Performance and Emission Characteristics of a Small Gas Turbine Engine Using Hexanol as a Biomass-Derived Fuel
Highlights
- Hexanol blends achieve high efficiency in turbines at high RPM.
- CO emissions drop significantly with hexanol at higher rotational speeds.
- Hexanol offers a sustainable alternative to fossil turbine fuels.
- NOx emissions peak at high engine loads with hexanol blends.
Abstract
1. Introduction
2. Production Methods of Hexanol
2.1. Renewable Production Methods
2.2. Non-Renewable Production Methods
3. The Use of Hexanol as a Fuel in Combustion Engine
3.1. Application of Hexanol in Diesel Engines
3.2. Literature Review on Alcohols in Gas Turbines
3.3. Motivation
4. Test Stand and Methodological Approach
4.1. Experimental Facility and Exhaust Gas Composition Measurement Measurements
4.2. Fuels
| JET A1 | He25 Hexanol | He50 Hexanol | |
|---|---|---|---|
| Molecular formula | C13H26 | ||
| Mass Percentage of Oxygen [%] | 0 | 3.92 | 7.83 |
| Molecular Weight [kg/kmol] | 182 | 162.0 | 142.1 |
| Density at 15 °C [kg/m³] | 810 | 812 | 815 |
| Viscosity at 15 °C [cP] | 3.80 | 4.22 | 4.65 |
| Lower Heating Value [MJ/kg] | 43.28 | 41.46 | 39.64 |
| Latent Heat of Vaporization [kJ/kg] | 330 | 355 | 380 |
| Boiling Point [°C] | 118 | 127.8 | 137.5 |
| Property | Methanol | Ethanol | n-Butanol | n-Hexanol |
|---|---|---|---|---|
| Molecular Formula | CH3OH | C2H5OH | C4H9OH | C6H13OH |
| Molecular Structure | ||||
| Density at 15 °C (g/mL) | 0.791 | 0.789 | 0.810 | 0.820 |
| Kinematic Viscosity at 40 °C (mm²/s) | 0.58 | 1.08 | 2.22 | 3.57 |
| Lower Heating Value (MJ/kg) | 20.1 | 26.9 | 33.1 | 36.0 |
| Heat of Vaporization (kJ/kg) | 1162 | 918 | 585 | 285 |
| Cetane Number | 3.8 | 5–8 | 17 | 25 |
| Boiling Point (°C) | 65 | 78 | 117 | 157 |
| Flash Point (°C) | 12 | 13 | 35 | 62 |
| Autoignition Temperature (°C) | 463 | 420 | 345 | 280 |
| Oxygen Content (% by weight) | 49.93 | 34.73 | 21.59 | 15.62 |
| Carbon content (wt%) | 37.49 | 52.15 | 64.82 |
- ○
- Temperature measurements (T1–T4): Type K thermocouples (provided by Czah, Katowice, Poland) were utilized to record the temperatures at the compressor and turbine inlets and outlets. These sensors operate within a range of 0 to 1100 °C with a precision of 1 °C and an uncertainty margin of ±1 °C.
- ○
- Pressure measurements (P1–P4): Digital pressure transducers (provided by Aplisense, Warsaw, Poland) measured the pressures at key points in the compressor and turbine. Sensor P1 covered a range from 0 to 0.98 bar, while sensors P2 to P4 spanned from 0 to 9.8 bar. The devices have a resolution of 0.01 bar and an uncertainty of ±1.0%.
- ○
- Static thrust: A strain gauge-based load cell assessed the static thrust produced by the engine, capable of measuring forces between 0 and 200 N with a resolution of 1 N and an uncertainty of ±1 mV/V.
- ○
- Fuel flow Rate: The volumetric flow rate of fuel was monitored using an oval gear flow meter, suitable for flows from 0.5 to 100 L per hour (L/h). The flow meter offers a resolution of 0.01 L/h and has an uncertainty of ±0.5%.
- ○
- Rotational speed: Engine speed was tracked using a magnetic pickup tachometer, effective over a range of 0 to 200,000 RPM. The instrument provides readings with a resolution of 1 RPM and an uncertainty of ±0.5%.
- ○
- Exhaust gas analysis (provided by Madur; Ga 40plus): Concentrations of oxygen, carbon monoxide, nitric oxide, and sulfur dioxide in the exhaust were measured using electrochemical gas analyzers. These devices offer high-resolution measurements and have specified absolute and relative uncertainties to ensure data accuracy.
4.3. Procedure
5. Results and Discussion of Gas Turbine Performance Characteristics
5.1. Thrust-Specific Fuel Consumption and Fuel Flow
5.2. Turbine Inlet Temperature
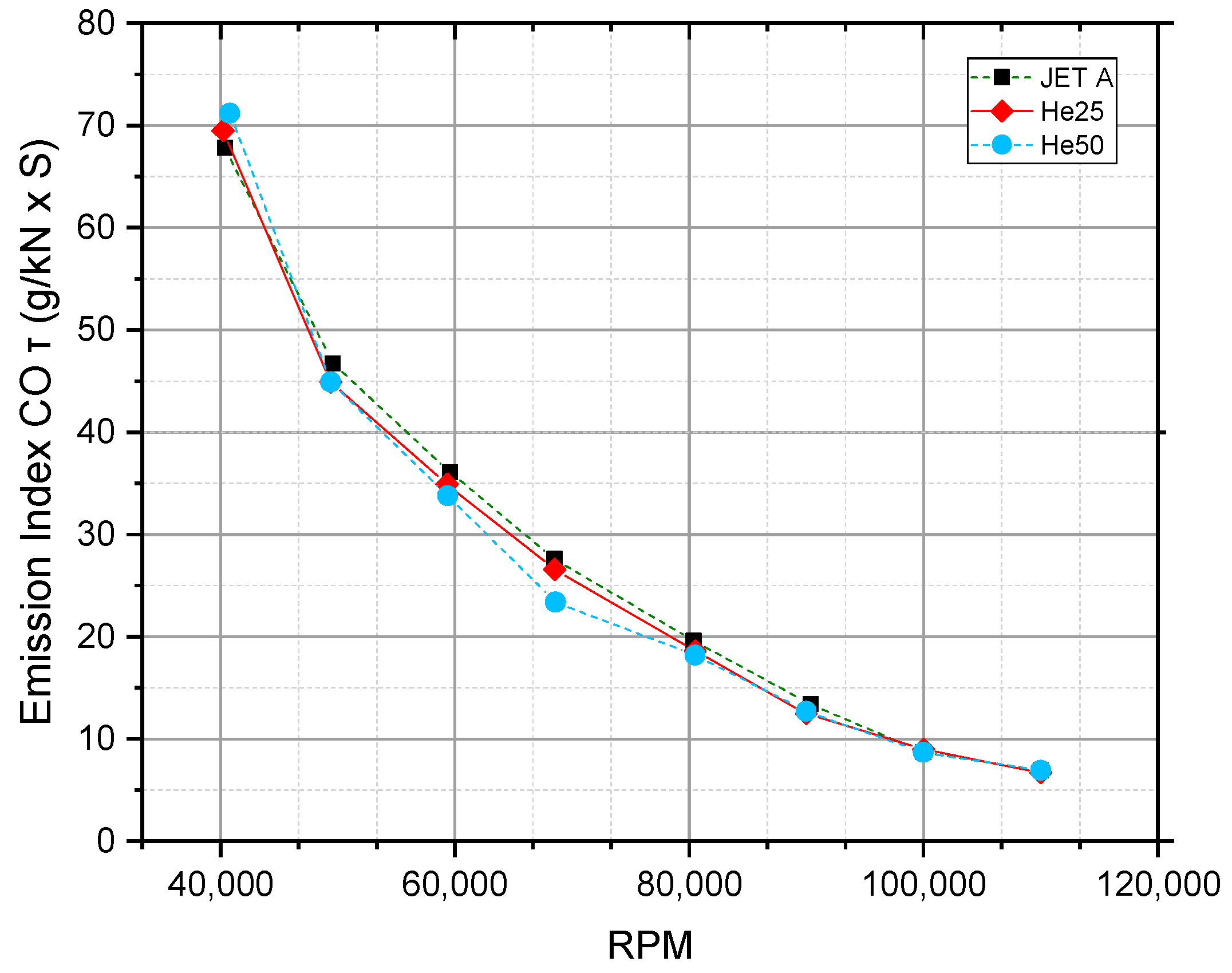
5.3. Gas Turbine Emission Index
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamperidou, V.; Terzopoulou, P.; Barboutis, I. Marginal lands providing tree-crop biomass as feedstock for solid biofuels. Biofuels Bioprod. Biorefin. 2021, 15, 1395–1405. [Google Scholar] [CrossRef]
- Rajak, A.K.; Harikrishna, M.; Mahato, D.L.; Anandamma, U.; Pothu, R.; Sarangi, P.K.; Sahoo, U.K.; Vennu, V.; Boddula, R.; Karuna, M.S.L. Valorising orange and banana peels: Green catalysts for transesterification and biodiesel production in a circular bioeconomy. J. Taiwan Inst. Chem. Eng. 2024, 105804. [Google Scholar] [CrossRef]
- Suchocki, T.; Kazimierski, P.; Lampart, P.; Januszewicz, K.; Białecki, T.; Gawron, B.; Janicka, A. A comparative study of pentanol (C5 alcohol) and kerosene blends in terms of gas turbine engine performance and exhaust gas emission. Fuel 2023, 334, 126741. [Google Scholar] [CrossRef]
- Yilmaz, N.; Atmanli, A.; Hall, M.J.; Vigil, F.M. Determination of the Optimum Blend Ratio of Diesel, Waste Oil Derived Biodiesel and 1-Pentanol Using the Response Surface Method. Energies 2022, 15, 5144. [Google Scholar] [CrossRef]
- Kurzawska-pietrowicz, P. A Review of Alternative Aviation Fuels. Energies 2024, 17, 3890. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Hu, C.; Bi, W. Determination of alcohols-diesel oil by near infrared spectroscopy based on gramian angular field image coding and deep learning. Fuel 2022, 309, 122121. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Wang, Z.; Liu, H. Combustion and emissions of compression ignition in a direct injection diesel engine fueled with pentanol. Energy 2015, 80, 575–581. [Google Scholar] [CrossRef]
- Çelebi, Y.; Aydın, H. An overview on the light alcohol fuels in diesel engines. Fuel 2019, 236, 890–911. [Google Scholar] [CrossRef]
- Campos-Fernández, J.; Arnal, J.M.; Gómez, J.; Dorado, M.P. A comparison of performance of higher alcohols/diesel fuel blends in a diesel engine. Appl. Energy 2012, 95, 267–275. [Google Scholar] [CrossRef]
- De Poures, M.V.; Gopal, K.; Sathiyagnanam, A.P.; Rajesh Kumar, B.; Rana, D.; Saravanan, S. Comparative account of the effects of two high carbon alcohols (C5 & C6) on combustion, performance and emission characteristics of a DI diesel engine. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 1772–1784. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, D.; Bautista, F.M.; Romero, A.A.; Estevez, R. Advanced Biofuels from ABE (Acetone/Butanol/Ethanol) and Vegetable Oils (Castor or Sunflower Oil) for Using in Triple Blends with Diesel: Evaluation on a Diesel Engine. Materials 2022, 15, 6493. [Google Scholar] [CrossRef]
- Obergruber, M.; Hönig, V.; Jenčík, J.; Hájek, J.; Schlehöfer, D.; Herink, T. Lignocellulosic bioethanol and biobutanol as a biocomponent for diesel fuel. Materials 2021, 14, 5597. [Google Scholar] [CrossRef]
- Kumar, A.N.; Ashok, B.; Nanthagopal, K.; Ong, H.C.; Geca, M.J.; Victor, J. Experimental analysis of higher alcohol–based ternary biodiesel blends in CI engine parameters through multivariate and desirability approaches. Biomass Convers. Biorefinery 2022, 12, 1525–1540. [Google Scholar] [CrossRef]
- Rajesh Kumar, B.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Che Mat, S.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and emissions of straight vegetable oils and its blends as a fuel in diesel engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Pandian, A.K.; Munuswamy, D.B.; Radhakrishnan, S.; Devarajan, Y.; Ramakrishnan, R.B.B.; Nagappan, B. Emission and performance analysis of a diesel engine burning cashew nut shell oil bio diesel mixed with hexanol. Pet. Sci. 2018, 15, 176–184. [Google Scholar] [CrossRef]
- Santhosh, K.; Kumar, G.N. Impact of 1-Hexanol/diesel blends on combustion, performance and emission characteristics of CRDI CI mini truck engine under the influence of EGR. Energy Convers. Manag. 2020, 217, 113003. [Google Scholar] [CrossRef]
- De Poures, M.V.; Sathiyagnanam, A.P.; Rana, D.; Rajesh Kumar, B.; Saravanan, S. 1-Hexanol as a sustainable biofuel in DI diesel engines and its effect on combustion and emissions under the influence of injection timing and exhaust gas recirculation (EGR). Appl. Therm. Eng. 2017, 113, 1505–1513. [Google Scholar] [CrossRef]
- Aucejo, A.; Burguet, M.C.; Muñoz, R. Densities, Viscosities, and Refractive Indices of Some Binary Liquid Systems of Methanol + Isomers of Hexanol at 298.15 K. J. Chem. Eng. Data 1996, 41, 508–510. [Google Scholar] [CrossRef]
- Thomas, J.J.; Nagarajan, G.; Sabu, V.R.; Manojkumar, C.V.; Sharma, V. Performance and emissions of hexanol-biodiesel fuelled RCCI engine with double injection strategies. Energy 2022, 253, 124069. [Google Scholar] [CrossRef]
- Dillikannan, D.; Dilipsingh, J.; Poures, M.V.D.; Kaliyaperumal, G.; Sathiyagnanam, A.P.; Babu, R.K. Effective utilization of waste plastic oil/n-hexanol in an off-road, unmodified DI diesel engine and evaluating its performance, emission, and combustion characteristics. Energy Sources Part Recover. Util. Environ. Eff. 2019, 42, 1375–1390. [Google Scholar] [CrossRef]
- Ramesh, A.; Ashok, B.; Nanthagopal, K.; Pathy, M.R.; Tambare, A.; Mali, P. Influence of hexanol as additive with Calophyllum Inophyllum biodiesel for CI engine applications. Fuel 2019, 249, 472–485. [Google Scholar] [CrossRef]
- Nour, M.; Sun, Z.; El-Seesy, A.I.; Li, X. Experimental evaluation of the performance and emissions of a direct-injection compression-ignition engine fueled with n-hexanol–diesel blends. Fuel 2021, 302, 121144. [Google Scholar] [CrossRef]
- Bhatt, A.N.; Shrivastava, N. Experimental Investigation and neural network modelling of diesel engine using hexanol blended ternary waste cooking oil biodiesel with moderate preheating. Sustain. Energy Technol. Assess. 2022, 52, 102285. [Google Scholar] [CrossRef]
- Oh, H.J.; Ko, J.K.; Gong, G.; Lee, S.M.; Um, Y. Production of Hexanol as the Main Product Through Syngas Fermentation by Clostridium carboxidivorans P7. Front. Bioeng. Biotechnol. 2022, 10, 850370. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. A comparative analysis of n-butanol/diesel and 1-pentanol/diesel blends in a compression ignition engine. Fuel 2018, 234, 161–169. [Google Scholar] [CrossRef]
- Atmanli, A. Comparative analyses of diesel–waste oil biodiesel and propanol, n-butanol or 1-pentanol blends in a diesel engine. Fuel 2016, 176, 209–215. [Google Scholar] [CrossRef]
- Enagi, I.I.; Al-attab, K.A.; Zainal, Z.A. Liquid biofuels utilization for gas turbines: A review. Renew. Sustain. Energy Rev. 2018, 90, 43–55. [Google Scholar] [CrossRef]
- Higashide, W.; Li, Y.; Yang, Y.; Liao, J.C. Metabolic Engineering of Clostridium cellulolyticum for Production of Isobutanol from Cellulose. Appl. Environ. Microbiol. 2011, 77, 2727–2733. [Google Scholar] [CrossRef]
- Bao, T.; Zhao, J.; Li, J.; Liu, X.; Yang, S.T. n-Butanol and ethanol production from cellulose by Clostridium cellulovorans overexpressing heterologous aldehyde/alcohol dehydrogenases. Bioresour. Technol. 2019, 285, 121316. [Google Scholar] [CrossRef]
- Formighieri, C. Cyanobacteria as a Platform for Direct Photosynthesis-to-Fuel Conversion BT. In Solar-to-Fuel Conversion in Algae and Cyanobacteria; Formighieri, C., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 31–38. ISBN 978-3-319-16730-5. [Google Scholar]
- Desai, S.H.; Rabinovitch-Deere, C.A.; Tashiro, Y.; Atsumi, S. Isobutanol production from cellobiose in Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 3727–3736. [Google Scholar] [CrossRef]
- Ofuonye, E.; Kutin, K.; Stuart, D.T. Engineering Saccharomyces cerevisiae fermentative pathways for the production of isobutanol. Biofuels 2013, 4, 185–201. [Google Scholar] [CrossRef]
- Konan, D.; Koffi, E.; Ndao, A.; Peterson, E.C.; Rodrigue, D.; Adjallé, K. An Overview of Extrusion as a Pretreatment Method of Lignocellulosic Biomass. Energies 2022, 15, 3002. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.L.; Sharma, V.; Sun, P.P.; Nargotra, P.; Bajaj, B.K.; Chen, C.W.; Dong, C. Di Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Ojo, A.O. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Hua, Y.; Zhu, C.; Zhang, L.; Dong, F. Designing Surface and Interface Structures of Copper-Based Catalysts for Enhanced Electrochemical Reduction of CO2 to Alcohols. Materials 2024, 17, 600. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kikhtyanin, O.; Kubička, D.; Dimitriadis, A. Refinery co-processing of renewable feeds. Prog. Energy Combust. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, D.K. Emerging Role of Biorefining of Heavier Crude Oils and Integration of Biorefining with Petroleum Refineries in the Future. Pet. Sci. Technol. 2006, 24, 1125–1159. [Google Scholar] [CrossRef]
- Gusevskaya, E.V.; Börner, A.; Jiménez-Pinto, J. Hydroformylation in the Realm of Scents. ChemCatChem 2014, 6, 382–411. [Google Scholar] [CrossRef]
- Çelebi, Y.; Cengiz, M.; Aydın, A.; Aydın, H. A comprehensive review of hexanol and its blends in diesel engines. Energy Convers. Manag. 2024, 321, 119004. [Google Scholar] [CrossRef]
- Vinayagam, M.; Balamurugan, G.; Vijayanand, G.; Hafeez, M.M.A. Emission study on alcohol-biodiesel propelled compression ignition engine. Int. J. Ambient. Energy 2021, 42, 1049–1054. [Google Scholar] [CrossRef]
- Prabhu Kishore, N.; Gugulothu, S.K.; Venkat Reddy, R.; Barmavatu, P. Trade-off study on environmental aspects of a reactivity controlled compression ignition engine using 1-hexanol and jatropha oil/diesel in dual fuel mode. Appl. Therm. Eng. 2024, 246, 122891. [Google Scholar] [CrossRef]
- Thomas, J.J.; Sabu, V.R.; Nagarajan, G.; Kumar, S.; Basrin, G. Influence of waste vegetable oil biodiesel and hexanol on a reactivity controlled compression ignition engine combustion and emissions. Energy 2020, 206, 118199. [Google Scholar] [CrossRef]
- Pradeepraj, R.; Rajan, K.; Nallusamy, S. Impact of 1-hexanol fumigation on diesel engine emissions using Moringa oleifera biodiesel. Int. J. Eng. Trends Technol. 2020, 68, 150–155. [Google Scholar] [CrossRef]
- Mendez, C.J.; Parthasarathy, R.N.; Gollahalli, S.R. Performance and emission characteristics of a small scale gas turbine engine fueled with propanol/jet a blends. In Proceedings of the ASME 2011 International Mechanical Engineering Congress and Exposition, Denver, CO, USA, 11–17 November 2011; Volume 4, pp. 1359–1370. [Google Scholar] [CrossRef]
- Mendez, C.; Parthasarathy, R.; Gollahalli, S. Performance and Emission Characteristics of a Small-Scale Gas Turbine Engine Fueled with Ethanol/Jet A Blends. In Proceedings of the 50th AIAA Aerospace Sciences Meeting including the New Horizons Forum and Aerospace Exposition, Nashville, TN, USA, 9–12 January 2012; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2012; pp. 1359–1370. [Google Scholar]
- Chen, L.; Zhang, Z.; Lu, Y.; Zhang, C.; Zhang, X.; Zhang, C.; Roskilly, A.P. Experimental study of the gaseous and particulate matter emissions from a gas turbine combustor burning butyl butyrate and ethanol blends. Appl. Energy 2017, 195, 693–701. [Google Scholar] [CrossRef]
- Buffi, M.; Cappelletti, A.; Rizzo, A.M.; Martelli, F.; Chiaramonti, D. Combustion of fast pyrolysis bio-oil and blends in a micro gas turbine. Biomass Bioenergy 2018, 115, 174–185. [Google Scholar] [CrossRef]
- Sallevelt, J.L.H.P.; Pozarlik, A.K.; Brem, G. Numerical study of pyrolysis oil combustion in an industrial gas turbine. Energy Convers. Manag. 2016, 127, 504–514. [Google Scholar] [CrossRef]
- Buffi, M.; Seljak, T.; Cappelletti, A.; Bettucci, L.; Valera-Medina, A.; Katrašnik, T.; Chiaramonti, D. Performance and emissions of liquefied wood as fuel for a small scale gas turbine. Appl. Energy 2018, 230, 1193–1204. [Google Scholar] [CrossRef]
- Mendez, C.J.; Parthasarathy, R.N.; Gollahalli, S.R. Performance and emission characteristics of butanol/Jet A blends in a gas turbine engine. Appl. Energy 2014, 118, 135–140. [Google Scholar] [CrossRef]
- Suchocki, T.K.; Kazimierski, P.; Januszewicz, K.; Lampart, P.; Zaniewski, D.; Klimaszewski, P.; Witanowski, Ł. Pyrolysis-derived waste polypropylene oils in gas turbine engines: A comprehensive performance and emission study. Arch. Thermodyn. 2023, 44, 157–183. [Google Scholar] [CrossRef]
- Suchocki, T.; Kazimierski, P.; Januszewicz, K.; Lampart, P.; Gawron, B.; Białecki, T. Exploring Performance of Pyrolysis-Derived Plastic Oils in Gas Turbine Engines. Energies 2024, 17, 3903. [Google Scholar] [CrossRef]
- Suchocki, T. Sustainable Energy Application of Pyrolytic Oils from Plastic Waste in Gas Turbine Engines: Performance, Environmental, and Economic Analysis. Sustainability 2024, 16, 8566. [Google Scholar] [CrossRef]
- Podwin, A.K.; Janicka, A.; Molska, J.; Zawiślak, M.; Lizanets, D.; Białecki, T.; Gawron, B.; Suchocki, T. Microfluidic-assisted toxicity studies of jet fuels on environmental microorganisms—Towards new lab-on-a-chip sensing applications. Meas. J. Int. Meas. Confed. 2022, 204, 112037. [Google Scholar] [CrossRef]
- Główka, M.; Wójcik, J.; Boberski, P.; Białecki, T.; Gawron, B.; Skolniak, M.; Suchocki, T. Sustainable aviation fuel—Comprehensive study on highly selective isomerization route towards HEFA based bioadditives. Renew. Energy 2024, 220, 119696. [Google Scholar] [CrossRef]
- Chmielewski, M.; Niszczota, P.; Gieras, M. Combustion efficiency of fuel-water emulsion in a small gas turbine. Energy 2020, 211, 118961. [Google Scholar] [CrossRef]
- Niszczota, P.; Gieras, M. Inlet injection as an alternative, simplified method of hydrogen application in a miniature gas turbine. Appl. Therm. Eng. 2024, 257, 124175. [Google Scholar] [CrossRef]
- Badami, M.; Nuccio, P.; Pastrone, D.; Signoretto, A. Performance of a small-scale turbojet engine fed with traditional and alternative fuels. Energy Convers. Manag. 2014, 82, 219–228. [Google Scholar] [CrossRef]
- Kamps, T. Model Jet Engines (Modeller’s World S.); Traplet Publications Ltd.: Malvern, UK, 2005. [Google Scholar]
- Gawron, B.; Białecki, T. Impact of a Jet A-1/HEFA blend on the performance and emission characteristics of a miniature turbojet engine. Int. J. Environ. Sci. Technol. 2018, 15, 1501–1508. [Google Scholar] [CrossRef]
- Gawron, B.; Białecki, T.; Janicka, A.; Suchocki, T. Combustion and emissions characteristics of the turbine engine fueled with HeFA blends from different feedstocks. Energies 2020, 13, 1277. [Google Scholar] [CrossRef]
- Przysowa, R.; Gawron, B.; Białecki, T.; Łęgowik, A.; Merkisz, J.; Jasiński, R. Performance and Emissions of a Microturbine and Turbofan Powered by Alternative Fuels. Aerospace 2021, 8, 25. [Google Scholar] [CrossRef]
- Suchocki, T.; Witanowski, Ł.; Lampart, P.; Kazimierski, P.; Januszewicz, K.; Gawron, B. Experimental investigation of performance and emission characteristics of a miniature gas turbine supplied by blends of kerosene and waste tyre pyrolysis oil. Energy 2021, 215, 119125. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion—Concepts and Applications; McGraw-Hill: New York, NY, USA, 2012; ISBN 9780073380193. [Google Scholar]
- Yilmaz, N.; Atmanli, A. Experimental evaluation of a diesel engine running on the blends of diesel and pentanol as a next generation higher alcohol. Fuel 2017, 210, 75–82. [Google Scholar] [CrossRef]
- 1-Hexanol. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 2 December 2024).

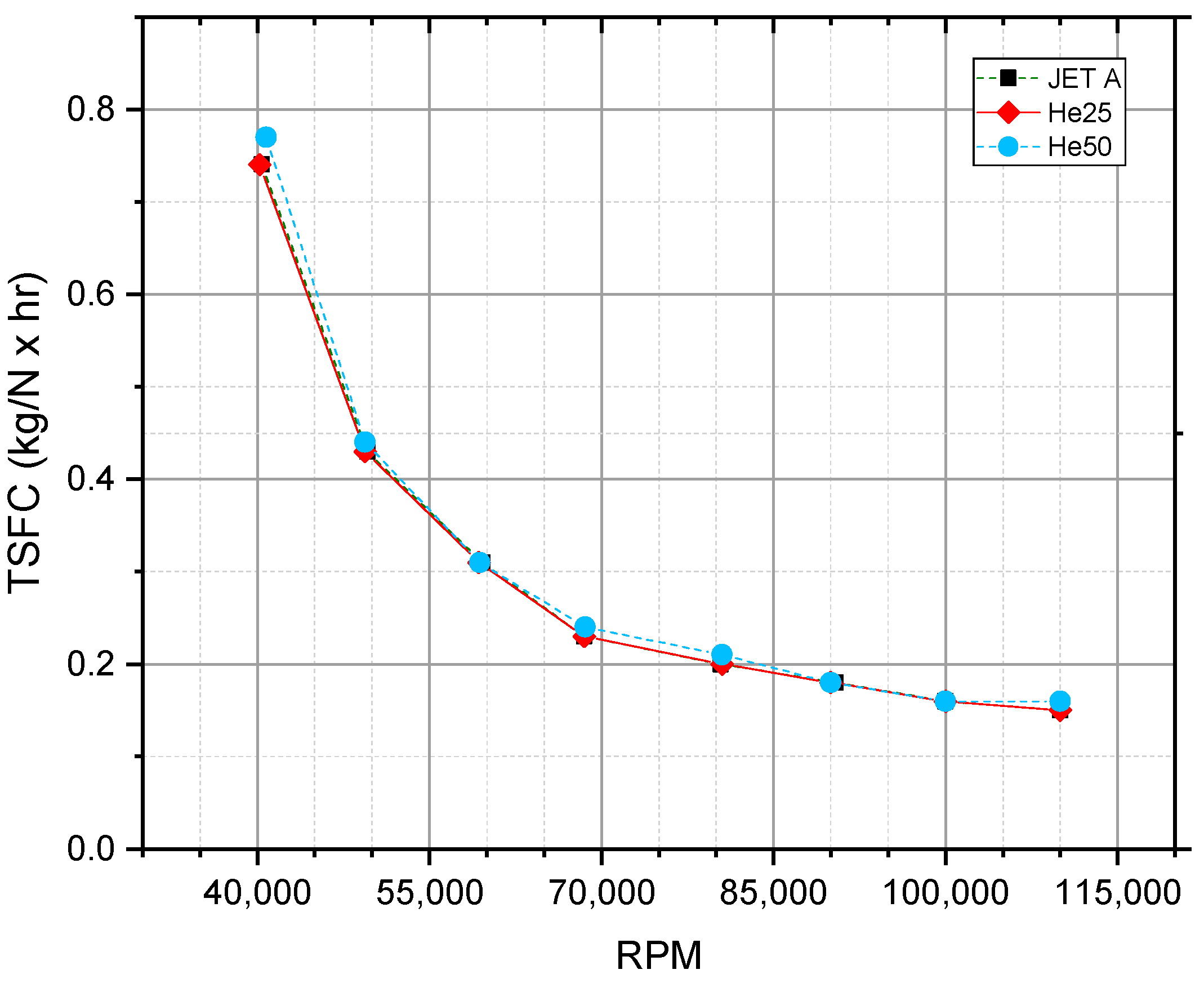
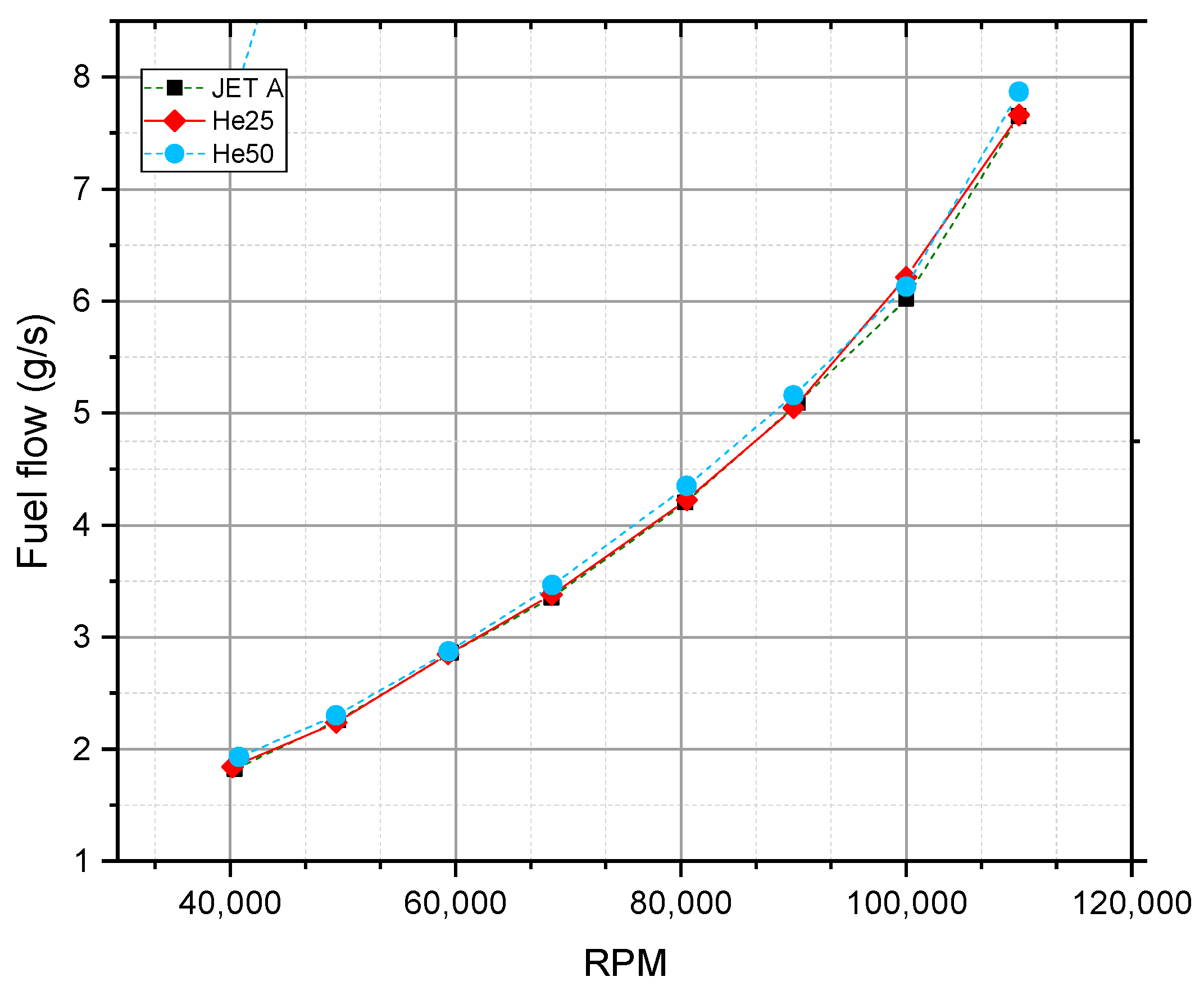
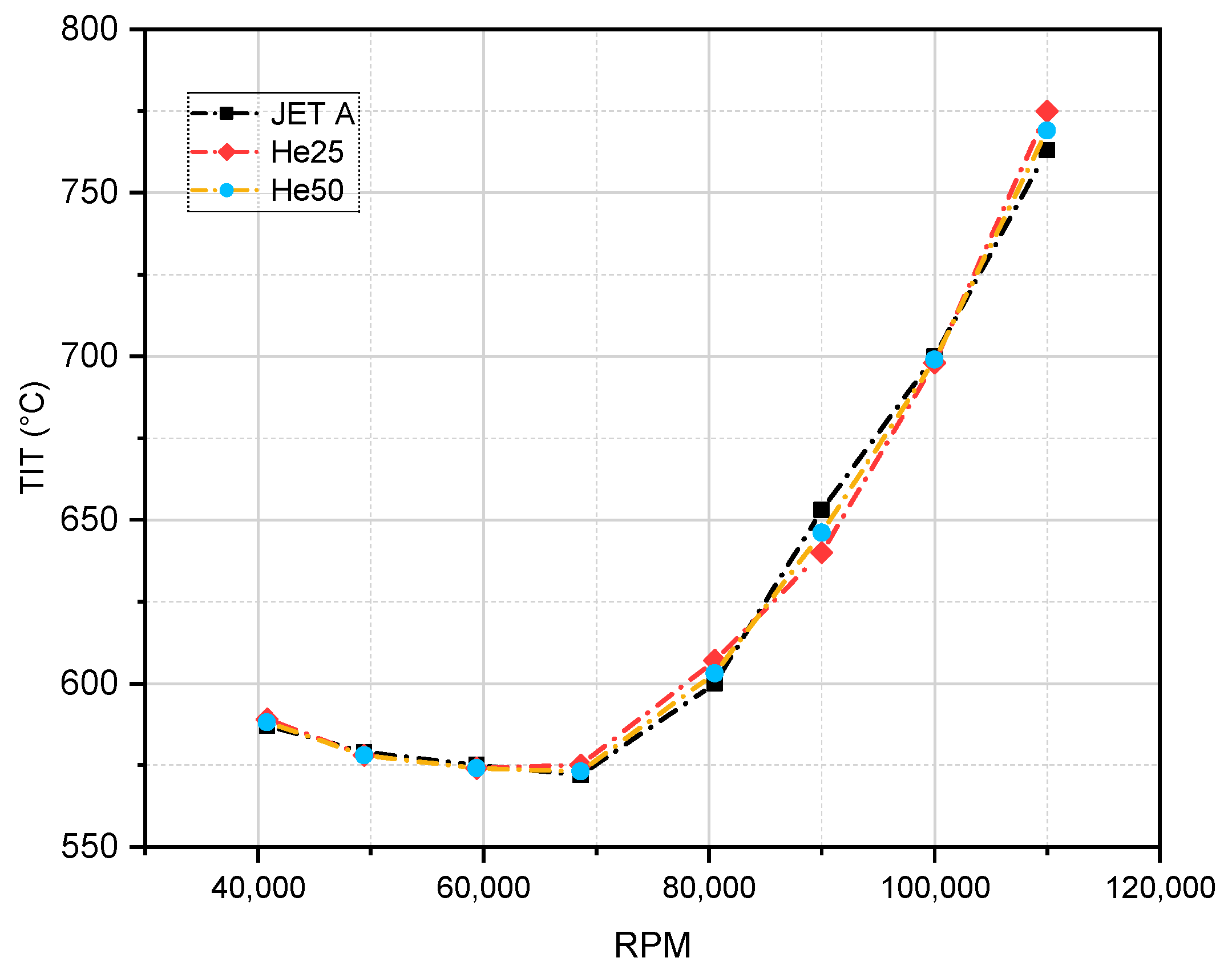
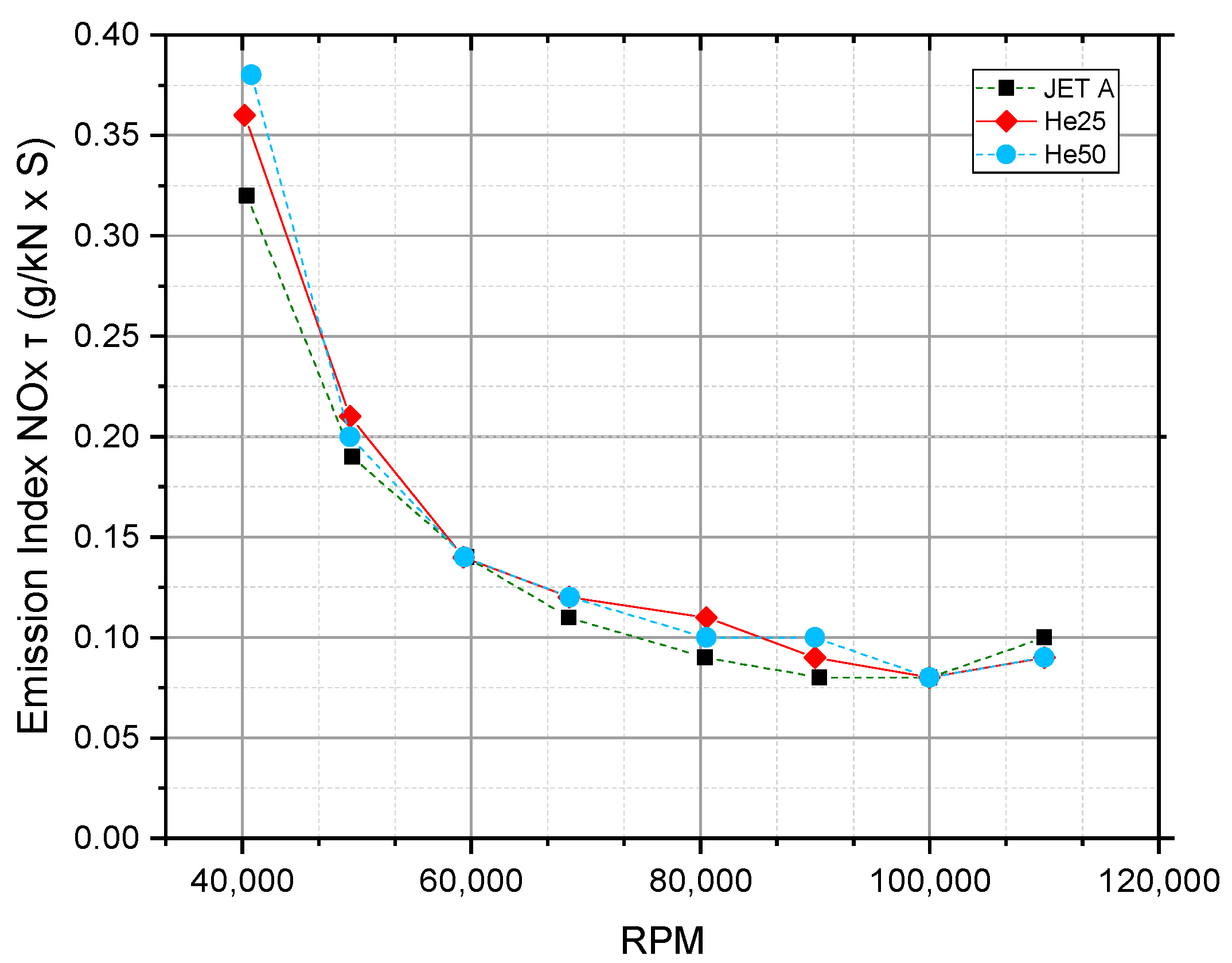
| Reference, Year | Tested Fuel | Test Setup | Conclusion Higher Alcohols Fraction Led to: | |
|---|---|---|---|---|
| Performance | Emission | |||
| Mendez et al. [48] 2012 | Ethanol (E25-E75) | Thrust ↑ TSFC ↑ TIT/TOT/EGT = | ↓ ↓ | |
| Sallevelt et al. [51] 2014 | Ethanol | OPRA’s 2 MWe class OP 16 gas turbine combustor Range 29–54 KW | Fully combusted Power ↑ | CO = NO ↓ |
| Buffi et al. [52] 2018 | Liquefied Wood + Denatured Ethanol (LW/EtOH 75/25; 50/50 wt%) | GTP 30–67 | TIT/TOT/EGT ↑ | ↓ ↓ ↑ |
| Chen et al. [49] 2017 | Butyl Butyrate/Ethanol (ethanol fraction 0–50%) | Combustion chamber is fabricated based on an aero-engine | TSFC ↑ TIT/TOT/EGT = | ↓ ↓ ↑ |
| Buffi et al. [50] 2018 | Pyrolysis Oil/Ethanol (PO/EtOH 20/80%; 50/50%) | GTP 30–67 | TSFC ↑ TIT/TOT/EGT = | ↓ ↓ |
| Mendez et al. [47] 2011 | Propanol (P25-P100) | Small-scale gas turbine 178 N | TSFC ↑ TIT/TOT/EGT ↑ | ↓ ↓ |
| Mendez et al. [53] 2014 | Butanol (B50-B100) | TSFC ↑ TIT/TOT/EGT = | ↓ ↓ | |
| Suchocki et al. [3] 2023 | Pentanol (Pe25-Pe100) | JETPOL GTM140 140 N | TSFC ↓ TIT/TOT/EGT ↓ | ↓ ↓ |
| This work 2024 | Hexanol (He25-He50) | JETPOL GTM160 180 N | TSFC = TIT/TOT/EGT ↓ | ↓ ↑ |
| Parameter | Specification |
|---|---|
| Design Maximum Thrust | 160 N, up to 170 N |
| Air Mass Flow Rate | 0.4 kg/s |
| Compression Ratio | 3:1 |
| Specific Fuel Consumption | 7.0 g/s (Experimental: 400 g/min at max thrust) |
| Compressor | Single-stage centrifugal flow (radial outflow) |
| Turbine | Single-stage axial flow |
| Maximum RPM | 120,000 RPM |
| Minimum RPM | 33,000 RPM (Experimental) |
| Max Turbine Inlet Temp (TIT) | 700 °C |
| Max Exhaust Gas Temp (EGT) | 550 °C |
| Lubrication | 3–5% Aeroshell in fuel (JET A) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchocki, T. Performance and Emission Characteristics of a Small Gas Turbine Engine Using Hexanol as a Biomass-Derived Fuel. Materials 2024, 17, 6011. https://doi.org/10.3390/ma17236011
Suchocki T. Performance and Emission Characteristics of a Small Gas Turbine Engine Using Hexanol as a Biomass-Derived Fuel. Materials. 2024; 17(23):6011. https://doi.org/10.3390/ma17236011
Chicago/Turabian StyleSuchocki, Tomasz. 2024. "Performance and Emission Characteristics of a Small Gas Turbine Engine Using Hexanol as a Biomass-Derived Fuel" Materials 17, no. 23: 6011. https://doi.org/10.3390/ma17236011
APA StyleSuchocki, T. (2024). Performance and Emission Characteristics of a Small Gas Turbine Engine Using Hexanol as a Biomass-Derived Fuel. Materials, 17(23), 6011. https://doi.org/10.3390/ma17236011






