the M5 cladding forms a passivation layer consisting of SPPs (β-Nb), Nb2O5, and ZrO2.
- (4)
- The dissolution behavior of M5 cladding in mixed acid is primarily influenced by the formation of the passivation layer. As dissolution and oxidation proceed, Nb oxide and β-Nb continuously accumulate in the outer region of the layer while Zr oxide forms on the surface of the M5 matrix. Meanwhile, the passivation layer restricts acid diffusion to the M5 matrix, thereby slowing down the dissolution of the M5 cladding and improving the overall uniformity of the dissolution process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azevedo, C.R.F. Selection of fuel cladding material for nuclear fission reactors. Eng. Fail. Anal. 2011, 18, 1943–1962. [Google Scholar] [CrossRef]
- Steinbrueck, M.; da Silva, F.O.; Grosse, M. Oxidation of zircaloy-4 in steam-nitrogen mixtures at 600–1200 °C. J. Nucl. Mater. 2017, 490, 226–237. [Google Scholar] [CrossRef]
- Steinbrück, M.; Vér, N.; Große, M. Oxidation of advanced zirconium cladding alloys in steam at temperatures in the range of 600–1200 °C. Oxid. Met. 2011, 76, 215–232. [Google Scholar] [CrossRef]
- Liu, S.-M.; Beyerlein, I.J.; Han, W.-Z. Two-dimensional vacancy platelets as precursors for basal dislocation loops in hexagonal zirconium. Nat. Commun. 2020, 11, 5766. [Google Scholar] [CrossRef]
- Fu, J.; Wang, J.; Li, F.; Cui, K.; Du, X.; Wu, Y. Effect of Nb addition on the microstructure and corrosion resistance of ferritic stainless steel. Appl. Phys. A 2020, 126, 194. [Google Scholar] [CrossRef]
- Liu, J.; He, G.; Callow, A.; Li, K.; Moore, K.L.; Nordin, H.; Moody, M.; Lozano-Perez, S.; Grovenor, C.R.M. The role of β-Zr in a Zr-2.5Nb alloy during aqueous corrosion: A multi-technique study. Acta Mater. 2021, 215, 117042. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, D.; Wei, Y.; Liu, H.; Liu, L.; Kuang, W. Corrosion of Zircaloy-4, Zr-1Nb and FeCrAl alloys in deaerated water at 330 °C and 14 MPa: Superior corrosion resistance and corrosion mechanism of FeCrAl alloy by experiments and molecular dynamics simulations. Chem. Eng. J. 2023, 462, 142143. [Google Scholar] [CrossRef]
- Jayaraj, J.; Thinaharan, C.; Ningshen, S.; Mallika, C.; Kamachi Mudali, U. Corrosion behavior and surface film characterization of TaNbHfZrTi high entropy alloy in aggressive nitric acid medium. Intermetallics 2017, 89, 123–132. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, H.; Wu, C.; Tian, H.; Wang, X. The role of alloying elements in the initiation of nanoscale porosity in oxide films formed on zirconium alloys. Corros. Sci. 2013, 77, 391–396. [Google Scholar] [CrossRef]
- Bell, B.D.C.; Murphy, S.T.; Burr, P.A.; Comstock, R.J.; Partezana, J.M.; Grimes, R.W.; Wenman, M.R. The influence of alloying elements on the corrosion of Zr alloys. Corros. Sci. 2016, 105, 36–43. [Google Scholar] [CrossRef]
- Stoll, U.; Slavinskaya, N. Corrosion behavior of zirconium alloys in the aqueous environment. Phenomenological aspects. Overview. J. Nucl. Sci. Technol. 2023, 60, 573–602. [Google Scholar] [CrossRef]
- Motta, A.T.; Couet, A.; Comstock, R.J. Corrosion of zirconium alloys used for nuclear fuel cladding. Annu. Rev. Mater. Res. 2015, 45, 311–343. [Google Scholar] [CrossRef]
- Charit, I.; Murty, K.L. Creep behavior of niobium-modified zirconium alloys. J. Nucl. Mater. 2008, 374, 354–363. [Google Scholar] [CrossRef]
- Lai, P.; Lu, J.; Zhang, H.; Liu, Q.; Zeng, Q.; Guo, X.; Zhang, L. The corrosion behavior of M5 (Zr–1Nb-0.12O) alloy in 360 °C water with dissolved oxygen. J. Nucl. Mater. 2020, 532, 152079. [Google Scholar] [CrossRef]
- Duriez, C.; Drouan, D.; Pouzadoux, G. Reaction in air and in nitrogen of pre-oxidised Zircaloy-4 and M5™ claddings. J. Nucl. Mater. 2013, 441, 84–95. [Google Scholar] [CrossRef]
- Yuan, R.; Xie, Y.-P.; Li, T.; Xu, C.-H.; Yao, M.-Y.; Xu, J.-X.; Guo, H.-B.; Zhou, B.-X. An origin of corrosion resistance changes of Zr alloys: Effects of Sn and Nb on grain boundary strength of surface oxide. Acta Mater. 2021, 209, 116804. [Google Scholar] [CrossRef]
- Zimmermann, H.G. Pickling of zirconium alloys. J. Nucl. Mater. 1964, 11, 247–248. [Google Scholar] [CrossRef]
- Lindell, D.; Pettersson, R. Pickling of process-oxidised austenitic stainless steels in HNO3-HF mixed acid. Steel Res. Int. 2010, 81, 542–551. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Y.; Li, M. Pickling behavior of 2205 duplex stainless steel hot-rolled strips in mixed solutions of HNO3-HF. Mater. Res. Express 2020, 7, 046502. [Google Scholar] [CrossRef]
- Rudisill, T.S. Decontamination of Zircaloy cladding hulls from spent nuclear fuel. J. Nucl. Mater. 2009, 385, 193–195. [Google Scholar] [CrossRef]
- Lewis, B.J.; Thompson, W.T.; Iglesias, F.C. Fission Product Chemistry in Oxide Fuels; Royal Military College: Kingston, ON, Canada, 2012; pp. 515–546. [Google Scholar]
- Collins, E.D.; DelCul, G.D.; Spencer, B.B.; Brunson, R.R.; Johnson, J.A.; Terekhov, D.S.; Emmanuel, N.V. Process development studies for zirconium recovery/recycle from used nuclear fuel cladding. Procedia Chem. 2012, 7, 72–76. [Google Scholar] [CrossRef]
- Fan, S.; Xin, Q.; Zhang, Y.; Zhao, X.; Liu, X.; Hao, X.; Hu, E.; Wang, H.; Lv, J.; Lei, Z.; et al. Efficient and clean release of uranium and zirconium in hazardous uranium purification waste by combined alkali decomposition and acid leaching process. J. Environ. Chem. Eng. 2023, 11, 109382. [Google Scholar] [CrossRef]
- Jahangiri, N.; Raraz, A.G.; Indacochea, J.E.; McDeavitt, S.M. Assessing corrosion of UNS S30403 stainless steel for applications in nuclear waste reprocessing systems. Corrosion 2013, 69, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S. Dissolution of Zr in a mixture of H2SO4 and HNO3. Corros. Sci. 2012, 65, 567–570. [Google Scholar] [CrossRef]
- El-Basiouny, M.S.; Mazhar, A.A.; El-Taib Heakal, F.; Ameer, M.A. Kinetic studies of the dissolution behaviour of anodic oxide films on Zr in H2SO4. J. Electroanal. Chem. Interfacial Electrochem. 1983, 147, 181–191. [Google Scholar] [CrossRef]
- Vanderwall, E.M.; Whitener, E.M. Concentrated nitric and dilute hydrofluoric acid mixtures in dissolution of zirconium metal. Ind. Eng. Chem. 1959, 51, 51–54. [Google Scholar]
- Smith, T.; Hill, G.R. A reaction rate study of the corrosion of low-hafnium zirconium in aqueous hydrofluoric acid solutions. J. Electrochem. Soc. 1958, 105, 117–121. [Google Scholar] [CrossRef]
- James, W.J.; Custead, W.G.; Straumanis, M.E. Chemical kinetics of the zirconium-hydrofluoric acid reaction. J. Phys. Chem. 1960, 64, 286–288. [Google Scholar] [CrossRef]
- Gu, H.; Li, G.; Liu, C.; Yuan, F.; Zhang, L.; Wang, L.; Li, X.; Wu, S.; Peng, S.; Gao, B. Characterization of the products obtained from the reactions of Zircaloy-4 with an acid mixture of concentrated HNO3 and dilute HF with the aim of understanding pickle salts of zirconium alloys. RSC Adv. 2016, 6, 109815–109825. [Google Scholar] [CrossRef]
- Klein, R. Dissolution of zirconium in nitric-hydrofluoric acid mixtures. Corrosion 1997, 53, 327–332. [Google Scholar] [CrossRef]
- Gu, H.F.; Zhang, L.F.; Li, M.Y.; Wang, L.; Li, X.N.; Wu, S.Q.; Peng, S.; Gao, B.; Li, G.P. Studies of the products from the reactions of co-acids containing concentrated HF and dilute HNO3 with Zircaloy-4. J. Fluor. Chem. 2014, 166, 28–33. [Google Scholar] [CrossRef]
- Hlawka, F.; Sutter, E.M.M. Reactivity of the surface of zirconium during pickling in nitric-hydrofluoric acid. Mater. Corros. 1991, 42, 428–436. [Google Scholar] [CrossRef]
- Sutter, E.M.M.; Hlawka, F.; Cornet, A. Comparative behavior of titanium and zirconium in hydrofluoric-nitric acid pickling solutions. Corrosion 1990, 46, 537–544. [Google Scholar] [CrossRef]
- Goncalves, Z.; Münzel, H. Dissolution kinetics of zircaloy in HNO3/HF mixtures. J. Nucl. Mater. 1990, 170, 261–269. [Google Scholar] [CrossRef]
- Gwinner, B.; Balbaud-Célérier, F.; Fauvet, P.; Gruet, N.; Laghoutaris, P.; Miserque, F.; Robin, R. A step towards a better understanding of corrosion of zirconium in nitric acid with additions of fluorine: Focus on the role of the presence of an initial oxide film. Corros. Sci. 2022, 201, 110284. [Google Scholar] [CrossRef]
- Covino, B.S.; Scalera, J.V.; Driscoll, T.J.; Carter, J.P. Dissolution behavior of 304 stainless steel in HNO3/HF mixtures. Metall. Trans. A 1986, 17, 137–149. [Google Scholar] [CrossRef]
- Amrutha, M.S.; Rao, M.T.; Ramanathan, S. Mechanistic analysis of anodic dissolution of Zr in acidic fluoride media. J. Electrochem. Soc. 2018, 165, C162. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Mandal, D.; Visweswara Rao, R.V.R.L.; Sairam, S.; Thakur, S. Recovery of zirconium from pickling solution, regeneration and its reuse. Nucl. Eng. Des. 2017, 316, 89–92. [Google Scholar] [CrossRef]
- Oskarsson, M.; Ahlberg, E.; Andersson, U.; Pettersson, K. Characterisation of pre-transition oxides on Zircaloys. J. Nucl. Mater. 2001, 297, 77–88. [Google Scholar] [CrossRef]
- Alvarez, R.B.; Martin, H.J.; Horstemeyer, M.F.; Chandler, M.Q.; Williams, N.; Wang, P.T.; Ruiz, A. Corrosion relationships as a function of time and surface roughness on a structural AE44 magnesium alloy. Corros. Sci. 2010, 52, 1635–1648. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding magnesium corrosion-A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Clark, R.N.; Humpage, J.; Burrows, R.; Godfrey, H.; Sagir, M.; Williams, G. A study into the localized corrosion of magnesium alloy magnox Al-80. Corrosion 2020, 77, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Werden, J.W.; Capps, N.A.; Linton, K.D.; Couet, A. (S)TEM/EDS study of native precipitates and irradiation induced Nb-rich platelets in high-burnup M5®. J. Nucl. Mater. 2021, 544, 152667. [Google Scholar] [CrossRef]
- He, G.; Liu, J.; Li, K.; Hu, J.; Mir, A.H.; Lozano-Perez, S.; Grovenor, C. Investigating the stability of second phase particles in Zr-Nb alloys under irradiation. J. Nucl. Mater. 2019, 526, 151738. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, M.Y.; Gao, C.Y.; Huang, J.; Chen, B.; Hu, L.J.; Lin, X.D.; Xie, Y.P.; Zhou, B.X. Oxide characteristics of 90Nb-10Zr alloy corroded under different water chemistry conditions. Nucl. Eng. Des. 2024, 419, 112931. [Google Scholar] [CrossRef]
- Huang, B.X.; Wang, K.; Church, J.S.; Li, Y.-S. Characterization of oxides on niobium by raman and infrared spectroscopy. Electrochim. Acta 1999, 44, 2571–2577. [Google Scholar] [CrossRef]
- Caliari, F.R.; Garcia, E.; Miranda, F.; Filho, G.P.; Sampath, S. Phase evolution in plasma sprayed Nb2O5 coatings. J. Eur. Ceram. Soc. 2021, 41, 5248–5257. [Google Scholar] [CrossRef]
- Efaw, C.M.; Vandegrift, J.L.; Reynolds, M.; McMurdie, S.; Jaques, B.J.; Hu, H.; Xiong, H.; Hurley, M.F. Characterization of zirconium oxides part I: Raman mapping and spectral feature analysis. Nucl. Mater. Energy 2019, 21, 100707. [Google Scholar] [CrossRef]
- Chen, L.; Qu, Y.; Wei, K.; Jin, X.; Liao, B.; Xue, W. Fabrication and characterization of microarc oxidation films on Ti-39Nb-6Zr alloy at different voltages in KOH electrolyte. J. Alloys Compd. 2017, 725, 1158–1165. [Google Scholar] [CrossRef]
- Jayaraj, J.; Nanda Gopala Krishna, D.; Mallika, C.; Kamachi Mudali, U. Passive film properties and corrosion behavior of Ni–Nb and Ni–Nb–Ta amorphous ribbons in nitric acid and fluorinated nitric acid environments. Mater. Chem. Phys. 2015, 151, 318–329. [Google Scholar] [CrossRef]





| Dissolution Rate (HF:HNO3 Ratio) | 50 s (×10−5 g cm−2 s−1) | 900 s (×10−5 g cm−2 s−1) | 1800 s (×10−5 g cm−2 s−1) | Reduction Between 900 s and 1800 s (×10−5 g cm−2 s−1) |
|---|---|---|---|---|
| 1:0 | 10.29 | 9.04 | 8.27 | 0.77 |
| 1:0.005 | 9.30 | 8.53 | 8.03 | 0.50 |
| 1:0.05 | 8.76 | 7.16 | 6.73 | 0.43 |
| 1:0.5 | 8.63 | 6.42 | 5.89 | 0.53 |
| 1:5 | 8.35 | 6.23 | 5.67 | 0.56 |
| Element | Photo Electron lines | Oxidation/Chemical State | Binding Energy (eV) | Atomic Concentration (%) |
|---|---|---|---|---|
| O | 1s | O2− (metal oxide) | 529.9 | 100 |
| Nb | 3d5/2; 3d3/2 | Nb5+ (Nb2O5) | 206.8; 209.6 | 79.6 |
| Nb | 201.7; 204.4 | 20.4 | ||
| Zr | 3d5/2; 3d3/2 | Zr4+ (ZrO2) | 182.1; 184.5 | 83.6 |
| Zr | 177.6; 179.7 | 16.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, Y.; Bai, Y.; Zhao, Z.; Wei, Z.; Liu, F.; Yuan, Z.; Yan, T.; Zheng, W. Dissolution Behavior of M5 Cladding in Hydrofluoric–Nitric Mixed Acid. Materials 2024, 17, 5771. https://doi.org/10.3390/ma17235771
Chen Y, Sun Y, Bai Y, Zhao Z, Wei Z, Liu F, Yuan Z, Yan T, Zheng W. Dissolution Behavior of M5 Cladding in Hydrofluoric–Nitric Mixed Acid. Materials. 2024; 17(23):5771. https://doi.org/10.3390/ma17235771
Chicago/Turabian StyleChen, Ying, Yandong Sun, Yang Bai, Ziqian Zhao, Zheng Wei, Fang Liu, Zhongwei Yuan, Taihong Yan, and Weifang Zheng. 2024. "Dissolution Behavior of M5 Cladding in Hydrofluoric–Nitric Mixed Acid" Materials 17, no. 23: 5771. https://doi.org/10.3390/ma17235771
APA StyleChen, Y., Sun, Y., Bai, Y., Zhao, Z., Wei, Z., Liu, F., Yuan, Z., Yan, T., & Zheng, W. (2024). Dissolution Behavior of M5 Cladding in Hydrofluoric–Nitric Mixed Acid. Materials, 17(23), 5771. https://doi.org/10.3390/ma17235771






