Small-Scale High-Pressure Hydrogen Storage Vessels: A Review
Highlights
- Different commercial types of high-pressure hydrogen storage vessels are compared.
- The advantages and disadvantages of the manufacturing process for high-pressure hydrogen storage vessels are discussed.
- The improvement approaches for high-pressure hydrogen storage vessels are sum-marized.
- Glass pressure vessels are a promising technology for high-pressure hydrogen stor-age.
- Ideas for the development of small hydrogen storage containers are provided.
Abstract
1. Introduction
2. Traditional High-Pressure Hydrogen Storage Vessels
2.1. Type I and II Vessels
2.2. Fiber Composite Winding Gas Vessel
2.2.1. Preparation Process of Filament-Wound Gas Vessels
Molding Process of the Liners
Molding Process of Filament Winding
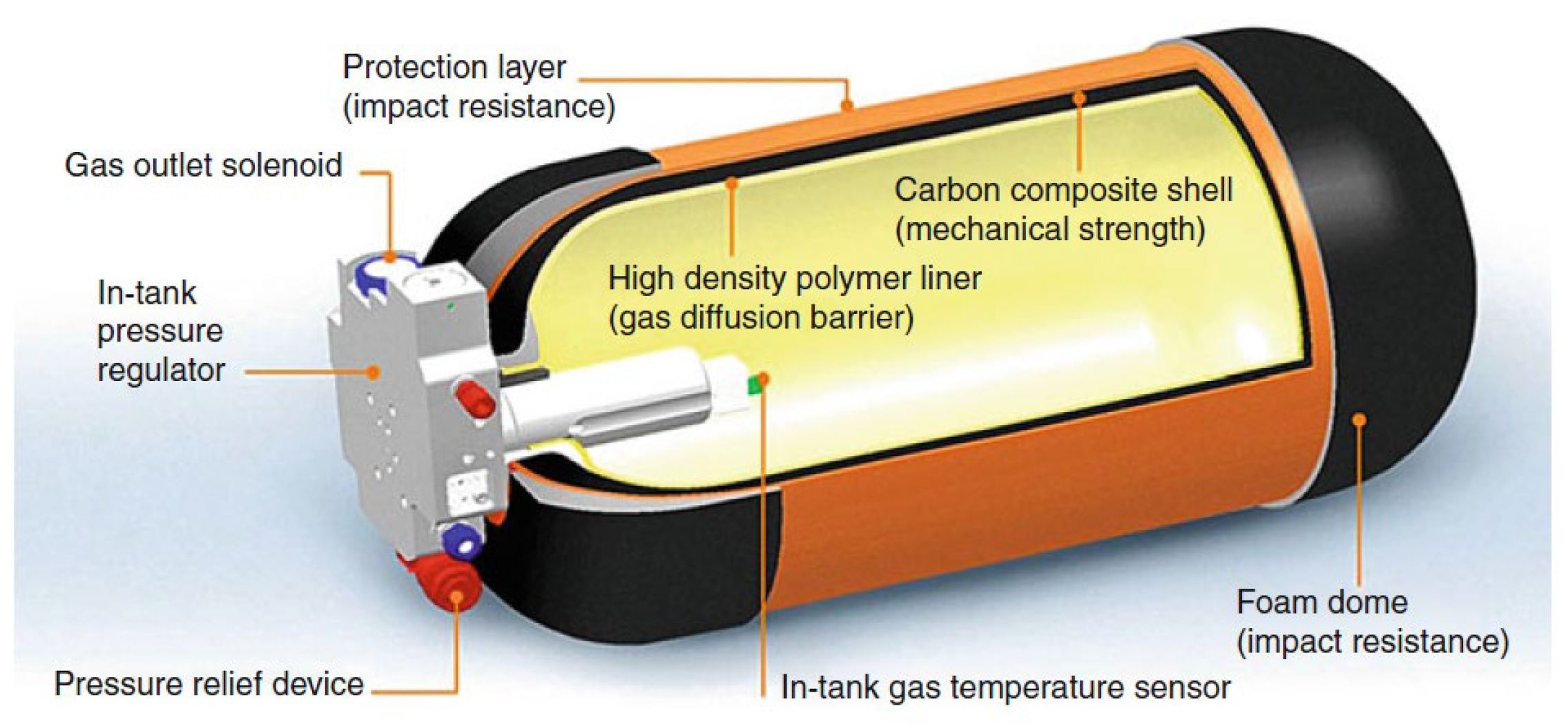
2.2.2. Material of Type IV Hydrogen Storage Vessels
- (i)
- The total thickness of the vessel wall is about 20~30 mm.
- (ii)
- Innermost gas barrier layer: In direct contact with hydrogen, with a thickness of about 2~3 mm, it is an olefin plastic polymer, which plays the role of the hydrogen barrier.
- (iii)
- Middle pressure-resistant layer: Carbon fiber-reinforced composite material (carbon fiber and epoxy resin) with the thickest layer, and the thickness of this layer is minimized to improve the hydrogen storage efficiency under the premise of ensuring the pressure-resistant grade.
- (iv)
- Outermost protective layer: Glass fiber-reinforced composite material (glass fiber and epoxy resin) with a thickness of about 2~3 mm.
Liner Materials
Carbon Fiber-Reinforced Polymer/Plastic (CFRP)
3. Novel High-Pressure Glass Hydrogen Storage Vessels
3.1. Hollow Glass Microspheres (HGM)
3.1.1. Hydrogen Charging and Discharging Mechanism of HGMs
3.1.2. Hydrogen Storage Efficiency of HGMs
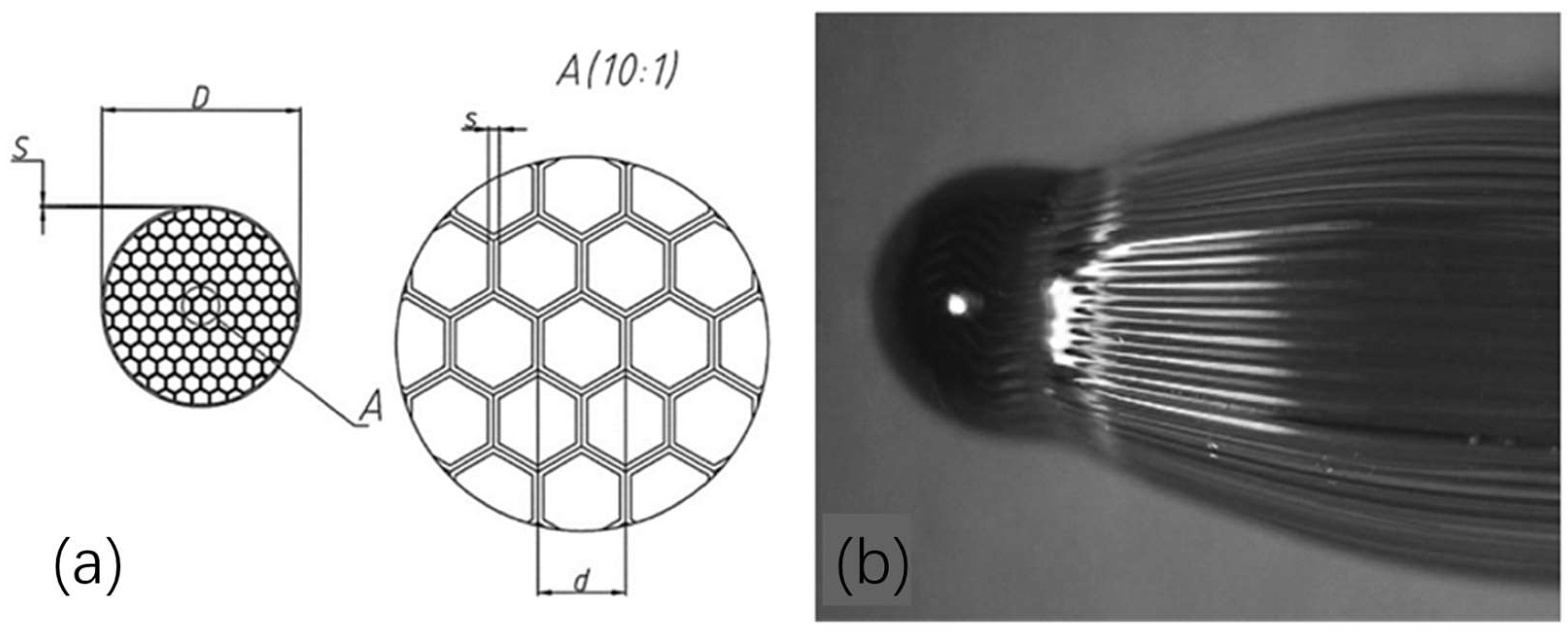
3.2. Glass Capillary Arrays
3.2.1. The Mode of Glass Capillary Array Charging and Discharging
3.2.2. Study on the Pressure Resistance of Glass Capillary Hydrogen Storage Vessels
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Jain, P.C. Greenhouse effect and climate change: Scientific basis and overview. Renew. Energy 1993, 3, 403–420. [Google Scholar] [CrossRef]
- Son, J.-Y.; Ma, K. Wind Energy Systems. Proc. IEEE 2017, 105, 2116–2131. [Google Scholar] [CrossRef]
- Schlögl, R. Put the sun in the tank: Future developments in sustainable energy systems. Angew. Chem. Int. Ed. 2019, 58, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.; Rahman, K.S.; Selvanathan, V.; Nuthammachot, N.; Suklueng, M.; Mostafaeipour, A.; Habib, A.; Akhtaruzzaman, M.; Amin, N.; Techato, K. Current trends and prospects of tidal energy technology. Environ. Dev. Sustain. 2021, 23, 8179–8194. [Google Scholar] [CrossRef] [PubMed]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable energy and fuels from biomass: A review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Mohammadshahi, S.S.; Gray EM, A.; Webb, C.J. A review of mathematical modelling of metal-hydride systems for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 3470–3484. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Zhang, X.; Han, F.; Tu, W.; Yang, W. Microalgal hydrogen production. Small Methods 2020, 4, 1900514. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zavabeti, A.; Chen, K.; Guo, Y.; Hu, G.; Fan, X.; Li, G.K. Hydrogen production from the air. Nat. Commun. 2022, 13, 5046. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Xu, P.; Liu, P.; Zhao, Y.; Yang, J. Development of high pressure gaseous hydrogen storage technologies. Int. J. Hydrogen Energy 2012, 37, 1048–1057. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Novikov, Y.V.; Rusakevich, I.V. Review on modern ways of insulation of reservoirs for liquid hydrogen storage. Int. J. Hydrogen Energy 2022, 47, 41046–41054. [Google Scholar] [CrossRef]
- Kumar, A.; Muthukumar, P.; Sharma, P.; Kumar, E.A. Absorption based solid state hydrogen storage system: A review. Sustain. Energy Technol. Assess. 2022, 52, 102204. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. Science 2021, 24, 102966. [Google Scholar] [CrossRef]
- Yamashita, A.; Kondo, M.; Goto, S.; Ogami, N. Development of High-Pressure Hydrogen Storage System for the Toyota “Mirai”. SAE Tech. Pap. 2015, 01, 1169. [Google Scholar] [CrossRef]
- The U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. 2015. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storagelightduty-vehicles (accessed on 25 January 2024).
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Ali, N.A.; Ismail, M. Advanced hydrogen storage of the Mg–Na–Al system: A review. J. Magnes. Alloys 2021, 9, 1111–1122. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The US Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, J.Y.; Liu, P.F. Finite element analysis of burst pressure of composite hydrogen storage vessels. Mater. Des. 2009, 30, 2295–2301. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, H.; Jia, X.; Zu, L.; Cheng, S.; Wang, H. Design of a 70 MPa type IV hydrogen storage vessel using accurate modeling techniques for dome thickness prediction. Compos. Struct. 2020, 236, 111915. [Google Scholar] [CrossRef]
- Magneville, B.; Gentilleau, B.; Villalonga, S.; Nony, F. Modeling, parameters identification and experimental validation of composite materials behavior law used in 700 bar type IV hydrogen high pressure storage vessel. Int. J. Hydrogen Energy 2015, 40, 13193–13205. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Barthélémy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Wulf, C.; Reuß, M.; Grube, T.; Zapp, P.; Robinius, M.; Hake, J.F.; Stolten, D. Life Cycle Assessment of hydrogen transport and distribution options. J. Clean. Prod. 2018, 199, 431–443. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Staykov, A.; Yamabe, J.; Somerday, B.P. Effect of hydrogen gas impurities on the hydrogen dissociation on iron surface. Int. J. Quantum Chem. 2014, 114, 626–635. [Google Scholar] [CrossRef]
- Mair, G.W.; Hoffmann, M. Regulations and research on RC&S for hydrogen storage relevant to transport and vehicle issues with special focus on composite containments. Int. J. Hydrogen Energy 2014, 39, 6132–6145. [Google Scholar] [CrossRef]
- ISO/TR 15916:2004; Basic Considerations for the Safety of Hydrogen Systems. ISO: Geneva, Switzerland, 2004.
- Young, K.S. Advanced composites storage containment for hydrogen. Int. J. Hydrogen Energy 1992, 17, 505–507. [Google Scholar] [CrossRef]
- Vasiliev, V.V.; Krikanov, A.A.; Razin, A.F. New generation of filament-wound composite pressure vessels for commercial applications. Compos. Struct. 2003, 62, 449–459. [Google Scholar] [CrossRef]
- Karkamkar, A.; Aardahl, C.; Autrey, T. Recent developments on hydrogen release from ammonia borane. Mater. Matters 2007, 2, 6–9. Available online: https://availabletechnologies.pnnl.gov/media/87_723200830003.pdf (accessed on 3 November 2023).
- Colom, S.; Weber, M.; Baarbier, F. Storhy: A European development of composite vessels for 70 MPa hydrogen storage. In Proceedings of the World Hydrogen Energy Conference, Brisbane, QLD, Australia, 15–19 June 2008. [Google Scholar]
- Toyota. Available online: https://global.toyota/en/newsroom/toyota/22740159.html (accessed on 25 January 2024).
- Zu, L.; Koussios, S.; Beukers, A.; Zhang, D. Development of filament wound composite isotensoidal pressure vessels. Polym. Polym. Compos. 2014, 22, 227–232. [Google Scholar] [CrossRef]
- Wang, D.; Liao, B.; Zheng, J.; Huang, G.; Hua, Z.; Gu, C.; Xu, P. Development of regulations, codes and standards on composite tanks for on-board gaseous hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 22643–22653. [Google Scholar] [CrossRef]
- Hopmann, C.; Kreimeier, S.; Schöngart, M. Laser transmission welding of foamed thermoplastic injection moulded parts. AIP Conf. Proc. 2017, 1914, 60004. [Google Scholar] [CrossRef]
- Xie, P.; Zhu, J. The trend in innovation and development of global injection molding industry from K-SHOW 2019. China Plast. 2020, 34, 111e6. [Google Scholar] [CrossRef]
- McWhorter, S. Load-Sharing Polymeric Liner for Hydrogen Storage Composite Tanks; Savannah River National Laboratory: Jackson, SC, USA, 2014. Available online: https://www.hydrogen.energy.gov/pdfs/review14/st112_mcwhorter_2014_p.pdf (accessed on 3 November 2023).
- Xu, G. Technology and application of rotational molding technology in steel lined plastic storage tank. China Rubber Plast. Technol. Equip. 2021, 47, 15–19. [Google Scholar] [CrossRef]
- Morozov, E.V. Application of the boundary-layer theory to the analysis of composite shells of revolution. Compos. Struct. 2001, 54, 261–265. [Google Scholar] [CrossRef]
- Cohen, D.; Mantell, S.C.; Zhao, L. The effect of fiber volume fraction on filament wound composite pressure vessel strength. Compos. Part B Eng. 2001, 32, 413–429. [Google Scholar] [CrossRef]
- Edgecombe, B. Next Generation Hydrogen Storage Vessels Enabled by Carbon Fiber Infusion with a Low Viscosity, High Toughness System; Materia, Inc.: Pasadena, CA, USA, 2018. [Google Scholar] [CrossRef]
- Kumar, A.A.; Alagar, M.; Rao, R.M. Studies on thermal and morphological behavior of siliconized epoxy bismaleimide matrices. J. Appl. Polym. Sci. 2001, 81, 2330–2346. [Google Scholar] [CrossRef]
- Ramirez, J.P.B.; Halm, D.; Grandidier, J.C.; Villalonga, S.; Nony, F. 700 bar type IV high pressure hydrogen storage vessel burst—Simulation and experimental validation. Int. J. Hydrogen Energy 2015, 40, 13183–13192. [Google Scholar] [CrossRef]
- Bie, H.; Li, X.; Liu, P.; Liu, Y.; Xu, P. Fatigue life evaluation of high pressure hydrogen storage vessel. Int. J. Hydrogen Energy 2010, 35, 2633–2636. [Google Scholar] [CrossRef]
- Son, D.S.; Chang, S.H. Evaluation of modeling techniques for a type III hydrogen pressure vessel (70 MPa) made of an aluminum liner and a thick carbon/epoxy composite for fuel cell vehicles. Int. J. Hydrogen Energy 2012, 37, 2353–2369. [Google Scholar] [CrossRef]
- Onder, A.; Sayman, O.; Dogan, T.; Tarakcioglu, N. Burst failure load of composite pressure vessels. Compos. Struct. 2009, 89, 159–166. [Google Scholar] [CrossRef]
- Mertiny, P.; Ellyin, F.; Hothan, A. An experimental investigation on the effect of multi-angle filament winding on the strength of tubular composite structures. Compos. Sci. Technol. 2004, 64, 1–9. [Google Scholar] [CrossRef]
- Krikanov, A.A. Composite pressure vessels with higher stiffness. Compos. Struct. 2000, 48, 119–127. [Google Scholar] [CrossRef]
- Rosenow, M.W.K. Wind angle effects in glass fibre-reinforced polyester filament wound pipes. Composites 1984, 15, 144–152. [Google Scholar] [CrossRef]
- Parnas, L.; Katirci, N. Design of fiber-reinforced composite pressure vessels under various loading conditions. Compos. Struct. 2002, 58, 83–95. [Google Scholar] [CrossRef]
- Adali, S.; Verijenko, V.E.; Tabakov, P.Y.; Walker, M. Optimization of Multilayered Composite Pressure Vessels Using Exact Elasticity Solution; American Society of Mechanical Engineers: New York, NY, USA, 1995. Available online: https://www.osti.gov/biblio/122539 (accessed on 3 November 2023).
- Messager, T.; Pyrz, M.; Gineste, B.; Chauchot, P. Optimal laminations of thin underwater composite cylindrical vessels. Compos. Struct. 2002, 58, 529–537. [Google Scholar] [CrossRef]
- Lin, D.T.W.; Wang, C.C.; Yang, C.Y.; Li, J.C. Inverse estimation of temperature boundary conditions with irregular shape of gas tank. Int. J. Heat Mass Transf. 2010, 53, 4651–4662. [Google Scholar] [CrossRef]
- Von Helmolt, R.; Eberle, U. Fuel cell vehicles: Status 2007. J. Power Sources 2007, 165, 833–843. [Google Scholar] [CrossRef]
- ISO 19881:2018; Gaseous Hydrogen d Land Vehicle Fuel Containers. ISO: Geneva, Switzerland, 2018.
- Melideo, D.; Baraldi, D.; Acosta-Iborra, B.; Cebolla, R.O.; Moretto, P. CFD simulations of filling and emptying of hydrogen tanks. Int. J. Hydrogen Energy 2017, 42, 7304–7313. [Google Scholar] [CrossRef]
- Saldi, Z.S.; Wen, J.X. Modeling thermal response of polymer composite hydrogen cylinders subjected to external fires. Int. J. Hydrogen Energy 2017, 42, 7513–7520. [Google Scholar] [CrossRef]
- Qin, Y.Q.; Gong, Y.; Yuan, Y.W.; Yang, Z.G. Failure analysis on leakage of hydrogen storage tank for vehicles occurring in oil circulation fatigue test. Eng. Fail. Anal. 2020, 117, 104830. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Nishimura, S. Degradation behavior of acrylonitrile butadiene rubber after cyclic high-pressure hydrogen exposure. Int. J. Hydrogen Energy 2015, 40, 2025–2034. [Google Scholar] [CrossRef]
- Baldwin, D. Development of High Pressure Hydrogen Storage Tank for Storage and Gaseous Truck Delivery; Hexagon Lincoln LLC: Lincoln, NE, USA, 2017. [Google Scholar] [CrossRef]
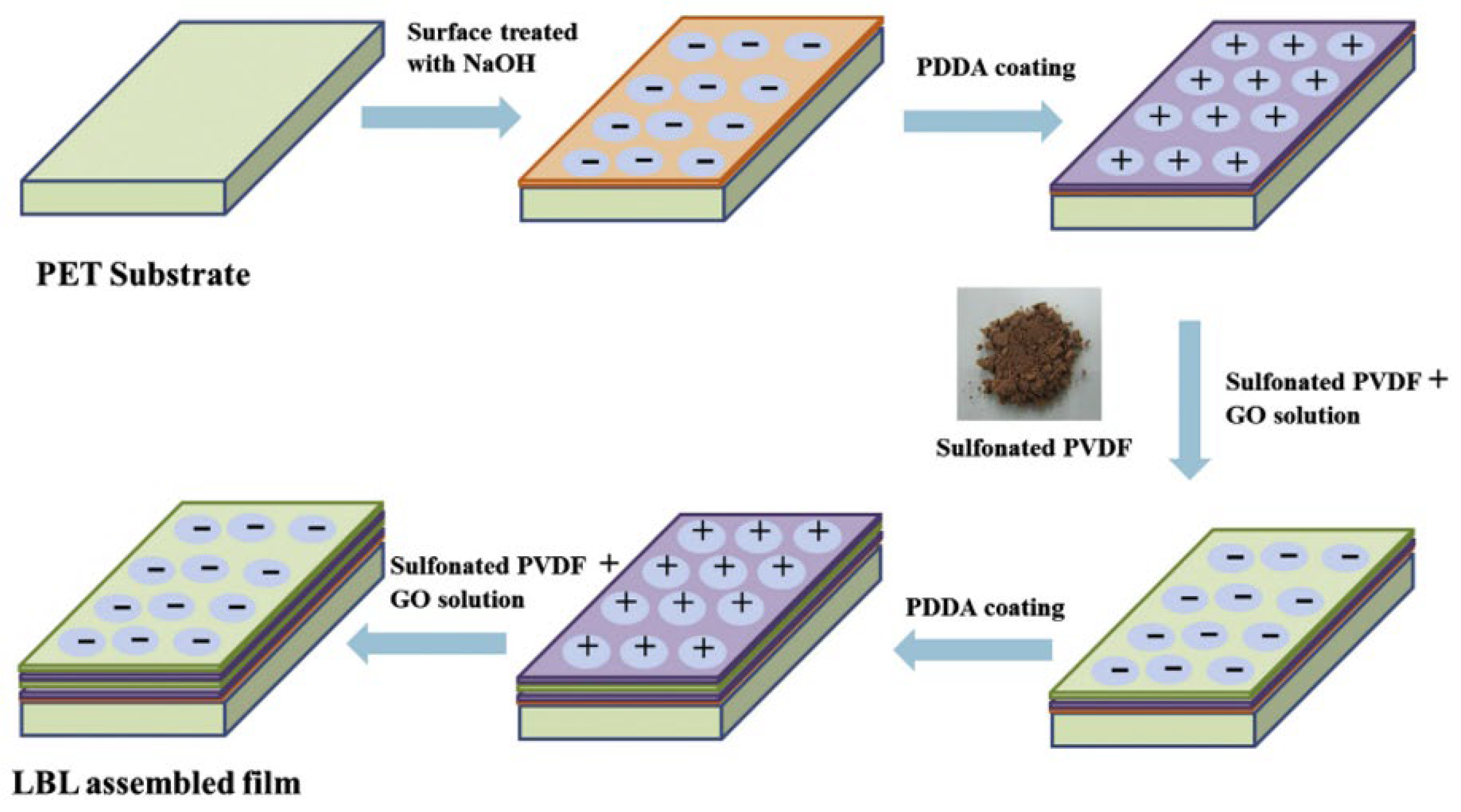
- Rajasekar, R.; Kim, N.H.; Jung, D.; Kuila, T.; Lim, J.K.; Park, M.J.; Lee, J.H. Electrostatically assembled layer-by-layer composites containing graphene oxide for enhanced hydrogen gas barrier application. Compos. Sci. Technol. 2013, 89, 167–174. [Google Scholar] [CrossRef]
- Carrera, M.C.; Erdmann, E.; Destéfanis, H.A. Barrier properties and structural study of nanocomposite of HDPE/montmorillonite modified with polyvinylalcohol. J. Chem. 2013, 2013, 679567. [Google Scholar] [CrossRef]
- Lee, S.; Choi, B.; Baek, U.; Choi, B.H. Deep learning-based detection method for analysis of high-pressure hydrogen induced damage in acrylonitrile butadiene rubber for hydrogen mobility. Mater. Des. 2023, 235, 112470. [Google Scholar] [CrossRef]
- Wolf, C.; Angellier-Coussy, H.; Gontard, N.; Doghieri, F.; Guillard, V. How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review. J. Membr. Sci. 2018, 556, 393–418. [Google Scholar] [CrossRef]
- Zhou, H. Sixteen main application areas and recent technical progress for the carbon fiber: Part 3. Tech. Text. 2017, 35, 1–7. [Google Scholar] [CrossRef]
- Kamegawa, A.; Okada, M. Hydrogen Storage Technology and High Pressure Science—Hydrogen Storage Tanks and Storage Materials. Sci. Technol. High Press. 2007, 17, 173–179. [Google Scholar] [CrossRef][Green Version]
- Ohtani, S. After the development of carbon material manufacturing technology and the present state of the art. J. Soc. Synth. Org. Chem. 1980, 38, 427–432. [Google Scholar] [CrossRef]
- Cha, J.; Jun, G.H.; Park, J.K.; Kim, J.C.; Ryu, H.J.; Hong, S.H. Improvement of modulus, strength and fracture toughness of CNT/Epoxy nanocomposites through the functionalization of carbon nanotubes. Compos. Part B Eng. 2017, 129, 169–179. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, E.; Lee, M.Y.; Lee, M.K.; Lee, Y.S. Improvement of the mechanical and thermal properties of polyethersulfone-modified epoxy composites. J. Ind. Eng. Chem. 2016, 33, 73–79. [Google Scholar] [CrossRef]
- Welter, T.; Müller, R.; Deubener, J.; Marzok, U. Hydrogen permeation through glass. Front. Mater. 2020, 6, 342. [Google Scholar] [CrossRef]
- Kohli, D.K.; Khardekr, R.K.; Singh, R.; Gupta, P.K. Glass micro-container based hydrogen storage scheme. Int. J. Hydrogen Energy 2008, 33, 417–422. [Google Scholar] [CrossRef]
- Zhevago, N.K.; Glebov, V.I. Hydrogen storage in capillary arrays. Energy Convers. Manag. 2007, 48, 1554–1559. [Google Scholar] [CrossRef]
- Budov, V.V. Hollow glass microspheres. Use, properties, and technology. Glass Ceram. 1994, 51, 230–235. [Google Scholar] [CrossRef]
- Dalai, S.; Vijayalakshmi, S.; Shrivastava, P.; Sivam, S.P.; Sharma, P. Preparation and characterization of hollow glass microspheres (HGMs) for hydrogen storage using urea as a blowing agent. Microelectron. Eng. 2014, 126, 65–70. [Google Scholar] [CrossRef]
- Shetty, S.; Hall, M. Facile production of optically active hollow glass microspheres for photo-induced outgassing of stored hydrogen. Int. J. Hydrogen Energy 2011, 36, 9694–9701. [Google Scholar] [CrossRef]
- Teitel, R.J. Microcavity Hydrogen Storage. Final Progress Report; Brookhaven National Lab.: Upton, NY, USA, 1981. [Google Scholar] [CrossRef]
- Das, L.M. On-board hydrogen storage systems for automotive application. Int. J. Hydrogen Energy 1996, 21, 789–800. [Google Scholar] [CrossRef]
- Tsugawa, R.T.; Moen, I.; Roberts, P.E.; Souers, P.C. Permeation of helium and hydrogen from glass-microsphere laser targets. J. Appl. Phys. 1976, 47, 1987–1993. [Google Scholar] [CrossRef]
- Rambach, G.D.; Hendricks, C. Hydrogen Transport and Storage in Engineered Glass Microspheres; Lawrence Livermore National Lab.: Livermore, CA, USA, 1994; pp. 237–241. Available online: https://xueshu.studiodahu.com/books?hl=zh-CN&lr=&id=7rNTAAAAMAAJ&oi=fnd&pg=PA237&dq=Hydrogen+transport+and+storage+in+engineered+glass+microspheres%5BR%5D.+Lawrence+Livermore+National+Lab.,+CA&ots=wtS5m1LH4H&sig=UEdltnz0fftTqKNanVj528sK7SM (accessed on 25 January 2024).
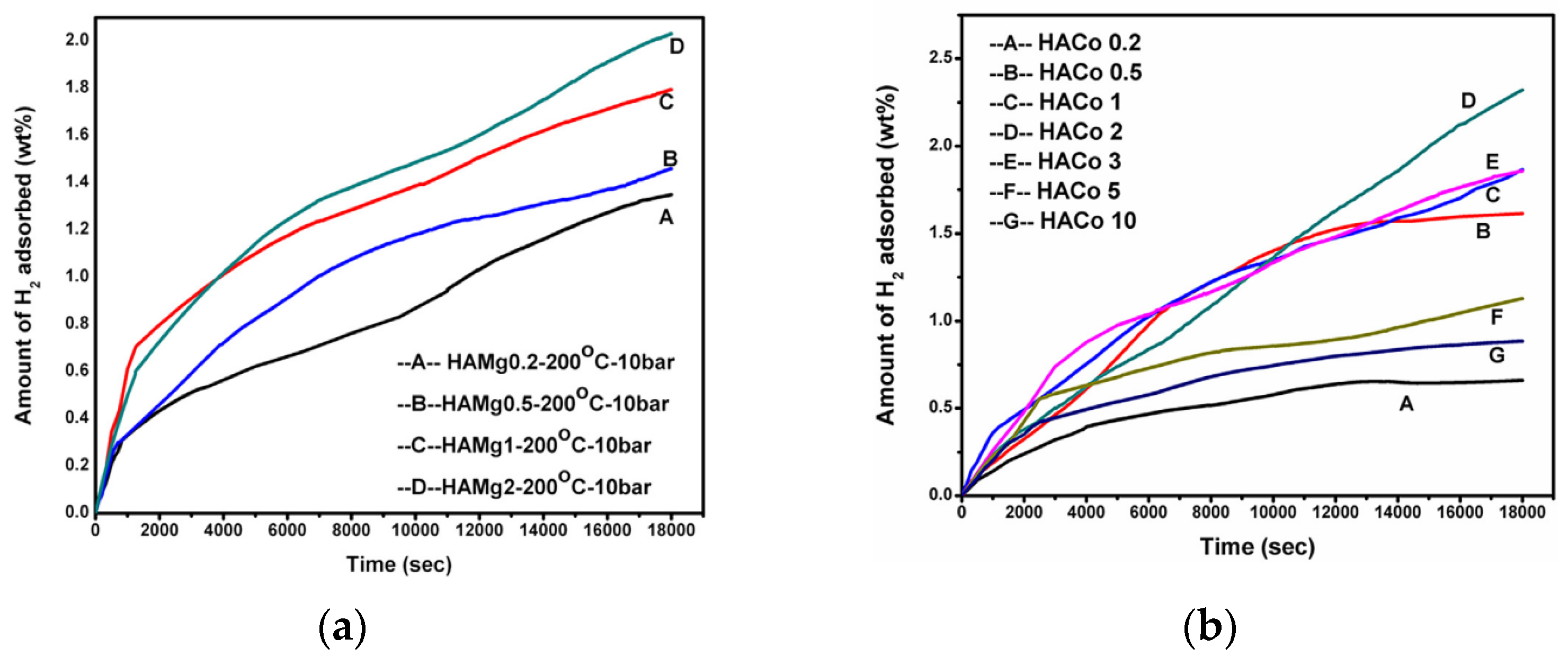
- Dalai, S.; Vijayalakshmi, S.; Sharma, P.; Choo, K.Y. Magnesium and iron loaded hollow glass microspheres (HGMs) for hydrogen storage. Int. J. Hydrogen Energy 2014, 39, 16451–16458. [Google Scholar] [CrossRef]
- Dalai, S.; Savithri, V.; Sharma, P. Investigating the effect of cobalt loading on thermal conductivity and hydrogen storage capacity of hollow glass microspheres (HGMs). Mater. Today Proc. 2017, 4, 11608–11616. [Google Scholar] [CrossRef]
- Dalai, S.; Vijayalakshmi, S.; Shrivastava, P.; Santosh, P.S.; Pratibha, S. Effect of Co loading on the hydrogen storage characteristics of hollow glass microspheres (HGMs). Int. J. Hydrogen Energy 2014, 39, 3304–3312. [Google Scholar] [CrossRef]
- Dalai, S.; Savithri, V.; Shrivastava, P.; Sivam, S.P.; Sharma, P. Fabrication of zinc-loaded hollow glass microspheres (HGMs) for hydrogen storage. Int. J. Energy Res. 2015, 39, 717–726. [Google Scholar] [CrossRef]
- Shelby, J.E.; Hall, M.M.; Snyder, M.J.; Wachtel, P.B. A Radically New Method for Hydrogen Storage in Hollow Glass Microspheres; Alfred University: Alfred, NY, USA, 2009. [Google Scholar] [CrossRef]
- Qin, F.; Brosseau, C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J. Appl. Phys. 2012, 111, 4. [Google Scholar] [CrossRef]
- Zhevago, N.K.; Gnedenko, V.; Goryachev, I.; Korobtsev, S.V. On-board hydrogen accumulator for vehicles. In Proceedings of the G8 International Forum: Hydrogen Technologies for Energy Production, Moscow, Russia, 6–10 February 2006. [Google Scholar]
- Zhevago, N.K. Other methods for the physical storage of hydrogen. In Compendium of Hydrogen Energy; Woodhead Publishing: Sawston, UK, 2016; pp. 189–218. [Google Scholar] [CrossRef]
- Meyer, R.; Holtappels, K.; Beckmann-Kluge, M.; Eliezer, D. A New Technology for Hydrogen Safety: Glass Structures as a Storage System. 2011. Available online: https://www.h2knowledgecentre.com/content/conference480 (accessed on 3 November 2023).
- Zhevago, N.K.; Chabak, A.F.; Denisov, E.I.; Glebov, V.I.; Korobtsev, S.V. Storage of cryo-compressed hydrogen in flexible glass capillaries. Int. J. Hydrogen Energy 2013, 38, 6694–6703. [Google Scholar] [CrossRef]
- Zhevago, N.K.; Denisov, E.I.; Glebov, V.I. Experimental investigation of hydrogen storage in capillary arrays. Int. J. Hydrogen Energy 2010, 35, 169–175. [Google Scholar] [CrossRef]
- Griffith, A.A. VI. The phenomena of rupture and flow in solids. Philos. Trans. R. Soc. Lond. Ser. A 1921, 221, 163–198. [Google Scholar] [CrossRef]
- Gröschl, C. Examination of Stress and Strain in Glass Structures during Pressure Treatment Using FEM Simulation and Experimental Tests. Ph.D. Dissertation, Universität Magdeburg, Magdeburg, Germany, 2016. Available online: https://d-nb.info/111857222X/34 (accessed on 3 November 2023).
- Meyer-Scherf, R. The Pressure Resistance of Hollow Glass Fibers at Internal Pressure Load. Ph.D. Dissertation, Universität Magdeburg, Magdeburg, Germany, 2015. Available online: https://141.48.10.209/bitstream/1981185920/12086/1/DissertationRMeyer-Scherf.pdf (accessed on 3 November 2023).
- Prewitz, M.; Gaber, M.; Müller, R.; Marotztke, C.; Holtappels, K. Polymer coated glass capillaries and structures for high-pressure hydrogen storage: Permeability and hydrogen tightness. Int. J. Hydrogen Energy 2018, 43, 5637–5644. [Google Scholar] [CrossRef]





| Technical Index | Hydrogen | Gasoline Vapor | Natural Gas |
|---|---|---|---|
| Explosion limit (%) | 4.1~75 | 1.4~7.6 | 5.3~15 |
| Burning point energy (MJ) | 0.02 | 0.2 | 0.29 |
| Diffusion coefficient in air (m2·s−1) | 6.11 × 10−5 | 0.55 × 10−5 | 1.61 × 10−5 |
| Energy density (MJ·kg−1) | 143 | 44 | 42 |
| Type |  |  |  |  |
|---|---|---|---|---|
| Material | All steel | Fiberglass hoop wrap, steel liner | All-carbon full wrap, metallic liner | Fiberglass/carbon full wrap, plastic liner |
| Working pressure (MPa) | 17.5–20 | 26.3–30 | 30–70 | >70 |
| Media compatibility | Hydrogen brittle, corrosive | Hydrogen brittle, corrosive | Hydrogen brittle, corrosive | Hydrogen brittle, corrosive |
| Mass hydrogen storage density (wt%) | ≈1 | ≈1.5 | ≈2.4–4.1 | ≈2.5–5.7 |
| Volumetric hydrogen storage density (g/L) | 14.28–17.23 | 14.28–17.23 | 35–40 | 38–40 |
| Service life (years) | 15 | 15 | 15–20 | 15–20 |
| Cost | Low | Moderate | Highest | High |
| Is the car available? | No | No | Yes | Yes |
| Metal | Extreme Hydrogen Embrittlement | Serious Hydrogen Embrittlement | Light Hydrogen Embrittlement | Negligible Hydrogen Embrittlement | |
|---|---|---|---|---|---|
| Aluminum | 1100 | ✔ | |||
| 6061-T6 | ✔ | ||||
| 7075-T73 | ✔ | ||||
| Be-Cu25 | ✔ | ||||
| Cu | ✔ | ||||
| Ni270 | ✔ | ||||
| Alloy steel 4140 | ✔ | ||||
| Carbon steel | 1020 | ✔ | |||
| 1042 (normalized) | ✔ | ||||
| 1042 (quenching and tempering) | ✔ | ||||
| Maraging steel, 18Ni-250 | ✔ | ||||
| Stainless | A286 | ✔ | |||
| 17-7PH | ✔ | ||||
| 304 ELC | ✔ | ||||
| 305 | ✔ | ||||
| 310 | ✔ | ||||
| 316 | ✔ | ||||
| 410 | ✔ | ||||
| 440C | ✔ | ||||
| Nickel alloys 718 | ✔ | ||||
| Ti and titanium alloys | Ti | ✔ | |||
| Ti-5Al-2.5SN (ELI) | ✔ | ||||
| Ti-6Al-4V (annealing) | ✔ | ||||
| Ti-6Al-4V (STA) | ✔ | ||||
| Molding Process | Injection Molding and Welding Molding | Blow Molding | Rotational Molding |
|---|---|---|---|
| Advantages | Dimensional stability Low cost Free design of sealing structures Thin-walled products can be prepared High impact toughness High resistance to environmental cracking | Uniform wall thickness Simple forming process Low tooling cost Large parts can be molded | High production efficiency High impact toughness High resistance to environmental cracking |
| Disadvantages | Welding required Many work processes Difficult to form large-sized structures | Poor dimensional stability Low denseness; easy to form defects High requirements for the melt flow rate High energy consumption for batch production Low production efficiency | Low tooling cost Uneven wall thickness High requirements for the melt flow rate Special structure with inserts and large-sized structures are difficult to mold The surface of the sealing part generally requires subsequent processing. |
| Winding Process | Wet Winding | Dry Winding | Semi-Dry Wingding |
|---|---|---|---|
| Advantages | Lower cost than dry windingGood parallelism of fiber alignment Less fiber wear during wet winding High production efficiency | Good quality of products Clean winding machine Good labor hygiene High product quality | It is easy to operate without prepreg molding process. Good product quality; low bubble content in resin. |
| Disadvantages | Resin waste and poor operating environment It is not easy to control the glue content and quality of the finished products Less variety of resin available for wet winding | The winding equipment is expensive and requires additional prepreg yarn manufacturing equipment, so the investment is larger. The interlayer shear strength of dry winding products is low. | Larger investment in winding equipment Poor process adaptability |
| High Performance Fibers | Modulus of Elasticity (GPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|
| E-Glass Fiber | 74 | 3510 | 1.38 |
| S-Glass Fiber | 84 | 4920 | 1.97 |
| Aramid fiber | 121 | 3790 | 2.61 |
| T300 Carbon Fiber | 230 | 3530 | 2.0 |
| T700 Carbon Fiber | 235 | 4900 | 2.74 |
| T1000 Carbon Fiber | 304 | 6330 | 3.54 |
| Glass Composition | Density (g/cm3) | Outside Diameter (μm) | Inside Diameter (μm) | Length (mm) | Pumax (MPa) | Pumin (MPa) |
|---|---|---|---|---|---|---|
| Soda-lime | 2.52 | 400 | 300 | 100 | 114.7 | 25.0 |
| Borosilicate | 2.33 | 400 | 360 | 200 | 124.2 | 73.7 |
| Aluminosilicate | 2.65 | 340 | 300 | 200 | 62.7 | 32.6 |
| Quartz | 2.2 | 400 | 300 | 200 | 109.1 | 39.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Chai, X.; Gu, Y.; Zhang, P.; Yang, X.; Wen, Y.; Xu, Z.; Jiang, B.; Wang, J.; Jin, G.; et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. https://doi.org/10.3390/ma17030721
Li J, Chai X, Gu Y, Zhang P, Yang X, Wen Y, Xu Z, Jiang B, Wang J, Jin G, et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials. 2024; 17(3):721. https://doi.org/10.3390/ma17030721
Chicago/Turabian StyleLi, Jian, Xingzai Chai, Yunpeng Gu, Pengyu Zhang, Xiao Yang, Yuhui Wen, Zhao Xu, Bowen Jiang, Jian Wang, Ge Jin, and et al. 2024. "Small-Scale High-Pressure Hydrogen Storage Vessels: A Review" Materials 17, no. 3: 721. https://doi.org/10.3390/ma17030721
APA StyleLi, J., Chai, X., Gu, Y., Zhang, P., Yang, X., Wen, Y., Xu, Z., Jiang, B., Wang, J., Jin, G., Qiu, X., & Zhang, T. (2024). Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials, 17(3), 721. https://doi.org/10.3390/ma17030721








