Impact of Water Purity and Oxygen Content in Gas Phase on Effectiveness of Surface Cleaning with Microbubbles
Abstract
1. Introduction
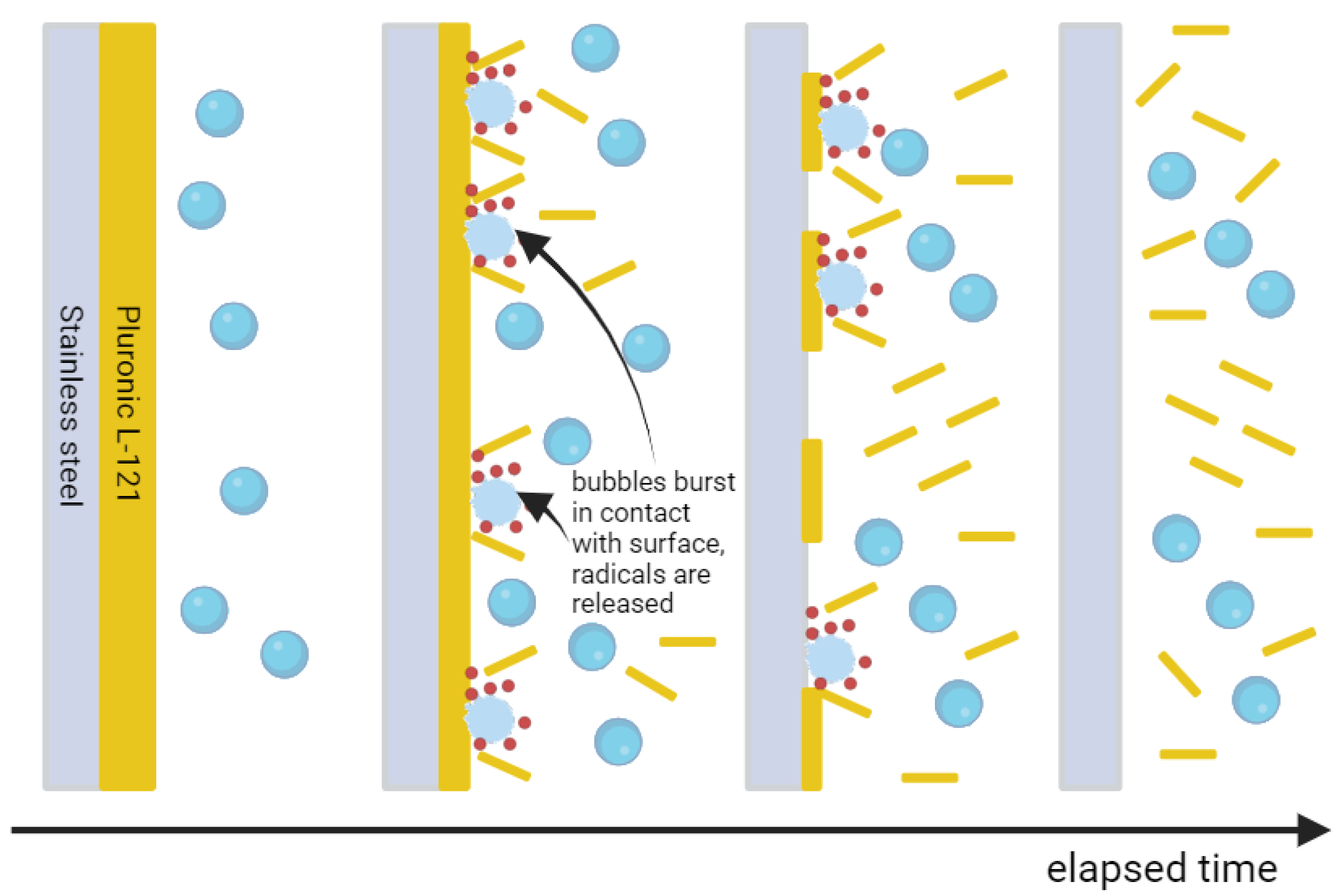
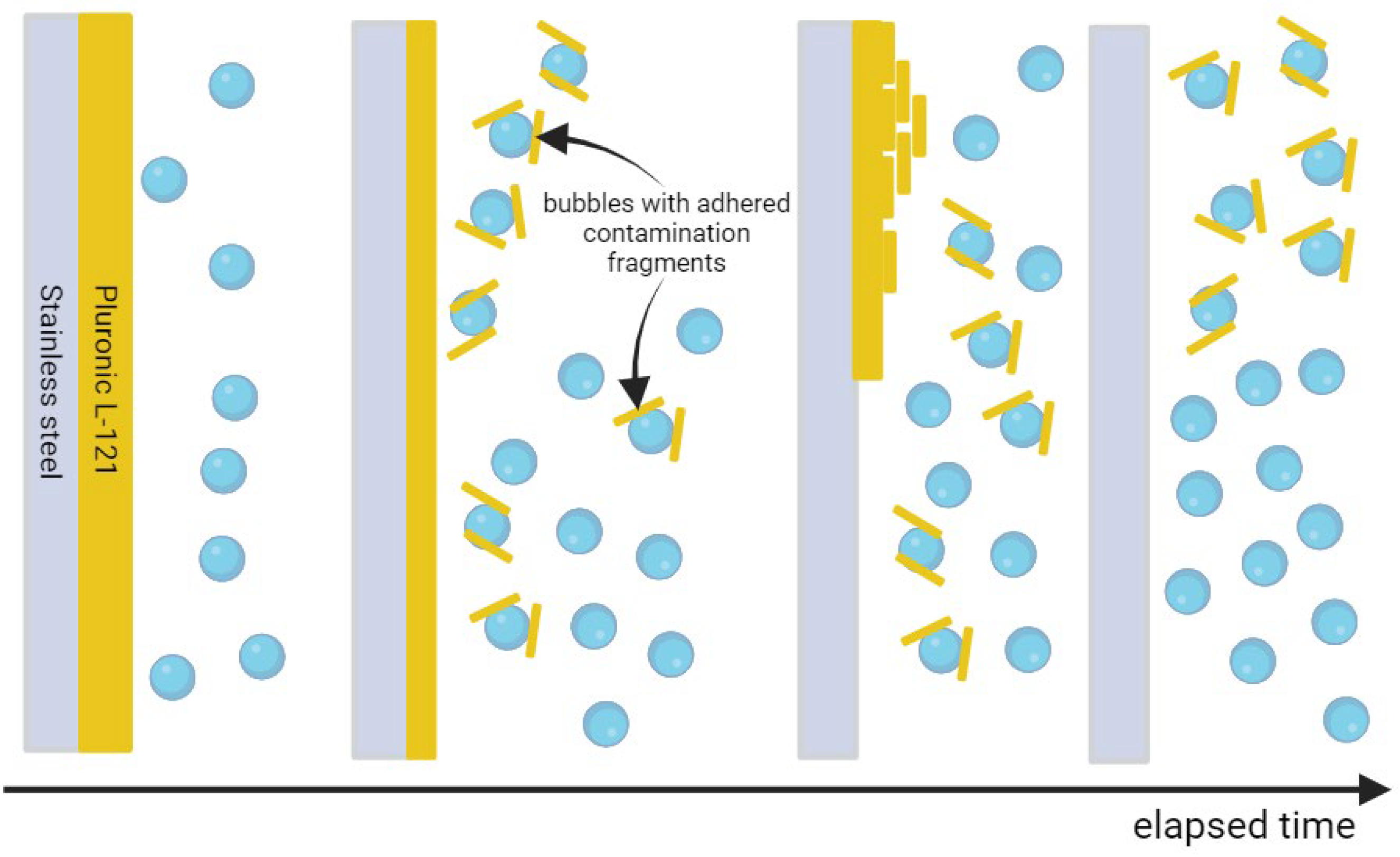
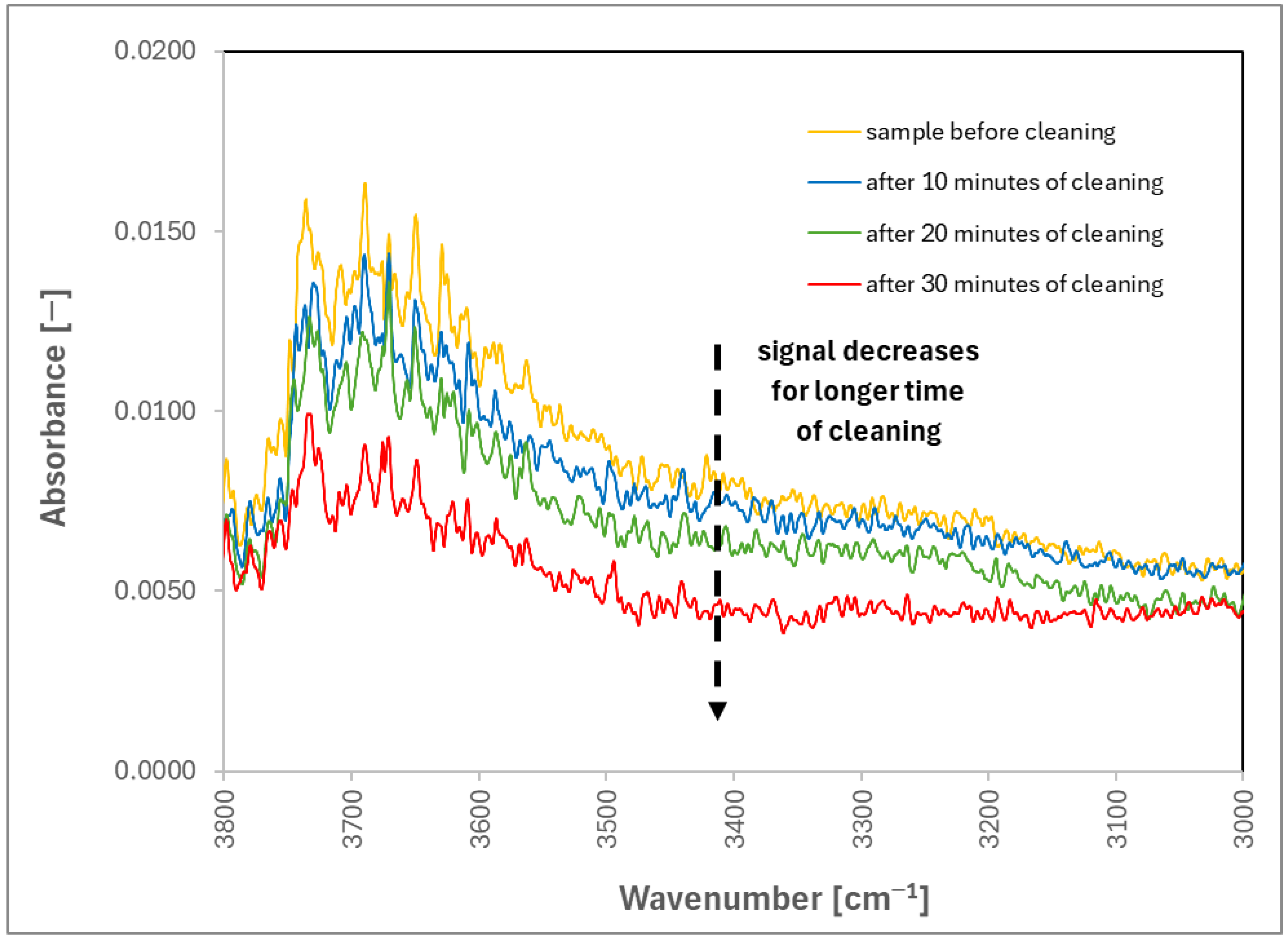
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Contaminant Solutions
3.2. Contaminated-Stainless-Steel Preparation
3.3. Water Preparation
3.4. Cleaning Procedure
3.5. Sample Range
3.6. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.H.; Zhang, X.H.; Zhang, X.D.; Sun, J.L.; Dong, Y.M.; Hu, J. In Situ AFM Observation of BSA Adsorption on HOPG with Nanobubble. Chin. Sci. Bull. 2007, 52, 1913–1919. [Google Scholar] [CrossRef]
- Wu, Z.H.; Zhang, X.; Zhang, X.; Li, G.; Sun, J.; Zhang, Y.; Li, M.; Hu, J. Nanobubbles Influence on BSA Adsorption on Mica Surface. Surf. Interface Anal. 2006, 38, 990–995. [Google Scholar] [CrossRef]
- Chen, H.; Mao, H.; Wu, L.; Zhang, J.; Dong, Y.; Wu, Z.; Hu, J. Defouling and Cleaning Using Nanobubbles on Stainless Steel. Biofouling 2009, 25, 353–357. [Google Scholar] [CrossRef]
- Zhu, J.; An, H.; Alheshibri, M.; Liu, L.; Terpstra, P.M.J.; Liu, G.; Craig, V.S.J. Cleaning with Bulk Nanobubbles. Langmuir 2016, 32, 11203–11211. [Google Scholar] [CrossRef]
- Ducker, W.A. Contact Angle and Stability of Interfacial Nanobubbles. Langmuir 2009, 25, 8907–8910. [Google Scholar] [CrossRef]
- Eslami, B.; Solares, S.D. Imaging of Surface Nanobubbles by Atomic Force Microscopy in Liquids: Influence of Drive Frequency on the Characterization of Ultrasoft Matter. Microsc. Res. Tech. 2017, 80, 41–49. [Google Scholar] [CrossRef]
- Tan, B.H.; An, H.; Ohl, C.D. Stability of Surface and Bulk Nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 53, 101428. [Google Scholar] [CrossRef]
- Manning, G.S. On the Thermodynamic Stability of Bubbles, Immiscible Droplets, and Cavities. Phys. Chem. Chem. Phys. 2020, 22, 17523–17531. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical Approach to Nanobubble Solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Attard, P. Pinning Down the Reasons for the Size, Shape, and Stability of Nanobubbles. Langmuir 2016, 32, 11138–11146. [Google Scholar] [CrossRef]
- Brenner, M.P.; Lohse, D. Dynamic Equilibrium Mechanism for Surface Nanobubble Stabilization. Phys. Rev. Lett. 2008, 101, 214505. [Google Scholar] [CrossRef]
- Xiao, Q.; Liu, Y.; Guo, Z.; Liu, Z.; Lohse, D.; Zhang, X. Solvent Exchange Leading to Nanobubble Nucleation: A Molecular Dynamics Study. Langmuir 2017, 33, 8090–8096. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of the Decrease in Surface Tension by Bulk Nanobubbles (Ultrafine Bubbles). Langmuir 2023, 39, 16574–16583. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sidorski, M.; Sobieszuk, P. Oil-Contaminated Surface Cleaning Using Oxygen and Nitrogen Nanobubbles. J. Phys. Conf. Ser. 2020, 1681, 012017. [Google Scholar] [CrossRef]
- Sayed-Ahmed, A.S. Nanobubble Column Flotation for More Efficient Coal Recovery; University of Kentucky: Lexington, KY, USA, 2013; Volume 507. [Google Scholar]
- Fan, M.; TAO, D.; Honaker, R.; Luo, Z. Nanobubble Generation and Its Applications in Froth Flotation (Part II): Fundamental Study and Theoretical Analysis. Min. Sci. Technol. 2010, 20, 159–177. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation Using a Multiphase Pump, Properties and Features in Flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, J.; Zhao, B.; Zhu, P. Microbubble Size Distribution Measurement in a DAF System. Ind. Eng. Chem. Res. 2015, 54, 5179–5183. [Google Scholar] [CrossRef]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of Ferric Hydroxide by Flotation with Micro and Nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M. Effective Parameters on Generation of Nanobubbles by Cavitation Method for Froth Flotation Applications. Physicochem. Probl. Miner. Process. 2017, 53, 920–942. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Electroflotation Process: A Review. J. Mol. Liq. 2016, 220, 657–664. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Historical Perspective Bulk Nanobubbles in the Mineral and Environmental Areas: Updating Research and Applications. Adv. Colloid Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Efthimiadou, E.K.; Mitropoulos, A.C.; Favvas, E.P. Fundamentals and Applications of Nanobubbles. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 69–99. ISBN 9780128141786. [Google Scholar]
- Gurung, A.; Dahl, O.; Jansson, K. The Fundamental Phenomena of Nanobubbles and Their Behavior in Wastewater Treatment Technologies. Geosyst. Eng. 2016, 19, 133–142. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Z.; Zeng, W.; Zhang, Y. Adhesion between Nanobubbles and Fine Cassiterite Particles. Int. J. Min. Sci. Technol. 2023, 33, 503–509. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bomis, G.; Kosheleva, R.I.; Efthimiadou, E.K.; Favvas, E.P.; Kostoglou, M.; Mitropoulos, A.C. Nanobubbles Effect on Heavy Metal Ions Adsorption by Activated Carbon. Chem. Eng. J. 2019, 356, 91–97. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Honaker, R.; Luo, Z. Nanobubble Generation and Its Application in Froth Flotation (Part I): Nanobubble Generation and Its Effects on Properties of Microbubble and Millimeter Scale Bubble Solutions. Min. Sci. Technol. 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Zhao, Y.; Honaker, R. Effect of Nanobubbles on the Flotation of Different Sizes of Coal Particle. Min. Metall. Explor. 2013, 30, 157–161. [Google Scholar] [CrossRef]
- Lu, B.; Xu, W.; Luo, C.; Li, W.; Su, X.; Song, Y.; Zhou, J.; Li, K. Effect of Nanobubbles on the Flotation Behavior of Microfine-Grained Serpentine. Minerals 2023, 13, 144–150. [Google Scholar] [CrossRef]
- Gomez-Flores, A.; Park, H.; Hong, G.; Nam, H.; Gomez-Flores, J.; Kang, S.; Heyes, G.W.; Leal Filho, L.d.S.; Kim, H.; Lee, J.M.; et al. Flotation Separation of Lithium–Ion Battery Electrodes Predicted by a Long Short-Term Memory Network Using Data from Physicochemical Kinetic Simulations and Experiments. J. Ind. Inf. Integr. 2024, 42, 100697. [Google Scholar] [CrossRef]
- Yasuda, K. Characteristics of Ultrafine Bubbles (Bulk Nanobubbles) and Their Application to Particle-Related Technology. KONA Powder Part. J. 2024, 2024, 183–196. [Google Scholar] [CrossRef]
- Qiu, J.; Zou, Z.; Wang, S.; Wang, X.; Wang, L.; Dong, Y.; Zhao, H.; Zhang, L.; Hu, J. Formation and Stability of Bulk Nanobubbles Generated by Ethanol–Water Exchange. ChemPhysChem 2017, 18, 1345–1350. [Google Scholar] [CrossRef]
- Millare, J.C.; Basilia, B.A. Nanobubbles from Ethanol-Water Mixtures: Generation and Solute Effects via Solvent Replacement Method. ChemistrySelect 2018, 3, 9268–9275. [Google Scholar] [CrossRef]
- Sharma, H.; Trivedi, M.; Nirmalkar, N. Do Nanobubbles Exist in Pure Alcohol? Langmuir 2024, 40, 1534–1543. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, Z.; Tan, T.; Wang, Y.; Zhang, L.; Hu, J.; Zhang, Y. Generating Bulk Nanobubbles in Alcohol Systems. ACS Omega 2021, 6, 2873–2881. [Google Scholar] [CrossRef]
- Sharifi, A.; Saghravani, S.F.; Ghasemipanah, K.; Dahrazma, B.; Rasekh, B. Evaluation of the Performance of Air Micro-Nano Bubbles for Cleaning in Place to Reduce the Reverse Osmosis Membrane Clogging. Desalin. Water Treat. 2024, 320, 100599. [Google Scholar] [CrossRef]
- Mo, J.; Lin, T.; Liu, W.; Zhang, Z.; Yan, Y. Cleaning Efficiency and Mechanism of Ozone Micro-Nano-Bubbles on Ceramic Membrane Fouling. Sep. Purif. Technol. 2024, 331, 125698. [Google Scholar] [CrossRef]
- Xie, B.; Zhou, C.; Huang, X.; Chen, J.; Ma, X.; Zhang, J. Microbubble Generation in Organic Solvents by Porous Membranes with Different Membrane Wettabilities. Ind. Eng. Chem. Res. 2021, 60, 8579–8587. [Google Scholar] [CrossRef]
- Kukizaki, M.; Wada, T. Effect of the Membrane Wettability on the Size and Size Distribution of Microbubbles Formed from Shirasu-Porous-Glass (SPG) Membranes. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 146–154. [Google Scholar] [CrossRef]
- Khirani, S.; Kunwapanitchakul, P.; Augier, F.; Guigui, C.; Guiraud, P.; Hébrard, G. Microbubble Generation through Porous Membrane under Aqueous or Organic Liquid Shear Flow. Ind. Eng. Chem. Res. 2012, 51, 1997–2009. [Google Scholar] [CrossRef]
- Terasaka, K.; Hirabayashi, A.; Nishino, T.; Fujioka, S.; Kobayashi, D. Development of Microbubble Aerator for Waste Water Treatment Using Aerobic Activated Sludge. Chem. Eng. Sci. 2011, 66, 3172–3179. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.H.; Liao, H.L.; Jiang, W.T.; Luo, Q.; Chu, G.W.; Luo, Y. HiGee Microbubble Generator: (II) Controllable Preparation of Microbubbles. Ind. Eng. Chem. Res. 2022, 61, 16832–16842. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Liao, Z.; Ueda, Y.; Yoshikawa, K.; Zhang, G. Microbubbles for Effective Cleaning of Metal Surfaces Without Chemical Agents. Langmuir 2022, 38, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Zhang, F.; Cui, Y.; Sun, L.; Gao, H.; Pu, Z.; Yang, W. Environment-Friendly Surface Cleaning Using Micro-Nano Bubbles. Particuology 2022, 66, 1–9. [Google Scholar] [CrossRef]
- Woo, J.; Kim, Y.; Park, H.; Kim, H. Effective and Environment-Friendly Oil Removal with Microbubble Jet. Sep. Purif. Technol. 2025, 357, 130076. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sobieszuk, P.; Mróz, A.; Ciach, T. Stability of Nanobubbles Generated in Water Using Porous Membrane System. Chem. Eng. Process. Process Intensif. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Jin, F.; Li, J.; Ye, X.; Wu, C. Effects of PH and Ionic Strength on the Stability of Nanobubbles in Aqueous Solutions of α-Cyclodextrin. J. Phys. Chem. B 2007, 111, 11745–11749. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K. Mechanism for Stability of Ultrafine Bubbles. Jpn. J. Multiph. Flow 2016, 30, 19–26. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W.; Kato, K. Dynamic Equilibrium Model for a Bulk Nanobubble and a Microbubble Partly Covered with Hydrophobic Material. Langmuir 2016, 32, 11101–11110. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Sun, P.; Wang, M.; Lin, F.; Fiallos, M.; Khu, S.T. Generation Mechanism of Hydroxyl Free Radicals in Micro–Nanobubbles Water and Its Prospect in Drinking Water. Processes 2024, 12, 683. [Google Scholar] [CrossRef]
- Weijs, J.H.; Seddon, J.R.T.; Lohse, D. Diffusive Shielding Stabilizes Bulk Nanobubble Clusters. ChemPhysChem 2012, 13, 2197–2204. [Google Scholar] [CrossRef] [PubMed]
- Bunkin, N.F.; Yurchenko, S.O.; Suyazov, N.V.; Shkirin, A.V. Structure of the Nanobubble Clusters of Dissolved Air in Liquid Media. J. Biol. Phys. 2012, 38, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Bunkin, N.F.; Bunkin, F.V. Bubbstons: Stable Microscopic Gas Bubbles in Very Dilute Electrolytic Solutions. Sov. Phys. JETP 1992, 74, 271–278. [Google Scholar]
- Takahashi, M. ζ Potential of Microbubbles in Aqueous Solutions: Electrical Properties of the Gas−Water Interface. J. Phys. Chem. B 2005, 109, 21858–21864. [Google Scholar] [CrossRef]
- Boshenyatov, B.V.; Kosharidze, S.I.; Levin, Y.K. On the Stability of Nanobubbles in Water. Russ. Phys. J. 2019, 61, 1914–1921. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average Size and Zeta Potential of Nanobubbles in Different Reagent Solutions. J. Nanopart. Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, M.G.; Kim, J.D. Zeta Potential of Nanobubbles Generated by Ultrasonication in Aqueous Alkyl Polyglycoside Solutions. J. Colloid Interface Sci. 2000, 223, 285–291. [Google Scholar] [CrossRef]
- Nassauer, J.; Kessler, H.G. The Effect of Electrostatic Phenomena on the Cleaning of Surfaces. Chem. Eng. Process. 1986, 20, 43–52. [Google Scholar] [CrossRef]
- Firouzi, M.; Howes, T.; Nguyen, A.V. A Quantitative Review of the Transition Salt Concentration for Inhibiting Bubble Coalescence. Adv. Colloid Interface Sci. 2015, 222, 305–318. [Google Scholar] [CrossRef]
- Christenson, H.K.; Bowen, R.E.; Carlton, J.A.; Denne, J.R.M.; Lu, Y. Electrolytes That Show a Transition to Bubble Coalescence Inhibition at High Concentrations. J. Phys. Chem. C 2008, 112, 794–796. [Google Scholar] [CrossRef]
- Craig, V.S.J.; Ninham, B.W.; Pashley, R.M. The Effect of Electrolytes on Bubble Coalescence in Water. J. Phys. Chem. 1993, 97, 10192–10197. [Google Scholar] [CrossRef]
- Sjogreen, C.A.; Landínez Téllez, D.A.; Rosas Pérez, J.E.; Plazas Hurtado, P.C.; Roa-Rojas, J. Experimental Study of Nanobubbles in Salt Solutions. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2018, 42, 41–48. [Google Scholar] [CrossRef]
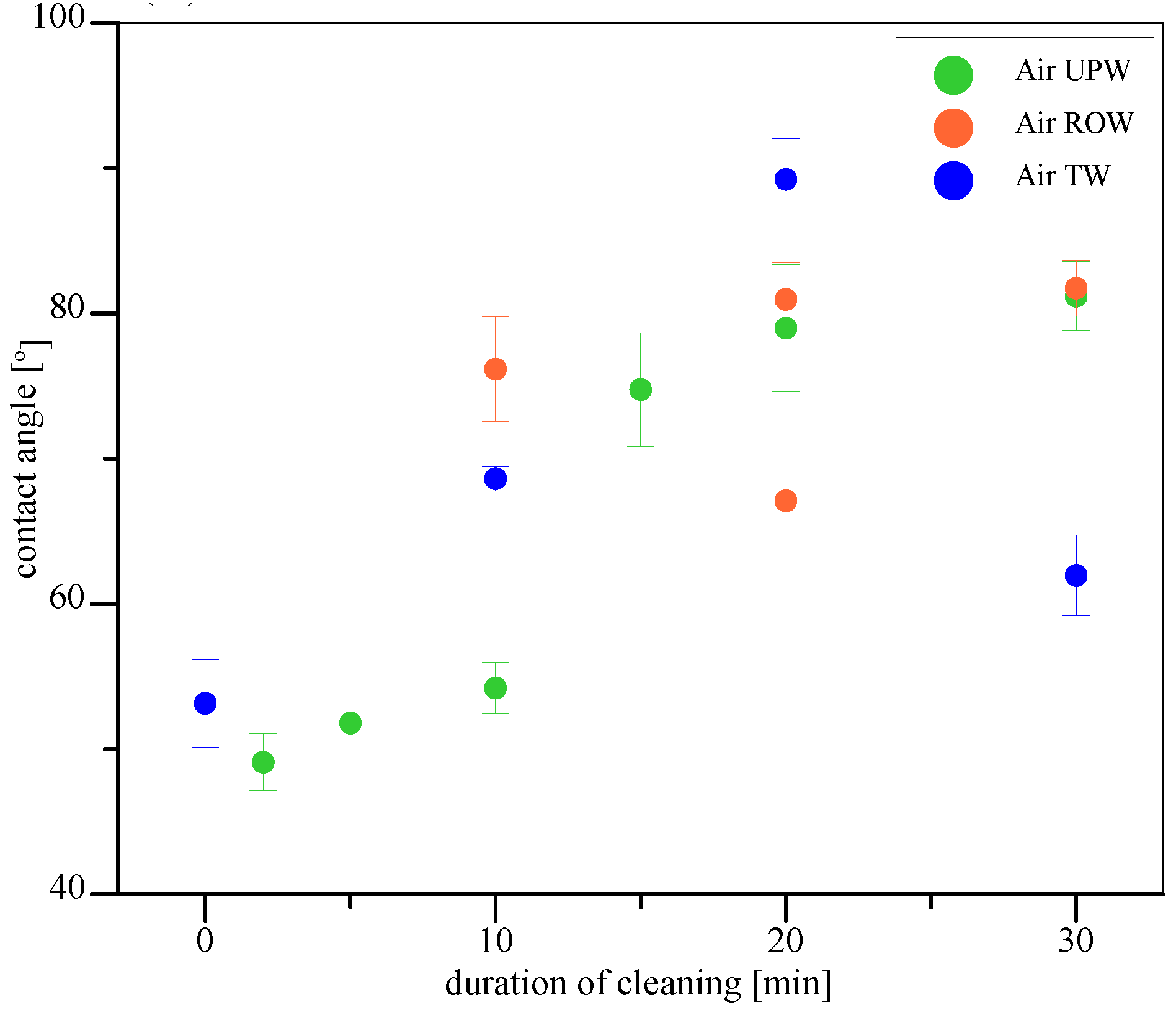

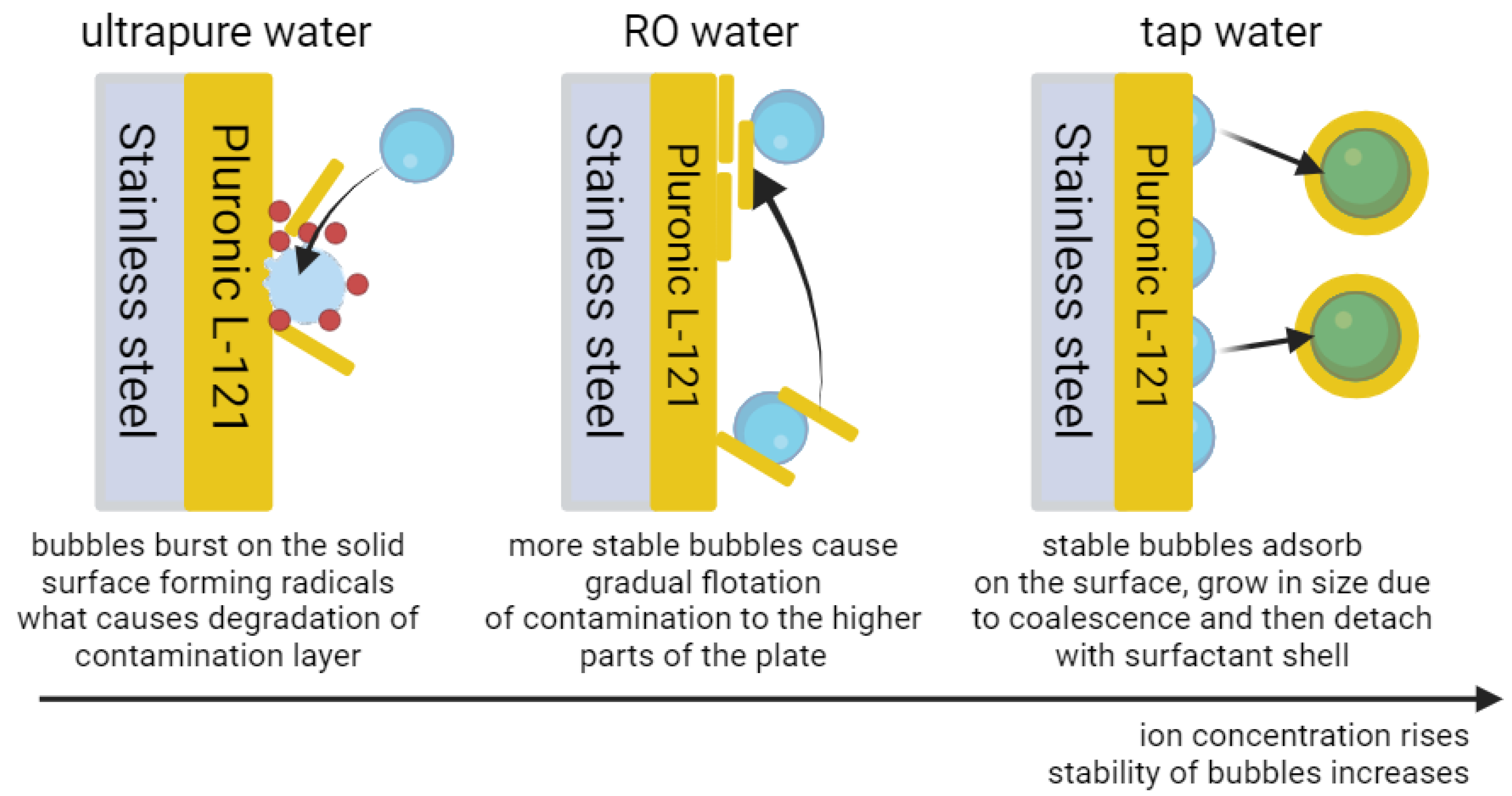
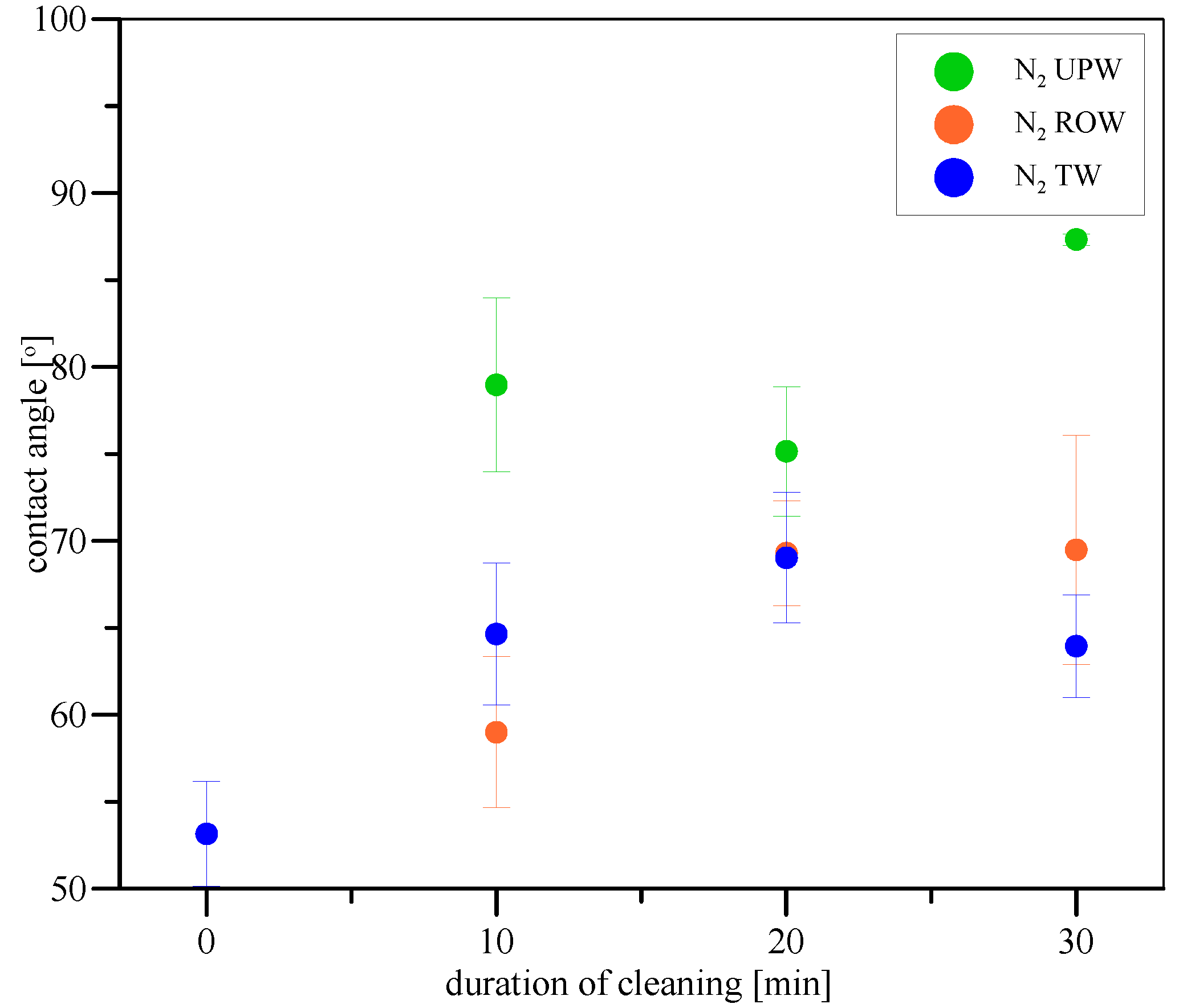

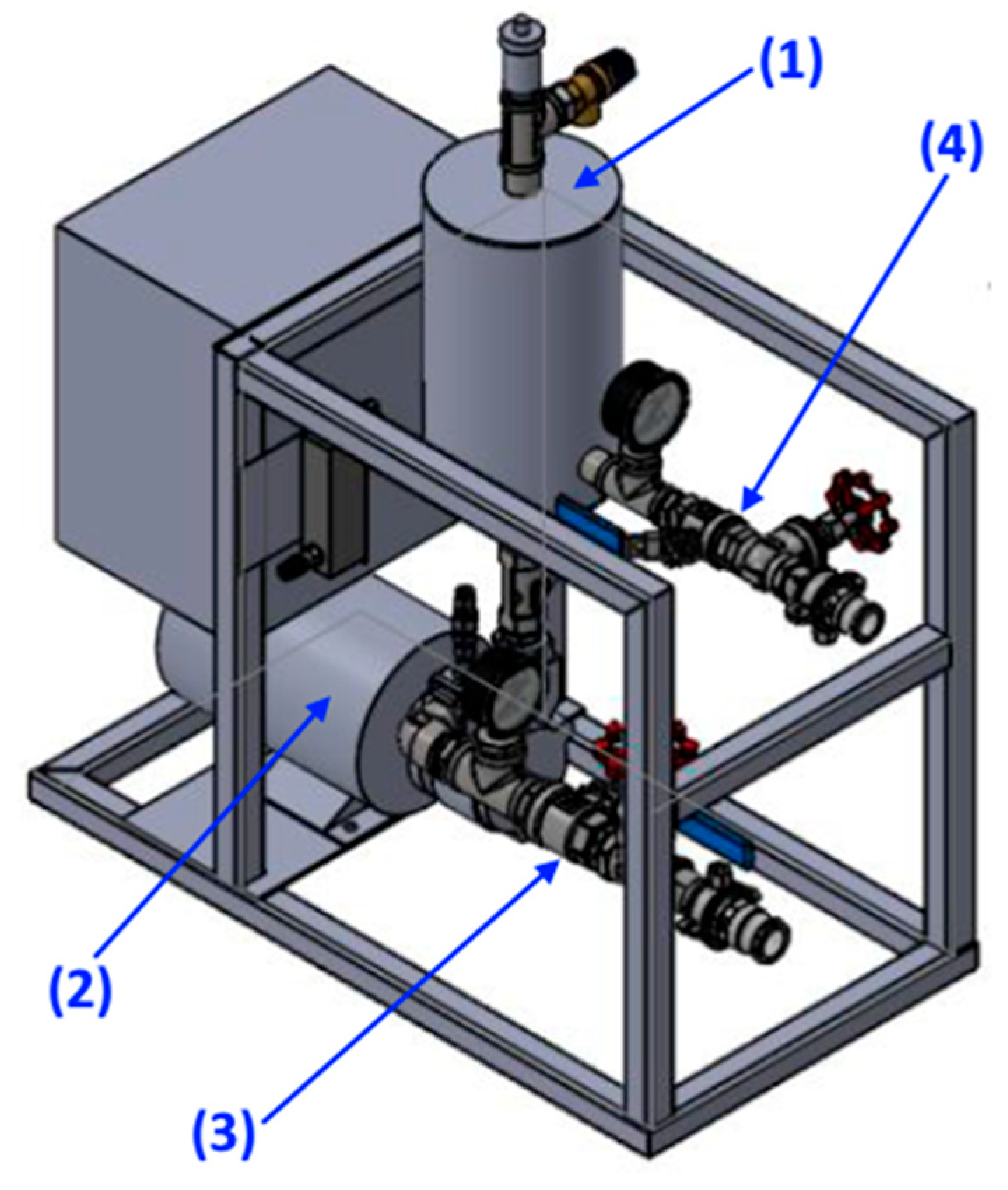
| Sample No. | Water Used | Microbubbles Used | Median Contact Angle [°] | Notes | |
|---|---|---|---|---|---|
| 1 | N/A | N/A | N/A | Purified stainless steel without contamination | |
| 2 | N/A | N/A | N/A | Samples with contamination before cleaning | |
| 3 | UPW | N/A | 30 | Contaminated samples immersed in given water without microbubble generation | |
| 4 | ROW | N/A | 30 | ||
| 5 | TW | N/A | 30 | ||
| 6 | UPW | 2 | |||
| 7 | UPW | 5 | |||
| 8 | UPW | 10 | |||
| 9 | UPW | 15 | |||
| 10 | UPW | 20 | |||
| 11 | UPW | 30 | |||
| 12 | ROW | 10 | |||
| 13 | ROW | 20 | Two distinct contact angle values were measured for different parts of the investigated plates | ||
| 14 | ROW | 30 | |||
| 15 | TW | 10 | |||
| 16 | TW | 20 | |||
| 17 | TW | 30 | |||
| 18 | UPW | 10 | |||
| 19 | UPW | 20 | |||
| 20 | UPW | 30 | |||
| 21 | ROW | 10 | |||
| 22 | ROW | 20 | |||
| 23 | ROW | 30 | |||
| 24 | TW | 10 | |||
| 25 | TW | 20 | |||
| 26 | TW | 30 | |||
| 27 | UPW | 10 | Two distinct contact angle values were measured for different parts of the investigated plates | ||
| 28 | UPW | 20 | Two distinct contact angle values were measured for different parts of the investigated plates | ||
| 29 | UPW | 30 | Three distinct contact angle values were measured for different parts of the investigated plates | ||
| 30 | ROW | 10 | |||
| 31 | ROW | 20 | |||
| 32 | ROW | 30 | |||
| 33 | TW | 10 | |||
| 34 | TW | 20 | Two distinct contact angle values were measured for different parts of the investigated plates | ||
| 35 | TW | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulatowski, K.; Szczygielski, P.; Sobieszuk, P. Impact of Water Purity and Oxygen Content in Gas Phase on Effectiveness of Surface Cleaning with Microbubbles. Materials 2024, 17, 6046. https://doi.org/10.3390/ma17246046
Ulatowski K, Szczygielski P, Sobieszuk P. Impact of Water Purity and Oxygen Content in Gas Phase on Effectiveness of Surface Cleaning with Microbubbles. Materials. 2024; 17(24):6046. https://doi.org/10.3390/ma17246046
Chicago/Turabian StyleUlatowski, Karol, Patryk Szczygielski, and Paweł Sobieszuk. 2024. "Impact of Water Purity and Oxygen Content in Gas Phase on Effectiveness of Surface Cleaning with Microbubbles" Materials 17, no. 24: 6046. https://doi.org/10.3390/ma17246046
APA StyleUlatowski, K., Szczygielski, P., & Sobieszuk, P. (2024). Impact of Water Purity and Oxygen Content in Gas Phase on Effectiveness of Surface Cleaning with Microbubbles. Materials, 17(24), 6046. https://doi.org/10.3390/ma17246046







