Iodine-Doped Hollow Carbon Nanocages without Templates Strategy for Boosting Zinc-Ion Storage by Nucleophilicity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
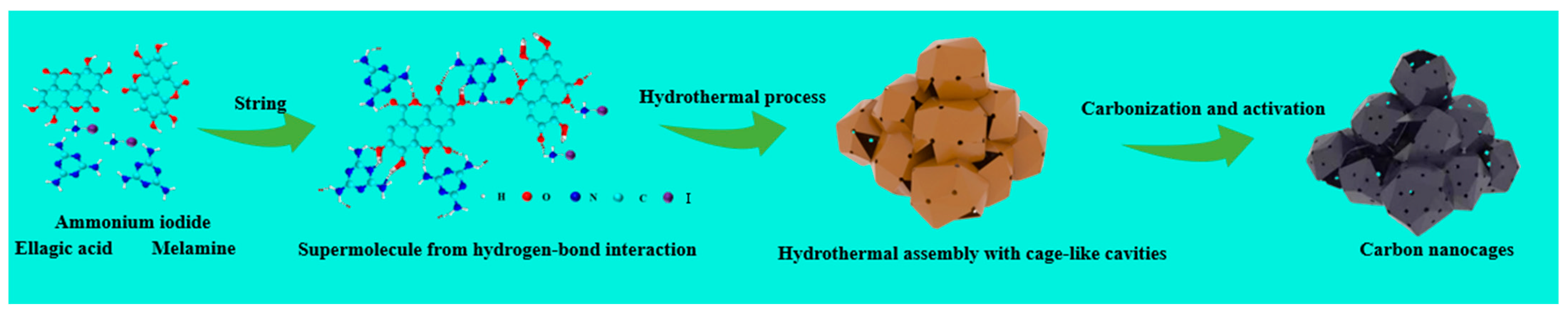
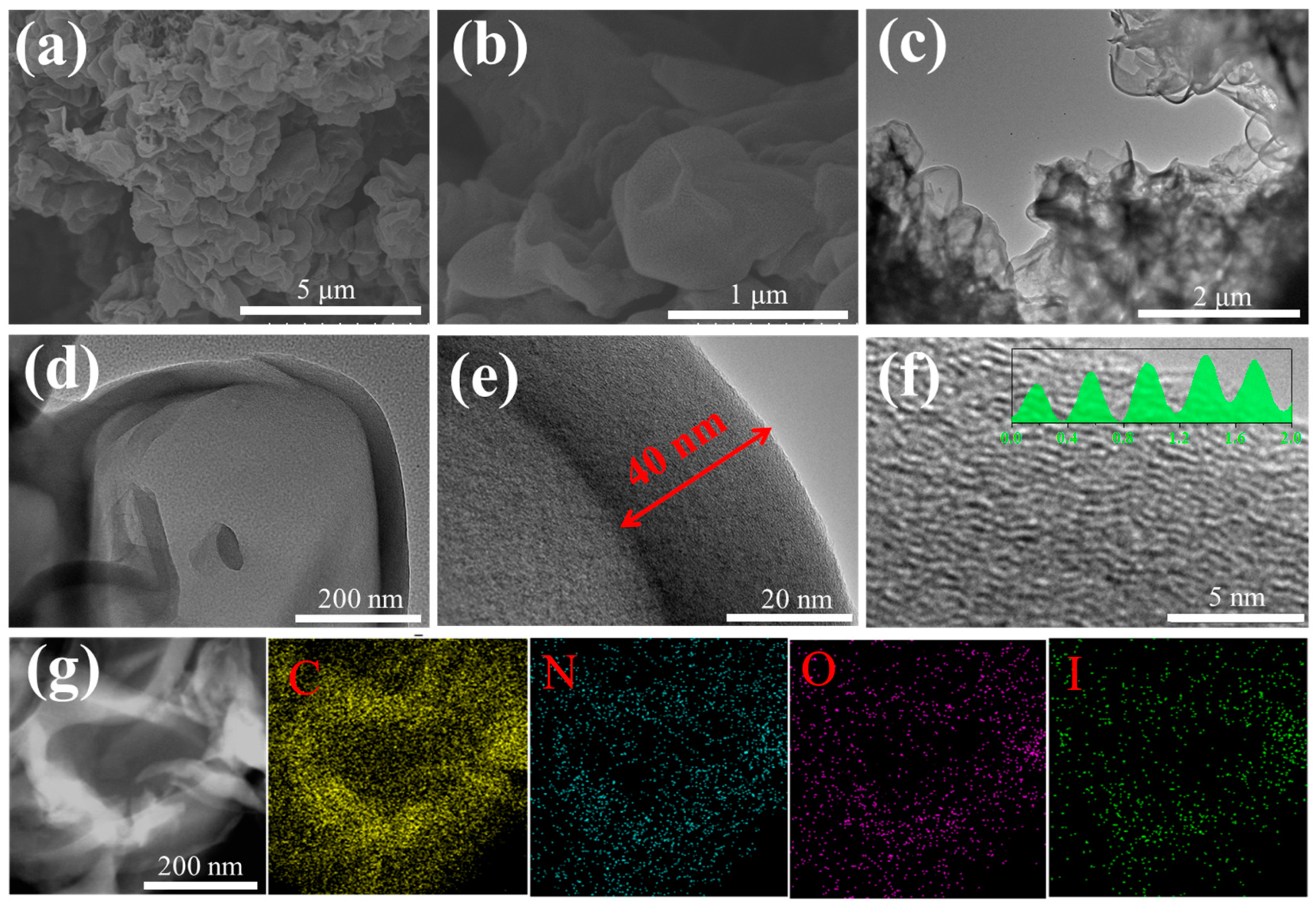
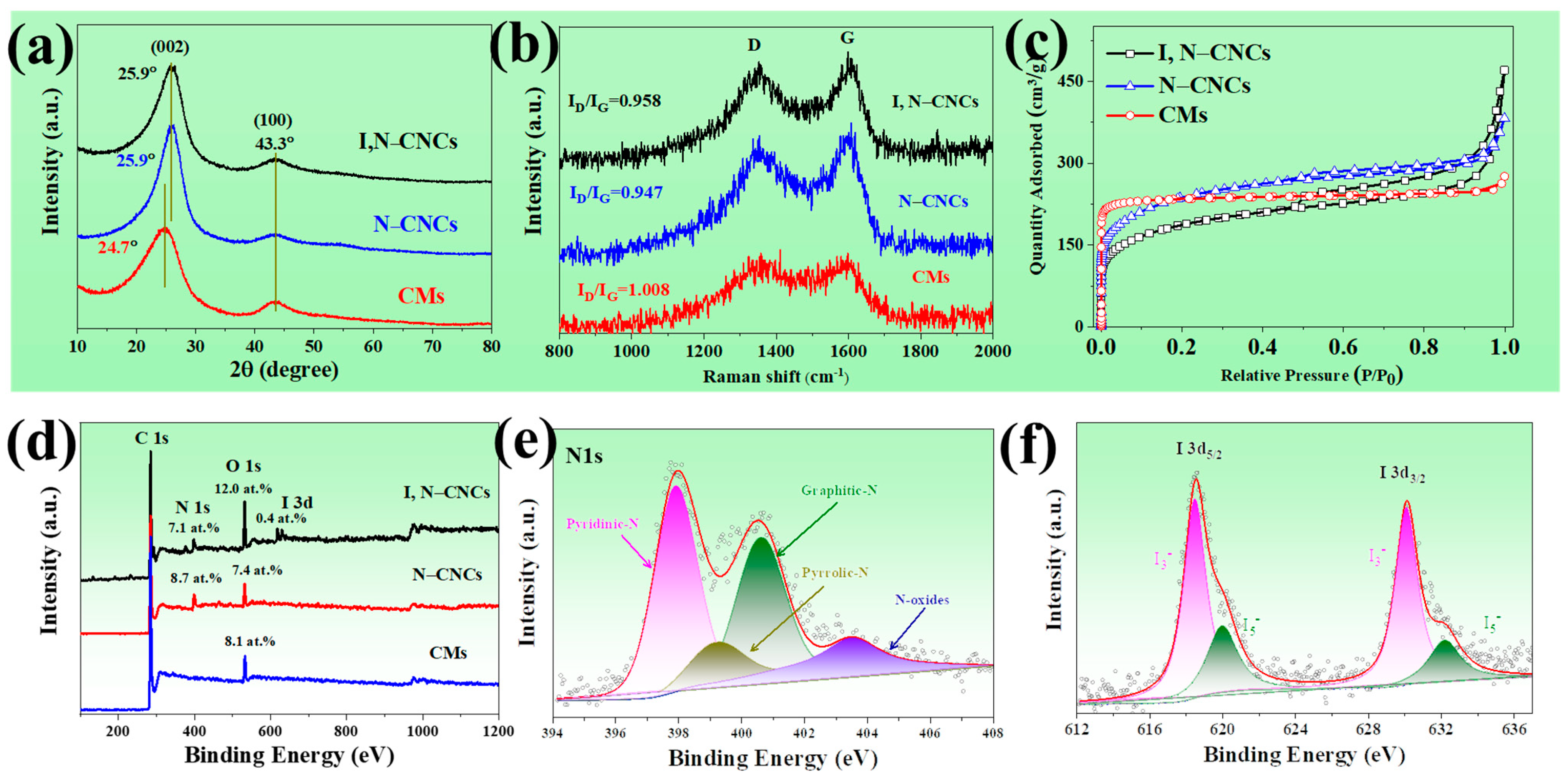
3.1. Structure Characterizations
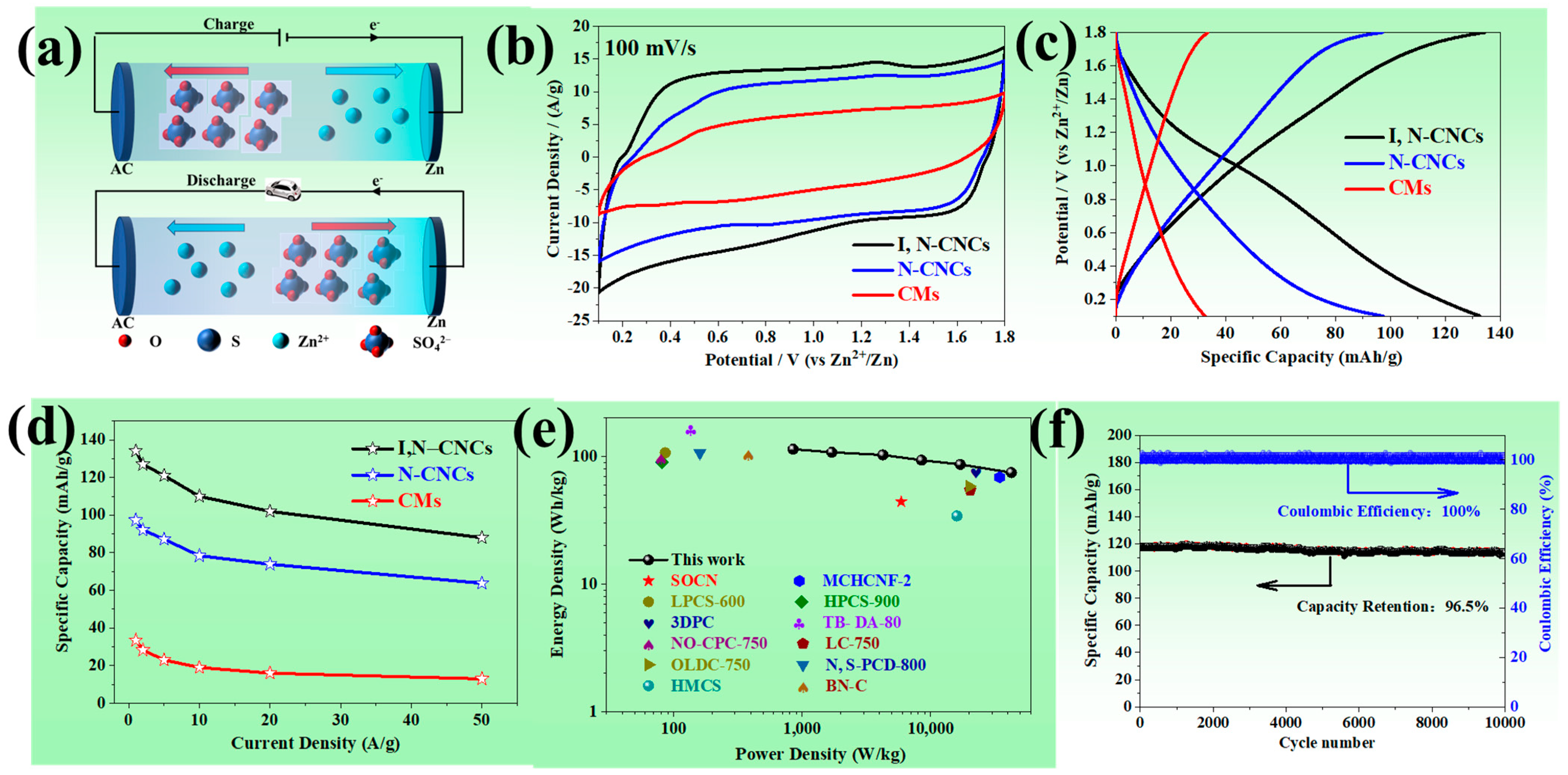
3.2. Electrochemical Performance of ZHCs
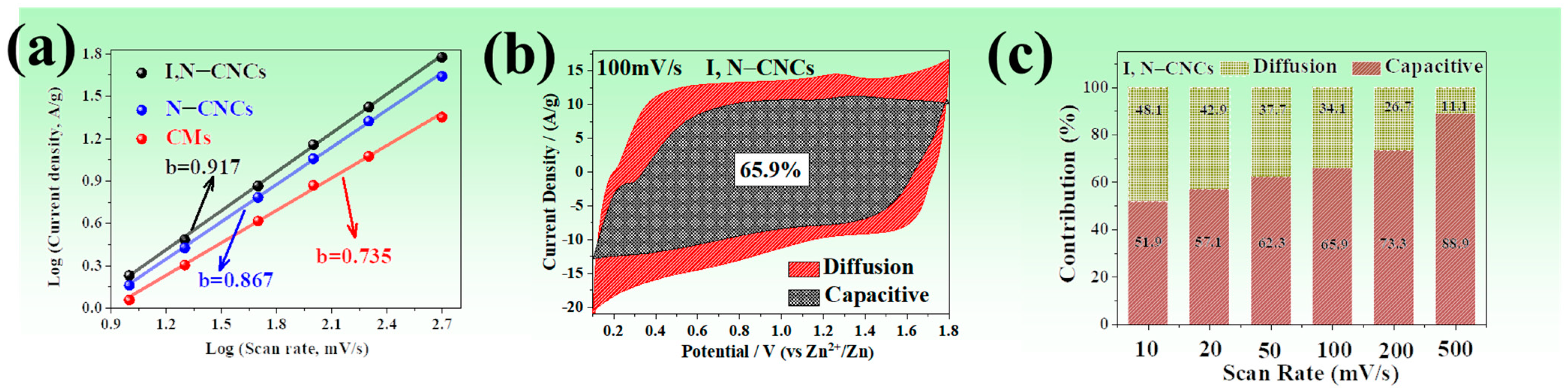
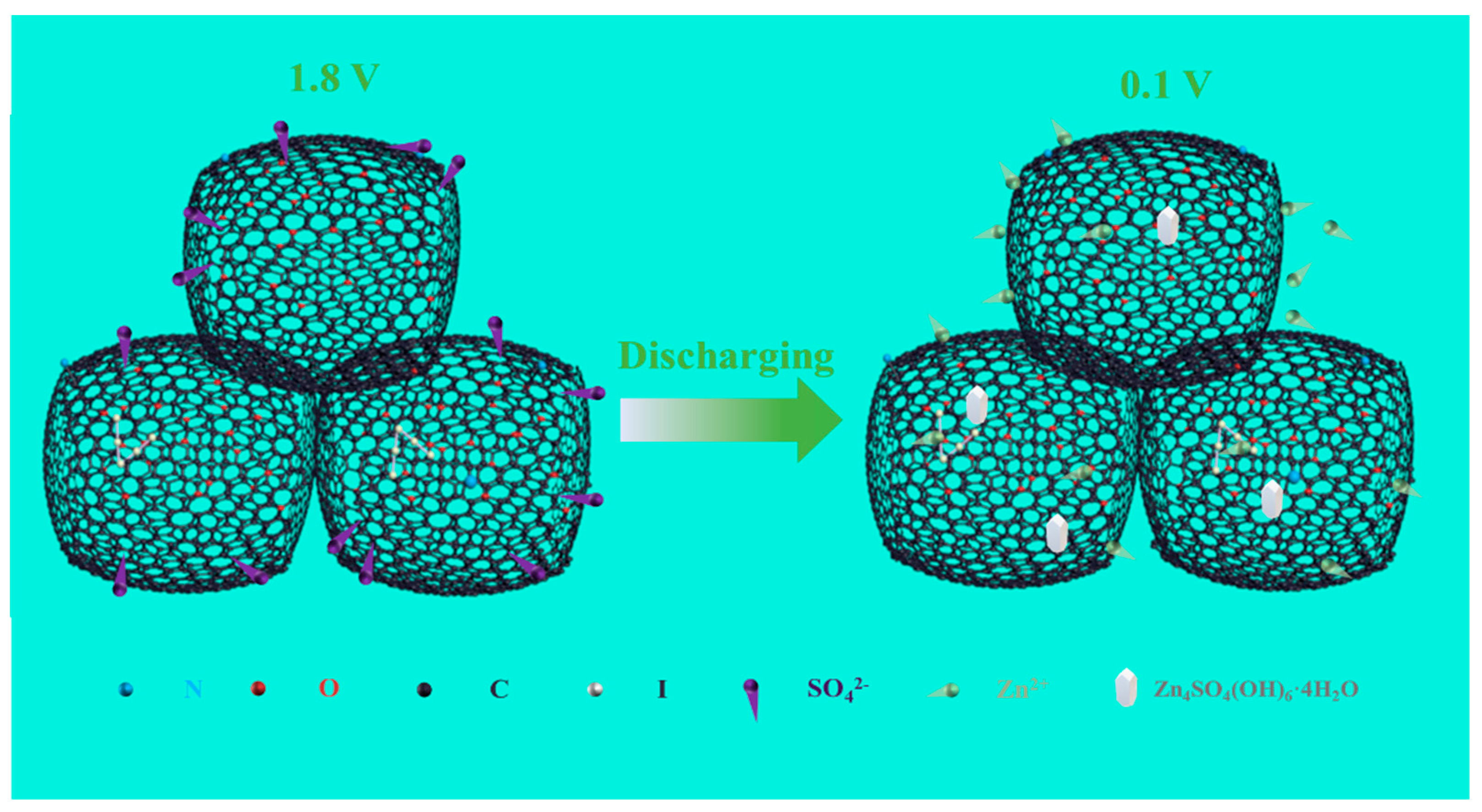
3.3. Energy Storage Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrogen Energy 2013, 38, 4034–4045. [Google Scholar]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Jagadale, A.D.; Rohit, R.C.; Shinde, S.K.; Kim, D.Y. Materials Development in Hybrid Zinc-Ion Capacitors. ChemNanoMat 2021, 7, 1082–1098. [Google Scholar] [CrossRef]
- Devi, M.; Moorthy, B.; Thangavel, R. Recent developments in zinc metal anodes, cathodes, and electrolytes for zinc-ion hybrid capacitors. Sustain. Energy Fuels 2023, 7, 3776–3795. [Google Scholar] [CrossRef]
- Naik, P.B.; Yadav, P.; Nagaraj, R.; Puttaswamy, R.; Beere, H.K.; Maiti, U.N.; Mondal, C.; Sanna Kotrappanavar, N.; Ghosh, D. Developing High-Performance Flexible Zinc Ion Capacitors from Agricultural Waste-Derived Carbon Sheets. ACS Sustain. Chem. Eng. 2022, 10, 1471–1481. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, S.; Chen, Q.; Gao, G.; Ban, Q.; Xu, Z.; He, F.; Hu, G.; Hu, X. Phosphorus in honeycomb-like carbon as a cathode boosting pseudocapacitive properties for Zn-ion storage. J. Power Sources 2021, 493, 229687. [Google Scholar] [CrossRef]
- Bhattacharjee, U.; Bhowmik, S.; Ghosh, S.; Vangapally, N.; Martha, S.K. Boron-doped graphene anode coupled with microporous activated carbon cathode for lithium-ion ultracapacitors. Chem. Eng. J. 2022, 430, 132835. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Sevilla, M.; Carro-Rodríguez, J.; Díez, N.; Fuertes, A.B. Straightforward synthesis of Sulfur/N,S-codoped carbon cathodes for Lithium-Sulfur batteries. Sci. Rep. 2020, 10, 4866. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zeng, Y.; Guo, Y.; Li, J.; Zhu, S.; Gao, S.; Zhang, H.; He, X. Recent progress on the heteroatom-doped carbon cathode for zinc ion hybrid capacitors. Chem. Eng. J. 2023, 468, 143576. [Google Scholar] [CrossRef]
- Lin, D.; Li, Y. Recent advances of aqueous rechargeable zinc-iodine batteries: Challenges, solutions, and prospects. Adv. Mater. 2022, 34, 2108856. [Google Scholar] [CrossRef]
- Nagaraju, G.; Tagliaferri, S.; Panagiotopoulos, A.; Och, M.; Quintin-Baxendale, R.; Mattevi, C. Durable Zn-ion hybrid capacitors using 3D printed carbon composites. J. Mater. Chem. A 2022, 10, 15665–15676. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Das, D.; Nanda, K.K. pH-dependent hydrogen evolution using spatially confined ruthenium on hollow N-doped carbon nanocages as a Mott–Schottky catalyst. J. Mater. Chem. A 2021, 9, 13958–13966. [Google Scholar] [CrossRef]
- Petala, E.; Georgiou, Y.; Kostas, V.; Dimos, K.; Karakassides, M.A.; Deligiannakis, Y.; Aparicio, C.; Tuček, J.; Zbořil, R. Magnetic Carbon Nanocages: An Advanced Architecture with Surface- and Morphology-Enhanced Removal Capacity for Arsenites. ACS Sustain. Chem. Eng. 2017, 5, 5782–5792. [Google Scholar] [CrossRef]
- Matsui, K.; Segawa, Y.; Namikawa, T.; Kamada, K.; Itami, K. Synthesis and properties of all-benzene carbon nanocages: A junction unit of branched carbon nanotubes. Chem. Sci. 2013, 4, 84–88. [Google Scholar] [CrossRef]
- Tsai, C.K.; Kang, H.Y.; Hong, C.I.; Huang, C.H.; Chang, F.C.; Wang, H.P. Preparation of hollow spherical carbon nanocages. J. Nanoparticle Res. 2012, 14, 1315. [Google Scholar] [CrossRef]
- Vinu, A.; Miyahara, M.; Mori, T.; Ariga, K. Carbon nanocage: A large-pore cage-type mesoporous carbon material as an adsorbent for biomolecules. J. Porous Mater. 2006, 13, 379–383. [Google Scholar] [CrossRef]
- Matsui, K.; Segawa, Y.; Itami, K. All-Benzene Carbon Nanocages: Size-Selective Synthesis, Photophysical Properties, and Crystal Structure. J. Am. Chem. Soc. 2014, 136, 16452–16458. [Google Scholar] [CrossRef]
- Vinu, A.; Miyahara, M.; Sivamurugan, V.; Mori, T.; Ariga, K. Large pore cage type mesoporous carbon, carbon nanocage: A superior adsorbent for biomaterials. J. Mater. Chem. 2005, 15, 5122–5127. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Cyr, M.; King, B.; Lessard, B.H.; Brusso, J.L. Exploring ellagic acid as a building block in the design of organic semiconductors. Dyes Pigments 2022, 199, 109998. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Xue, Y.; Zhang, Y.; Zhao, H.; He, Y.; Li, X.; Yuan, Z. Colorimetric detection of melamine based on the interruption of the synthesis of gold nanoparticles. Anal. Methods 2013, 5, 1930–1934. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical Sensors Based on the Electropolymerized Natural Phenolic Antioxidants and Their Analytical Application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef]
- Sevilla, M.; Sanchís, C.; Valdés-Solís, T.; Morallón, E.; Fuertes, A.B. Synthesis of Graphitic Carbon Nanostructures from Sawdust and Their Application as Electrocatalyst Supports. J. Phys. Chem. C 2007, 111, 9749–9756. [Google Scholar] [CrossRef]
- Arunpandian, R.; Kumar, M.; Yang, P.-Y.; Chiang, C.-H.; Chang, J.-H. Enhancing graphitization with desirable porosity for CO2 adsorption through a one-step strategy and a novel stepwise pyrolysis method from Terminalia boivinii tul tree dead leaves. Surf. Interfaces 2024, 44, 103696. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Xiong, Y.; Gao, Z.; Hu, W.; Shi, J.; Chen, J.; Tian, W.; Wu, J.; Huang, M.; et al. Multi-Channel Hollow Carbon Nanofibers with Graphene-Like Shell-Structure and Ultrahigh Surface Area for High-Performance Zn-Ion Hybrid Capacitors. Small Methods 2023, 7, e2300714. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, S.; Cheong, W.-C.; Wang, K.; Xu, H.; Huang, A.; Ma, J.; Li, J.; Ip, W.-F.; San Hui, K.; et al. Topological defect and sp3/sp2 carbon interface derived from ZIF-8 with linker vacancies for oxygen reduction reaction. Carbon 2023, 203, 76–87. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, Z.; Tian, Z.; Li, S.; Wu, G.; Wang, M.; Tong, X.; Shen, F.; Xia, Z.; Tung, V.; et al. Regulating Oxygen Substituents with Optimized Redox Activity in Chemically Reduced Graphene Oxide for Aqueous Zn-Ion Hybrid Capacitor. Adv. Funct. Mater. 2020, 31, 2007843. [Google Scholar] [CrossRef]
- Dey, S.; Paul, S.; Debsharma, K.; Sinha, C. A highly emissive Zn(ii)-pyridyl-benzimidazolyl-phenolato-based chemosensor: Detection of H2PO4−via “use” and “throw” device fabrication. Anal. Methods 2021, 13, 5282–5292. [Google Scholar] [CrossRef]
- Li, Y.; Lu, P.; Shang, P.; Wu, L.; Wang, X.; Dong, Y.; He, R.; Wu, Z.-S. Pyridinic nitrogen enriched porous carbon derived from bimetal organic frameworks for high capacity zinc ion hybrid capacitors with remarkable rate capability. J. Energy Chem. 2021, 56, 404–411. [Google Scholar] [CrossRef]
- Datta, K.K.; Balasubramanian, V.V.; Ariga, K.; Mori, T.; Vinu, A. Highly Crystalline and Conductive Nitrogen-Doped Mesoporous Carbon with Graphitic Walls and Its Electrochemical Performance. Chem. Eur. J. 2011, 17, 3390–3397. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.; Wang, F.; Zhao, X.-L.; Wei, X.-P.; Wang, X.-W.; Tian, Y. One-step and low-temperature synthesis of iodine-doped graphene and its multifunctional applications for hydrogen evolution reaction and electrochemical sensing. Electrochim. Acta 2017, 246, 1155–1162. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, Y.; Guo, M.; Zheng, Q.; Lin, D. Novel sustainable nitrogen, iodine-dual-doped hierarchical porous activated carbon as a superior host material for high performance lithium-sulfur batteries. Int. J. Hydrogen Energy 2018, 43, 20022–20032. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Huang, C.; Fan, H.; Yuan, D.; Luo, W.-B.; Hu, A.; Tang, Q.; Chen, X. Understanding the effect of I/N dual-doped hard carbon for high performance K-ion storage. Electrochim. Acta 2021, 394, 139146. [Google Scholar] [CrossRef]
- Li, G.; Blake, G.R.; Palstra, T.T.M. Vacancies in functional materials for clean energy storage and harvesting: The perfect imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef]
- Singal, S.; Joshi, A.; Tomar, A.K.; Sahu, V.; Singh, G.; Sharma, R.K. Vacancies and edges: Enhancing supercapacitive performance metrics of electrode materials. J. Energy Storage 2020, 31, 101614. [Google Scholar] [CrossRef]
- Gorji, B.; Khosrozadeh, A.; Doja, S.; Tao, L.; Miller, M.B.; Bichler, L.; Arjmand, M.; Liu, J. Critical evaluation of hybrid and organic electrolytes for supercapacitors with optimized porous carbon. Electrochim. Acta 2023, 441, 141778. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Liu, S.; Che, X.; He, S.; Liu, Z.; Wang, M.; Wang, X.; Qiu, J. 3D graphene-like oxygen and sulfur-doped porous carbon nanosheets with multilevel ion channels for high-performance aqueous Zn-ion storage. Carbon 2023, 201, 624–632. [Google Scholar] [CrossRef]
- Borchardt, L.; Leistenschneider, D.; Haase, J.; Dvoyashkin, M. Revising the concept of pore hierarchy for ionic transport in carbon materials for supercapacitors. Adv. Energy Mater. 2018, 8, 1800892. [Google Scholar] [CrossRef]
- Wang, L.; Peng, M.; Chen, J.; Hu, T.; Yuan, K.; Chen, Y. Eliminating the Micropore Confinement Effect of Carbonaceous Electrodes for Promoting Zn-Ion Storage Capability. Adv. Mater. 2022, 34, e2203744. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Liu, K.; Li, B.; Li, C.; Shi, Z. Di-ionic multifunctional porous organic frameworks for efficient CO2 fixation under mild and co-catalyst free conditions. Green Chem. 2018, 20, 5285–5291. [Google Scholar] [CrossRef]
- Oda, A.; Torigoe, H.; Itadani, A.; Ohkubo, T.; Yumura, T.; Kobayashi, H.; Kuroda, Y. An Important Factor in CH4 Activation by Zn Ion in Comparison with Mg Ion in MFI: The Superior Electron-Accepting Nature of Zn2+. J. Phys. Chem. C 2014, 118, 15234–15241. [Google Scholar] [CrossRef]
- Zhu, C.; Long, R.; Zhu, L.; Zou, W.; Zhang, Y.; Gao, Z.; Shi, J.; Tian, W.; Wu, J.; Wang, H. Sulfate template induced S/O doped carbon nanosheets enabling rich physi/chemi-sorption sites for high-performance zinc ion hybrid capacitors. J. Colloid. Interface Sci. 2023, 652, 590–598. [Google Scholar] [CrossRef]
- Wen, F.; Yan, Y.; Sun, S.; Li, X.; He, X.; Meng, Q.; Zhe Liu, J.; Qiu, X.; Zhang, W. Synergistic effect of nitrogen and oxygen dopants in 3D hierarchical porous carbon cathodes for ultra-fast zinc ion hybrid supercapacitors. J. Colloid. Interface Sci. 2023, 640, 1029–1039. [Google Scholar] [CrossRef]
- Shang, K.; Liu, Y.; Cai, P.; Li, K.; Wen, Z. N, P, and S co-doped 3D porous carbon-architectured cathode for high-performance Zn-ion hybrid capacitors. J. Mater. Chem. A 2022, 10, 6489–6498. [Google Scholar] [CrossRef]
- Qin, Y.; Song, Z.; Miao, L.; Hu, C.; Chen, Y.; Liu, P.; Lv, Y.; Gan, L.; Liu, M. Hydrogen-bond-mediated micelle aggregating self-assembly towards carbon nanofiber networks for high-energy and long-life zinc ion capacitors. Chem. Eng. J. 2023, 470, 144256. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Wu, M.; Guo, C.; Bai, L.; Li, J.; Li, Y.; Luo, D.; Duan, J.; Li, X.; et al. Fabrication and morphological effect of waxberry-like carbon for high-performance aqueous zinc-ion electrochemical storage. Carbon 2023, 205, 226–235. [Google Scholar] [CrossRef]
- Li, H.X.; Shi, W.J.; Liu, L.Y.; Zhang, X.; Zhang, P.F.; Zhai, Y.J.; Wang, Z.Y.; Liu, Y. Fabrication of dual heteroatom-doped graphitic carbon from waste sponge with “killing two birds with one stone” strategy for advanced aqueous zinc-ion hybrid capacitors. J. Colloid. Interface Sci. 2023, 647, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Su, P.; Liao, Q.; Liu, Y.; Li, Y.; Niu, X.; Liu, X.; Wang, K. Olive Leaves-Derived Hierarchical Porous Carbon as Cathode Material for Anti-Self-Discharge Zinc-Ion Hybrid Capacitor. Small 2023, 19, e2304172. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, D.; Wang, H.; Ye, P.; Ping, Z.; Ning, J.; Zhong, Y.; Hu, Y. Two-step nitrogen and sulfur doping in porous carbon dodecahedra for Zn-ion hybrid supercapacitors with long term stability. Chem. Eng. J. 2022, 431, 133250. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Zhao, X.; Wang, N.; Luo, T.; Chen, X.; Wu, T.; Jiang, S.; van Aken, P.A.; Qu, S.; et al. Hollow Mesoporous Carbon Spheres for High Performance Symmetrical and Aqueous Zinc-Ion Hybrid Supercapacitor. Front. Chem. 2020, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-L.; Wang, H.-L.; Fan, W.-J.; Zhai, S.-L.; Wang, X.-J.; Shi, J.; Huang, M.-H.; Liu, S.; Li, Z.; Chen, J.-W. Large-scale doping-engineering enables boron/nitrogen dual-doped porous carbon for high-performance zinc ion capacitors. Rare Met. 2022, 41, 2505–2516. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, R.; Fan, H.; Ban, Q.; Zhou, D.; Zhao, L.; Yu, J.; Chen, Q.; Hu, X. Iodine-Doped Hollow Carbon Nanocages without Templates Strategy for Boosting Zinc-Ion Storage by Nucleophilicity. Materials 2024, 17, 838. https://doi.org/10.3390/ma17040838
Niu R, Fan H, Ban Q, Zhou D, Zhao L, Yu J, Chen Q, Hu X. Iodine-Doped Hollow Carbon Nanocages without Templates Strategy for Boosting Zinc-Ion Storage by Nucleophilicity. Materials. 2024; 17(4):838. https://doi.org/10.3390/ma17040838
Chicago/Turabian StyleNiu, Ruiting, Huailin Fan, Qingfu Ban, Dezhi Zhou, Lekang Zhao, Jiayuan Yu, Qifeng Chen, and Xun Hu. 2024. "Iodine-Doped Hollow Carbon Nanocages without Templates Strategy for Boosting Zinc-Ion Storage by Nucleophilicity" Materials 17, no. 4: 838. https://doi.org/10.3390/ma17040838
APA StyleNiu, R., Fan, H., Ban, Q., Zhou, D., Zhao, L., Yu, J., Chen, Q., & Hu, X. (2024). Iodine-Doped Hollow Carbon Nanocages without Templates Strategy for Boosting Zinc-Ion Storage by Nucleophilicity. Materials, 17(4), 838. https://doi.org/10.3390/ma17040838





