Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
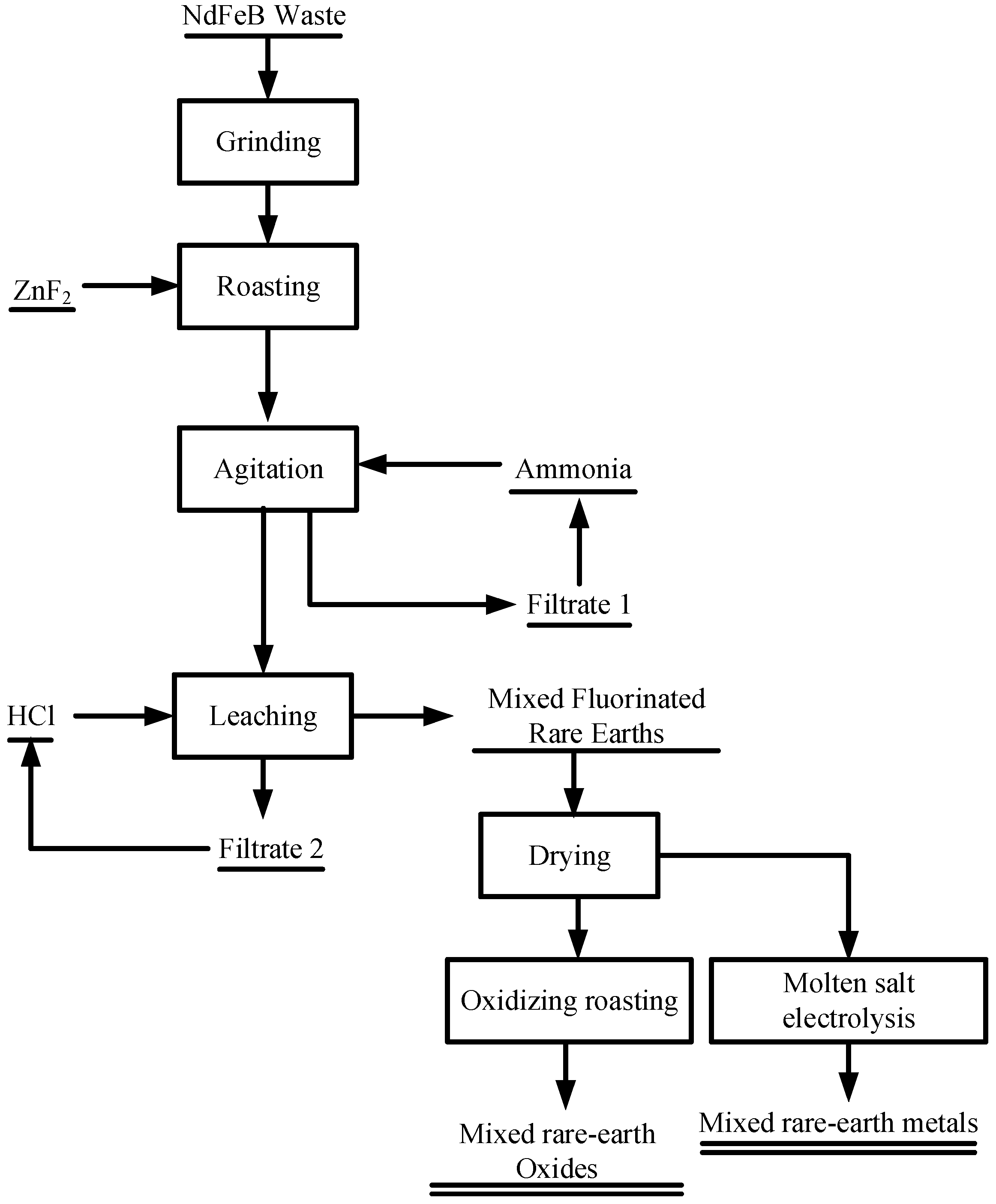
2.2. Procedure
2.3. RSM Optimization Process Based on Box–Behnken Design
3. Results and Discussion
3.1. Thermodynamic Calculation
3.2. Single-Factor Condition Experiments
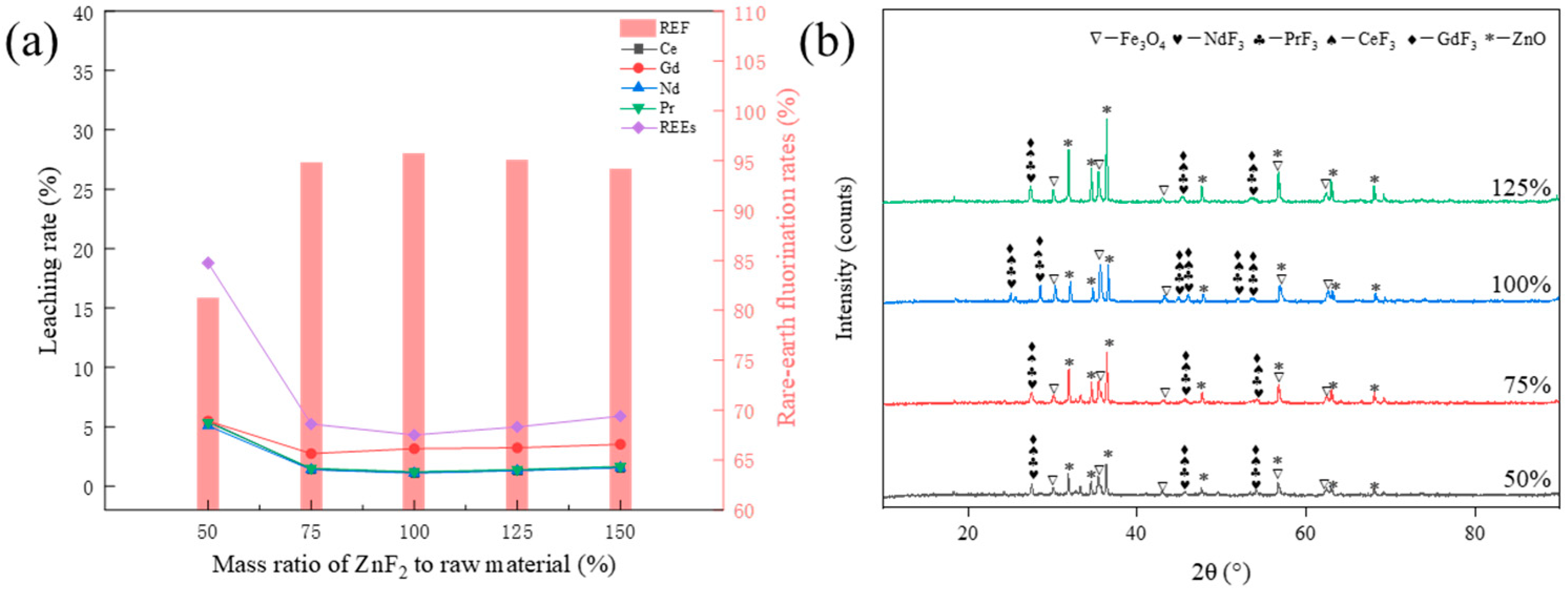
3.2.1. Effect of ZnF2 Addition on the Fluorination Rate of Rare Earths
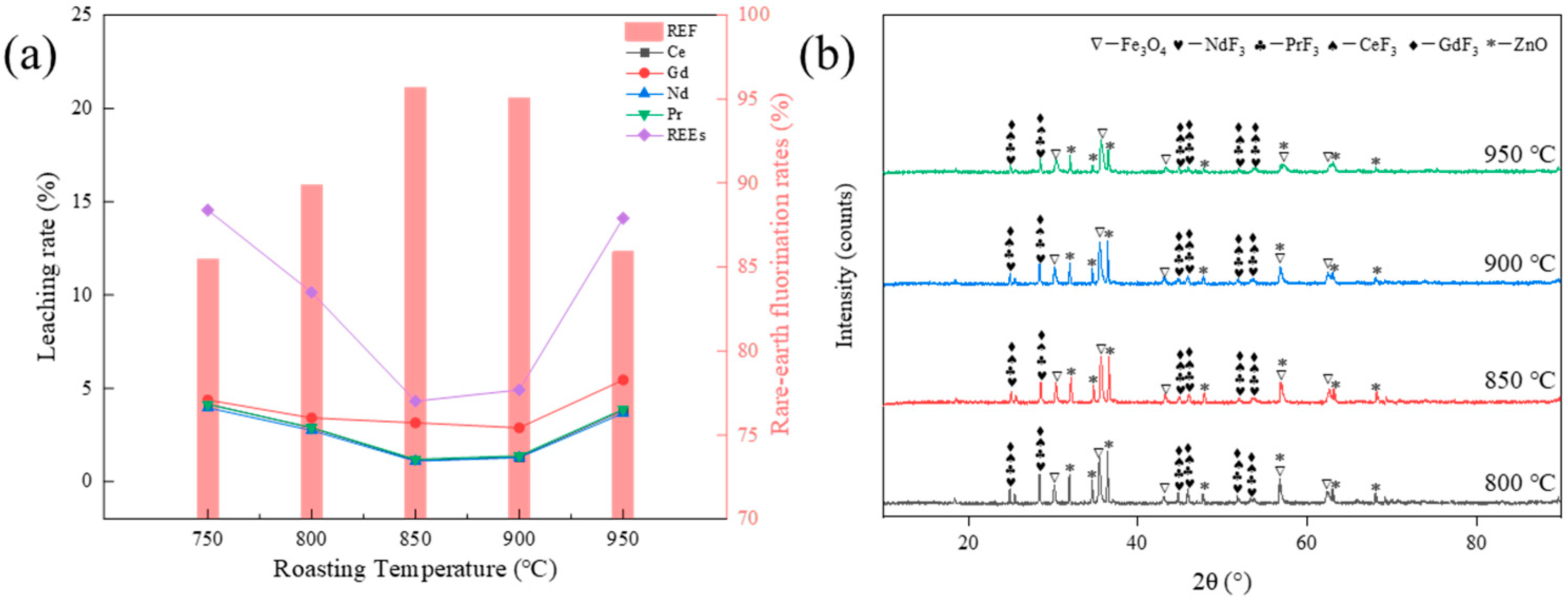
3.2.2. Effect of Temperature on the Fluorination Rate of Rare Earths
3.2.3. Effect of Roasting Time on Fluorination Rate
3.3. Box–Behnken Experimental Design
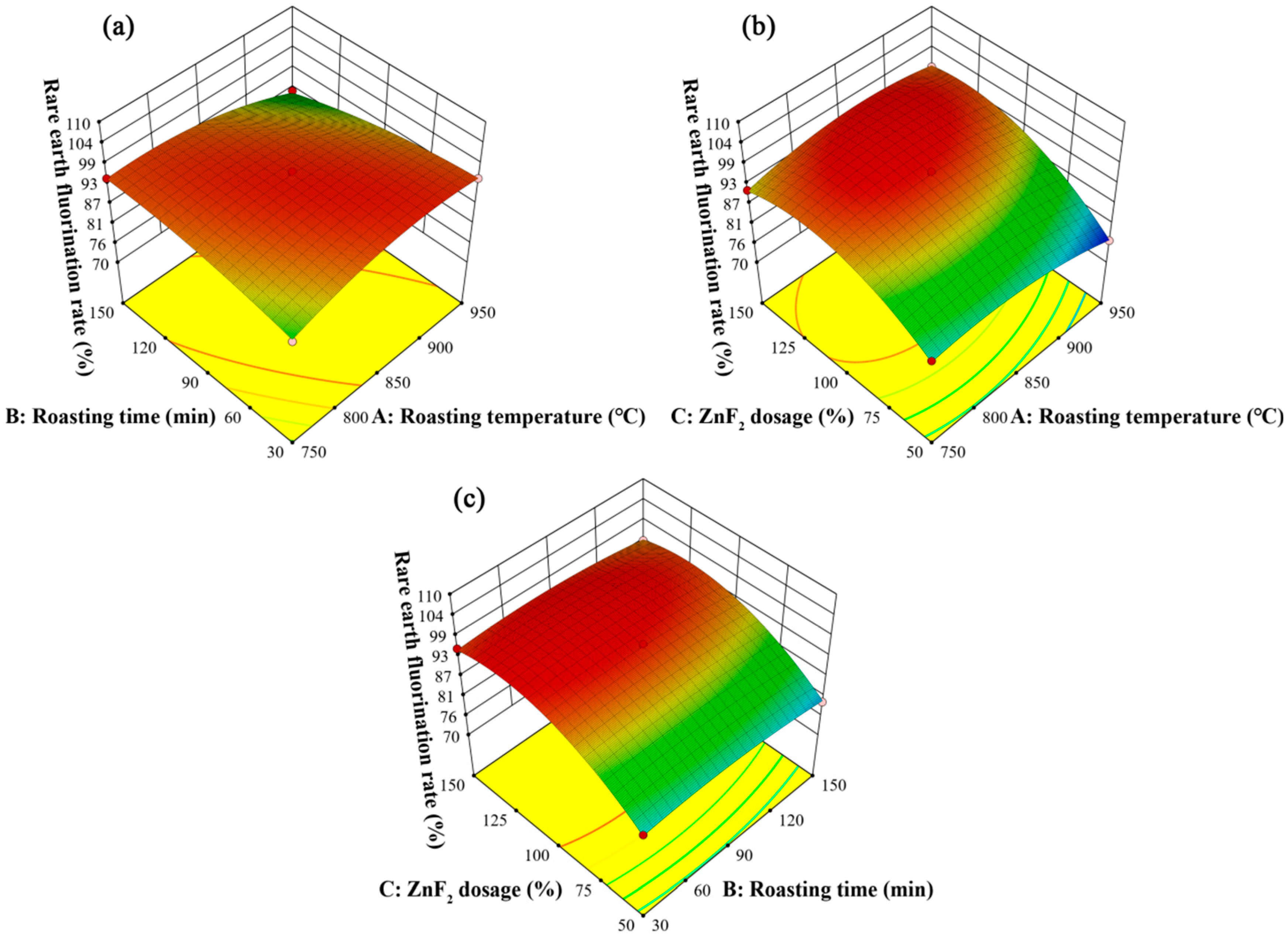
3.3.1. Statistical Analysis and ANOVA
3.3.2. Response Surface Optimization Analysis
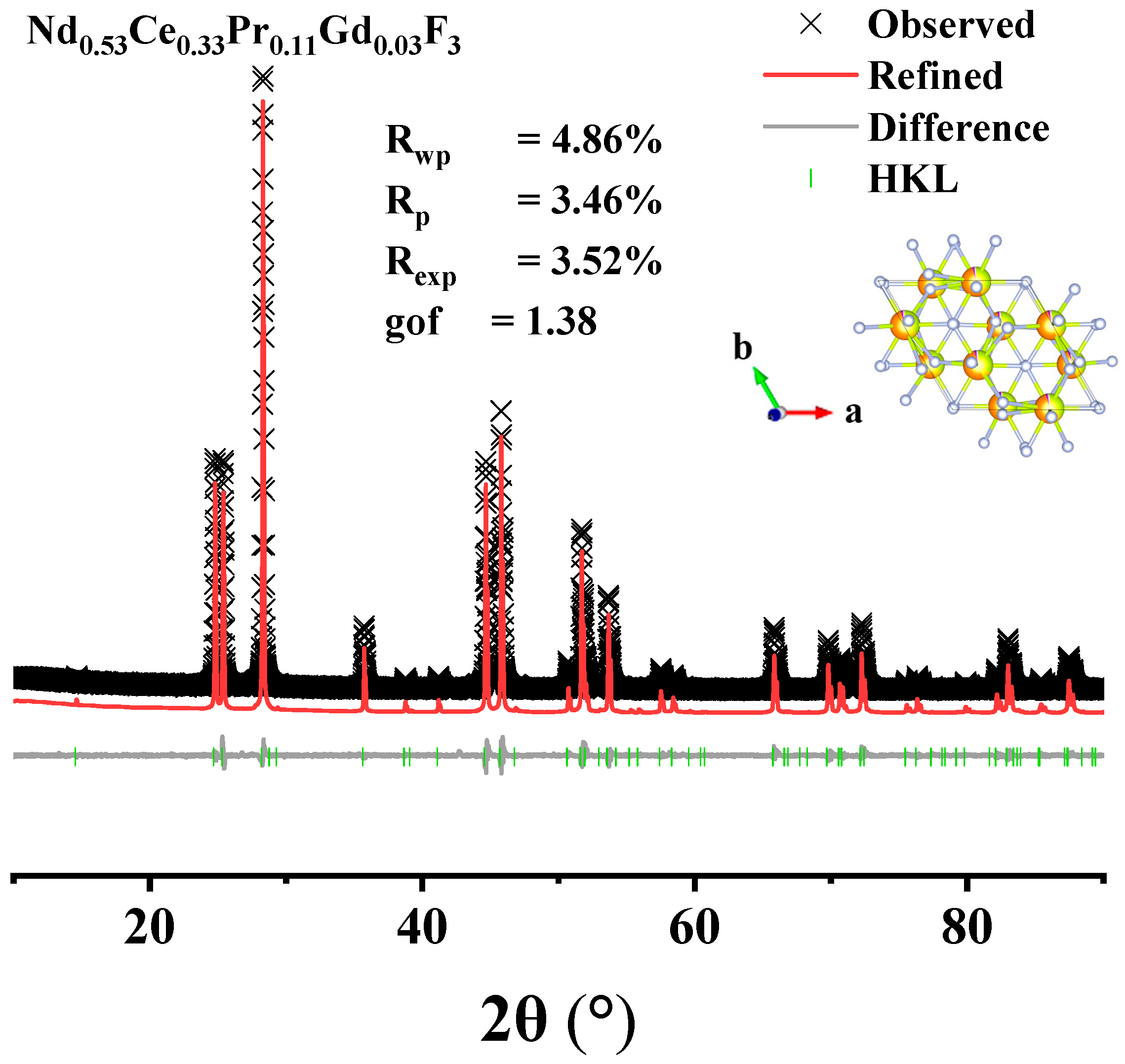
3.4. Compositional Analysis of Acid Leach Products
3.5. Recommendations for the Process
4. Conclusions
- The thermodynamic analysis results show that all the rare earth elements in NdFeB waste can react with ZnF2 to produce fluoride. However, increasing the roasting temperature is unfavorable to the fluoride reaction.
- The optimal roasting process was obtained through one-factor experiments: NdFeB waste was added with 100% ZnF2 and then reacted at 850 °C for 90 min, and the recovery rate of rare earths reached 95.69%. In addition, by analyzing the phase composition of the calcined products, the conclusion of the thermodynamic calculations was verified: an excessive calcination temperature is not conducive to the fluorination reaction, which reduces the recovery of rare earths.
- The BBD model was constructed according to the RSM criterion. It was found that the ZnF2 addition had the greatest effect on the rare earth fluorination rate, followed by roasting temperature and roasting time. In addition, the optimal roasting conditions were determined as follows: a roasting temperature of 828 °C, a roasting time of 91 min, and a ZnF2 dosage of 119%. The verification experiment demonstrated that the rare earth recovery rate could reach 97.29% under optimal process conditions.
- Some aspects remain that require further in-depth research and improvement, including the following: (1) detailed experiments on hydrochloric acid purification of fluorinated rare earths and precise control of leaching conditions, which can increase the efficiency of the subsequent fluorinated rare earth purification work; (2) further research on the separation of fluorinated rare earths, which is conducive to improving the value of the product; and (3) optimization of the process conditions and flow to improve the process reliability and practical applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orefice, M.; Bulck DV, A.; Blanpain, B.; Binnemans, K. Selective Roasting of Nd-Fe-B Permanent Magnets as a Pretreatment Step for Intensified Leaching with an Ionic Liquid. J. Sustain. Metall. 2020, 6, 91–102. [Google Scholar] [CrossRef]
- Du, C.; Ma, S.; Xie, M.; Yang, F.; Zhao, Z.; Chen, Y.; Ma, Y. Recovery of high-value rare earth elements from waste NdFeB by the water-soluble ammonium salt [Hbet]cl. Sep. Purif. Technol. 2023, 308, 122946. [Google Scholar] [CrossRef]
- Gebregiorgis, T.A.; Mentore, V.; Duarte, F.C.; Prasad, S.; Rtimi, S. Emerging technologies for the recovery of rare earth elements (REEs) from the end-of-life electronic wastes: A review on progress, challenges, and perspectives. Environ. Sci. Pollut. Res. Int. 2020, 27, 36052–36074. [Google Scholar]
- Binnemans, K.; Jones, P.T.; Muller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Yadav, J.; Sarker, K.S.; Bruckard, W.; Jegatheesan, V.; Haque, N.; Singh, N.; Pramanik, B.K. Greening the supply chain: Sustainable approaches for rare earth element recovery from neodymium iron boron magnet waste. J. Environ. Chem. Eng. 2024, 12, 113169. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, C.; Guo, L.; An, Z.; Zhao, Z.; Li, B. Recovery of rare-earth element from rare-earth permanent magnet waste by electro-refining in molten fluorides. Sep. Purif. Technol. 2020, 233, 116030. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Su, Z.; Liu, S.; Anderson, C.; Jiang, T. Hydrometallurgical Recovery of Rare Earth Elements from NdFeB Permanent Magnet Scrap: A Review. Metals 2020, 10, 841. [Google Scholar] [CrossRef]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Ji, B.; Li, Q.; Zhang, W. Leaching recovery of rare earth elements from the calcination product of a coal coarse refuse using organic acids. J. Rare Earths 2022, 40, 318–327. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, S.; Xu, Y.; Tang, K.; Lu, X.; Ding, W. Recovery of rare earth elements from permanent magnet scraps by pyrometallurgical process. Rare Met. 2022, 41, 1697–1702. [Google Scholar] [CrossRef]
- Yin, T.; Xue, Y.; Yan, Y.; Ma, Z.; Ma, F.; Zhang, M.; Wang, G.; Qiu, M. Recovery and separation of rare earth elements by molten salt electrolysis. Int. J. Miner. Metall. Mater. 2021, 28, 899–914. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, N.; Li, Y.; Wu, P.; Dang, Z.; Ke, Y. Efficient recovery of rare earth elements from discarded NdFeB magnets. Process Saf. Environ. Prot. 2019, 124, 317–325. [Google Scholar] [CrossRef]
- Shirayama, S.; Okabe, T.H. Selective Extraction and Recovery of Nd and Dy from Nd-Fe-B Magnet Scrap by Utilizing Molten MgCl2. Metall. Mater. Trans. B 2018, 49, 1067. [Google Scholar] [CrossRef]
- Venkatesan, P.; Hoogerstraete, T.V.; Binnemans, K.; Sun, Z.; Sietsma, J.; Yang, Y. Selective Extraction of Rare-Earth Elements from NdFeB Magnets by a Room-Temperature Electrolysis Pretreatment Step. ACS Sustain. Chem. Eng. 2018, 6, 9375–9382. [Google Scholar] [CrossRef]
- Kumari, A.; Sahu, S.K. A comprehensive review on recycling of critical raw materials from spent neodymium iron boron (NdFeB) magnet. Sep. Purif. Technol. 2023, 317, 123527. [Google Scholar] [CrossRef]
- Hua, Z.; Wang, J.; Wang, L.; Zhao, Z.; Li, X.; Xiao, Y.; Yang, Y. Selective Extraction of Rare Earth Elements from NdFeB Scrap by Molten Chlorides. ACS Sustain. Chem. Eng. 2014, 2, 2536–2543. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Tsubouchi, N.; Sugawara, K. Selective Recovery of Rare Earth Elements from Dy containing NdFeB Magnets by Chlorination. ACS Sustain. Chem. Eng. 2013, 1, 655–662. [Google Scholar] [CrossRef]
- Lorenz, T.; Bertau, M. Recycling of rare earth elements from FeNdB-Magnets via solid-state chlorination. J. Clean. Prod. 2019, 215, 131–143. [Google Scholar] [CrossRef]
- Lee, S.E.A.; Sinclai, C.W. The effect of precipitation on recrystallization in a Mg-Nd alloy. Materialia 2020, 10, 100643. [Google Scholar]
- Sun, M.; Hu, X.; Peng, L.; Fu, P.; Ding, W.; Peng, Y. On the production of Mg-Nd master alloy from NdFeB magnet scraps. J. Mater. Process. Technol. 2015, 218, 57–61. [Google Scholar] [CrossRef]
- Heo, S.G.; Yang, J.Y.; Oh, S.J.; Seo, S.-J.; Lee, M.H.; Park, K.-T. Extraction of rare earth elements from neodymium (NdFeB) magnet scrap using magnesium halides. J. Rare Earths 2024, in press. [Google Scholar] [CrossRef]
- Hua, Z.S.; Wang, L.; Wang, J.; Xiao, Y.P.; Yang, Y.X.; Zhao, Z.; Liu, M.J. Extraction of rare earth elements from NdFeB scrap by AlF3–NaF melts. Mater. Sci. Technol. 2015, 31, 1007–1010. [Google Scholar] [CrossRef]
- Önal, M.A.R.; Riaño, S. Binnemans, Alkali baking and solvometallurgical leaching of NdFeB magnets. Hydrometallurgy 2020, 191, 105213. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Y.; Wang, T.; Zhang, Y.; Shi, T.; Li, J.; Xu, H.; Xia, Z.; Sun, H.; Zhao, Z. Separation of neodymium from FeNd alloy and the preparation of porous iron alloy by chemical dealloying in molten chlorides: Application to the recovery of NdFeB wastes. Sep. Purif. Technol. 2023, 311, 123185. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Ma, B.; Wang, C.; Chen, Y. Strengthening extraction of lithium and rubidium from activated α-spodumene concentrate via sodium carbonate roasting. J. Ind. Eng. Chem. 2023, 123, 248–259. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Lu, Y.; Gao, Q.; Wu, Y.; Wang, Y.; Zhang, C. Phase diagram thermodynamic calculation of KNO3-NaNO2-KNO2 ternary system molten salt and its thermophysical properties investigation for thermal energy storage. J. Energy Storage 2024, 96, 112422. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, X.; Tan, C.; Yao, Q.R.; Wang, J.; Rao, G.H.; Zhou, H.Y. Experimental determination and thermodynamic calculation of phase equilibria in the Sm-Fe-B ternary system. Calphad 2024, 85, 102706. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, O.J. Optimization of turbidity removal and floc formation for ballasted flocculation using magnetite-based agents by response surface methodology. Chemosphere 2024, 363, 142932. [Google Scholar] [CrossRef]
- Anaklı, D.; Erşan, M. Optimization of reduced graphene oxide yield using response surface methodology. Diam. Relat. Mater. 2024, 148, 111524. [Google Scholar] [CrossRef]
- Battestini-Vives, M.; Xiao, X.; Lipnizki, F.; Rudolph-Schöpping, G. Response surface methodology to optimize membrane cleaning in nanofiltration of kraft black liquor. Sep. Purif. Technol. 2025, 354, 128626. [Google Scholar] [CrossRef]
- Abeng, D.; Sutaryo, S.; Purnomoadi, A.; Susanto, S.; Purbowati, E.; Adiwinarti, R.; Purwasih, R.; Widiharih, T. Optimization of methane production from dairy cow manure and germinated papaya seeds using response surface methodology. Case Stud. Chem. Environ. Eng. 2024, 10, 100927. [Google Scholar] [CrossRef]




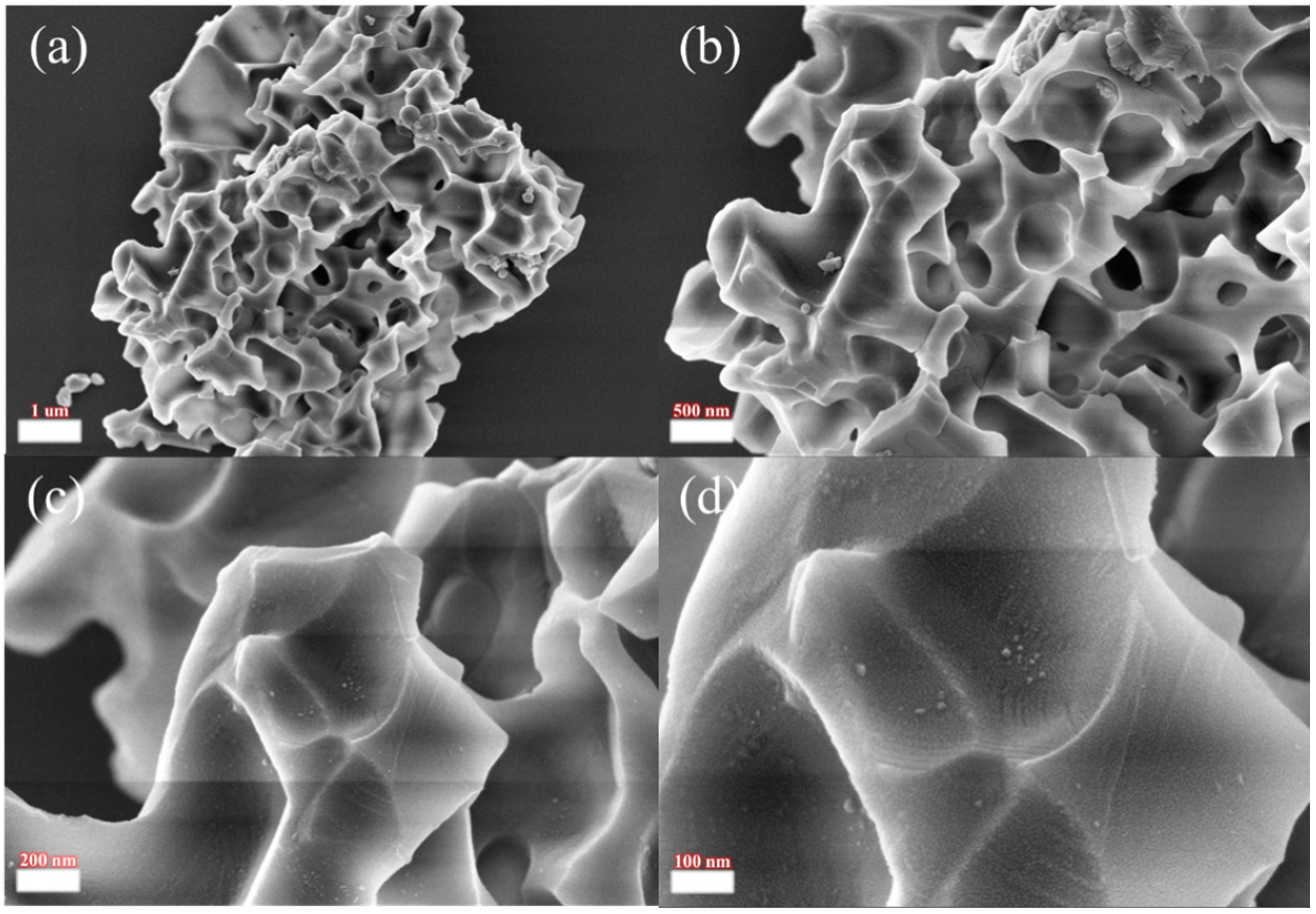
| Component | CeO2 | Pr6O11 | Nd2O3 | Sm2O3 | Gd2O3 | Dy2O3 | Ho2O3 | La2O3 | Eu2O3 | Tb2O3 | Er2O3 | Y2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 28.82 | 10.90 | 51.57 | 1.91 | 3.53 | 2.29 | 0.79 | 0.18 | <0.2 | <0.2 | <0.2 | <0.2 |
| Component | Fe * | Al | Co | K | Ca | Zn | Cu | Ni | Na | Mg | Pb | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 48.22 | 0.96 | 0.50 | 0.41 | 0.27 | 0.22 | 0.18 | 0.15 | 0.13 | 0.12 | 0.12 | 0.09 |
| Levels | Factors | ||
|---|---|---|---|
| Roasting Temperature (°C) | Roasting Time (min) | ZnF2 Dosage (%) | |
| −1 | 750 | 30 | 50 |
| 0 | 850 | 90 | 100 |
| 1 | 950 | 150 | 150 |
| No. | Reactions |
|---|---|
| 1 | 3ZnF2 + Nd2O3 = 2NdF3 + 3ZnO |
| 2 | 3ZnF2 + Ce2O3 = 2CeF3 +3ZnO |
| 3 | 3ZnF2 + Gd2O3 = 2GdF3 + 3ZnO |
| 4 | 3ZnF2 + La2O3 = 2LaF3 + 3ZnO |
| 5 | 3ZnF2 + Ho2O3 = 2HoF3 + 3ZnO |
| 6 | 3ZnF2 + Dy2O3 = 2DyF3 + 3ZnO |
| 7 | 9ZnF2 + Pr6O11 = 6PrF3 + 9ZnO + O2 (g) |
| 8 | 3ZnF2 + Fe2O3 = 2FeF3 + 3ZnO |
| Run | Roasting Temperature (°C) | Roasting Time (min) | ZnF2 Dosage (%) | Rare Earth Element Fluorination Rate (%) |
|---|---|---|---|---|
| 1 | 950 | 150 | 100 | 86.52 |
| 2 | 850 | 150 | 50 | 79.98 |
| 3 | 950 | 30 | 100 | 94.79 |
| 4 | 850 | 90 | 100 | 95.96 |
| 5 | 750 | 90 | 50 | 82.82 |
| 6 | 850 | 90 | 100 | 95.64 |
| 7 | 850 | 90 | 100 | 96.14 |
| 8 | 850 | 90 | 100 | 95.47 |
| 9 | 750 | 30 | 100 | 88.53 |
| 10 | 750 | 90 | 150 | 91.43 |
| 11 | 950 | 90 | 150 | 93.47 |
| 12 | 850 | 30 | 150 | 95.36 |
| 13 | 850 | 150 | 150 | 93.08 |
| 14 | 850 | 90 | 100 | 96.69 |
| 15 | 750 | 150 | 100 | 94.75 |
| 16 | 850 | 30 | 50 | 82.41 |
| 17 | 950 | 90 | 50 | 76.65 |
| Sum of | Mean | F | p-Value | |||
|---|---|---|---|---|---|---|
| Source | Squares | df | Square | Value | Prob > F | Significant |
| Model | 671.37 | 9 | 74.60 | 215.03 | <0.0001 | |
| A—Roasting temperature (°C) | 4.66 | 1 | 4.66 | 13.42 | 0.0080 | |
| B—Roasting time (min) | 5.69 | 1 | 5.69 | 16.41 | 0.0049 | |
| C—Mass ratio of ZnF2 to raw material (%) | 331.06 | 1 | 331.06 | 954.29 | <0.0001 | |
| AB | 52.51 | 1 | 52.51 | 151.37 | <0.0001 | |
| AC | 16.83 | 1 | 16.83 | 48.51 | 0.0002 | |
| BC | 0.0054 | 1 | 0.0054 | 0.0157 | 0.9039 | |
| A2 | 43.73 | 1 | 43.73 | 126.04 | <0.0001 | |
| B2 | 10.92 | 1 | 10.92 | 31.47 | 0.0008 | |
| C2 | 186.96 | 1 | 186.96 | 538.92 | <0.0001 | |
| Residual | 2.43 | 7 | 0.3469 | |||
| Lack of fit | 1.53 | 3 | 0.5091 | 2.26 | 0.2236 | Not significant |
| Pure error | 0.9011 | 4 | 0.2253 | |||
| Cor total | 673.80 | 16 | ||||
| Fit statistics | ||||||
| Std. Dev. | 0.5890 | R2 | 0.9964 | |||
| Mean | 90.57 | Adjusted R2 | 0.9918 | |||
| C.V. | 0.6503 | Predicted R2 | 0.9616 | |||
| PRESS | 25.85 | Adeq Precision | 42.3543 |
| REF | CeF4 | PrF3 | NdF3 | SmF3 | GdF3 | DyF3 | HoF3 | LaF3 | EuF3 | TbF3 | ErF3 | YF3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 30.97 | 10.28 | 50.26 | 1.74 | 3.04 | 1.70 | 0.54 | 0.18 | 0.08 | 0.10 | 0.01 | 0.01 | 98.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhong, Y.; Lei, X.; Wang, J. Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method. Materials 2024, 17, 5807. https://doi.org/10.3390/ma17235807
Liu Y, Zhong Y, Lei X, Wang J. Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method. Materials. 2024; 17(23):5807. https://doi.org/10.3390/ma17235807
Chicago/Turabian StyleLiu, Youwei, Yuan Zhong, Xiang Lei, and Jinliang Wang. 2024. "Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method" Materials 17, no. 23: 5807. https://doi.org/10.3390/ma17235807
APA StyleLiu, Y., Zhong, Y., Lei, X., & Wang, J. (2024). Mechanism and Experimental Study on the Recovery of Rare Earth Elements from Neodymium Iron Boron Waste Using the ZnF2 Fluorination Method. Materials, 17(23), 5807. https://doi.org/10.3390/ma17235807




