The Effect of Exposure to Candida Albicans Suspension on the Properties of Silicone Dental Soft Lining Material
Abstract
1. Introduction
2. Materials
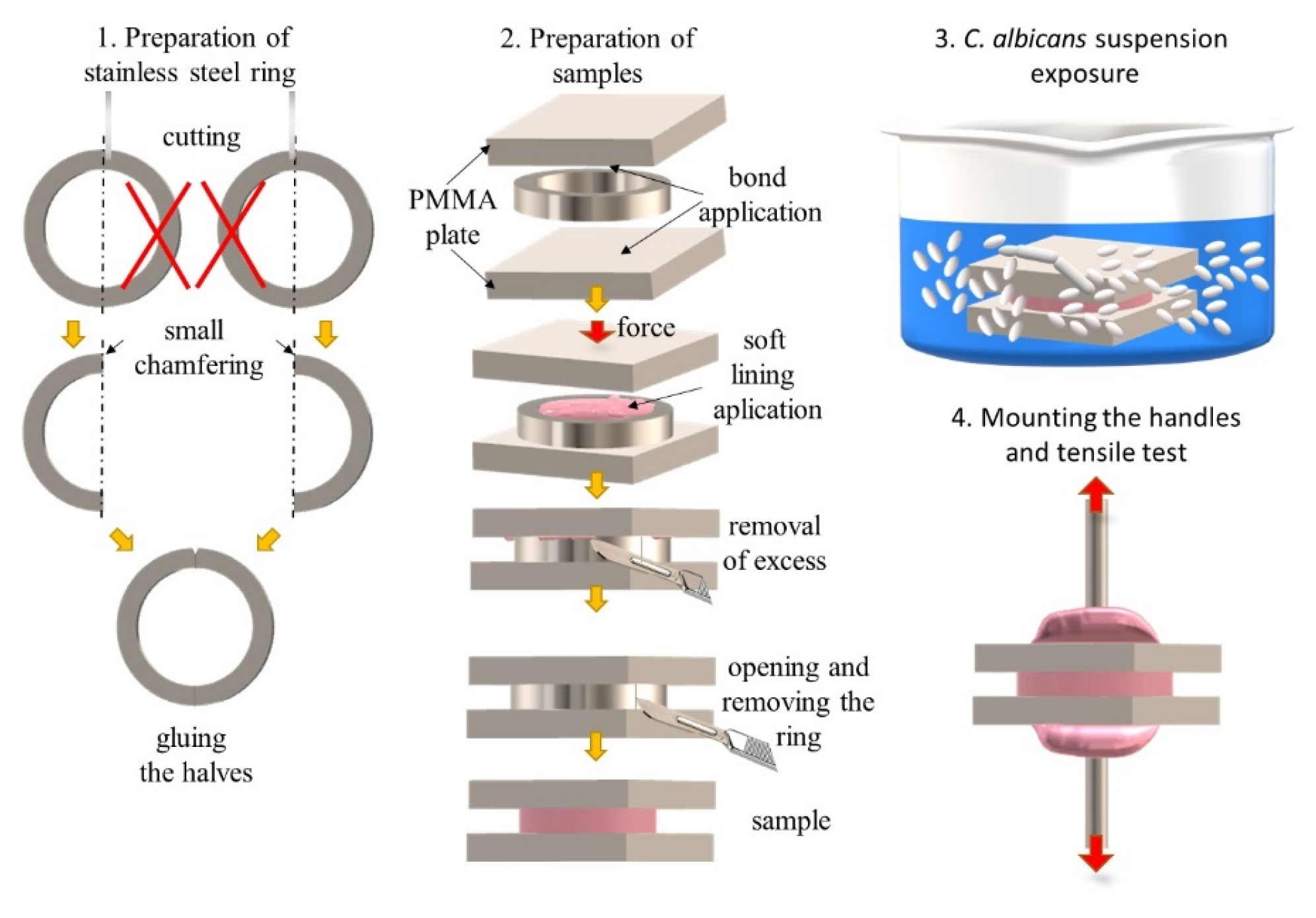
2.1. Materials and Samples Preparation
2.2. Exposure to Suspension of Candida albicans
2.3. Mechanical Properties Tests
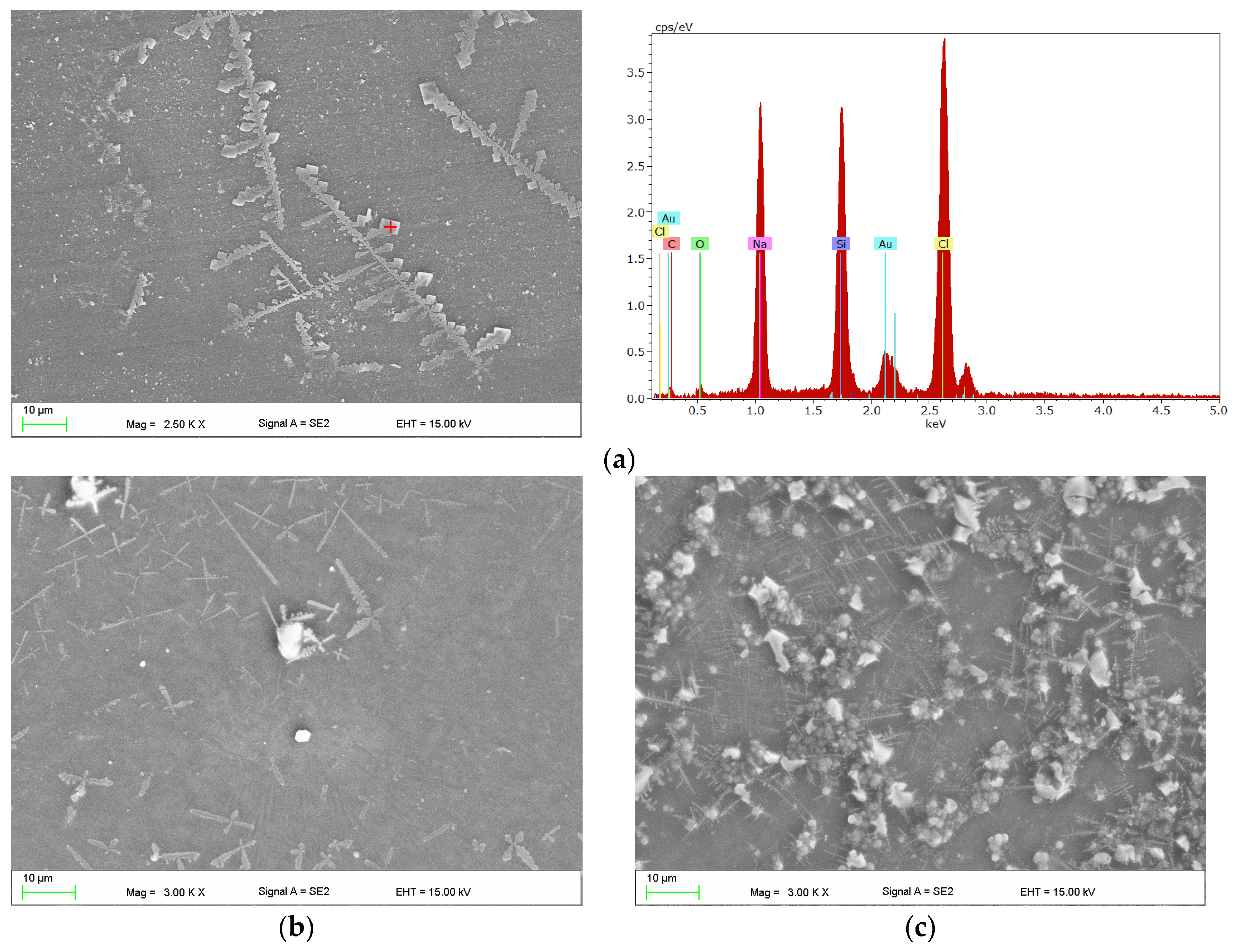
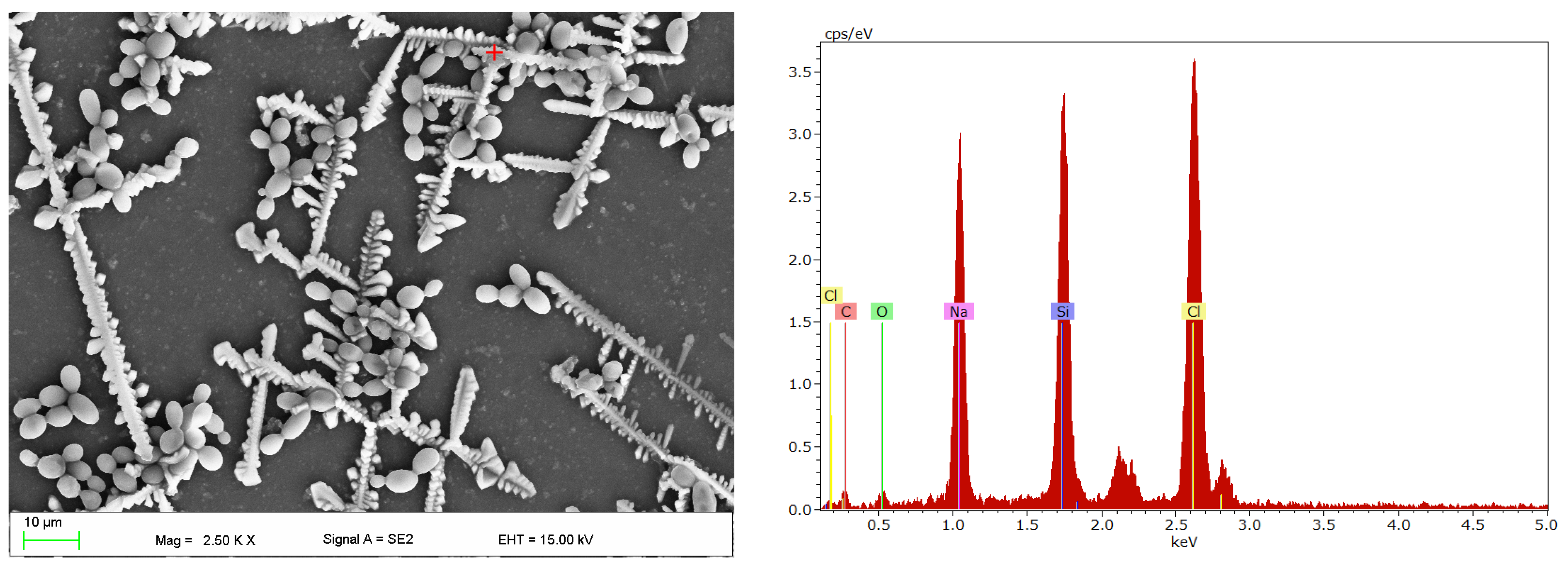
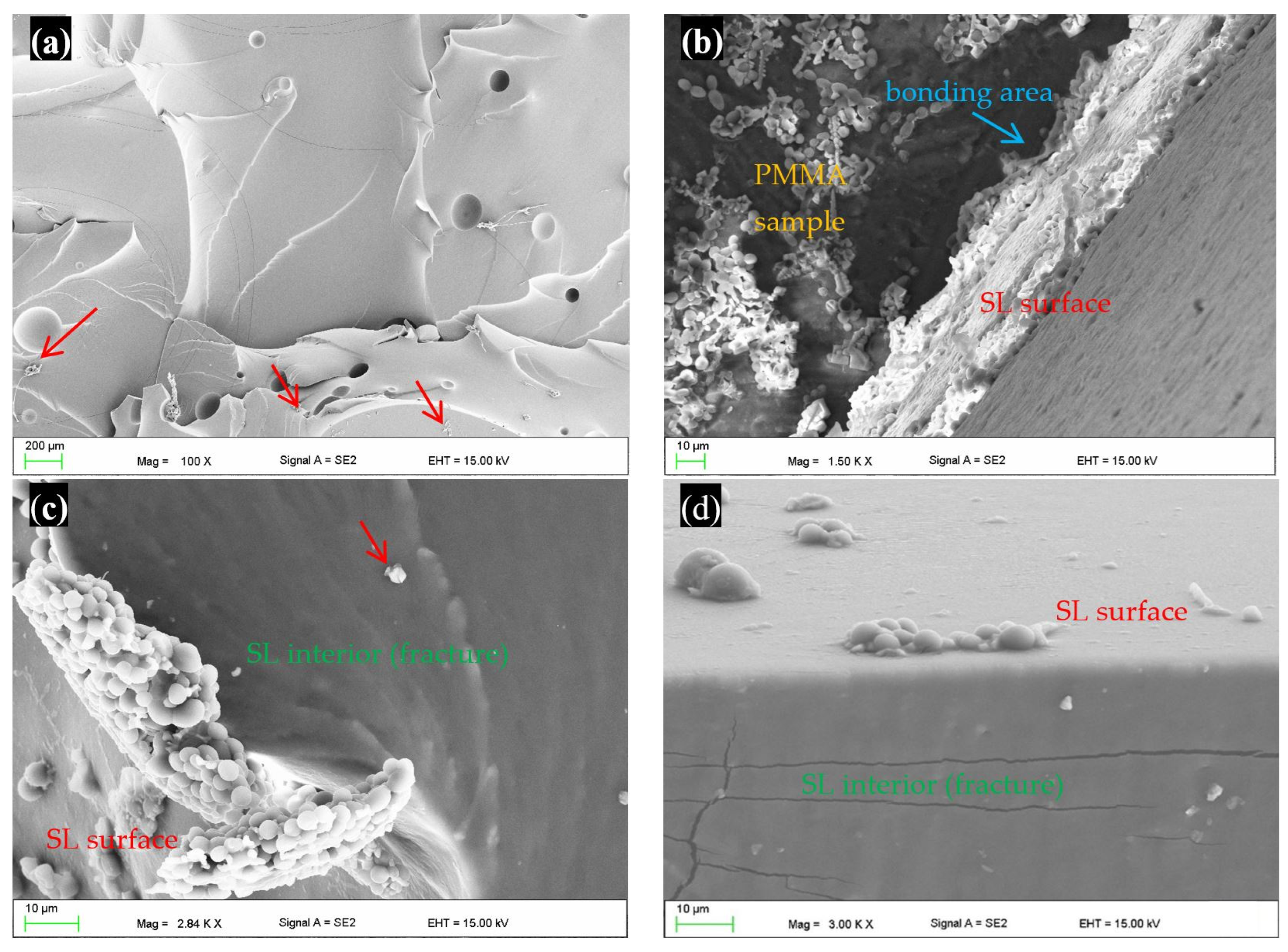
2.4. Scanning Electron Microscopy Investigations
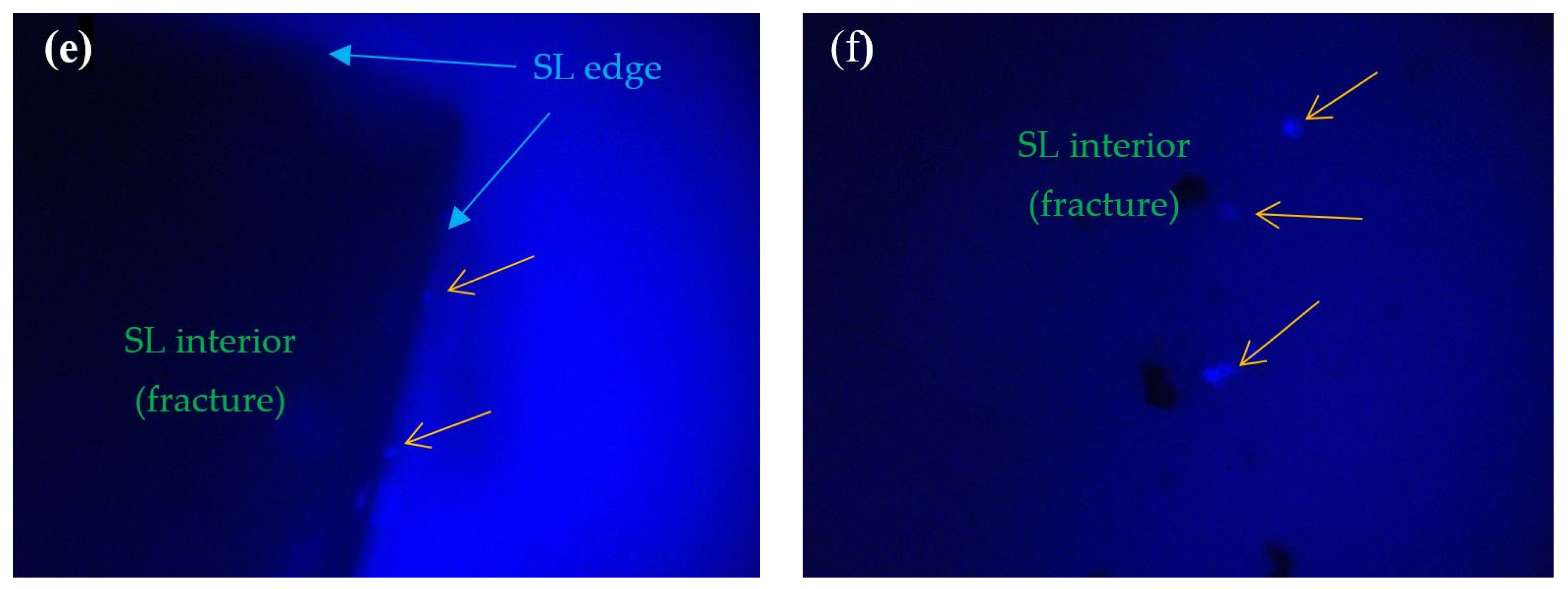
2.5. Fluorescence Microscope Investigations
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peltzer, K.; Hewlett, S.; Yawson, A.E.; Moynihan, P.; Preet, R.; Wu, F.; Guo, G.; Arokiasamy, P.; Snodgrass, J.J.; Chatterji, S.; et al. Prevalence of Loss of All Teeth (Edentulism) and Associated Factors in Older Adults in China, Ghana, India, Mexico, Russia and South Africa. Int. J. Environ. Res. Public Health 2014, 11, 11308–11324. [Google Scholar] [CrossRef]
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. Edentulism Prevalence Following 5 Decades of Decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef]
- Bukleta, M.S.; Bukleta, D.; Selmani, M.; Kuhar, M. Frequency of Complete and Removable Partial Denture Treatment in the Primary Health Centres in Three Different Regions of Kosovo from 2002 to 2013. Slov. J. Public Health 2019, 58, 104. [Google Scholar] [CrossRef]
- Ni, S.C.; Thomas, C.; Yonezawa, Y.; Hojo, Y.; Nakamura, T.; Kobayashi, K.; Sato, H.; Da Silva, J.D.; Kobayashi, T.; Ishikawa-Nagai, S. Comprehensive Assessment of the Universal Healthcare System in Dentistry Japan: A Retrospective Observational Study. Healthcare 2022, 10, 2173. [Google Scholar] [CrossRef] [PubMed]
- Braden, M.; Wright, P.S.; Parker, S. Soft Lining Materials—A Review. Eur. J. Prosthodont. Restor. Dent. 1995, 3, 163–174. [Google Scholar] [PubMed]
- Shen, C.; Rawls, H.R.; Esquivel-Upshaw, J. (Eds.) Phillips’ Science of Dental Materials, 13th ed.; Saunders: St. Louis, MO, USA, 2021; ISBN 978-0-323-69755-2. [Google Scholar]
- ELsyad, M.A.; Shaheen, N.H.; Ashmawy, T.M. Long-Term Clinical and Prosthetic Outcomes of Soft Liner and Clip Attachments for Bar/Implant Overdentures: A Randomised Controlled Clinical Trial. J. Oral Rehabil. 2017, 44, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kreve, S.; Dos Reis, A.C. Denture Liners: A Systematic Review Relative to Adhesion and Mechanical Properties. Available online: https://www.hindawi.com/journals/tswj/2019/6913080/ (accessed on 29 October 2019).
- Furuya, Y.; Kimoto, S.; Furuse, N.; Furokawa, S.; Igarashi, K.; Suzuki, A.; Kawai, Y. Effectiveness of Silicone-Based Resilient Denture Liners on the Patient-Reported Chewing Ability: A Randomized Controlled Trial. J. Prosthodont. Res. 2022, 66, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Tripathi, A.; Fatima, T. A Comparative Study of Masticatory Performance in Complete Denture Patients before and after Application of Soft Liner. Med. J. Armed Forces India 2019, 75, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Srivastava, S.; Gaur, A.; Dupare, A.; Rastogi, S.; Kamatagi, L. Electromyographic Evaluation of the Effect of Lined Dentures on Masticatory Muscle Activity in Edentulous Subjects. J. Clin. Diagn. Res. 2015, 9, ZC80–ZC83. [Google Scholar] [CrossRef]
- Mohsen, Y.H.; Kader, M.A.; Abdel Nabi, N.; Radi, I.A.W. Satisfaction with Resilient Denture Liner versus Acrylic Resin Telescopic Prostheses for Patients with Ectodermal Dysplasia: A Nonrandomized Crossover Clinical Trial. J. Prosthet. Dent. 2022, 128, 656–663. [Google Scholar] [CrossRef]
- Sônego, M.V.; Neto, C.L.M.M.; Dos Santos, D.M.; de Melo Moreno, A.L.; de M. Bertoz, A.P.; Goiato, M.C. Quality of Life, Satisfaction, Occlusal Force, and Halitosis after Direct and Indirect Relining of Inferior Complete Dentures. Eur. J. Dent. 2022, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Alnazzawi, A.A.; Farghal, A.E.; Bakr, R.M.; Mahmoud, I.I. Impact of Acrylic and Silicone-Based Soft-Liner Materials on Biting Force and Quality of Life of the Complete Denture Wearers: A Randomized Clinical Trial. J. Clin. Med. 2023, 12, 2073. [Google Scholar] [CrossRef] [PubMed]
- Shinde, J.; Mowade, T.; Gupta, P.; Tekale, R.; Pande, N.; Deshmukh, K.; Lokhande, T.; Radke, U. Satisfaction in Conventional Acrylic Complete Denture Patient with and without Denture Liners—A Systematic Review. Pan. Afr. Med. J. 2022, 42, 296. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Luz, M.S.; Boscato, N.; Pereira-Cenci, T. Biofilm Formation on Denture Liners in a Randomised Controlled in Situ Trial. J. Dent. 2013, 41, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Tasopoulos, T.; Vrioni, G.; Naka, O.; Diamantatou, T.; Zoidis, P.; Tsakris, A. Adherence of Candida Albicans to Five Long-Term Silicone-Based Denture Lining Materials Bonded to CAD-CAM Denture Base. J. Prosthodont. 2022, 32, 292–297. [Google Scholar] [CrossRef]
- Mutluay, M.M.; Oguz, S.; Fløystrand, F.; Saxegaard, E.; Dogan, A.; Bek, B.; Ruyter, I.E. A Prospective Study on the Clinical Performance of Polysiloxane Soft Liners: One-Year Results. Dent. Mater. J. 2008, 27, 440–447. [Google Scholar] [CrossRef]
- Smith, A.; Williams, D.; Bradshaw, D.; Milward, P.; Kutubi, S.A.; Rowe, W. The Effect of Residual Food Stain on Candida Albicans Colonisation of Denture Acrylics. Dent. Oral Biol. Craniofacial Res. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Gleiznys, A.; Zdanavičienė, E.; Žilinskas, J. Candida Albicans Importance to Denture Wearers. A Literature Review. Stomatologija 2015, 17, 54–66. [Google Scholar]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Eskandarion, S.; Marashi, S.M.A.; Yunesi, G.; Kharazifard, M. Antifungal Efficacy of a Permanent Silicon Soft Liner Containing Silver Nanoparticles. Front. Dent. 2021, 18, 8. [Google Scholar] [CrossRef]
- Jabłońska-Stencel, E.; Pakieła, W.; Mertas, A.; Bobela, E.; Kasperski, J.; Chladek, G. Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material. Materials 2018, 11, 318. [Google Scholar] [CrossRef]
- Deng, J.; Ren, L.; Pan, Y.; Gao, H.; Meng, X. Antifungal Property of Acrylic Denture Soft Liner Containing Silver Nanoparticles Synthesized in Situ. J. Dent. 2021, 106, 103589. [Google Scholar] [CrossRef]
- Ahmed, A.Q.; Al-Hmedat, S.J.A.-Z.; Hanweet, D.M.; Haider, J. Assessing the Antifungal Activity of a Soft Denture Liner Loaded with Titanium Oxide Nanoparticles (TiO2 NPs). Dent. J. 2023, 11, 90. [Google Scholar] [CrossRef]
- Ansarifard, E.; Zareshahrabadi, Z.; Sarafraz, N.; Zomorodian, K. Evaluation of Antimicrobial and Antibiofilm Activities of Copper Oxide Nanoparticles within Soft Denture Liners against Oral Pathogens. Bioinorg. Chem. Appl. 2021, 2021, 9939275. [Google Scholar] [CrossRef] [PubMed]
- Songsang, N.; Anunmana, C.; Pudla, M.; Eiampongpaiboon, T. Effects of Litsea Cubeba Essential Oil Incorporated into Denture Soft Lining Materials. Polymers 2022, 14, 3261. [Google Scholar] [CrossRef]
- AlHamdan, E.M. Soft Denture Liner and Microbial Disinfection with Contemporary and Conventional Agents. Photodiagn. Photodyn. Ther. 2022, 38, 102768. [Google Scholar] [CrossRef] [PubMed]
- Hahnel, S.; Rosentritt, M.; Bürgers, R.; Handel, G.; Lang, R. Candida Albicans Biofilm Formation on Soft Denture Liners and Efficacy of Cleaning Protocols. Gerodontology 2012, 29, e383–e391. [Google Scholar] [CrossRef]
- Mańka-Malara, K.; Trzaskowski, M.; Gawlak, D. The Influence of Decontamination Procedures on the Surface of Two Polymeric Liners Used in Prosthodontics. Polymers 2021, 13, 4340. [Google Scholar] [CrossRef] [PubMed]
- Salloum, A.M. Effect of Aging on Bond Strength of Two Soft Lining Materials to a Denture Base Polymer. J. Indian Prosthodont. Soc. 2014, 14, 155–160. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Hallikerimath, R.B. An In-Vitro Evaluation of Retention, Colonization and Penetration of Commonly Used Denture Lining Materials By Candida Albicans. J. Clin. Diagn. Res. 2016, 10, ZC84–ZC88. [Google Scholar] [CrossRef]
- Tari, B.F.; Nalbant, D.; Dogruman Al, F.; Kustimur, S. Surface Roughness and Adherence of Candida Albicans on Soft Lining Materials as Influenced by Accelerated Aging. J. Contemp. Dent. Pract. 2007, 8, 18–25. [Google Scholar] [CrossRef]
- Taylor, R.L.; Bulad, K.; Verran, J.; McCord, J.F. Colonization and Deterioration of Soft Denture Lining Materials in Vivo. Eur. J. Prosthodont. Restor. Dent. 2008, 16, 50–55. [Google Scholar] [CrossRef]
- Kang, S.-H.; Lee, H.-J.; Hong, S.-H.; Kim, K.-H.; Kwon, T.-Y. Influence of Surface Characteristics on the Adhesion of Candida Albicans to Various Denture Lining Materials. Acta Odontol. Scand. 2013, 71, 241–248. [Google Scholar] [CrossRef]
- Mutluay, M.M.; Oğuz, S.; Ørstavik, D.; Fløystrand, F.; Doğan, A.; Söderling, E.; Närhi, T.; Olsen, I. Experiments on in Vivo Biofilm Formation and in Vitro Adhesion of Candida Species on Polysiloxane Liners. Gerodontology 2010, 27, 283–291. [Google Scholar] [CrossRef]
- De Foggi, C.C.; Ayres, M.S.B.; Feltrin, G.P.; Jorge, J.H.; Machado, A.L. Effect of Surface Characteristics of Soft Liners and Tissue Conditioners and Saliva on the Adhesion and Biofilm Formation. Am. J. Dent. 2018, 31, 45–52. [Google Scholar]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and Penetration of Denture Soft Lining Materials by Candida Albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Rodger, G.; Taylor, R.L.; Pearson, G.J.; Verran, J. In Vitro Colonization of an Experimental Silicone by Candida Albicans. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 226–235. [Google Scholar] [CrossRef] [PubMed]
- ISO 7619-1:2010; Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1: Durometer Method (Shore Hardness). ISO: Geneva, Switzerland, 2010.
- EN ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO International Organization for Standardization: Geneva, Switzerland, 2017.
- EN ISO 10139-2:2016; Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use. ISO International Organization for Standardization: Geneva, Switzerland, 2016.
- Shirkavand, S.; Moslehifard, E. Effect of TiO2 Nanoparticles on Tensile Strength of Dental Acrylic Resins. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 197–203. [Google Scholar] [CrossRef]
- Cavalcanti, Y.W.; Wilson, M.; Lewis, M.; Williams, D.; Senna, P.M.; Del-Bel-Cury, A.A.; da Silva, W.J. Salivary Pellicles Equalise Surfaces’ Charges and Modulate the Virulence of Candida Albicans Biofilm. Arch. Oral Biol. 2016, 66, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Gacon, I.; Loster, J.E.; Wieczorek, A. Relationship between Oral Hygiene and Fungal Growth in Patients: Users of an Acrylic Denture without Signs of Inflammatory Process. Clin. Interv. Aging 2019, 14, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.I. Advances in Soft Denture Liners: An Update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar]
- Chladek, G.; Żmudzki, J.; Kasperski, J. Long-Term Soft Denture Lining Materials. Materials 2014, 7, 5816–5842. [Google Scholar] [CrossRef]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3029. [Google Scholar] [CrossRef]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.C.; Tahseen, H.; Sajja, N.P. Invitro Antifungal Evaluation of Denture Soft Liner Incorporated with Tea Tree Oil: A New Therapeutic Approach Towards Denture Stomatitis. J. Clin. Diagn. Res. 2015, 9, ZC62–ZC64. [Google Scholar] [CrossRef]
- da Silva, W.J.; Seneviratne, J.; Samaranayake, L.P.; Del Bel Cury, A.A. Bioactivity and Architecture of Candida Albicans Biofilms Developed on Poly(Methyl Methacrylate) Resin Surface. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 149–156. [Google Scholar] [CrossRef]
- Faot, F.; Cavalcanti, Y.W.; de Mendonça e Bertolini, M.; de Rezende Pinto, L.; da Silva, W.J.; Cury, A.A.D.B. Efficacy of Citric Acid Denture Cleanser on the Candida Albicans Biofilm Formed on Poly(Methyl Methacrylate): Effects on Residual Biofilm and Recolonization Process. BMC Oral Health 2014, 14, 77. [Google Scholar] [CrossRef]
- Altinci, P.; Mutluay, M.; Söderling, E.; Tezvergil-Mutluay, A. Antimicrobial Efficacy and Mechanical Properties of BAC-Modified Hard and Soft Denture Liners. Odontology 2018, 106, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Oral Cavity and Candida Albicans: Colonisation to the Development of Infection. Pathogens 2022, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Microbial Shifts during Dental Biofilm Re-Development in the Absence of Oral Hygiene in Periodontal Health and Disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Evren, B.A.; Uludamar, A.; Işeri, U.; Ozkan, Y.K. The Association between Socioeconomic Status, Oral Hygiene Practice, Denture Stomatitis and Oral Status in Elderly People Living Different Residential Homes. Arch. Gerontol. Geriatr. 2011, 53, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.R.; Burns, D.A.; DiPietro, G.J.; Gregory, R.L. Response of Processed Resilient Denture Liners to Candida Albicans. J. Prosthet. Dent. 1987, 57, 507–512. [Google Scholar] [CrossRef]
- Khiyani, S.; Mahajan, A.B.; Sarda, A.; Srivastava, H.; Sanap, A.; Bhalerao, N.N. Comparison of Penetration of Candida Albicans in Two Denture Base Materials. J. Res. Adv. Dent. 2022, 13, 1–3. [Google Scholar]
- Chladek, G.; Nowak, M.; Pakieła, W.; Mertas, A. Effect of Candida Albicans Suspension on the Mechanical Properties of Denture Base Acrylic Resin. Materials 2022, 15, 3841. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; Zuccolotti, B.C.R.; Moreno, A.; dos Santos, D.M.; Pesqueira, A.A. Effect of Thermocycling on Hardness, Absorption, Solubility and Colour Change of Soft Liners. Gerodontology 2012, 29, e215–e219. [Google Scholar] [CrossRef]
- Mese, A.; Guzel, K.G. Effect of Storage Duration on the Hardness and Tensile Bond Strength of Silicone- and Acrylic Resin-Based Resilient Denture Liners to a Processed Denture Base Acrylic Resin. J. Prosthet. Dent. 2008, 99, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Białożyt-Bujak, E.; Wyszyńska, M.; Chladek, G.; Czelakowska, A.; Gala, A.; Orczykowska, M.; Białożyt, A.; Kasperski, J.; Skucha-Nowak, M. Analysis of the Hardness of Soft Relining Materials for Removable Dentures. Int. J. Environ. Res. Public Health 2021, 18, 9491. [Google Scholar] [CrossRef] [PubMed]
- Wyszyńska, M.; Białożyt-Bujak, E.; Chladek, G.; Czelakowska, A.; Rój, R.; Białożyt, A.; Gruca, O.; Nitsze-Wierzba, M.; Kasperski, J.; Skucha-Nowak, M. Analysis of Changes in the Tensile Bond Strenght of Soft Relining Material with Acrylic Denture Material. Materials 2021, 14, 6868. [Google Scholar] [CrossRef] [PubMed]
- Mutahar, M.; Al Ahmari, N.M.; Gadah, T.S.; Kariri, M.A.M.; Madkhli, H.Y.; Somaili, D.M.; Mobarki, Y.M.Y.; Darraj, O.A.; Halawi, S.M.; Al Moaleem, M.M. Comparative Evaluation of Hardness and Energy Absorption of Some Commercially Available Chairside Silicone-Based Soft Denture Liners and a Heat-Cured Soft Denture Liner. Clin. Cosmet. Investig. Dent. 2023, 15, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Vuksic, J.; Pilipovic, A.; Poklepovic Pericic, T.; Kranjcic, J. Tensile Bond Strength between Different Denture Base Materials and Soft Denture Liners. Materials 2023, 16, 4615. [Google Scholar] [CrossRef]
- Panda, S.K.; Reddy, N.; Manual, L.; Krishna, C.; Jagadeesh, K.N.; Saidath, K.; Babaji, P. An In Vitro Evaluation of Tensile Bond Strength of Soft Liners Bonded to Different Denture Base Resins. Ann. Afr. Med. 2021, 20, 116–120. [Google Scholar]
- Al Taweel, S.M.; Al-Otaibi, H.N.; Labban, N.; AlFouzan, A.; Shehri, H.A. Soft Denture Liner Adhesion to Conventional and CAD/CAM Processed Poly(Methyl Methacrylate) Acrylic Denture Resins-An In-Vitro Study. Materials 2021, 14, 6614. [Google Scholar] [CrossRef]
- Wemken, G.; Burkhardt, F.; Spies, B.C.; Kleinvogel, L.; Adali, U.; Sterzenbach, G.; Beuer, F.; Wesemann, C. Bond Strength of Conventional, Subtractive, and Additive Manufactured Denture Bases to Soft and Hard Relining Materials. Dent. Mater. 2021, 37, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Mutluay, M.M.; Ruyter, I.E. Evaluation of Bond Strength of Soft Relining Materials to Denture Base Polymers. Dent. Mater. 2007, 23, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Valentijn-Benz, M.; Nazmi, K.; Brand, H.S.; van’t Hof, W.; Veerman, E.C.I. Growth of Candida Albicans in Human Saliva Is Supported by Low-Molecular-Mass Compounds. FEMS Yeast Res. 2015, 15, fov088. [Google Scholar] [CrossRef][Green Version]
- Pollack, J.H.; Hashimoto, T. The Role of Glucose in the pH Regulation of Germ-Tube Formation in Candida Albicans. J. Gen. Microbiol. 1987, 133, 415–424. [Google Scholar] [CrossRef]
- Thompson, D.S.; Carlisle, P.L.; Kadosh, D. Coevolution of Morphology and Virulence in Candida Species. Eukaryot. Cell 2011, 10, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.G.; Shafiq, A.; Hakim, S.T.; Anjum, Y.; Kazm, S.U. Effect of Growth Media, pH and Temperature on Yeast to Hyphal Transition in Candida Albicans. Open J. Med. Microbiol. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Nikolopoulou, F.; Tzortzopoulou, E. Salivary pH in Edentulous Patients Before and After Wearing Conventional Dentures and Implant Overdentures: A Clinical Study. Implant. Dent. 2007, 16, 397. [Google Scholar] [CrossRef]
- Alshahrani, F.A.; AlToraibily, F.; Alzaid, M.; Mahrous, A.A.; Al Ghamdi, M.A.; Gad, M.M. An Updated Review of Salivary pH Effects on Polymethyl Methacrylate (PMMA)-Based Removable Dental Prostheses. Polymers 2022, 14, 3387. [Google Scholar] [CrossRef]
- Ramos-Pardo, A.; Castro-Álvarez, R.; Quindós, G.; Eraso, E.; Sevillano, E.; Kaberdin, V.R. Assessing pH-dependent Activities of Virulence Factors Secreted by Candida Albicans. Microbiologyopen 2023, 12, e1342. [Google Scholar] [CrossRef]
- Nikawa, H.; Hamada, T.; Yamamoto, T. Denture Plaque—Past and Recent Concerns. J. Dent. 1998, 26, 299–304. [Google Scholar] [CrossRef]
- Gedik, H.; Ozkan, Y.K. The Effect of Surface Roughness of Silicone-Based Resilient Liner Materials on the Adherence of Candida Albicans and Inhibition of Candida Albicans with Different Disinfectants. Oral Health Prev. Dent. 2009, 7, 347–353. [Google Scholar]
- Nevzatoğlu, E.U.; Ozcan, M.; Kulak-Ozkan, Y.; Kadir, T. Adherence of Candida Albicans to Denture Base Acrylics and Silicone-Based Resilient Liner Materials with Different Surface Finishes. Clin. Oral Investig. 2007, 11, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Bhandari, A.; Sachdev, A.; Khan, F.; Malhotra, S.; Bashir, T. A Clinical Study of Adherence of Candida Albicans to an Improved NBR Denture Soft Lining Material. J. Health Allied Sci. NU 2015, 05, 036–039. [Google Scholar] [CrossRef]
- Jackson, S.; Coulthwaite, L.; Loewy, Z.; Scallan, A.; Verran, J. Biofilm Development by Blastospores and Hyphae of Candida Albicans on Abraded Denture Acrylic Resin Surfaces. J. Prosthet. Dent. 2014, 112, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Badaró, M.M.; Prates, T.P.; Leite-Fernandes, V.M.F.; de Cássia Oliveira, V.; de Freitas Oliveira Paranhos, H.; Silva-Lovato, C.H. In Vitro Evaluation of Resilient Liner after Brushing with Conventional and Experimental Ricinus Communis-Based Dentifrices. J. Prosthodont. 2019, 28, e857–e862. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Wada, T.; Kubo, K.; Ueda, T.; Sakurai, K. Effect of Mechanical and Chemical Cleaning on Surface Roughness of Silicone Soft Relining Material. J. Prosthodont. Res. 2020, 64, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Kubo, K.; Saito, T.; Obata, T.; Wada, T.; Yanagisawa, K.; Sakurai, K. Surface Morphology of Silicone Soft Relining Material after Mechanical and Chemical Cleaning. J. Prosthodont. Res. 2018, 62, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Luz, M.S.; Boscato, N.; Pereira-Cenci, T. Surface Roughness Changes in Denture Liners in Denture Stomatitis Patients. Int. J. Prosthodont. 2017, 30, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-E.; Liu, Y.; Dhall, A.; Bawazir, M.; Koo, H.; Hwang, G. Synergism of Streptococcus Mutans and Candida Albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study. Front. Cell Infect. Microbiol. 2020, 10, 623980. [Google Scholar] [CrossRef]
- Moraes, G.S.; Cachoeira, V.S.; Alves, F.M.C.; Kiratcz, F.; Albach, T.; Bueno, M.G.; Neppelenbroek, K.H.; Urban, V.M. Is There an Optimal Method to Detach Candida Albicans Biofilm from Dental Materials? J. Med. Microbiol. 2021, 70, 001436. [Google Scholar] [CrossRef] [PubMed]

| Time, Days | Shore A Hardness, Shore A | Tensile Bond Strength, MPa | Tensile Strength, MPa | |||
|---|---|---|---|---|---|---|
| CM | CAS | CM | CAS | CM | CAS | |
| BE | 31.4 ± 0.9 A | 31.4 ± 0.9 A | 1.43 ± 0.15 | 1.43 ± 0.15 | 3.14 ± 0.5 | 3.14 ± 0.5 |
| 30 | 33.1 ± 0.6 B | 33.6 ± 0.9 B | 1.56 ± 0.10 | 1.51 ± 0.12 | 3.15 ± 0.37 | 3.23 ± 0.44 |
| 60 | 33.0 ± 1.1 B | 33.7 ± 0.9 B | 1.42 ± 0.17 | 1.61 ± 0.25 | 3.34 ± 0.40 | 3.29 ± 0.41 |
| 90 | 33.5 ± 0.6 B | 33.2 ± 0.9 B | 1.50 ± 0.10 | 1.41 ± 0.15 | 3.2 ± 0.58 | 3.15 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Nowak, M.; Pakieła, W.; Barszczewska-Rybarek, I.; Żmudzki, J.; Mertas, A. The Effect of Exposure to Candida Albicans Suspension on the Properties of Silicone Dental Soft Lining Material. Materials 2024, 17, 723. https://doi.org/10.3390/ma17030723
Chladek G, Nowak M, Pakieła W, Barszczewska-Rybarek I, Żmudzki J, Mertas A. The Effect of Exposure to Candida Albicans Suspension on the Properties of Silicone Dental Soft Lining Material. Materials. 2024; 17(3):723. https://doi.org/10.3390/ma17030723
Chicago/Turabian StyleChladek, Grzegorz, Michał Nowak, Wojciech Pakieła, Izabela Barszczewska-Rybarek, Jarosław Żmudzki, and Anna Mertas. 2024. "The Effect of Exposure to Candida Albicans Suspension on the Properties of Silicone Dental Soft Lining Material" Materials 17, no. 3: 723. https://doi.org/10.3390/ma17030723
APA StyleChladek, G., Nowak, M., Pakieła, W., Barszczewska-Rybarek, I., Żmudzki, J., & Mertas, A. (2024). The Effect of Exposure to Candida Albicans Suspension on the Properties of Silicone Dental Soft Lining Material. Materials, 17(3), 723. https://doi.org/10.3390/ma17030723











