Associating Physical and Photocatalytic Properties of Recyclable and Reusable Blast Furnace Dust Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of the BFWD Powder
2.3. Photocatalytic Assay
3. Results and Discussion
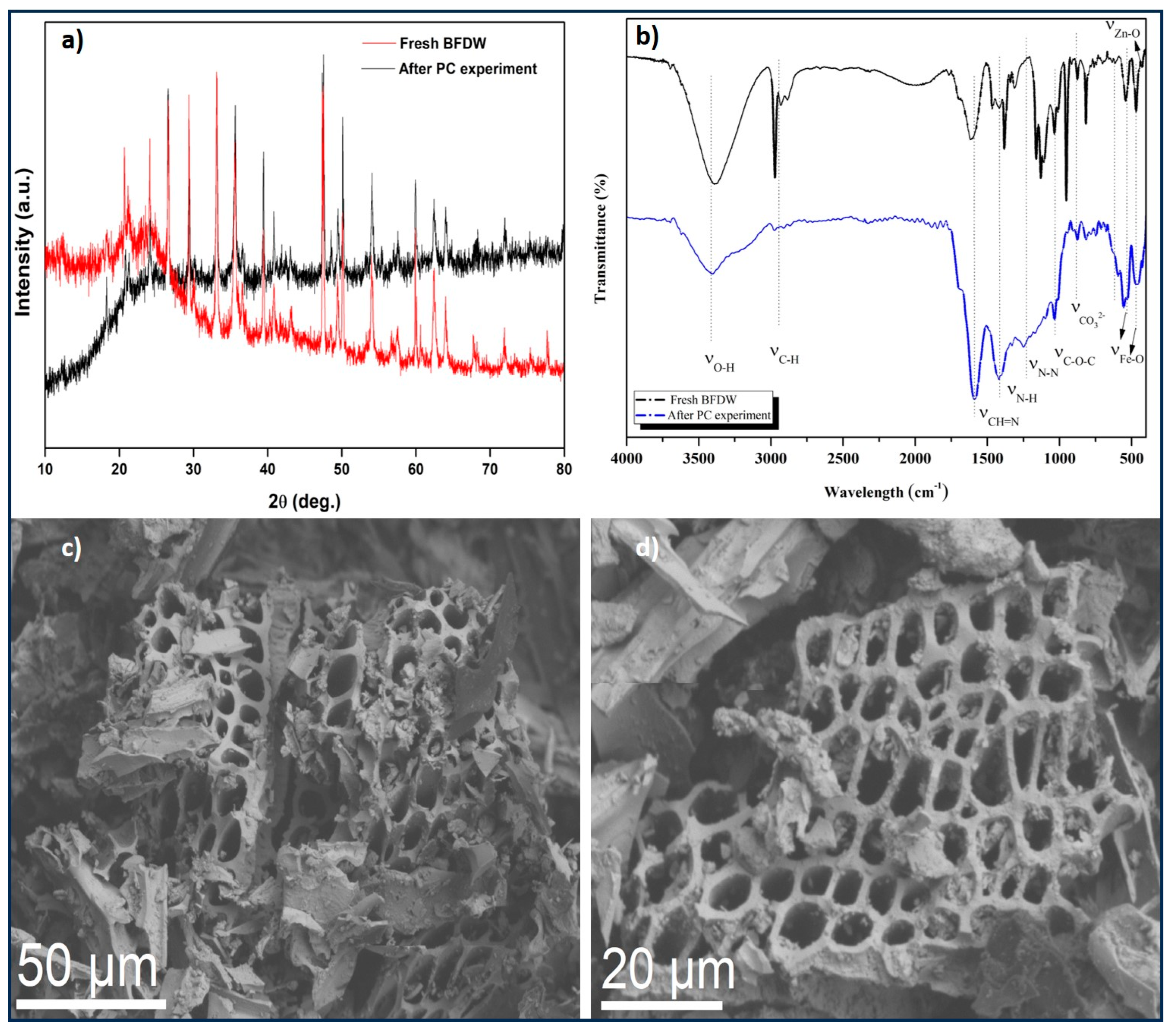
3.1. Characterization of BFDW Powder
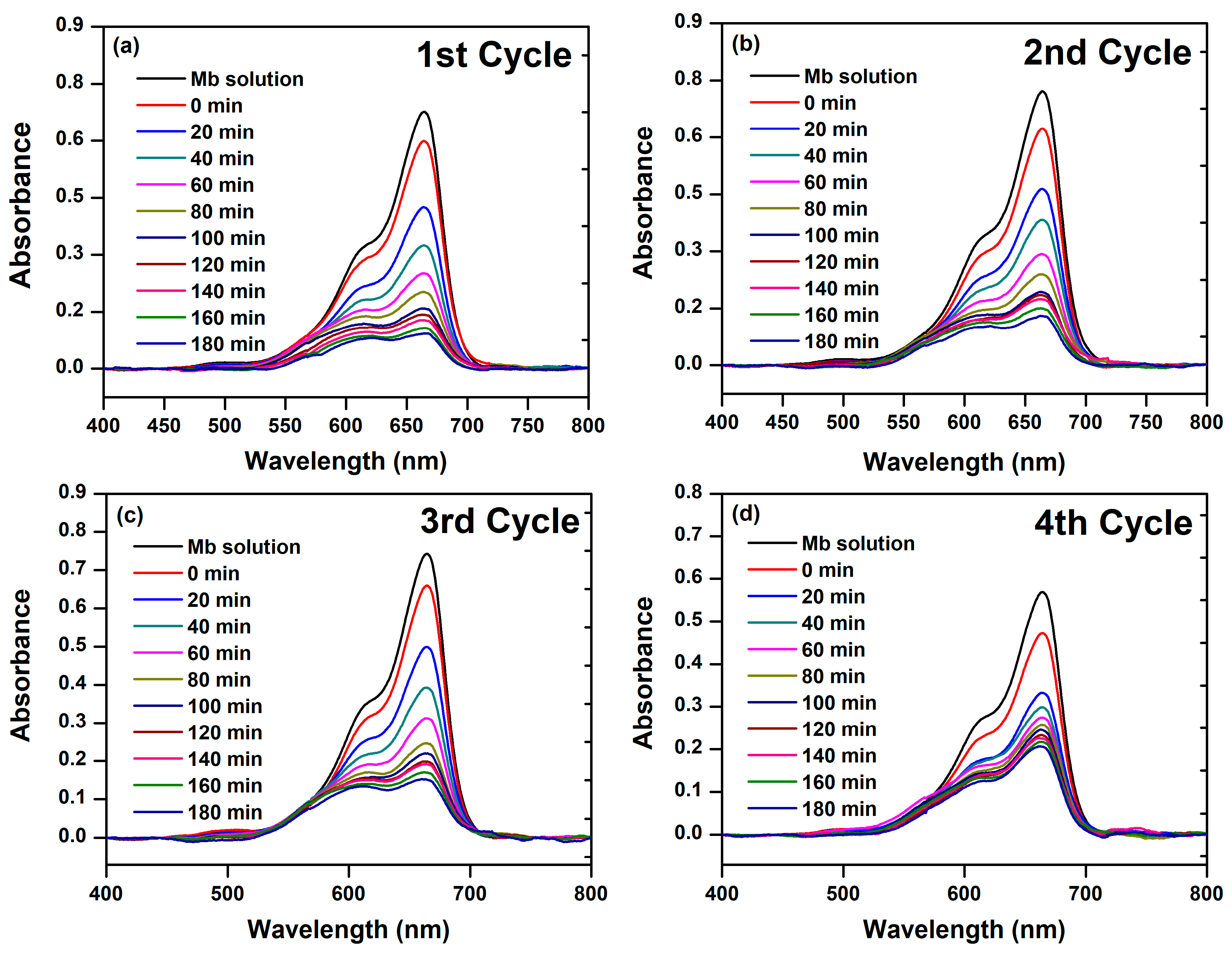
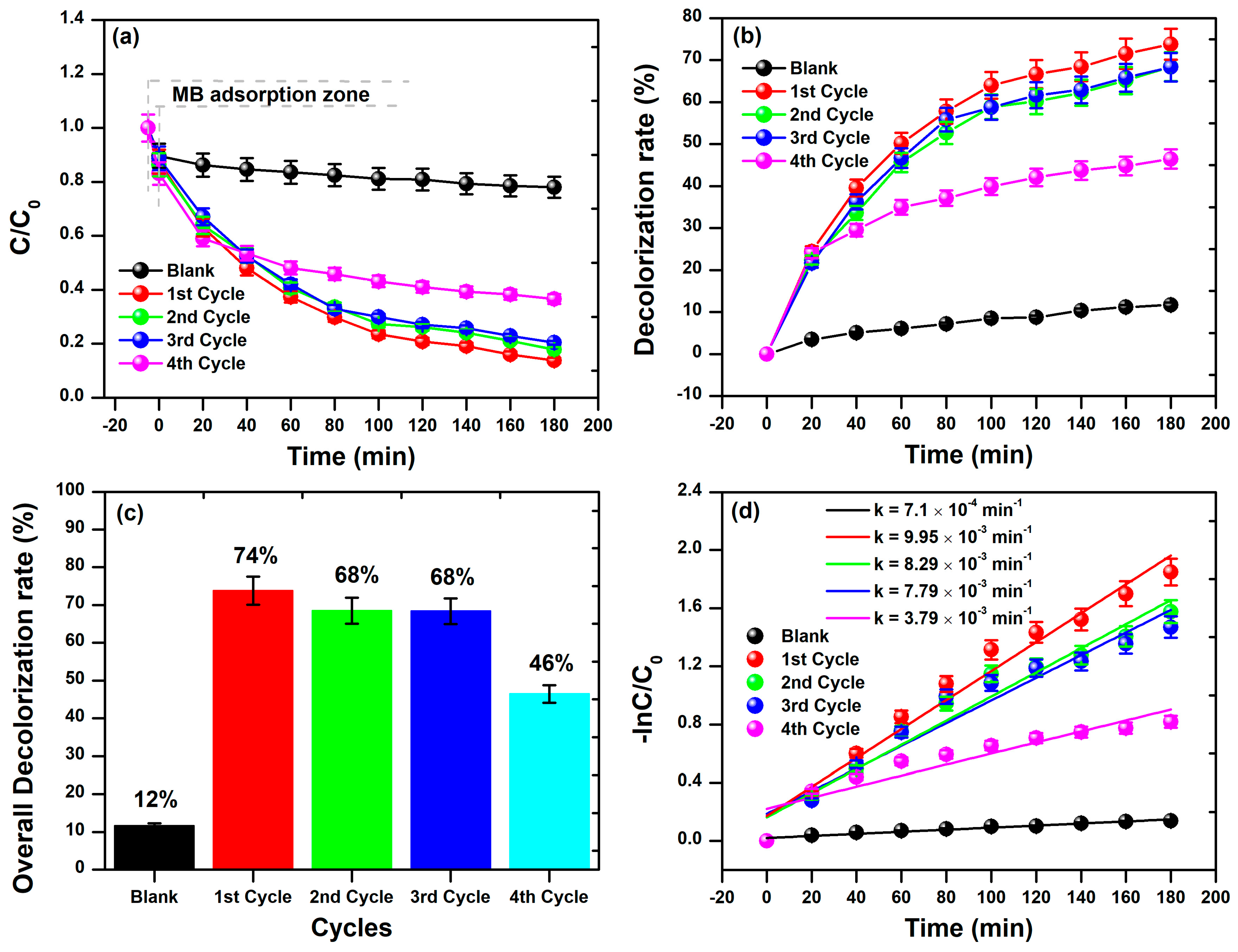
3.2. Photocatalytic Activity of BFDW
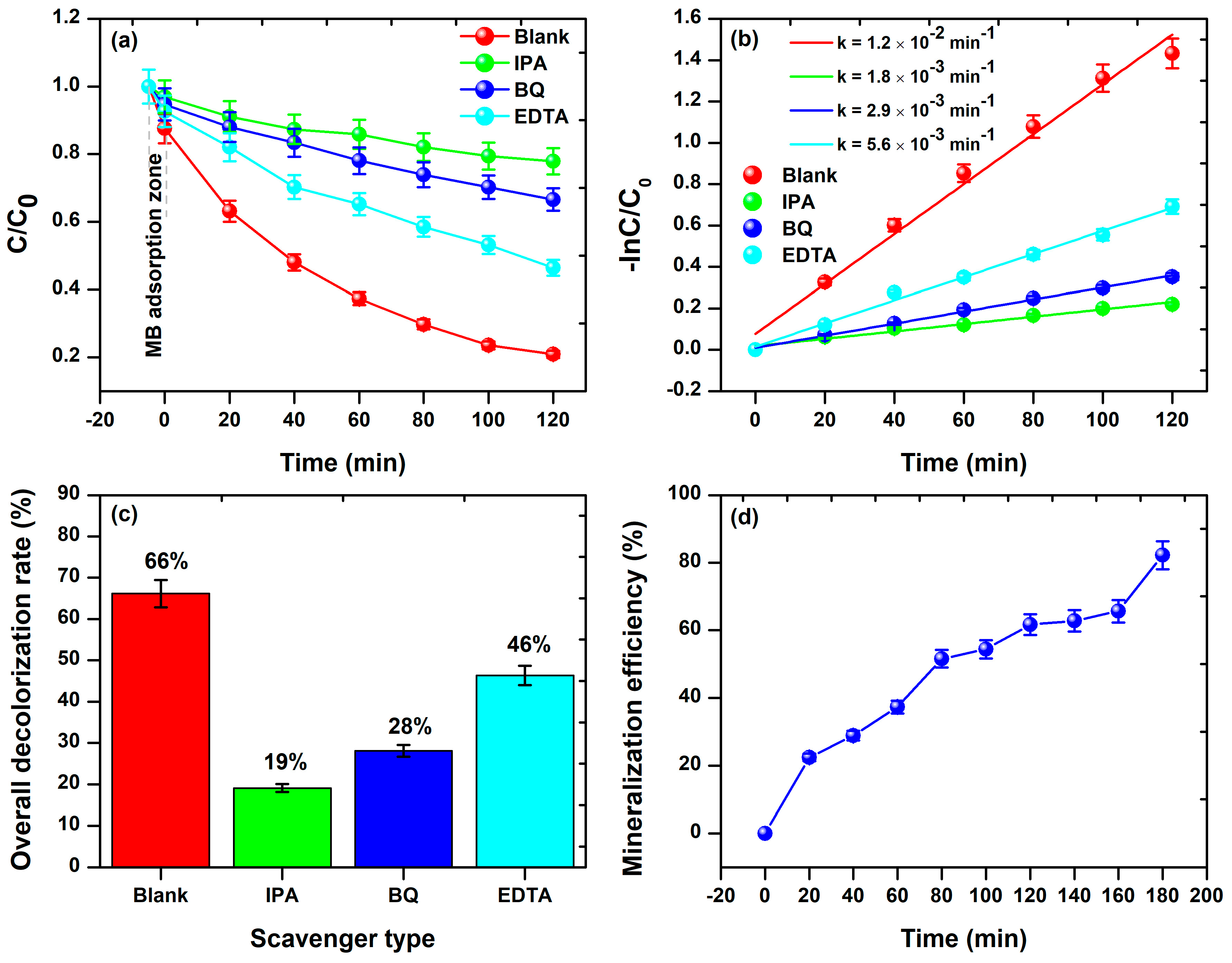
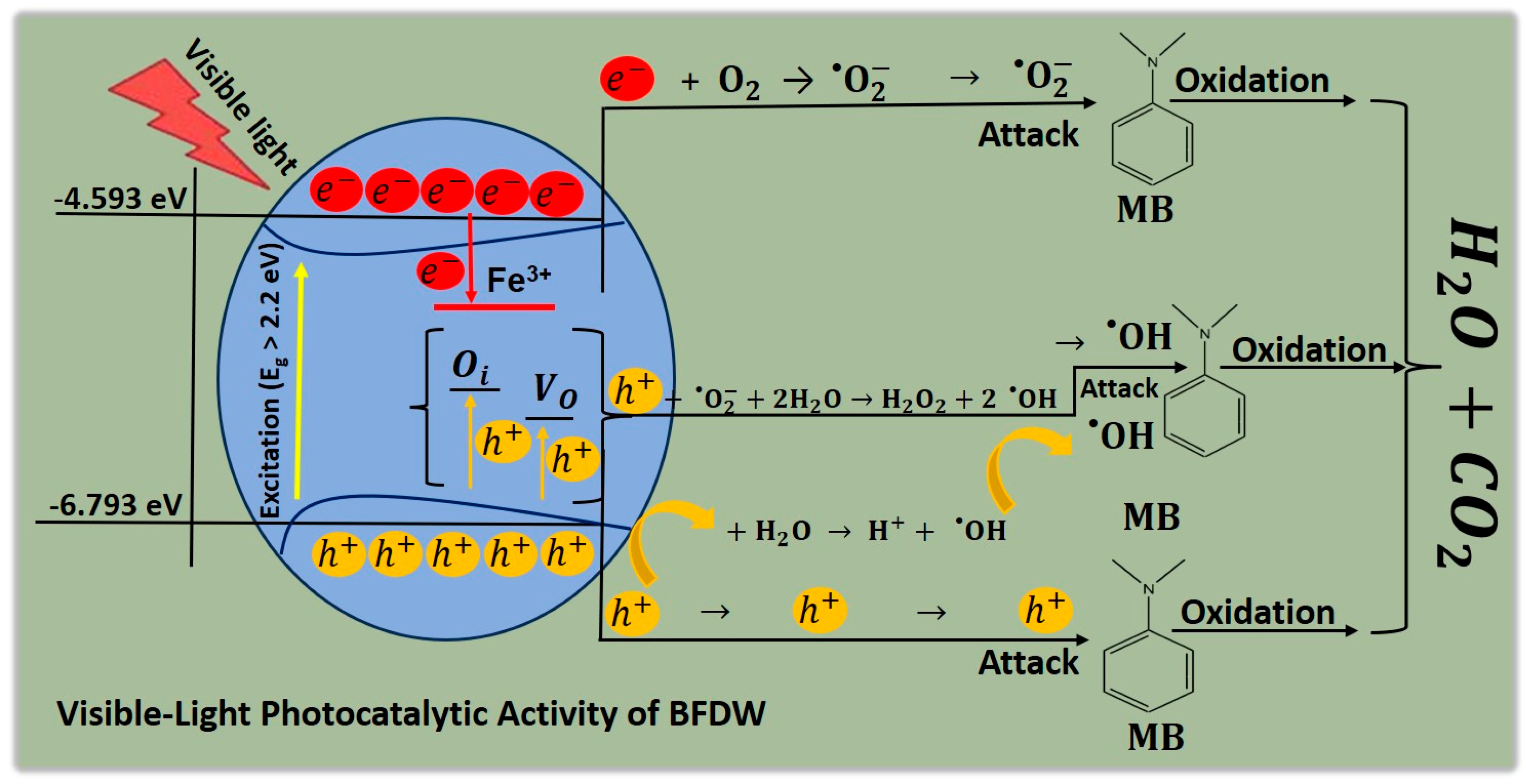
3.3. Probable Mechanism of the Photocatalytic Activity of BFDW
3.4. Comparing BFDW’s Photocatalytic Activity to Other Residues
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, X.; Zhang, S.; Sher, F.; Chen, J.; Xin, Y.; You, Z.; Wen, L.; Hu, M.; Qiu, G. A Review on Recycling and Reutilization of Blast Furnace Dust as a Secondary Resource. J. Sustain. Metall. 2021, 7, 340–357. [Google Scholar] [CrossRef]
- Nayak, N.P. Characterization of Blast Furnace Flue Dust—An Assessment for Its Utilization. Mater. Today Proc. 2022, 50, 2078–2083. [Google Scholar] [CrossRef]
- Soria-Aguilar, M.d.J.; Martínez-Luévanos, A.; Sánchez-Castillo, M.A.; Carrillo-Pedroza, F.R.; Toro, N.; Narváez-García, V.M. Removal of Pb(II) from Aqueous Solutions by Using Steelmaking Industry Wastes: Effect of Blast Furnace Dust’s Chemical Composition. Arab. J. Chem. 2021, 14, 103061. [Google Scholar] [CrossRef]
- Xu, J.; Wang, N.; Zhou, Z.; Chen, M.; Wang, P. Experimental and Numerical Studies of the Gas-Molten Reduction Behavior of Blast Furnace Dust Particles during in-Flight Process. Powder Technol. 2020, 361, 226–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Huang, W.; Fan, K.; Li, J.; Zhong, M.; Li, Z.; Li, C.; Zhang, Q. Research on Modified Blast Furnace Dust in Demulsification: The Synergistic Effect of Ferric Oxide, Hydrophobic Carbon, and Polysilicate. J. Air Waste Manag. Assoc. 2022, 72, 403–419. [Google Scholar] [CrossRef]
- Xu, J.; Wang, N.; Chen, M.; Zhou, Z.; Yu, H. Comparative Investigation on the Reduction Behavior of Blast Furnace Dust Particles during In-Flight Process in Hydrogen-Rich and Carbon Monoxide Atmospheres. Powder Technol. 2020, 366, 709–721. [Google Scholar] [CrossRef]
- Das, R.; Mondal, M.K.; Pramanik, S. Enhancement of Strength of Blast Furnace Flue Dust—Iron Oxide—Fly Ash Composite Briquette Using ANOVA-Based Mathematical Model. Ironmak. Steelmak. 2023, 50, 1085–1093. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Javed, S.; Javed, H.M.A.; Jamshaid, M.; Ali, U.; Akram, M.A. Systematic Investigation of Structural, Morphological, Thermal, Optoelectronic, and Magnetic Properties of High-Purity Hematite/Magnetite Nanoparticles for Optoelectronics. Nanomaterials 2022, 12, 1635. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a Photocatalytic Material: A Review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, R.; Raizada, P.; Khan, A.A.P.; Singh, A.; Van Le, Q.; Nguyen, V.-H.; Selvasembian, R.; Thakur, S.; Singh, P. Current Status of Hematite (α-Fe2O3) Based Z-Scheme Photocatalytic Systems for Environmental and Energy Applications. J. Environ. Chem. Eng. 2022, 10, 107427. [Google Scholar] [CrossRef]
- Liu, T.; Morelli, M.; Li, Y. Hematite Materials for Solar-Driven Photoelectrochemical Cells. In Photoelectrochemical Solar Cells; Sankir, N.D., Sankir, M., Eds.; Wiley: Hoboken, NJ, USA, 2018; ISBN 9781119459934. [Google Scholar]
- Katsuki, T.; Zahran, Z.N.; Tanaka, K.; Eo, T.; Mohamed, E.A.; Tsubonouchi, Y.; Berber, M.R.; Yagi, M. Facile Fabrication of a Highly Crystalline and Well-Interconnected Hematite Nanoparticle Photoanode for Efficient Visible-Light-Driven Water Oxidation. ACS Appl. Mater. Interfaces 2021, 13, 39282–39290. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef] [PubMed]
- Fawzi Suleiman Khasawneh, O.; Palaniandy, P. Removal of Organic Pollutants from Water by Fe2O3/TiO2 Based Photocatalytic Degradation: A Review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Xiao, C.; Li, J.; Zhang, G. Synthesis of Stable Burger-like α-Fe2O3 Catalysts: Formation Mechanism and Excellent Photo-Fenton Catalytic Performance. J. Clean. Prod. 2018, 180, 550–559. [Google Scholar] [CrossRef]
- Matos, R.S.; Monteiro, M.D.S.; Silva, R.S.; Macêdo, M.A.; Paz, S.P.A.; Angélica, R.S.; Oliveira, R.M.P.B.; Ferreira, N.S. Novel Amapá Latex-Mediated Synthesis of Defective α-Fe2O3 Nanoparticles with Enhanced Ferromagnetism and Sunlight Photocatalytic Activity. Ceram. Int. 2022, 48, 28496–28511. [Google Scholar] [CrossRef]
- Leonel, A.G.; Mansur, A.A.P.; Mansur, H.S. Advanced Functional Nanostructures Based on Magnetic Iron Oxide Nanomaterials for Water Remediation: A Review. Water Res. 2021, 190, 116693. [Google Scholar] [CrossRef]
- Abdulkadir, I.; Abdallah, H.M.I.; Jonnalagadda, S.B.; Martincigh, B.S. The Effect of Synthesis Method on the Structure, and Magnetic and Photocatalytic Properties of Hematite (α-Fe2O3) Nanoparticles—Research Article. S. Afr. J. Chem. 2018, 71, 68–78. [Google Scholar] [CrossRef]
- Ma, T.; Zheng, L.; Zhao, Y.; Xu, Y.; Zhang, J.; Liu, X. Highly Porous Double-Shelled Hollow Hematite Nanoparticles for Gas Sensing. ACS Appl. Nano Mater. 2019, 2, 2347–2357. [Google Scholar] [CrossRef]
- Jung, W.-S.; Park, S.-H.; Kadam, A.N.; Kim, H.; Lee, S.-W. Direct Hydrothermal Synthesis of Amine-Functionalized Cubic Hematite (C-Fe2O3) and Sonochemical Deposition of Nanosized Au for Its Application as a Visible-Light Photocatalyst. Dalton Trans. 2020, 49, 2924–2932. [Google Scholar] [CrossRef]
- Lai, F.; Feng, J.; Heil, T.; Wang, G.-C.; Adler, P.; Antonietti, M.; Oschatz, M. Strong Metal Oxide-Support Interactions in Carbon/Hematite Nanohybrids Activate Novel Energy Storage Modes for Ionic Liquid-Based Supercapacitors. Energy Storage Mater. 2019, 20, 188–195. [Google Scholar] [CrossRef]
- Ikram, H.; Al Rashid, A.; Koç, M. Synthesis and Characterization of Hematite (α-Fe2O3) Reinforced Polylactic Acid (PLA) Nanocomposites for Biomedical Applications. Compos. Part C Open Access 2022, 9, 100331. [Google Scholar] [CrossRef]
- Lunin, A.V.; Lizunova, A.A.; Mochalova, E.N.; Yakovtseva, M.N.; Cherkasov, V.R.; Nikitin, M.P.; Kolychev, E.L. Hematite Nanoparticles from Unexpected Reaction of Ferrihydrite with Concentrated Acids for Biomedical Applications. Molecules 2020, 25, 1984. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Sun, D.; Xu, Y.; Liu, P.; Zhang, G.; Sun, Y.; Gao, D. Hematite Nanoplates: Controllable Synthesis, Gas Sensing, Photocatalytic and Magnetic Properties. J. Colloid Interface Sci. 2016, 462, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Pan, Z.; Chong, Y.; Ye, C.; Jin, X.; Wu, Q.; Hu, Z.; Ye, D.; Waterhouse, G.I.N.; Qiu, Y.; et al. Boosting the Electrochemical Performance of Hematite Nanorods via Quenching-Induced Metal Single Atom Functionalization. J. Mater. Chem. A 2021, 9, 3492–3499. [Google Scholar] [CrossRef]
- Tofanello, A.; Shen, S.; de Souza, F.L.; Vayssieres, L. Strategies to Improve the Photoelectrochemical Performance of Hematite Nanorod-Based Photoanodes. APL Mater. 2020, 8, 040905. [Google Scholar] [CrossRef]
- R Ochoa Díaz, A.L.D. Electric Arc Furnace Slag and Blast Furnace Dust, Use for the Manufacture of Asphalt Concrete for Roads. Civ. Eng. Infrastruct. J. 2019, 52, 155–166. [Google Scholar] [CrossRef]
- Ochoa, R.; López, A.; Grimaldo, G. Behavior of the Dynamic Modulus and Fatigue in Asphalt Mixtures with Blast Oxygen Furnace Slag and Blast Furnace Dust. Period. Polytech. Civ. Eng. 2021, 65, 840–851. [Google Scholar] [CrossRef]
- Xie, B.; Geng, N.; Yu, Q.; He, D.; Wang, F.; Liu, T.; Gao, J.; Ning, P.; Song, X.; Jia, L. Removal of SO2 from Flue Gas Using Blast Furnace Dust as an Adsorbent. Environ. Sci. Pollut. Res. 2022, 29, 15642–15653. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Fernandes, T.; Silva, R.; Galvão, D.F.; Flores, I.V. Mill Scale and Flue Dust Briquettes as Alternative Burden to Low Height Blast Furnaces. J. Clean. Prod. 2020, 276, 124332. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Yue, Q.; Zhang, P.; Wang, Y.; Gao, B. Prepartion and Application of Novel Blast Furnace Dust Based Catalytic-Ceramic-Filler in Electrolysis Assisted Catalytic Micro-Electrolysis System for Ciprofloxacin Wastewater Treatment. J. Hazard. Mater. 2020, 383, 121215. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Wen, H.; Jia, H.; Wang, J. A Novel Recycling Way of Blast Furnace Dust from Steelworks: Electrocoagulation Coupled Micro-Electrolysis System in Indigo Wastewater Treatment. Chemosphere 2023, 327, 138416. [Google Scholar] [CrossRef]
- Kurniawan, A.; Sutiono, H.; Indraswati, N.; Ismadji, S. Removal of Basic Dyes in Binary System by Adsorption Using Rarasaponin–Bentonite: Revisited of Extended Langmuir Model. Chem. Eng. J. 2012, 189–190, 264–274. [Google Scholar] [CrossRef]
- Daâssi, D.; Frikha, F.; Zouari-Mechichi, H.; Belbahri, L.; Woodward, S.; Mechichi, T. Application of Response Surface Methodology to Optimize Decolourization of Dyes by the Laccase-Mediator System. J. Environ. Manag. 2012, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Lalnunhlimi, S.; Krishnaswamy, V. Decolorization of Azo Dyes (Direct Blue 151 and Direct Red 31) by Moderately Alkaliphilic Bacterial Consortium. Braz. J. Microbiol. 2016, 47, 39–46. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Chen, L.; Chuang, Y.; Nguyen, T.B.; Chang, J.-H.; Lam, S.S.; Chen, C.-W.; Dong, C.-D. Novel Molybdenum Disulfide Heterostructure Nanohybrids with Enhanced Visible-Light-Induced Photocatalytic Activity towards Organic Dyes. J. Alloys Compd. 2020, 848, 156448. [Google Scholar] [CrossRef]
- Chen, L.; Tsai, M.-L.; Chuang, Y.; Chen, C.-W.; Dong, C.-D. Construction of Carbon Nanotubes Bridged MoS2/ZnO Z-Scheme Nanohybrid towards Enhanced Visible Light Driven Photocatalytic Water Disinfection and Antibacterial Activity. Carbon N. Y. 2022, 196, 877–889. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Wang, L.-C.; Gao, Z.-F.; Liu, W.-M.; Wu, X.-R. Recycling Blast Furnace Dust into Metals (Al, Zn and Ti)-Doped Hematite with Enhanced Photocatalytic Activity. J. Environ. Chem. Eng. 2016, 4, 341–345. [Google Scholar] [CrossRef]
- de O. Pereira, L.; de Moura, S.G.; Coelho, G.C.M.; Oliveira, L.C.A.; de Almeida, E.T.; Magalhães, F. Magnetic Photocatalysts from Industrial Residues and TiO2 for the Degradation of Organic Contaminants. J. Environ. Chem. Eng. 2019, 7, 102826. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.-W.; Dong, C.-D. Direct Z-Scheme Heterostructures Based on MoSSe Quantum Dots for Visible Light-Driven Photocatalytic Tetracycline Degradation. ACS Appl. Nano Mater. 2021, 4, 1038–1047. [Google Scholar] [CrossRef]
- Chen, L.; Chen, C.-W.; Huang, C.-P.; Chuang, Y.; Nguyen, T.-B.; Dong, C.-D. A Visible-Light Sensitive MoSSe Nanohybrid for the Photocatalytic Degradation of Tetracycline, Oxytetracycline, and Chlortetracycline. J. Colloid Interface Sci. 2022, 616, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Iaria, C.; Scalisi, E.M.; Brundo, M.V.; Gulino, A.; Spitaleri, L.; Nicotra, G.; Dattilo, S.; et al. Preferential Removal of Pesticides from Water by Molecular Imprinting on TiO2 Photocatalysts. Chem. Eng. J. 2020, 379, 122309. [Google Scholar] [CrossRef]
- Zeshan, M.; Bhatti, I.A.; Mohsin, M.; Iqbal, M.; Amjed, N.; Nisar, J.; AlMasoud, N.; Alomar, T.S. Remediation of Pesticides Using TiO2 Based Photocatalytic Strategies: A Review. Chemosphere 2022, 300, 134525. [Google Scholar] [CrossRef]
- Matos, R.S.; Attah-Baah, J.M.; Monteiro, M.D.S.; Costa, B.F.O.; Mâcedo, M.A.; Silva Junior, R.S.; da Fonseca Filho, H.D.; Oliveira, R.M.P.B.; Ferreira, N.S. Effect of the Amapá-Latex Chelating Agent Contents on the Microstructure and Photocatalytic Properties of ZnO Nanoparticles. J. Mater. Res. Technol. 2023, 22, 2673–2689. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Shi, X.; Li, X.; Wei, C.; Li, M.; Deng, Z. Selective Separation of Zinc and Iron/Carbon from Blast Furnace Dust via a Hydrometallurgical Cooperative Leaching Method. Waste Manag. 2022, 139, 116–123. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, F.; Ning, P.; Li, K.; Jia, L.; Feng, J.; Xia, F. Resource Utilization of Hazardous Solid Waste Blast Furnace Dust: Efficient Wet Desulfurization and Metal Recovery. Chemosphere 2023, 314, 137592. [Google Scholar] [CrossRef]
- Geerdes, M.; Chaigneau, R.; Lingiardi, O.; Molenaar, R.; van Opbergen, R.; Sha, Y.; Warren, J. Modern Blast Furnace Ironmaking: An Introduction, 4th ed.; IOS Press BV: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ashrit, S.S.; Sarkar, S.; Singh, R.; Yadav, S.Z.; Chatti, R.V. Characterization of Blast Furnace Flue Dust—A Multi Analytical Techniques Approach. Metall. Res. Technol. 2020, 117, 606. [Google Scholar] [CrossRef]
- Trinkel, V.; Mallow, O.; Thaler, C.; Schenk, J.; Rechberger, H.; Fellner, J. Behavior of Chromium, Nickel, Lead, Zinc, Cadmium, and Mercury in the Blast Furnace—A Critical Review of Literature Data and Plant Investigations. Ind. Eng. Chem. Res. 2015, 54, 11759–11771. [Google Scholar] [CrossRef]
- Butt, A.R.; Butt, I.A.; Nazir, A.; Ikram, M.; Sadiq, S.; Rashid, K.; Shujah, T.; Ali, S. Molecular Imaging of CaO Nanowhiskers in Living Organs. Nucleus 2015, 52, 159–164. [Google Scholar]
- Ikram, M.; Muhammad Khan, A.; Haider, A.; Haider, J.; Naz, S.; Ul-Hamid, A.; Shahzadi, A.; Nabgan, W.; Shujah, T.; Shahzadi, I.; et al. Facile Synthesis of La- and Chitosan-Doped CaO Nanoparticles and Their Evaluation for Catalytic and Antimicrobial Potential with Molecular Docking Studies. ACS Omega 2022, 7, 28459–28470. [Google Scholar] [CrossRef] [PubMed]
- Albayatı, S.H.M.; Deveci, P. PH and GSH Dual Responsive Smart Silica Nanocarrier for Doxorubicin Delivery. Mater. Res. Express 2019, 6, 065705. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zeng, Y.-N.; Jiang, L.-Q.; Wang, Y.-T.; Li, J.-G.; Kang, L.-L.; Ji, R.; Gao, D.; Wang, F.-P.; Yu, Q.; et al. Highly Stable NaFeO2-Fe3O4 Composite Catalyst from Blast Furnace Dust for Efficient Production of Biodiesel at Low Temperature. Ind. Crops Prod. 2022, 182, 114937. [Google Scholar] [CrossRef]
- Koodynska, D.; Ryczkowski, J.; Hubicki, Z. FT-IR/PAS Studies of Chelates Adsorption on Anion Exchangers. Eur. Phys. J. Spec. Top. 2008, 154, 339–343. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sasaki, J.M.; Silva JR, R.S.; Attah-Baah, J.M.; Macêdo, M.A. Visible-Light-Responsive Photocatalytic Activity Significantly Enhanced by Active [V Zn + V O +] Defects in Self-Assembled ZnO Nanoparticles. Inorg. Chem. 2021, 60, 4475–4496. [Google Scholar] [CrossRef] [PubMed]
- Helmi, M.; Hemmati, A. Synthesis of Magnetically Solid Base Catalyst of NaOH/Chitosan-Fe3O4 for Biodiesel Production from Waste Cooking Oil: Optimization, Kinetics and Thermodynamic Studies. Energy Convers. Manag. 2021, 248, 114807. [Google Scholar] [CrossRef]
- Fang, Y.; Zou, T.; Liang, X.; Wang, S.; Liu, X.; Gao, X.; Zhang, Z. Self-Assembly Synthesis and Properties of Microencapsulated n-Tetradecane Phase Change Materials with a Calcium Carbonate Shell for Cold Energy Storage. ACS Sustain. Chem. Eng. 2017, 5, 3074–3080. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Owolabi, J.O. Synthesis and Characterization of Alumina Supported Coconut Chaff Catalyst for Biodiesel Production from Waste Frying Oil. S. Afr. J. Chem. Eng. 2019, 30, 42–49. [Google Scholar] [CrossRef]
- Thipperudra, A.; Nagaraja, N.; PrashanthKumar, M.; Arunkumar, B. DSC and FTIR Studies in Potassium, Strontium Doped Boro-Phosphate Glasses. Chem. Mater. Res. 2020, 12, 1–8. [Google Scholar]
- Paulson, E.; Jothibas, M. Significance of Thermal Interfacing in Hematite (α-Fe2O3) Nanoparticles Synthesized by Sol-Gel Method and Its Characteristics Properties. Surf. Interfaces 2021, 26, 101432. [Google Scholar] [CrossRef]
- Fouad, D.E.; Zhang, C.; Bi, C.; Chand, K.; Lv, J. A Facile Approach towards Large-Scale Synthesis of Pristine Hematite Nanoparticles with Enhanced Photo/Catalytic Properties via Annealing of Iron Sulfate Precursor in Presence of Treated Egg Shell Wastes as Desulfurizer. Mater. Charact. 2020, 169, 110592. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, J.; Wang, G.; Conejo, A.N.; Xu, R.; Wang, H.; Zhong, J. Structure Characteristics and Combustibility of Carbonaceous Materials from Blast Furnace Flue Dust. Appl. Therm. Eng. 2016, 108, 1168–1177. [Google Scholar] [CrossRef]
- Martins, F.M.; Neto, J.M.d.R.; Cunha, C.J. da Mineral Phases of Weathered and Recent Electric Arc Furnace Dust. J. Hazard. Mater. 2008, 154, 417–425. [Google Scholar] [CrossRef]
- Robinson, R. High Temperature Properties of By-Product Cold Bonded Pellets Containing Blast Furnace Flue Dust. Thermochim. Acta 2005, 432, 112–123. [Google Scholar] [CrossRef]
- Al-harahsheh, M.; Al-Nu’airat, J.; Al-Otoom, A.; Al-hammouri, I.; Al-jabali, H.; Al-zoubi, M.; Abu Al’asal, S. Treatments of Electric Arc Furnace Dust and Halogenated Plastic Wastes: A Review. J. Environ. Chem. Eng. 2019, 7, 102856. [Google Scholar] [CrossRef]
- Oustadakis, P.; Tsakiridis, P.E.; Katsiapi, A.; Agatzini-Leonardou, S. Hydrometallurgical Process for Zinc Recovery from Electric Arc Furnace Dust (EAFD). J. Hazard. Mater. 2010, 179, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Reduction of Hematite (Fe2O3) to Wüstite (FeO) by Carbon Monoxide (CO) for Chemical Looping Combustion. Chem. Eng. J. 2014, 242, 204–210. [Google Scholar] [CrossRef]
- Aldegs, Y.; Elbarghouthi, M.; Elsheikh, A.; Walker, G. Effect of Solution PH, Ionic Strength, and Temperature on Adsorption Behavior of Reactive Dyes on Activated Carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Nabil, K.; Abdelmonem, N.; Nogami, M.; Ismail, I. Preparation of Composite Monolith Supercapacitor Electrode Made from Textile-Grade Polyacrylonitrile Fibers and Phenolic Resin. Materials 2020, 13, 655. [Google Scholar] [CrossRef]
- Basri, N.H.; Deraman, M.; Kanwal, S.; Talib, I.A.; Manjunatha, J.G.; Aziz, A.A.; Farma, R. Supercapacitors Using Binderless Composite Monolith Electrodes from Carbon Nanotubes and Pre-Carbonized Biomass Residues. Biomass Bioenergy 2013, 59, 370–379. [Google Scholar] [CrossRef]
- Bhuyan, M.A.H.; Gebre, R.K.; Finnilä, M.A.J.; Illikainen, M.; Luukkonen, T. Preparation of Filter by Alkali Activation of Blast Furnace Slag and Its Application for Dye Removal. J. Environ. Chem. Eng. 2022, 10, 107051. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.-D.; Lee, J.; Park, J.H.; Chun, J.H. Influences of Surface Capping on Particle Size and Optical Characteristics of ZnS:Cu Nanocrystals. Mater. Sci. Eng. B 2006, 131, 13–17. [Google Scholar] [CrossRef]
- Kumar, S.V.; Ganesan, S. Preparation and Characterization of Gold Nanoparticles with Different Capping Agents. Int. J. Green Nanotechnol. 2011, 3, 47–55. [Google Scholar] [CrossRef]
- Parveen, R.; Datta, A.; Kumar Maiti, P. Concentration of Capping Agent Controls Size Selection, Agglomeration and Antimicrobial Action of Silver Nanoparticles. J. Surf. Sci. Technol. 2021, 36, 137–145. [Google Scholar] [CrossRef]
- Mizuno, S.; Yao, H. On the Electronic Transitions of α-Fe2O3 Hematite Nanoparticles with Different Size and Morphology: Analysis by Simultaneous Deconvolution of UV–Vis Absorption and MCD Spectra. J. Magn. Magn. Mater. 2021, 517, 167389. [Google Scholar] [CrossRef]
- Mansour, H.; Omri, K.; Ammar, S. Structural, Optical and Magnetic Properties of Cobalt Doped Hematite Nanoparticles. Chem. Phys. 2019, 525, 110400. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Parida, K.M. Fabrication, Growth Mechanism, and Characterization of α-Fe2O3 Nanorods. ACS Appl. Mater. Interfaces 2011, 3, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.A.; Xavier, M.J.S.; Rodrigues, E.S.; de Oliveira, C.A.; Farias, P.M.A.; Stingl, A.; Ferreira, N.S.; Silva, M.S. Green Synthesis of ZnO Nanoparticles Using Whey as an Effective Chelating Agent. Mater. Lett. 2020, 259, 126853. [Google Scholar] [CrossRef]
- Ai, M.; Li, X.; Pan, L.; Xu, X.; Yang, J.; Zou, J.-J.; Zhang, X. Surface States Modulation of Hematite Photoanodes for Enhancing Photoelectrochemical Catalysis. Chem. Eng. Sci. 2022, 250, 117397. [Google Scholar] [CrossRef]
- Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Musić, S.; Stanković, D.; Krehula, S. Influence of Sn Doping on the Structural, Magnetic, Optical and Photocatalytic Properties of Hematite (α-Fe2O3) Nanoparticles. J. Phys. Chem. Solids 2022, 161, 110372. [Google Scholar] [CrossRef]
- Rufus, A.; Sreeju, N.; Philip, D. Size Tunable Biosynthesis and Luminescence Quenching of Nanostructured Hematite (α-Fe2O3) for Catalytic Degradation of Organic Pollutants. J. Phys. Chem. Solids 2019, 124, 221–234. [Google Scholar] [CrossRef]
- Hjiri, M.; Alonizan, N.H.; Althubayti, M.M.; Alshammari, S.; Besbes, H.; Aida, M.S. Preparation and Photoluminescence of NiFe2O4 Nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 15379–15387. [Google Scholar] [CrossRef]
- Sundara Selvam, P.S.; Govindan, S.; Perumal, B.; Kandan, V. Screening of In Vitro Antibacterial Property of Hematite (α-Fe2O3) Nanoparticles: A Green Approach. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 177–187. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Malhotra, T.; Verma, S. Characterization of Structural, Optical and Photocatalytic Properties of Silver Modified Hematite (α-FeO) Nanocatalyst. J. Alloys Compd. 2022, 904, 164006. [Google Scholar] [CrossRef]
- Rahmani, M.B.; Ghasemi, E.; Rezaii, F. Synthesis of Wormlike α-Fe2O3 Nanostructure: Characterization and Antibacterial Application. J. Electron. Mater. 2020, 49, 6087–6095. [Google Scholar] [CrossRef]
- Rada, S.; Culea, E.; Rada, M. Novel ZrO2 Based Ceramics Stabilized by Fe2O3, SiO2 and Y2O3. Chem. Phys. Lett. 2018, 696, 92–99. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the Shape and Size of Iron Oxide (α-Fe2O3) Nanoparticles Synthesized through the Chemical Precipitation Method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Abo-Naf, S.M.; Zayed, H.A.; Hassan, N.S. Photoluminescence and Semiconducting Behavior of Fe, Co, Ni and Cu Implanted in Heavy Metal Oxide Glasses. J. Mater. Res. Technol. 2016, 5, 226–233. [Google Scholar] [CrossRef]
- Fonseca Filho, H.D.; Pires, M.P.; Souza, P.L.; Matos, R.S.; Prioli, R. Investigation of the Morphological and Fractal Behavior at Nanoscale of Patterning Lines by Scratching in an Atomic Force Microscope. Microsc. Res. Tech. 2022, 85, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Yang, Y.; Zhang, Z.; Bao, X.; Yang, R.; Li, S.; Sun, L. Photoluminescence and Defect Evolution of Nano-ZnO Thin Films at Low Temperature Annealing. Sci. China Technol. Sci. 2013, 56, 25–31. [Google Scholar] [CrossRef]
- Ravichandran, A.T.; Karthick, R. Enhanced Photoluminescence, Structural, Morphological and Antimicrobial Efficacy of Co-Doped ZnO Nanoparticles Prepared by Co-Precipitation Method. Results Mater. 2020, 5, 100072. [Google Scholar] [CrossRef]
- Justus, J.S.; Roy, S.D.D.; Raj, A.M.E. Influence of Lanthanum Doping on the Structural and Optical Properties of Hematite Nanopowders. J. Appl. Sci. Eng. Methodol. 2016, 2, 272–277. [Google Scholar]
- Wang, J.; White, W.B.; Adair, J.H. Optical Properties of Hydrothermally Synthesized Hematite Particulate Pigments. J. Am. Ceram. Soc. 2005, 88, 3449–3454. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Xue, J. Phase Transition, Ferroelectric Behaviors and Domain Structures of (Na1/2Bi1/2)1−xTiPbxO3 Thin Films. Acta Mater. 2006, 54, 1691–1698. [Google Scholar] [CrossRef]
- Ling, Y.; Wheeler, D.A.; Zhang, J.Z.; Li, Y. Optical Properties and Applications of Hematite (α-Fe2O3) Nanostructures. In One-Dimensional Nanostructures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 167–184. [Google Scholar]
- Fakhri, A.; Naji, M.; Nejad, P.A. Adsorption and Photocatalysis Efficiency of Magnetite Quantum Dots Anchored Tin Dioxide Nanofibers for Removal of Mutagenic Compound: Toxicity Evaluation and Antibacterial Activity. J. Photochem. Photobiol. B Biol. 2017, 173, 204–209. [Google Scholar] [CrossRef]
- Mathevula, L.E.; Noto, L.L.; Mothudi, B.M.; Chithambo, M.; Dhlamini, M.S. Structural and Optical Properties of Sol-Gel Derived α-Fe2O3 Nanoparticles. J. Lumin. 2017, 192, 879–887. [Google Scholar] [CrossRef]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal Synthesis of Hematite (α-Fe2O3) Nanoparticle Forms: Synthesis Conditions, Structure, Particle Shape Analysis, Cytotoxicity and Magnetic Properties. J. Alloys Compd. 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J. Hysteresis and Coercivity of Hematite. J. Geophys. Res. Solid Earth 2014, 119, 2582–2594. [Google Scholar] [CrossRef]
- Smart, T.J.; Ping, Y. Effect of Defects on the Small Polaron Formation and Transport Properties of Hematite from First-Principles Calculations. J. Phys. Condens. Matter 2017, 29, 394006. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Van San, E.; De Grave, E.; Da Costa, G.M. About the Morin Transition in Hematite in Relation with Particle Size and Aluminium Substitution. Czechoslov. J. Phys. 2001, 51, 663–675. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.; Albiss, B.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Letcher, T. Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Sarma, S.K.; Mohan, R.; Shukla, A. Structural, Opto-Electronic and Photoelectrochemical Properties of Tin Doped Hematite Nanoparticles for Water Splitting. Mater. Sci. Semicond. Process. 2020, 108, 104873. [Google Scholar] [CrossRef]
- Niu, Y.; Zhou, Y.; Niu, P.; Shen, H.; Ma, Y. Effects of Ti Doping on Hematite Photoanodes: More Surface States. J. Nanosci. Nanotechnol. 2019, 19, 3437–3446. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Zhang, Z.; Baek, M.; Yong, K. Photoelectrochemical Enhancement of ZnO/BiVO4/ZnFe2O4/Rare Earth Oxide Hetero-Nanostructures. Appl. Surf. Sci. 2018, 429, 29–36. [Google Scholar] [CrossRef]
- Lee, S.F.; Jimenez-Relinque, E.; Martinez, I.; Castellote, M. Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2. Catalysts 2023, 13, 1000. [Google Scholar] [CrossRef]
- Lee, S.F.; Jimenez-Relinque, E.; Martinez, I.; Castellote, M. Photoelectrochemical Global Approach to the Behaviour of Nanostructured Anatase under Different Irradiation Conditions. Catal. Today 2022, 397–399, 286–295. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Qiu, Y.; Mitsuzak, N.; Chen, Z. The Influence of the Hydrothermal Temperature and Time on Morphology and Photoelectrochemical Response of α-Fe2O3 Photoanode. J. Alloys Compd. 2017, 696, 980–987. [Google Scholar] [CrossRef]
- Wodka, D.; Socha, R.P.; Bielańska, E.; Elżbieciak-Wodka, M.; Nowak, P.; Warszyński, P. Photocatalytic Activity of Titanium Dioxide Modified by Fe2O3 Nanoparticles. Appl. Surf. Sci. 2014, 319, 173–180. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G. Silkworm Exuviae—A New Non-Conventional and Low-Cost Adsorbent for Removal of Methylene Blue from Aqueous Solutions. J. Hazard. Mater. 2011, 186, 1320–1327. [Google Scholar] [CrossRef]
- Falletta, E.; Galloni, M.G.; Mila, N.; bin Roslan, M.N.; Abd Ghani, N.; Cerrato, G.; Giordana, A.; Magni, M.; Spriano, S.; Boffito, D.C.; et al. Fast and Efficient Piezo-Photocatalytic Mineralization of Ibuprofen by BiOBr Nanosheets under Solar Light Irradiation. ACS Photonics 2023, 10, 3929–3943. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.K.; Nguyen, V.-H.; Singh, P. C-, N-Vacancy Defect Engineered Polymeric Carbon Nitride towards Photocatalysis: Viewpoints and Challenges. J. Mater. Chem. A 2021, 9, 111–153. [Google Scholar] [CrossRef]
- Ozin, G.A. Nanochemistry Views; University of Toronto: Toronto, ON, USA, 2014. [Google Scholar]
- Alshehri, A.A.; Malik, M.A. Biogenic Fabrication of ZnO Nanoparticles Using Trigonella Foenum-Graecum (Fenugreek) for Proficient Photocatalytic Degradation of Methylene Blue under UV Irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 16156–16173. [Google Scholar] [CrossRef]
- Alharbi, A.; Abdelrahman, E.A. Efficient Photocatalytic Degradation of Malachite Green Dye Using Facilely Synthesized Hematite Nanoparticles from Egyptian Insecticide Cans. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117612. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Nassar, H.N.; El-Gendy, N.S. Green Synthesis of α-Fe2O3 Using Citrus Reticulum Peels Extract and Water Decontamination from Different Organic Pollutants. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1425–1434. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Z.; Zhen, M.; Gu, B.; Shen, B.; Dong, F. Insights for Optimum Cation Defects in Photocatalysis: A Case Study of Hematite Nanostructures. Appl. Catal. B Environ. 2020, 264, 118506. [Google Scholar] [CrossRef]
- Feng, H.; Wang, Y.; Wang, C.; Diao, F.; Zhu, W.; Mu, P.; Yuan, L.; Zhou, G.; Rosei, F. Defect-Induced Enhanced Photocatalytic Activities of Reduced α-Fe2O3 Nanoblades. Nanotechnology 2016, 27, 295703. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 Nm Rutile Titanium Dioxide Nanoparticles for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Wu, M.; Chen, J.; Zhao, W.; Tian, Y.; Ding, T.; Zhang, J.; Jiang, Z.; Li, X. Rational Construction of Oxygen Vacancies onto Tungsten Trioxide to Improve Visible Light Photocatalytic Water Oxidation Reaction. Appl. Catal. B Environ. 2018, 239, 398–407. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ai, Z.; Jia, F.; Zhang, L. Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed. 2018, 57, 122–138. [Google Scholar] [CrossRef]
- Haseena, S.; Shanavas, S.; Ahamad, T.; Alshehri, S.M.; Baskaran, P.; Duraimurugan, J.; Acevedo, R.; Khan, M.A.M.; Anbarasan, P.M.; Jayamani, N. Investigation on Photocatalytic Activity of Bio-Treated α-Fe2O3 Nanoparticles Using Phyllanthus Niruri and Moringa Stenopetala Leaf Extract against Methylene Blue and Phenol Molecules: Kinetics, Mechanism and Stability. J. Environ. Chem. Eng. 2021, 9, 104996. [Google Scholar] [CrossRef]
- Weldegebrieal, G.K.; Sibhatu, A.K. Photocatalytic Activity of Biosynthesized α-Fe2O3 Nanoparticles for the Degradation of Methylene Blue and Methyl Orange Dyes. Optik 2021, 241, 167226. [Google Scholar] [CrossRef]
- Maji, S.K.; Jana, A. Two-Dimensional Nanohybrid (RGS@AuNPs) as an Effective Catalyst for the Reduction of 4-Nitrophenol and Photo-Degradation of Methylene Blue Dye. New J. Chem. 2017, 41, 3326–3332. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Santos, L.V.S.; Jacob, R.S. Remediation of Methylene Blue from Aqueous Solution by Chlorella Pyrenoidosa and Spirulina Maxima Biosorption: Equilibrium, Kinetics, Thermodynamics and Optimization Studies. J. Environ. Chem. Eng. 2018, 6, 6680–6690. [Google Scholar] [CrossRef]
- Baruah, R.; Yadav, A.; Das, A.M. Livistona Jekinsiana Fabricated ZnO Nanoparticles and Their Detrimental Effect towards Anthropogenic Organic Pollutants and Human Pathogenic Bacteria. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119459. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K. Photocatalytic Activity of Metal Oxides—The Role of Holes and OH Radicals. Appl. Catal. B Environ. 2011, 107, 150–157. [Google Scholar] [CrossRef]
- Liu, D.; Tian, R.; Wang, J.; Nie, E.; Piao, X.; Li, X.; Sun, Z. Photoelectrocatalytic Degradation of Methylene Blue Using F Doped TiO2 Photoelectrode under Visible Light Irradiation. Chemosphere 2017, 185, 574–581. [Google Scholar] [CrossRef]
- Geng, K.; Wu, Y.; Jiang, G.; Liu, K.; Jiang, L. RuC@g-C3N4(H+)/TiO2 Visible Active Photocatalyst: Facile Fabrication and Z-Scheme Carrier Transfer Mechanism. Mol. Catal. 2018, 458, 33–42. [Google Scholar] [CrossRef]
- Ghanei-Zare, S.; Moghadasi, M.; Khajavian, R.; Akbarzadeh-T, N.; Mirzaei, M. A Metal-Organic Framework-Derived CuO Microrods for Fast Photocatalytic Degradation of Methylene Blue. J. Mol. Struct. 2023, 1286, 135563. [Google Scholar] [CrossRef]
- Kumar, A.; Luxmi, V. Development of an Efficient Eco-Friendly Photo-Catalyst Using Agro-Waste Turmeric Leaves and Its Characterizations. Optik 2021, 242, 167057. [Google Scholar] [CrossRef]
- Sapiña, M.; Jimenez-Relinque, E.; Castellote, M. Turning Waste into Valuable Resource: Potential of Electric Arc Furnace Dust as Photocatalytic Material. Environ. Sci. Pollut. Res. 2014, 21, 12091–12098. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.M.; Saczk, A.A.; Felix, F.d.S.; Penido, E.S.; Santos, T.A.R.; de Souza Teixeira, A.; Magalhães, F. Characterization of Electric Arc Furnace Dust and Its Application in Photocatalytic Reactions to Degrade Organic Contaminants in Synthetic and Real Samples. J. Photochem. Photobiol. A Chem. 2023, 438, 114585. [Google Scholar] [CrossRef]
- Alcaraz, L.; Urbieta, A.; Rabanal, M.E.; Fernández, P.; López, F.A. Photocatalytic Activity of Electric-Arc Furnace Flue Dusts. J. Mater. Res. Technol. 2020, 9, 1261–1272. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zhang, Z.; Zhang, X.; Qiu, F.; Liu, Z.; Li, W. Treatment of Methylene Blue Wastewater with Nano-PbCrO4 Photocatalyst Prepared from Chromite Ore Processing Residue. J. Clean. Prod. 2022, 379, 134352. [Google Scholar] [CrossRef]
- Elanthikkal, S.; Mohamed, H.H.; Alomair, N.A. Extraction of Biosilica from Date Palm Biomass Ash and Its Application in Photocatalysis. Arab. J. Chem. 2023, 16, 104522. [Google Scholar] [CrossRef]
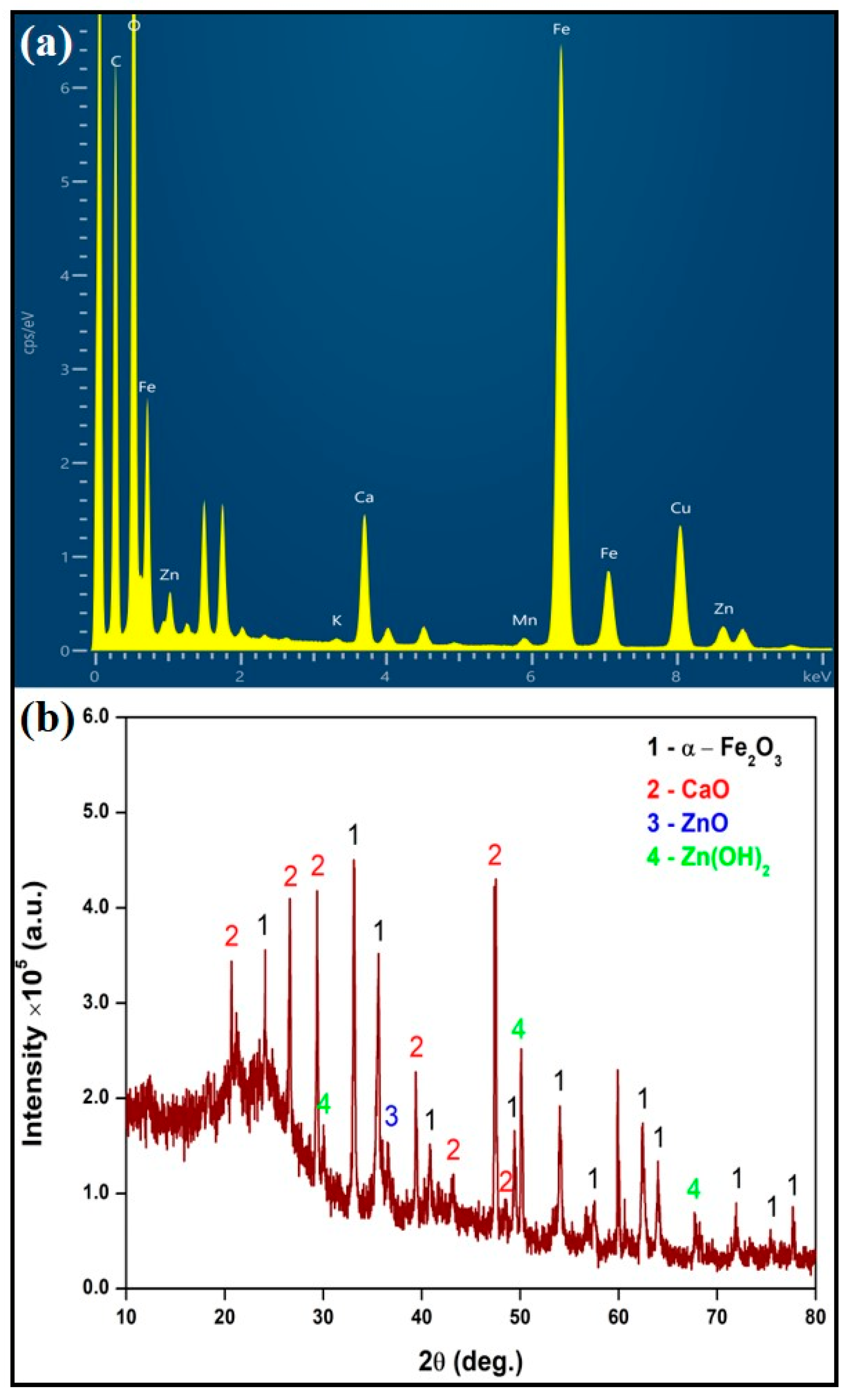
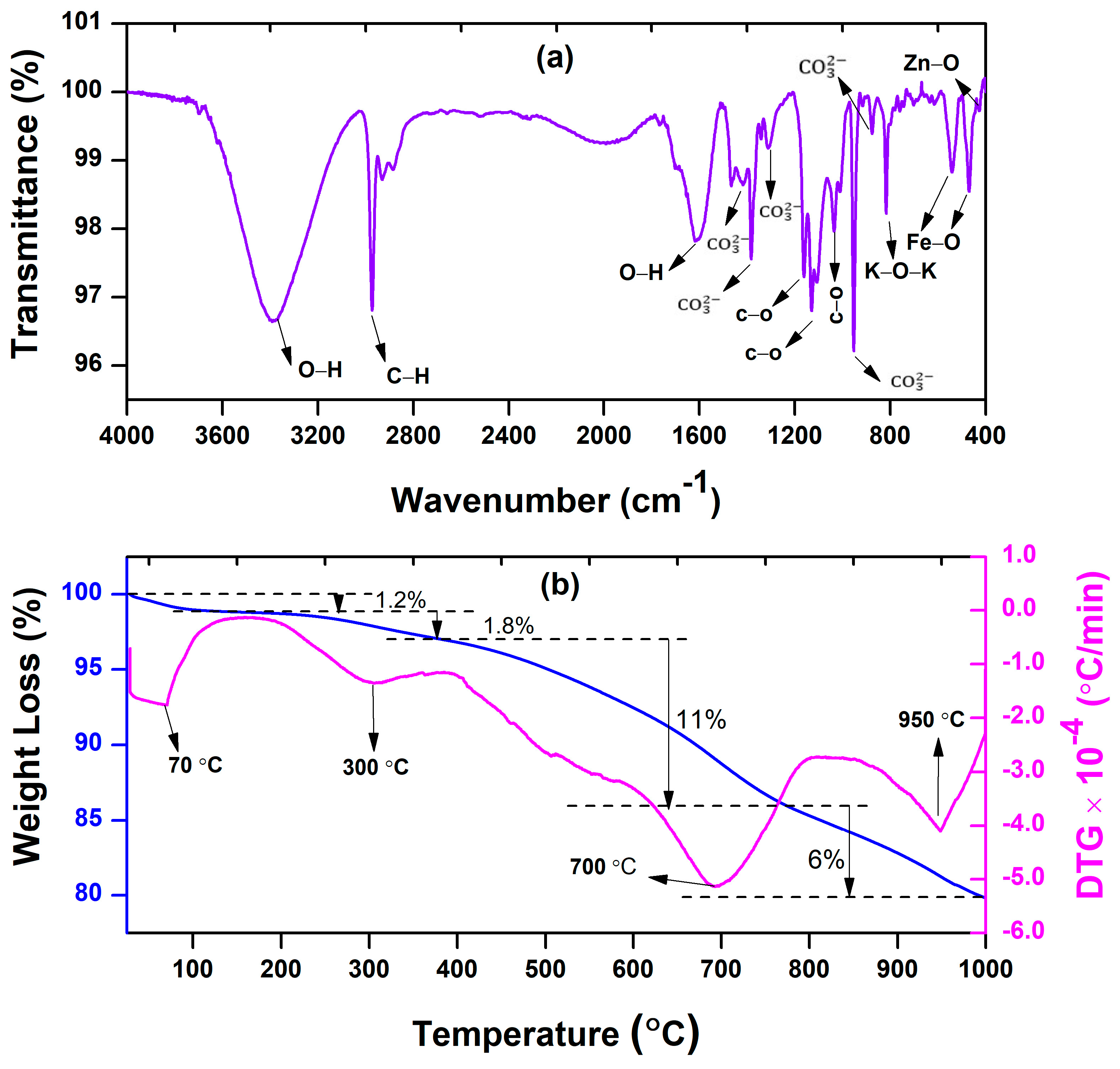
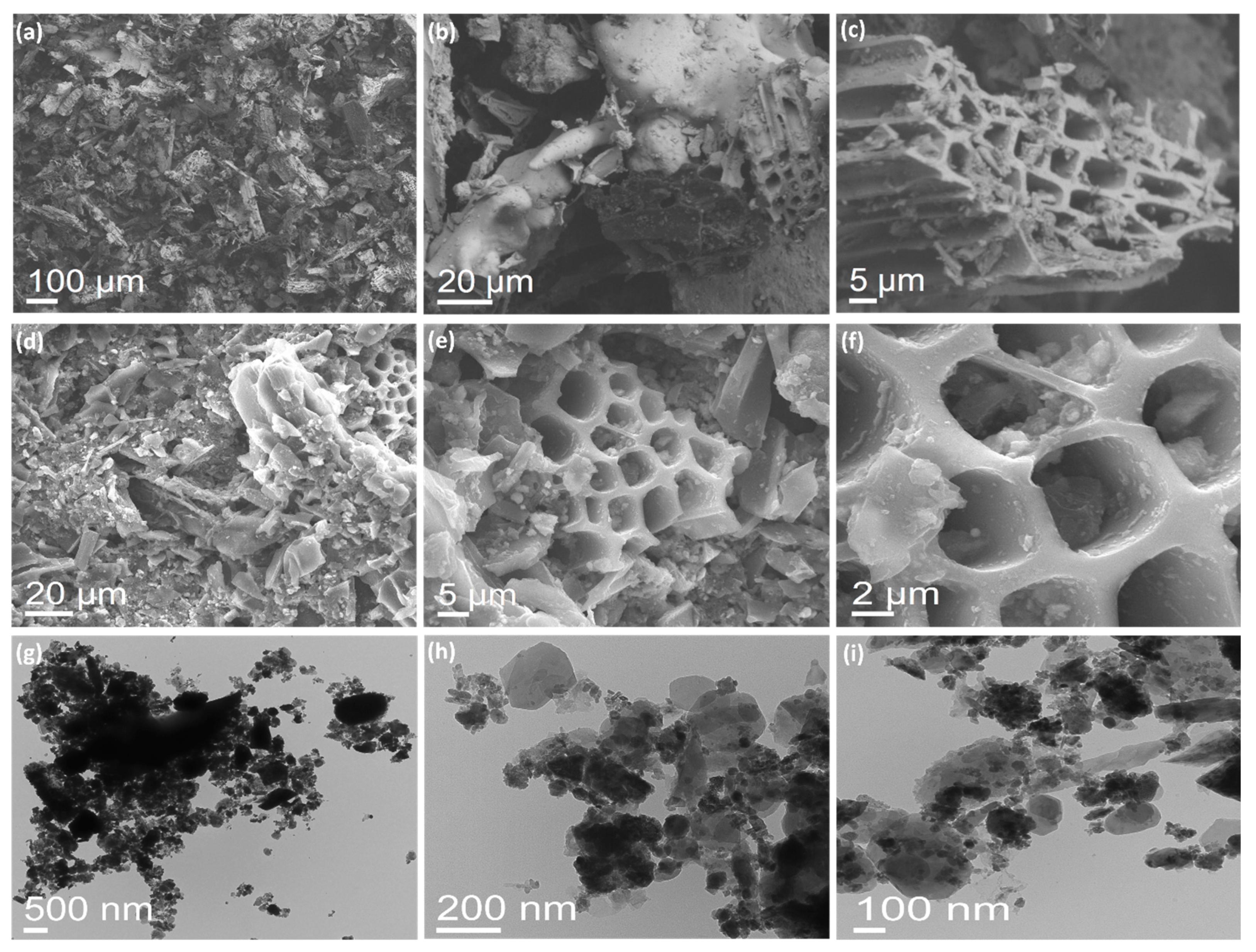

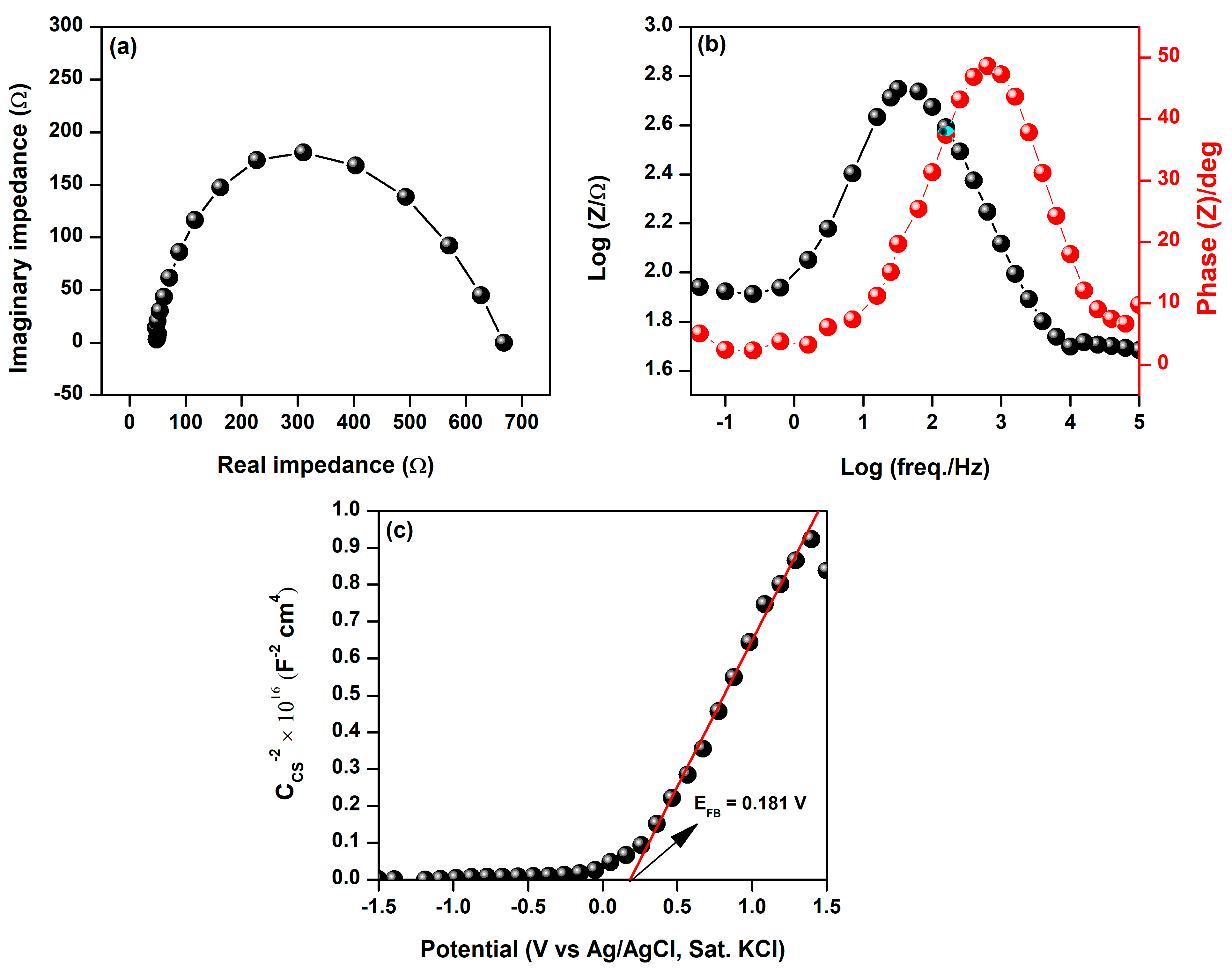

| Sample | Structure | Composition (%) |
|---|---|---|
| BFDW | α-Fe2O3 | 94.30 |
| CaO | 2.74 | |
| K2O | 1.6 | |
| ZnO | 0.73 | |
| MnO | 0.63 |
| Parameter | Unit | Values |
|---|---|---|
| Surface area | m2 g−1 | 284 |
| Pore volume | cm3 g−1 | 0.18 |
| Average pore diameter | Å | 15.27 |
| Sample | Peak Position (nm) | Energy Level (eV) | Defect | References |
|---|---|---|---|---|
| BFWD | 285 | 4.35 | MBE | [95,96] |
| 302 | 4.11 | MBE | [97] | |
| 345 | 3.59 | MBE, VO | [89] | |
| 374 | 3.32 | VB → CB | [90,98] | |
| 389 | 3.19 | NBE, | [17,99] | |
| 405 | 3.06 | VO | [17,100] | |
| 420 | 2.95 | Oi, VO | [91,92,93] | |
| 468 | 2.65 | VO | [84,94] |
| Waste | PT | PM | PC | RT | IS | PP | Reference |
|---|---|---|---|---|---|---|---|
| - | mg | mg∙L−1 | min | - | % | - | |
| BFD | methylene blue | 10 | 10 | 180 | Visible light | 74 | Here |
| Electric arc furnace dust | rhodamine B | 150 | 1 | 140 | UV light | 70 | [136] |
| Electric arc furnace dust | remazol black | 120 | 40 | 150 | UV light | 72 | [137] |
| Electric arc furnace dust | methylene blue | 50 | 2,5 | 120 | UV light | 80 | [138] |
| Chromite ore process waste | methylene blue | 70 | 10 | 80 | UV light | 77 | [139] |
| Turmeric leaves powder | methyl orange | 10 | 10 | 120 | Visible light | 46 | [135] |
| TiO2 supported on tar pitch and red mud | remazol black B | 200 | 40 | 240 | UV light | 71 | [41] |
| Biosilica from date palm biomass ash | bromophenol Blue | 50 | 20 | 129 | UV light | 74 | [140] |
| Metals-doped α-Fe2O3 prepared from BFD | methylene blue | 50 | 10 | 150 | UV light | 72 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, N.O.; Lima, L.S.; Monteiro, M.D.S.; Sobrinho, R.A.L.; Ferreira, N.S.; Ramos, G.Q.; da Fonseca Filho, H.D.; Oliveira, R.M.P.B.; Matos, R.S. Associating Physical and Photocatalytic Properties of Recyclable and Reusable Blast Furnace Dust Waste. Materials 2024, 17, 818. https://doi.org/10.3390/ma17040818
Chaves NO, Lima LS, Monteiro MDS, Sobrinho RAL, Ferreira NS, Ramos GQ, da Fonseca Filho HD, Oliveira RMPB, Matos RS. Associating Physical and Photocatalytic Properties of Recyclable and Reusable Blast Furnace Dust Waste. Materials. 2024; 17(4):818. https://doi.org/10.3390/ma17040818
Chicago/Turabian StyleChaves, Nayane O., Lucas S. Lima, Michael D. S. Monteiro, Raimundo A. L. Sobrinho, Nilson S. Ferreira, Glenda Q. Ramos, Henrique D. da Fonseca Filho, Rosane M. P. B. Oliveira, and Robert S. Matos. 2024. "Associating Physical and Photocatalytic Properties of Recyclable and Reusable Blast Furnace Dust Waste" Materials 17, no. 4: 818. https://doi.org/10.3390/ma17040818







