Biomimetic Use of Food-Waste Sources of Calcium Carbonate and Phosphate for Sustainable Materials—A Review
Abstract
:1. Introduction
2. Biological (Biogenic) Calcium Carbonate
2.1. The Interest in Obtaining Calcium Carbonate from Biological Species
2.2. The Problem of Interspecies Differences in Biological Calcium Carbonate
3. Application of Bio-Sources of Calcium Carbonate and Phosphate into Composite Materials and Biomaterials: Some Case Studies
3.1. Land Snail Shells
3.1.1. General Considerations
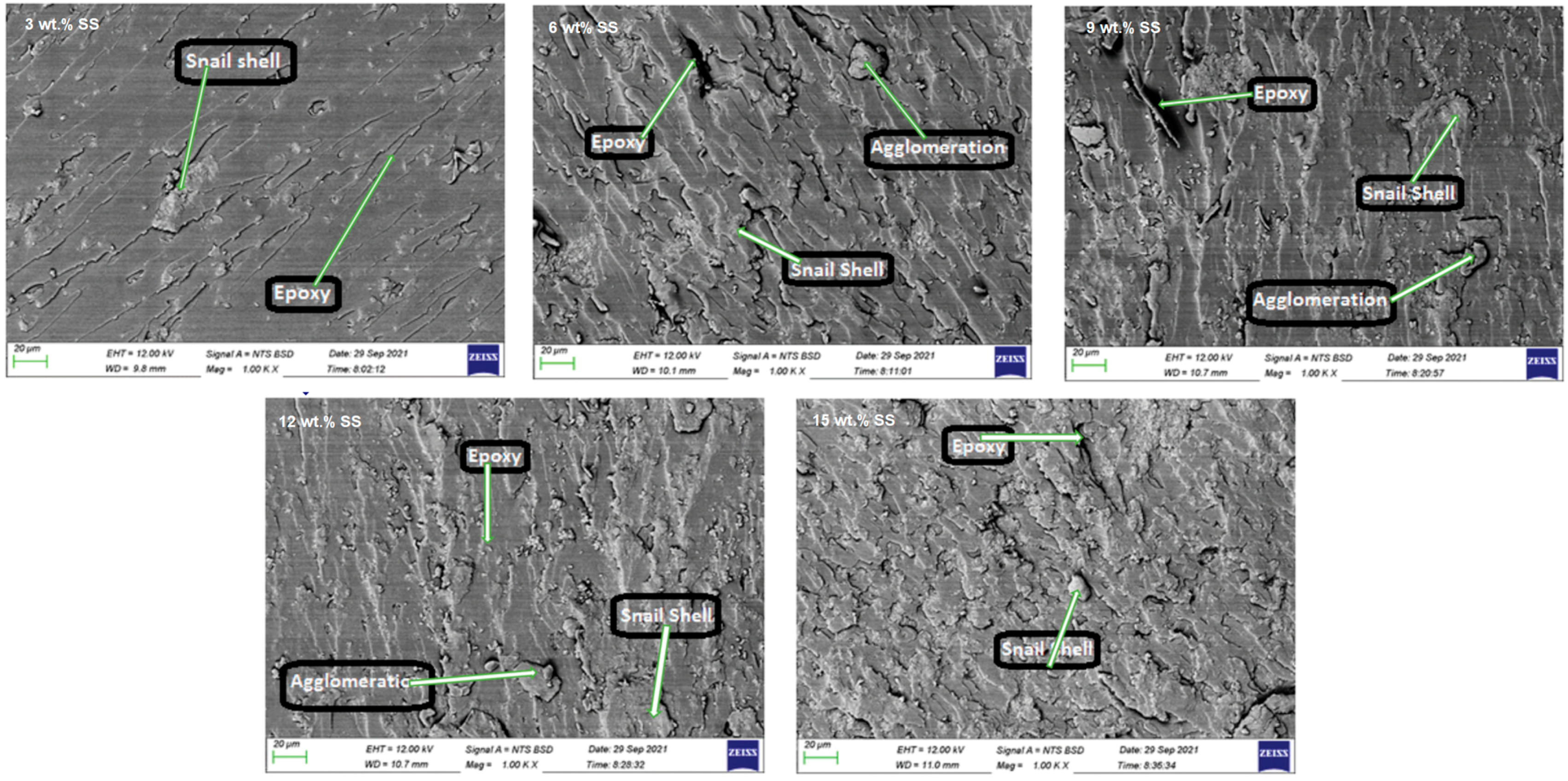
3.1.2. Applications of Snail Shells in Composites
3.1.3. Applications of Snail Shells in Biomaterials
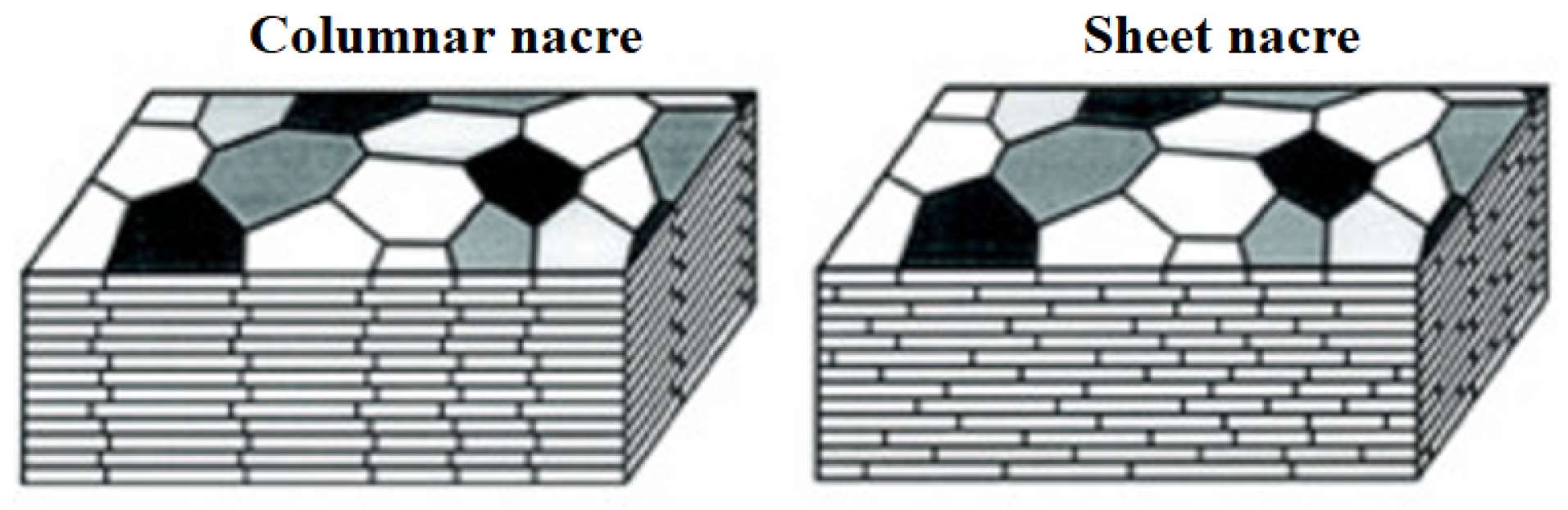
3.2. Clam Shells
3.2.1. General Considerations
3.2.2. Applications of Clam Shells in Composites
3.2.3. Applications of Clam Shells in Biomaterials
3.3. Mussel Shells
3.3.1. General Considerations
3.3.2. Applications of Mussel Shells in Composites
3.3.3. Applications of Mussel Shells in Biomaterials
3.4. Oyster Shells
3.4.1. Application of Oyster Shells in Composites
3.4.2. Application of Oyster Shells in Biomaterials
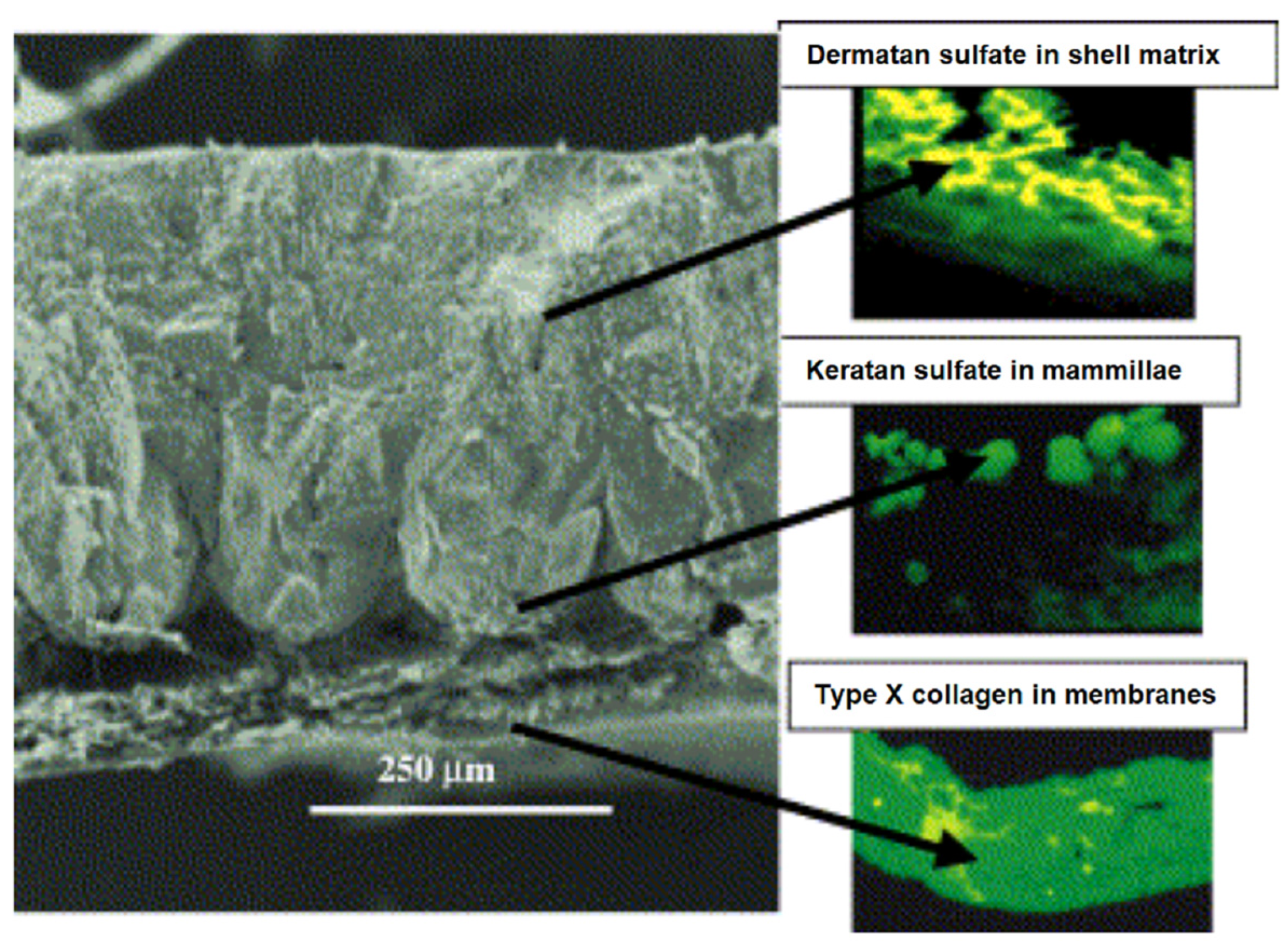
3.5. Eggshells
3.5.1. General Considerations
- 1.
- Production of active carbons
- 2.
- Production of catalysts for biodiesel
- 3.
- Various engineering materials
3.5.2. Applications in Composites
3.5.3. Applications of Eggshells as a Source of Hydroxyapatite
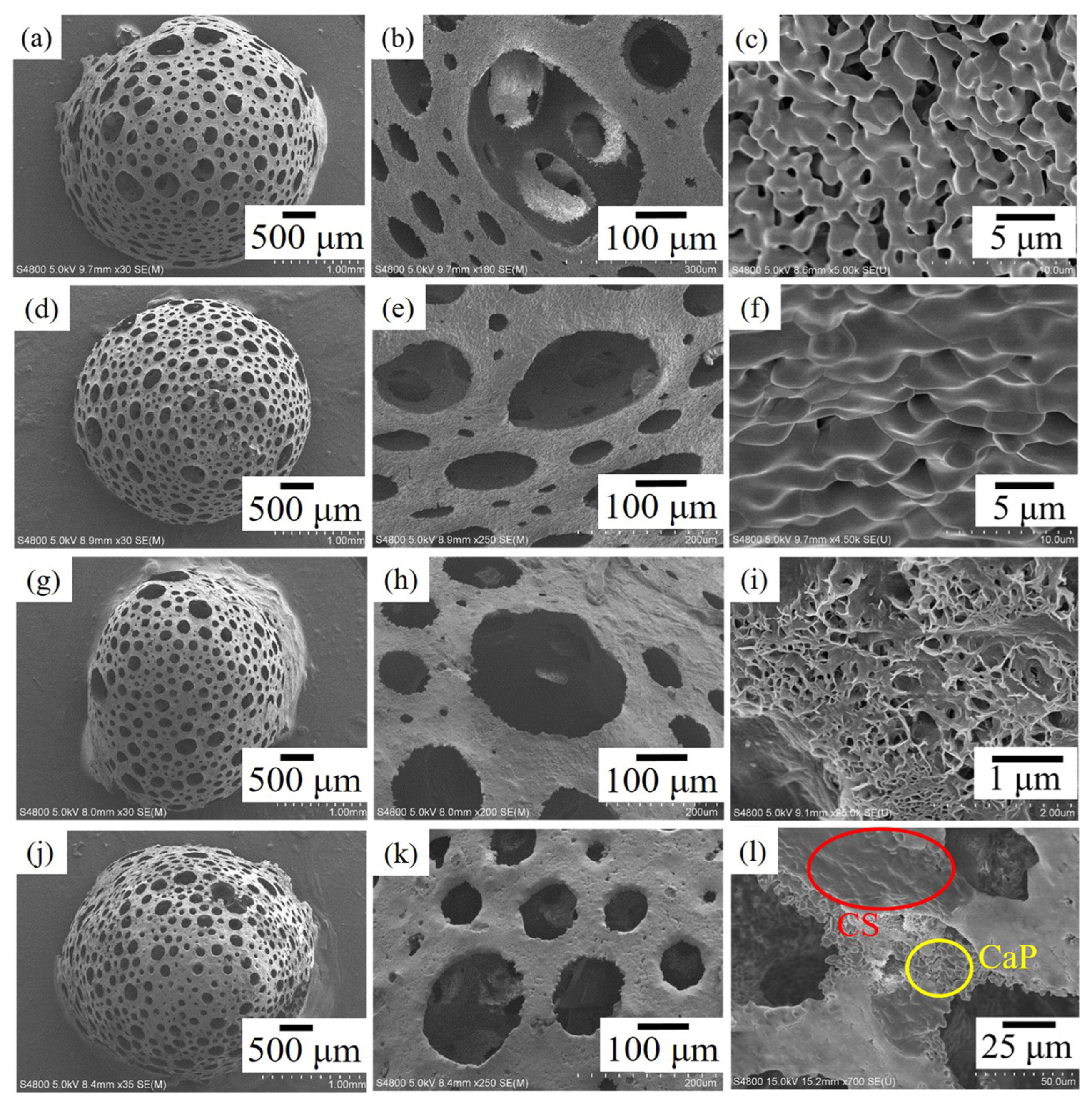
3.6. Cuttlefish Bone
3.6.1. General Considerations
3.6.2. Applications of Cuttlefish Bone in Biomedical Engineering
3.6.3. 3D Printing and Additive Manufacturing of Cuttlefish Composites
3.6.4. Applications in Other Functional Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, M.; Ming, X.; He, K.; Li, L.; Shen, S. Effect of macro-, micro- and nano-calcium carbonate on properties of cementitious composites—A review. Materials 2019, 12, 781. [Google Scholar] [CrossRef]
- Devi, K.S.; Lakshmi, V.V.; Alakanandana, A. Impacts of cement industry on environment—An overview. Asia Pac. J. Res. 2017, 1, 156–161. [Google Scholar]
- Thenepalli, T.; Jun, A.Y.; Han, C.; Ramakrishna, C.; Ahn, J.W. A strategy of precipitated calcium carbonate (CaCO3) fillers for enhancing the mechanical properties of polypropylene polymers. Korean J. Chem. Eng. 2015, 32, 1009–1022. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Khan, K.; Ahmad, W.; Amin, M.N.; Deifalla, A.F. Investigating the feasibility of using waste eggshells in cement-based materials for sustainable construction. J. Mater. Res. Technol. 2023, 23, 4059–4074. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Corporate Statistical Database. 2023. Available online: http://www.fao.org/faostat/en/#data/CL (accessed on 25 January 2024).
- Arias, J.L.; Fernandez, M.S. Biomimetic processes through the study of mineralized shells. Mater. Charact. 2003, 50, 189–195. [Google Scholar] [CrossRef]
- Xiang, J.; Cao, H.; Warner, J.H.; Watt, A.A. Crystallization and self-assembly of calcium carbonate architectures. Cryst. Growth Des. 2008, 8, 4583–4588. [Google Scholar] [CrossRef]
- Pu’ad, N.M.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar]
- Tanniru, M.; Misra, R.D.K.; Berbrand, K.; Murphy, D. The determining role of calcium carbonate on surface deformation during scratching of calcium carbonate-reinforced polyethylene composites. Mater. Sci. Eng. A 2005, 404, 208–220. [Google Scholar] [CrossRef]
- Owuamanam, S.; Cree, D. Progress of bio-calcium carbonate waste eggshell and seashell fillers in polymer composites: A review. J. Compos. Sci. 2020, 4, 70. [Google Scholar] [CrossRef]
- Dong, W.; Cheng, H.; Yao, Y.; Zhou, Y.; Tong, G.; Yan, D.; Lai, Y.; Li, W. Bioinspired synthesis of calcium carbonate hollow spheres with a nacre-type laminated microstructure. Langmuir 2011, 27, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B. Hierarchical structure and mechanical properties of nacre: A review. RSC Adv. 2012, 2, 7617–7632. [Google Scholar] [CrossRef]
- Goswami, A. A comparative study on the mechanical and structural design of nacre in gastropod and bivalve molluscs. J. Mech. Behav. Biomed. Mater. 2021, 114, 104212. [Google Scholar] [CrossRef] [PubMed]
- Azarian, M.H.; Sutapun, W. Biogenic calcium carbonate derived from waste shells for advanced material applications: A review. Front. Mater. 2022, 9, 1024977. [Google Scholar] [CrossRef]
- Minakshi, M.; Visbal, H.; Mitchell, D.R.; Fichtner, M. Bio-waste chicken eggshells to store energy. Dalton Trans. 2018, 47, 16828–16834. [Google Scholar] [CrossRef]
- Apalangya, V.; Rangari, V.; Tiimob, B.; Jeelani, S.; Samuel, T. Development of antimicrobial water filtration hybrid material from bio source calcium carbonate and silver nanoparticles. Appl. Surf. Sci. 2014, 295, 108–114. [Google Scholar] [CrossRef]
- Wang, H.; Yan, K.; Chen, J. Preparation of hydroxyapatite microspheres by hydrothermal self-assembly of marine shell for effective adsorption of Congo Red. Mater. Lett. 2021, 304, 130573. [Google Scholar] [CrossRef]
- Putkham, A.I.; Chuakham, S.; Chaiyachet, Y.; Suwansopa, T.; Putkham, A. Production of bio-calcium oxide derived from hatchery eggshell waste using an industrial-scale car bottom furnace. J. Renew. Mater. 2022, 10, 1137. [Google Scholar] [CrossRef]
- Hamza, M.; Ayoub, M.; Bin Shamsuddin, R.; Mukhtar, A.; Saqib, S.; Zahid, I.; Ameen, M.; Ullah, S.; Al-Sehemi, A.G.; Ibrahim, M. A review on the waste biomass derived catalysts for biodiesel production. Environ. Technol. Innov. 2021, 21, 101200. [Google Scholar] [CrossRef]
- Gorna, K.; Hund, M.; Vučak, M.; Gröhn, F.; Wegner, G. Amorphous calcium carbonate in form of spherical nanosized particles and its application as fillers for polymers. Mater. Sci. Eng. A 2008, 477, 217–225. [Google Scholar] [CrossRef]
- Kato, T. Polymer/calcium carbonate layered thin-film composites. Adv. Mater. 2000, 12, 1543–1546. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Azwar, E.; Fazhan, H.; Peng, W.; Ishak, S.D.; Tabatabaei, M.; Yek, P.N.Y.; Almomani, F.; Aghbashlo, M.; et al. A state-of-the-art review on producing engineered biochar from shellfish waste and its application in aquaculture wastewater treatment. Chemosphere 2022, 288, 132559. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Chen, T.; Li, H.Y.; Xia, M.S.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with modified shell waste. J. Hazard. Mater. 2013, 262, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Aguila-Almanza, E.; Hernandez-Cocoletzi, H.; Rubio-Rosas, E.; Calleja-Gonzalez, M.; Ren Lim, H.; Shiong Khoo, K.; Singh, V.; Maldonado-Montiel, J.C.; Loke Show, P. Recuperation and characterization of calcium carbonate from residual oyster and clamshells and their incorporation into a residential finish. Chemosphere 2022, 288, 132550. [Google Scholar] [CrossRef]
- Gumus, R.H.; Okpeku, I. Production of activated carbon and characterization from snail shell waste (Helix pomatia). Adv. Chem. Eng. Sci. 2014, 5, 51. [Google Scholar] [CrossRef]
- Berillis, P.; Hatziioannou, M.; Panagiotopoulos, N.; Neofitou, C. Similar shell features between rear and wild Cornu aspersum snails. World J. Agric. Res. 2013, 1, 1–4. [Google Scholar]
- Öksüz, K.E.; Şereflişan, H. Microstructure of Eobania vermiculata (Müller, 1774): SEM, F-TIR and XRD methods. J. Agric. Prod. 2022, 3, 42–47. [Google Scholar] [CrossRef]
- Puspitasari, P.; Utomo, D.M.; Zhorifah, H.F.N.; Permanasari, A.A.; Gayatri, R.W. Physicochemical determination of Calcium Carbonate (CaCO3) from chicken eggshell. Key Eng. Mater. 2020, 840, 478–483. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Kamil, F.H.; Hasso, A.A.; Abduljawaad, A.N.; Saleh, T.F.; Mahmood, S.K. Calcium carbonate nanoparticles of quail’s egg shells: Synthesis and characterizations. J. Mech. Behav. Mater. 2022, 31, 1–7. [Google Scholar] [CrossRef]
- Keller, N.; Del Piero, D.; Longinelli, A. Isotopic composition, growth rates and biological behaviour of Chamelea gallina and Callista chione from the Bay of Trieste (Italy). Mar. Biol. 2002, 140, 9–15. [Google Scholar]
- Liang, Y.; Zhao, Q.; Li, X.; Zhang, Z.; Ren, L. Study of the microstructure and mechanical properties of white clam shell. Micron 2016, 87, 10–17. [Google Scholar] [CrossRef]
- Nikolayev, D.; Lychagina, T.; Pakhnevich, A. Experimental neutron pole figures of minerals composing the bivalve mollusc shells. SN Appl. Sci. 2019, 1, 344. [Google Scholar] [CrossRef]
- Thongkam, M.; Saelim, J.; Boonchom, B.; Seesanong, S.; Chaiseeda, K.; Laohavisuti, N.; Bunya-Atichart, K.; Boonmee, W.; Taemchuay, D. Simple and rapid synthesis of calcium acetate from scallop shells to reduce environmental issues. Adsorp. Sci. Technol. 2021, 2021, 6450289. [Google Scholar] [CrossRef]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Kannan, S.; Valério, P.; Ferreira, J.M.F. Hydrothermal growth of hydroxyapatite scaffolds from aragonitic cuttlefish bones. J. Biomed. Mater. Res. A 2006, 77, 160–168. [Google Scholar] [CrossRef]
- Triunfo, C.; Gartner, S.; Marchini, C.; Fermani, S.; Maoloni, G.; Goffredo, S.; Gomez Morales, J.; Cölfen, H.; Falini, G. Recovering and exploiting aragonite and calcite single crystals with biologically controlled shapes from mussel shells. ACS Omega 2022, 7, 43992–43999. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G. Molluscan biomineralization: The proteinaceous shell constituents of Pinna nobilis L. Mater. Sci. Eng. C 2005, 25, 105–111. [Google Scholar] [CrossRef]
- Barthelat, F.; Rim, J.E.; Espinosa, H.D. A review on the structure and mechanical properties of mollusk shells–perspectives on synthetic biomimetic materials. In Applied Scanning Probe Methods XIII: Biomimetics and Industrial Applications; Bhushan, B., Fuchs, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–44. [Google Scholar]
- Ozyegin, L.; Sima, F.; Ristoscu, C.; Kiyici, I.A.; Mihailescu, I.N.; Meydanoglu, O.; Agathopoulos, S.; Oktar, F. Sea snail: An alternative source for nano-bioceramic production. Key Eng. Mater. 2012, 493, 781–786. [Google Scholar] [CrossRef]
- Miranda-Hernández, J.G.; González-Morán, C.O.; Herrera-Hernández, H.; Sánchez, E.H.; José de Jesús, A.; Ortega-Avilés, M. Sea snail shells for synthesis of ceramic compounds reinforced with metallic oxide: Microstructural, mechanical and electrical behavior. Mater. Today Comm. 2021, 28, 102656. [Google Scholar] [CrossRef]
- Şahin, Y.; Gündüz, O.; Bulut, B.; Özyeğin, L.; Gökçe, H.; Ağaoğulları, D.; Chou, J.; Kayalı, E.; Ben-Nissan, B.; Oktar, F.A.İ.K. Nano-bioceramic synthesis from tropical sea snail shells (tiger cowrie-Cypraea tigris) with simple chemical treatment. Acta Phys. Pol. A 2015, 127, 1055–1058. [Google Scholar] [CrossRef]
- Parveen, S.; Chakraborty, A.; Chanda, D.K.; Pramanik, S.; Barik, A.; Aditya, G. Microstructure analysis and chemical and mechanical characterization of the shells of three freshwater snails. ACS Omega 2020, 5, 25757–25771. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, R.; Maeda, H.; Kasuga, T. Water wettability dependence on surface structure of a snail shell. Bioinspir. Biomim. 2020, 15, 036001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, D.; Xu, X.; Yang, H.; Wyman, I.; Wang, J.; Wu, X. Versatile snail-inspired superamphiphobic coatings with repeatable adhesion and recyclability. Chem. Eng. Sci. 2021, 230, 116182. [Google Scholar] [CrossRef]
- Kolawole, M.Y.; Aweda, J.O.; Abdulkareem, S. Archachatina marginata bio-shells as reinforcement material in metal matrix composites. Int. J. Automot. Mech. Eng. 2022, 14, 4068–4079. [Google Scholar] [CrossRef]
- Karaoui, M.; Hsissou, R.; Alami, M.; Assouag, M. Thermal, flow, and mechanical properties of composites based on polystyrene (PS) and snail shell powder (SSP) biofiller (PS/SSP). Iran. Polym. J. 2023, 32, 621–631. [Google Scholar] [CrossRef]
- Chris-Okafor, P.U.; Nwokoye, J.N.; Oyom, P.O.; Ilodigwe, C.B. Effects of snail shell powder on the mechanical properties of low-density polyethylene (LDPE). Lond. J. Res. Sci. Nat. Formal. 2018, 18, 7–12. [Google Scholar]
- Syamimi, N.F.; Islam, M.R.; Sumdani, M.G.; Rashidi, N.M. Mechanical and thermal properties of snail shell particles-reinforced bisphenol-A bio-composites. Polym. Bull. 2020, 77, 2573–2589. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B. The investigation of reinforcement properties of nano-CaCO3 synthesized from Achatina fulica snail shell through mechanochemical methods on epoxy nanocomposites. Nanocomposites 2021, 7, 79–86. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B.; Onwubu, S. Optimization of milling procedures for synthesizing nano-CaCO3 from Achatina fulica shell through mechanochemical techniques. J. Nanomater. 2020, 2020, 4370172. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B.; Awogbemi, O. Studies on the mechanical and absorption properties of achatina fulica snail and eggshells reinforced composite materials. Compos. Struct. 2020, 239, 112043. [Google Scholar] [CrossRef]
- Oladele, I.O.; Onuh, L.; Taiwo, A.S.; Borisade, S.; Agbeboh, N.I.; Lephuthing, S.S. Mechanical, wear and thermal conductivity characteristics of snail shell-derived hydroxyapatite reinforced epoxy bio-composites for adhesive biomaterials applications. Int. J. Sustain. Eng. 2022, 15, 122–135. [Google Scholar] [CrossRef]
- Ngouoko, J.J.K.; Tajeu, K.Y.; Fotsop, C.G.; Tamo, A.K.; Doungmo, G.; Temgoua, R.C.T.; Kamgaing, T.; Tonle, I.K. Calcium carbonate originating from snail shells for synthesis of hydroxyapatite/l-lysine composite: Characterization and application to the electroanalysis of toluidine blue. Crystals 2022, 12, 1189. [Google Scholar] [CrossRef]
- Kumar, G.S.; Sathish, L.; Govindan, R.; Girija, E.K. Utilization of snail shells to synthesise hydroxyapatite nanorods for orthopedic applications. RSC Adv. 2015, 5, 39544–39548. [Google Scholar] [CrossRef]
- Ahmed, H.Y.; Safwat, N.; Shehata, R.; Althubaiti, E.H.; Kareem, S.; Atef, A.; Qari, S.H.; Aljahani, A.H.; Al-Meshal, A.S.; Youssef, M.; et al. Synthesis of natural nano-hydroxyapatite from snail shells and its biological activity: Antimicrobial, antibiofilm, and biocompatibility. Membranes 2022, 12, 408. [Google Scholar] [CrossRef]
- Singh, A. Hydroxyapatite, a biomaterial: Its chemical synthesis, characterization and study of biocompatibility prepared from shell of garden snail, Helix aspersa. Bull. Mater. Sci. 2012, 35, 1031–1038. [Google Scholar] [CrossRef]
- Lin, A.Y.M.; Meyers, M.A.; Vecchio, K.S. Mechanical properties and structure of Strombus gigas, Tridacna gigas, and Haliotis rufescens sea shells: A comparative study. Mater. Sci. Eng. C 2006, 26, 1380–1389. [Google Scholar] [CrossRef]
- Mu, G.; Duan, F.; Zhang, G.; Li, X.; Ding, X.; Zhang, L. Microstructure and mechanical property of Ruditapes philippinarum shell. J. Mech. Behav. Biomed. Mater. 2018, 85, 209–217. [Google Scholar] [CrossRef]
- Li, T.; Zeng, K. Nano-hierarchical structure and electromechanical coupling properties of clamshell. J. Struct. Biol. 2012, 180, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A. Size-based physical properties of hard-shell clam (Mercenaria mercenaria) shell relevant to the design of mechanical processing equipment. Aquacult Eng. 2020, 89, 102056. [Google Scholar] [CrossRef]
- Ahmad Khiri, M.Z.; Matori, K.A.; Zaid, M.H.M.; Abdullah, A.C.; Zainuddin, N.; Jusoh, W.N.W.; Jalil, R.A.; Abdul Rahman, N.A.; Kul, E.; Wahab, S.A.A.; et al. Soda lime silicate glass and clam shell act as precursor in synthesize calcium fluoroaluminosilicate glass to fabricate glass ionomer cement with different ageing time. J. Mater. Res. Technol. 2020, 9, 6125–6134. [Google Scholar] [CrossRef]
- Kassim, U.; Ong, B.P. Performance of concrete incorporating of clam shell as partially replacement of ordinary Portland cement (OPC). J. Adv. Res. Appl. Mech. 2019, 55, 12–21. [Google Scholar]
- Moon, H.; Kim, J.H.; Lee, J.Y.; Chung, C.W. Strength, absorption and interfacial properties of mortar using waste shells as fine aggregates. J. Korea Inst. Build. Constr. 2014, 14, 523–529. [Google Scholar] [CrossRef]
- Mahshuri, Y.; Amalina, M.A. Hardness and compressive properties of calcium carbonate derived from clam shell filled unsaturated polyester composites. Mater. Res. Innov. 2014, 18 (Suppl. S6), S6-291–S6-294. [Google Scholar] [CrossRef]
- Xia, M.S.; Yao, Z.T.; Ge, L.Q.; Chen, T.; Li, H.Y. A potential bio-filler: The substitution effect of furfural modified clam shell for carbonate calcium in polypropylene. J. Compos. Mater. 2015, 49, 807–816. [Google Scholar] [CrossRef]
- Jia, X.; Ling, X.; Tang, D. Microstructures and friction-wear characteristics of bivalve shells. Tribol. Int. 2006, 39, 657–662. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hashimoto, S. Cold sintering of calcium carbonate derived from seashells. Open Ceramics 2022, 12, 100302. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, W.; Wang, Z.; Lyu, X. Effect of clam shell from kitchen waste on the synthesis, performance, and hydration of cementitious clinker. Const. Build. Mater. 2022, 323, 126588. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Ismail, R.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Al Mulqi, M.S. Hydroxyapatite synthesis from clam shell using hydrothermal method: A review. In Proceedings of the 2019 International Biomedical Instrumentation and Technology Conference (IBITeC), Special Region of Yogyakarta, Indonesia, 23–24 October 2019; IEEE: Piscataway, NJ, USA, 2019; Volume 1, pp. 7–11. [Google Scholar]
- Bramhe, S.; Kim, T.N.; Balakrishnan, A.; Chu, M.C. Conversion from biowaste Venerupis clam shells to hydroxyapatite nanowires. Mater. Lett. 2014, 135, 195–198. [Google Scholar] [CrossRef]
- Núñez, D.; Serrano, J.A.; Mancisidor, A.; Elgueta, E.; Varaprasad, K.; Oyarzún, P.; Cáceres, R.; Ide, W.; Rivas, B.L. Heavy metal removal from aqueous systems using hydroxyapatite nanocrystals derived from clam shells. RSC Adv. 2019, 9, 22883–22890. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef]
- Pal, A.; Nasker, P.; Paul, S.; Chowdhury, A.R.; Sinha, A.; Das, M. Strontium doped hydroxyapatite from Mercenaria clam shells: Synthesis, mechanical and bioactivity study. J. Mech. Behav. Biomed. Mater. 2019, 90, 328–336. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Kuo, B.T.; Ho, W.F. Effects of cooling conditions and chitosan coating on the properties of porous calcium phosphate granules produced from hard clam shells. Adv. Powder Technol. 2022, 33, 103774. [Google Scholar] [CrossRef]
- Sindhya, A.; Jeyakumar, S.J.; Jothibas, M.; Pugalendhi, P. Synthesis and characterization of nanohydroxyapatite (nHAp) from Meretrix meretrix Clam shells and its in-vitro studies for biomedical applications. Vacuum 2022, 204, 111341. [Google Scholar] [CrossRef]
- Pal, A.; Metya, A.K.; Roy Chowdhury, A.; Sinha, A. Structural and mechanical behavior of mechanochemically synthesized nanocrystalline hydroxyapatite from mercenaria clam shells. Trans. Ind. Ceram. Soc. 2020, 79, 175–181. [Google Scholar] [CrossRef]
- Khiri, M.Z.A.; Matori, K.A.; Zainuddin, N.; Abdullah, C.A.C.; Alassan, Z.N.; Baharuddin, N.F.; Zaid, M.H.M. The usability of ark clam shell (Anadara granosa) as calcium precursor to produce hydroxyapatite nanoparticle via wet chemical precipitate method in various sintering temperature. SpringerPlus 2016, 5, 1206. [Google Scholar] [CrossRef] [PubMed]
- Murugiah, K.; Zakaria, M.I.; Suhaimi, H.; Caesarendra, W.; Sambudi, N.S. Synthesis and Characterisation of Hydroxyapatite (HAp) from Asiatic Hard Clam (Meretrix meretrix) and Blood Cockle Clam (Anadara granosa) Using Wet Precipitation Process. In Proceedings of the 2021 IEEE National Biomedical Engineering Conference (NBEC), Kuala Lumpur, Malaysia, 9–10 November 2021; IEEE: Piscataway, NJ, USA, 2021; pp. 1–6. [Google Scholar]
- Hussain, S.; Sabiruddin, K.; Patidar, P.; Solanki, K.; Baig, M.S. In vitro bioactivity and biocompatibility behaviour of atmospheric plasma sprayed Indian clam seashell derived hydroxyapatite coating on Ti-alloy. J. Alloys Compd. 2024, 976, 173132. [Google Scholar] [CrossRef]
- Ghazali, S.R.; Rosli, N.H.; Hassan, L.S.; Rozaini, M.Z.H.; Hamzah, H. Biocompatibility of Hydroxyapatite (HAp) derived from clamshell as active ingredients in sunscreen product. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 646, p. 012059. [Google Scholar]
- Maire, O.; Amouroux, J.M.; Duchêne, J.C.; Grémare, A. Relationship between filtration activity and food availability in the Mediterranean mussel Mytilus galloprovincialis. Mar. Biol. 2007, 152, 1293–1307. [Google Scholar] [CrossRef]
- Chakraborty, A.; Parveen, S.; Chanda, D.K.; Aditya, G. An insight into the structure, composition and hardness of a biological material: The shell of freshwater mussels. RSC Adv. 2020, 10, 29543–29554. [Google Scholar] [CrossRef] [PubMed]
- Manoj Nair, R.; Appukuttan, K.K. Effect of temperature on the development, growth, survival and settlement of green mussel Perna viridis (Linnaeus, 1758). Aquac. Res. 2003, 34, 1037–1045. [Google Scholar] [CrossRef]
- Ismail, R.; Cionita, T.; Shing, W.L.; Fitriyana, D.F.; Siregar, J.P.; Bayuseno, A.P.; Nugraha, F.W.; Muhamadin, R.C.; Junid, R.; Endot, N.A. Synthesis and Characterization of Calcium Carbonate Obtained from Green Mussel and Crab Shells as a Biomaterials Candidate. Materials 2022, 15, 5712. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Staden, R.-I.S.-V.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2021, 20, 25. [Google Scholar] [CrossRef]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-Hydroxyapatite Derived from Biogenic and Bioinspired Calcium Carbonates: Synthesis and In Vitro Bioactivity. Nanomater. 2021, 11, 264. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997, 7, 689–702. [Google Scholar] [CrossRef]
- Zamani, K.; Kocaman, S.; Işık, M.; Soydal, U.; Özmeral, N.; Ahmetli, G. Water sorption, thermal, and fire resistance properties of natural shell-based epoxy composites. J. Appl. Polym. Sci. 2022, 139, e52835. [Google Scholar] [CrossRef]
- Koçhan, C. Mechanical properties of waste mussel shell particles reinforced epoxy composites. Mater. Test. 2019, 61, 149–154. [Google Scholar] [CrossRef]
- Kocaman, S.; Ahmetli, G.; Cerit, A.; Yucel, A.; Gozukucuk, M. Characterization of biocomposites based on mussel shell wastes. Int. J. Mater. Metall. Eng. 2016, 10, 438–444. [Google Scholar]
- Gigante, V.; Cinelli, P.; Righetti, M.C.; Sandroni, M.; Tognotti, L.; Seggiani, M.; Lazzeri, A. Evaluation of mussel shells powder as reinforcement for PLA-based biocomposites. Int. J. Molec Sci. 2020, 21, 5364. [Google Scholar] [CrossRef] [PubMed]
- Kochan, C.; Selli, F.; Erdogan, U.H. Using mussel shell wastes as an additive for the production of thermally improved polypropylene composite monofilaments. J. Text. Inst. 2021, 112, 683–690. [Google Scholar] [CrossRef]
- Sahin, A.E.; Cetin, B.; Sinmazcelik, T. Effect of mussel shell reinforcement on mechanical and tribological behavior of polyphenylene sulfide composites. J. Thermoplast. Compos. Mater. 2022, 35, 1279–1302. [Google Scholar] [CrossRef]
- Kholil, A.; Dwiyati, S.T.; Randika, H.P. Performance testing of motorcycle centrifugal clutch lining made from composite wood powder, coconut fibre, and green mussel shell. J. Phys. Conf. Ser. 2021, 2019, 012065. [Google Scholar] [CrossRef]
- Reimer, T.; Dempster, T.; Wargelius, A.; Fjelldal, P.G.; Hansen, T.; Glover, K.A.; Solberg, M.F.; Swearer, S.E. Rapid growth causes abnormal vaterite formation in farmed fish otoliths. J. Exp. Biol. 2017, 220, 2965–2969. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Vichi, L.; Sandri, M. Influence of synthesis and sintering parameters on the characteristics of carbonate apatite. Biomaterials 2004, 25, 1763–1770. [Google Scholar] [CrossRef]
- Jiang, D.; Cai, L.; Ji, L.; Zhang, H.; Song, W. Nano-Bi2MoO6/calcined mussel shell composites with enhanced photocatalytic performance under visible-light irradiation. Micro. Nano Lett. 2018, 13, 1021–1025. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Sancho Vaquer, A.; Schmahl, W.W.; Ismail, R.; Jamari, J.; Bayuseno, A.P. In-depth knowledge of the low-temperature hydrothermal synthesis of nanocrystalline hydroxyapatite from waste green mussel shell (Perna viridis). Environ. Technol. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.D.A.; Ali, A.; Sun, Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater. Chem. Phys. 2015, 149, 607–616. [Google Scholar] [CrossRef]
- Shariffuddin, J.H.; Jones, M.I.; Patterson, D.A. Greener photocatalysts: Hydroxyapatite derived from waste mussel shells for the photocatalytic degradation of a model azo dye wastewater. Chem. Eng. Res. Des. 2013, 91, 1693–1704. [Google Scholar] [CrossRef]
- Amin, M.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Use of hydroxyapatite powder derived from green mussel shell wastes for coating on AISI 316L. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1268, p. 012035. [Google Scholar]
- Yamaguchi, K. Shell structure and behaviour related to cementation in oysters. Mar. Biol. 1994, 118, 89–100. [Google Scholar] [CrossRef]
- Iwase, K.; Harunari, Y.; Teramoto, M.; Mori, K. Crystal structure, microstructure, and mechanical properties of heat-treated oyster shells. J. Mech. Behav. Biomed. Mater. 2023, 147, 106107. [Google Scholar] [CrossRef] [PubMed]
- Bellei, P.; Torres, I.; Solstad, R.; Flores-Colen, I. Potential Use of Oyster Shell Waste in the Composition of Construction Composites: A Review. Buildings 2023, 13, 1546. [Google Scholar] [CrossRef]
- Hamester, M.R.R.; Balzer, P.S.; Becker, D. Characterization of calcium carbonate obtained from oyster and mussel shells and incorporation in polypropylene. Mater. Res. 2012, 15, 204–208. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Tsou, C.H.; Wu, C.S.; Hung, W.S.; De Guzman, M.R.; Gao, C.; Wang, R.Y.; Chen, J.; Wan, N.; Peng, Y.J.; Suen, M.C. Rendering polypropylene biocomposites antibacterial through modification with oyster shell powder. Polymer 2019, 160, 265–271. [Google Scholar] [CrossRef]
- Li, L.; Zeng, Z.; Wang, Z.; Peng, Z.; She, X.; Li, S.; Zhong, J. Effect of oyster shell powder loading on the mechanical and thermal properties of natural rubber/oyster shell composites. Polym. Polym. Compos. 2017, 25, 17–22. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, H.T.; Tsou, C.H.; Wang, C.C.; Gu, J.H.; Suen, M.C. Preparation and characterization of biodegradable polyurethane composites containing oyster shell powder. Polym. Bull. 2020, 77, 3325–3347. [Google Scholar] [CrossRef]
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Tseng, C.P.; Ho, W.F. Preparation and characterization of hydroxyapatite synthesized from oyster shell powders. Adv. Powder Technol. 2017, 28, 1154–1158. [Google Scholar] [CrossRef]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Q.; Pu, X.; Hou, Z.; Zhang, Q. Biphasic calcium phosphate macroporous scaffolds derived from oyster shells for bone tissue engineering. Chem. Eng. J. 2011, 173, 837–845. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Teixeira, S.; Santos, J.D.; Monteiro, F.J. Production of porous hydroxyapatite with potential for controlled drug delivery. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2004; Volume 455, pp. 358–360. [Google Scholar]
- Brundavanam, R.K.; Fawcett, D.; Poinern, G.E.J. Synthesis of a bone like composite material derived from waste pearl oyster shells for potential bone tissue bioengineering applications. Int. J. Res. Med. Sci. 2017, 5, 2454–2461. [Google Scholar] [CrossRef]
- Gurbuz, S.N.; Dericioglu, A.F. Effect of reinforcement surface functionalization on the mechanical properties of nacre-like bulk lamellar composites processed by a hybrid conventional method. Mater. Sci. Eng. C 2013, 33, 2011–2019. [Google Scholar] [CrossRef]
- Chen, T.Y.; Huang, H.C.; Cao, J.L.; Xin, Y.J.; Luo, W.F.; Ao, N.J. Preparation and characterization of alginate/HACC/oyster shell powder biocomposite scaffolds for potential bone tissue engineering applications. RSC Adv. 2016, 6, 35577–35588. [Google Scholar] [CrossRef]
- Jubier, N.J.; Hassani, R.H.; Hathot, S.F.; Salim, A.A. A new type of carbonate hydroxyapatite nanoparticles made from PMMA and oyster shells: Evaluation of structure, morphology and biocompatible properties. Polym. Bull. 2023, 80, 13263–13277. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Wu, L.; Younes, M.; Hincke, M. Biotechnological applications of eggshell: Recent advances. Front. Bioeng. Biotechnol. 2021, 9, 675364. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Sun, C.J.; Lian, L.; Zheng, J.X.; Xu, G.Y.; Yang, N. Effect of uniformity of eggshell thickness on eggshell quality in chickens. J. Poult. Sci. 2014, 51, 338–342. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Daniel, T.; Henry, J.; Sivakumar, G.; Mohanraj, K. Electrochemical performances of activated carbon prepared using eggshell waste. SN Appl. Sci. 2020, 2, 127. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M. Structural, morphological, and optical properties of country egg shell derived activated carbon for dye removal. Mater. Today Proc. 2021, 43, 1491–1495. [Google Scholar] [CrossRef]
- Guijarro-Aldaco, A.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A.; Mendoza-Castillo, D.I. Improving the adsorption of heavy metals from water using commercial carbons modified with egg shell wastes. Ind. Eng. Chem. Res. 2011, 50, 9354–9362. [Google Scholar] [CrossRef]
- Goli, J.; Sahu, O. Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew. En. 2018, 128, 142–154. [Google Scholar] [CrossRef]
- Nadeem, F.; Bhatti, I.A.; Ashar, A.; Yousaf, M.; Iqbal, M.; Mohsin, M.; Nisar, J.; Tamam, N.; Alwadai, N. Eco-benign biodiesel production from waste cooking oil using eggshell derived MM-CaO catalyst and condition optimization using RSM approach. Arab. J. Chem. 2021, 14, 103263. [Google Scholar] [CrossRef]
- Saparuddin, D.I.; Mohd Zaid, M.H.; Aziz, S.H.A.; Matori, K.A. Reuse of eggshell waste and recycled glass in the fabrication porous glass–ceramics. Appl. Sci. 2020, 10, 5404. [Google Scholar] [CrossRef]
- Imron, M.A.; Ahkam, D.N.I.; Hidayat, A.W. DECO FRECASE (drywall eco-friendly from eggshell and cane bagasse) as an innovation of eco-friendly interior construction. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 282, p. 012009. [Google Scholar]
- Özğan, A.O.; Özdemir, F. Investigation of mechanical properties of plastic composites produced using wood and eggshell. J. Compos. Mater. 2023, 57, 4709–4717. [Google Scholar] [CrossRef]
- Toro, P.; Quijada, R.; Yazdani-Pedram, M.; Arias, J.L. Eggshell, a new bio-filler for polypropylene composites. Mater. Lett. 2007, 61, 4347–4350. [Google Scholar] [CrossRef]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Sunardi, S.; Ariawan, D.; Surojo, E.; Prabowo, A.R.; Akbar, H.I.; Cao, B.; Carvalho, H. Assessment of eggshell-based material as a green-composite filler: Project milestones and future potential as an engineering material. J. Mech. Behav. Mater. 2023, 32, 20220269. [Google Scholar] [CrossRef]
- Feng, Y.; Ashok, B.; Madhukar, K.; Zhang, J.; Zhang, J.; Reddy, K.O.; Rajulu, A.V. Preparation and characterization of polypropylene carbonate bio-filler (eggshell powder) composite films. Int. J. Polym. Anal. Charact. 2014, 19, 637–647. [Google Scholar] [CrossRef]
- Vandeginste, V. Food waste eggshell valorization through development of new composites: A review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Jibrin, M.N.; Mohammed, S. From garbage to biomaterials: An overview on egg shell based hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar] [CrossRef]
- Ohshima, Y.; Takada, D.; Namai, S.; Sawai, J.; Kikuchi, M.; Hotta, M. Antimicrobial characteristics of heated eggshell powder. Biocontrol Sci. 2015, 20, 239–246. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Girija, E.K. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater. Lett. 2012, 76, 198–200. [Google Scholar] [CrossRef]
- Goloshchapov, D.L.; Kashkarov, V.M.; Rumyantseva, N.A.; Seredin, P.V.; Lenshin, A.S.; Agapov, B.L.; Domashevskaya, E.P. Synthesis of nanocrystalline hydroxyapatite by precipitation using hen’s eggshell. Ceram. Int. 2013, 39, 4539–4549. [Google Scholar] [CrossRef]
- Ho, W.-F.; Hsu, H.-C.; Hsu, S.-K.; Hung, C.-W.; Wu, S.-C. Calcium phosphate bioceramics synthesized from eggshell powders through a solid state reaction. Ceram. Int. 2013, 39, 6467–6473. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Zhang, W. Conversion of egg-shell to hydroxyapatite for highly sensitive detection of endocrine disruptor bisphenol A. Mater. Chem. 2011, 21, 14428–14431. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like agglomerates of hydroxyapatite crystals formed on an egg-shell membrane. Colloid. Surf. B 2011, 82, 490–496. [Google Scholar] [CrossRef]
- Naga, S.M.; El-Maghraby, H.F.; Sayed, M.; Saad, E.A. Highly porous scaffolds made of nanosized hydroxyapatite powder synthesized from eggshells. J. Ceram. Sci. Technol. 2015, 6, 237–244. [Google Scholar]
- Elline, E.; Ismiyatin, K.; Budhy, T.I. Novel biodegradable hydrogel scaffold based on hydroxyapatite eggshell, collagen, and epigallocatechin-3-gallate. Dent. Res. J. 2023, 20, 38. [Google Scholar]
- Acharjee, D.; Mandal, S.; Samanta, S.K.; Roy, M.; Kundu, B.; Roy, S.; Basak, P.; Nandi, S.K. In Vitro and In Vivo Bone Regeneration Assessment of Titanium-Doped Waste Eggshell-Derived Hydroxyapatite in the Animal Model. ACS Biomat Sci. Eng. 2023, 9, 4673–4685. [Google Scholar] [CrossRef] [PubMed]
- North, L.; Labonte, D.; Oyen, M.L.; Coleman, M.P.; Caliskan, H.B.; Johnston, R.E. Interrelated chemical-microstructural-nanomechanical variations in the structural units of the cuttlebone of Sepia officinalis. APL Mater. 2017, 5, 116103. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Melzner, F.; Pörtner, H.O.; Meier, S. Cuttlebone calcification increases during exposure to elevated seawater pCO2 in the cephalopod Sepia officinalis. Mar. Biol. 2010, 157, 1653–1663. [Google Scholar] [CrossRef]
- Currey, J.D. Mechanical properties of bone tissues with greatly differing functions. J. Biomech. 1979, 12, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gadipudi, V.K.; Salem, D.R. Topology optimization of lightweight lattice structural composites inspired by cuttlefish bone. Appl. Comp. Mater. 2018, 26, 15–27. [Google Scholar] [CrossRef]
- Chongcharoenchaikul, T.; Miyaji, K.; Junkong, P.; Poompradub, S.; Ikeda, Y. Effects of organic components in cuttlebone on the morphological and mechanical properties of peroxide cross-linked cuttlebone/natural rubber composites. RSC Adv. 2022, 12, 13557. [Google Scholar] [CrossRef]
- Ressler, A.; Gudelj, A.; Zadro, K.; Antunović, M.; Cvetnić, M.; Ivanković, M.; Ivanković, H. From bio-waste to bone substitute: Synthesis of biomimetic hydroxyapatite and its use in chitosan-based composite scaffold preparation. Chem. Biochem. Eng. Q. 2020, 34, 59–71. [Google Scholar] [CrossRef]
- Balu, S.; Sundaradoss, M.V.; Andra, S.; Jeevanandam, J. Facile biogenic fabrication of hydroxyapatite nanorods using cuttlefish bone and their bactericidal and biocompatibility study. Beilstein J. Nanotechnol. 2020, 11, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Thum, J.Y.; Sin, L.T.; Bee, S.T.; Lim, J.V.; Bee, S.L. Investigation of calcination of Sepia officinalis cuttlefish bone for reinforcement of polyvinyl alcohol added nano-size montmorillonite. Polymers 2022, 14, 1089. [Google Scholar] [CrossRef]
- Curti, F.; Drăgușin, D.-M.; Serafim, A.; Sorescu, A.; Stancu, I.-C.; Iovu, H.; Marinescu, R. Cuttlefish bone-based ink for 3D printing of scaffolds for orthopedic applications. UPB Sci. Bull. Ser. B 2021, 83, 1–14. [Google Scholar]
- Curti, F.; Serafim, A.; Olaret, E.; Dinescu, S.; Samoila, I.; Vasile, B.S.; Iovu, H.; Lungu, A.; Stancu, I.C.; Marinescu, R. Development of biocomposite alginate-cuttlebone-gelatin 3d printing inks designed for scaffolds with bone regeneration potential. Mar. Drugs 2022, 20, 670. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, W.; Shenton, W.; Davis, S.A.; Mann, S. Template mineralization of ordered macroporous chitin-silica composites using a cuttlebone-derived organic matrix. Chem. Mater. 2000, 12, 2835–2837. [Google Scholar] [CrossRef]
- Milovac, D.; Weigand, I.; Kovacic, M.; Ivankovic, M.; Ivankovic, H. Highly porous hydroxyapatite derived from cuttlefish bone as TiO2 catalyst support. Proc. Appl. Ceram. 2018, 12, 136–142. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, H.J.; Lee, K.S. Evaluation of biocompatibility of porous hydroxyapatite developed from edible cuttlefish bone. Key Eng. Mater. 2008, 361, 155–158. [Google Scholar]
- Tattanon, T.; Pongprayoon, T.; Arpornmaeklong, P.; Ummartyotin, S. Development of hydroxyapatite from cuttlebone and gelatin-based hydrogel composite for medical materials. J. Polym. Res. 2022, 29, 364. [Google Scholar] [CrossRef]
- Jalageri, M.B.; Mohan Kumar, G.C. Hydroxyapatite reinforced polyvinyl alcohol/polyvinyl pyrrolidone based hydrogel for cartilage replacement. Gels 2022, 8, 555. [Google Scholar] [CrossRef]
- Vibhatabandhu, P.; Srithongouthai, S. Removal of Copper (II) from Aqueous Solutions Using Cuttlebone as Bio-adsorbent. Appl. Environ. Res. 2016, 38, 39–48. [Google Scholar] [CrossRef]

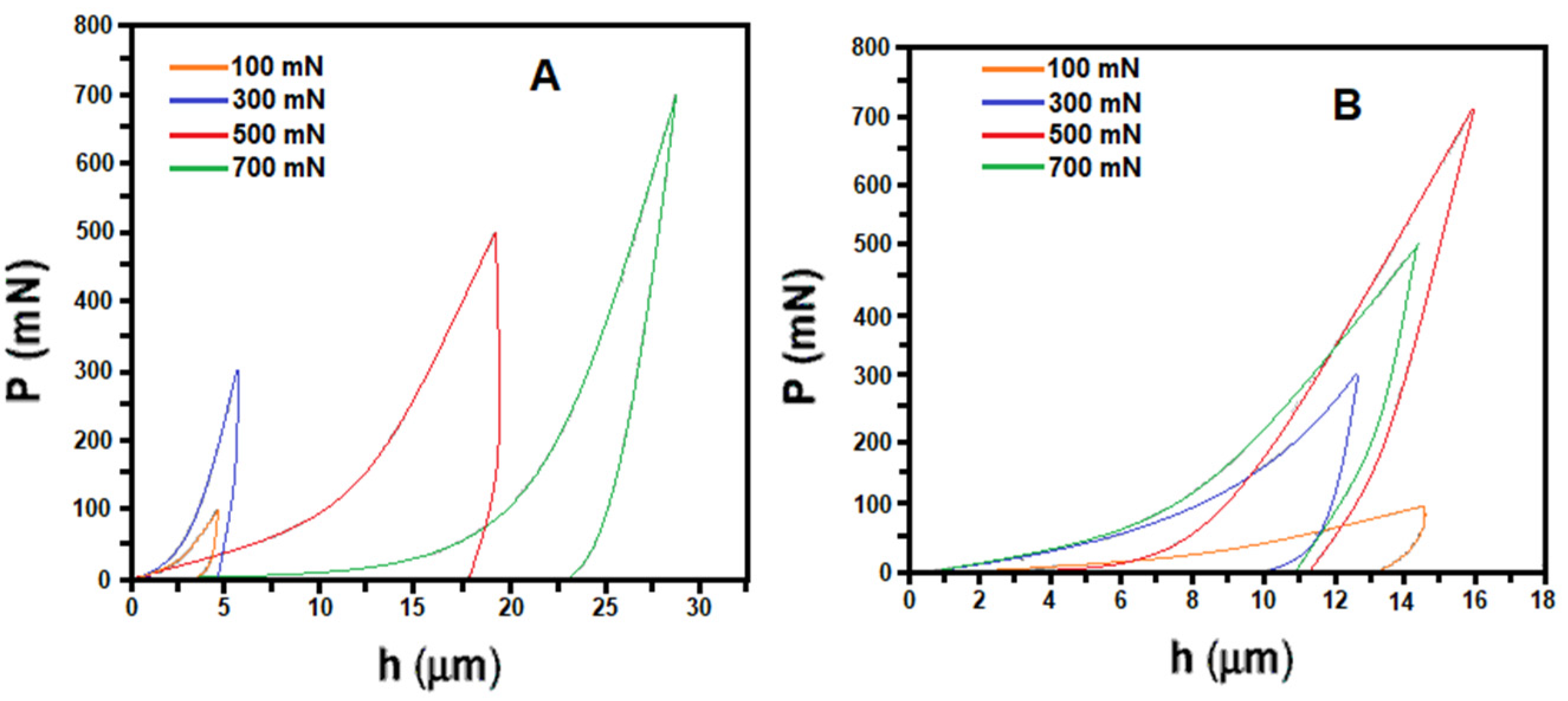

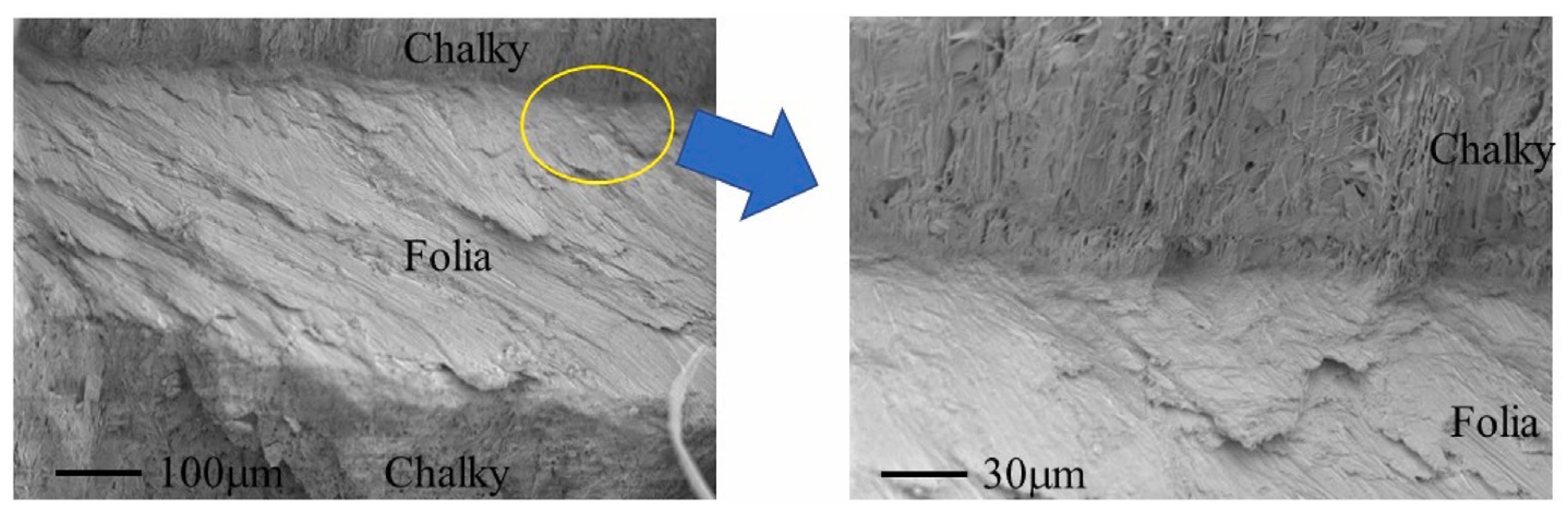

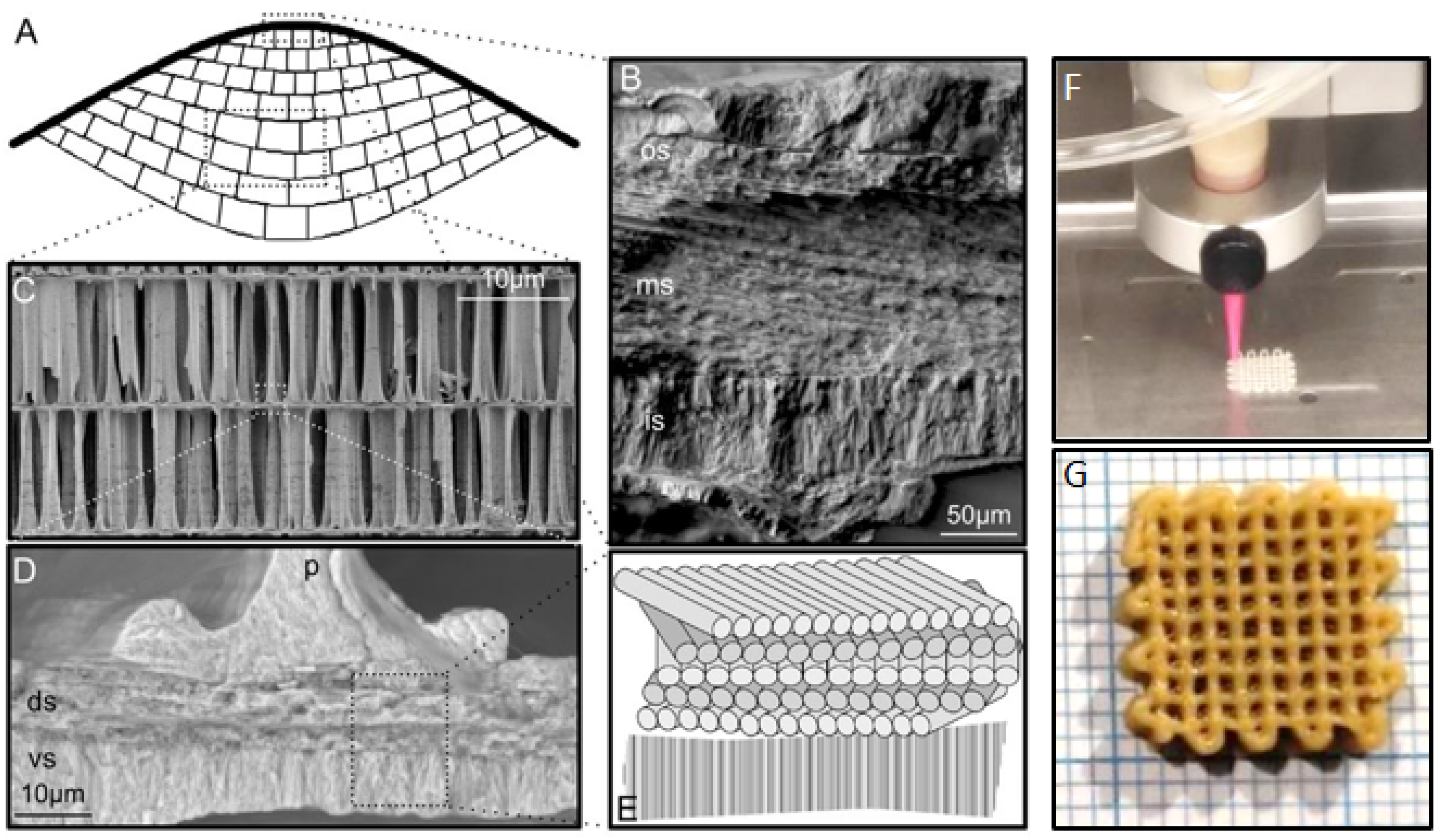
| Bio-Calcium Carbonate Source | Species | Composition | CaCO3 Content |
|---|---|---|---|
| Snail shell | Helix pomatia Cornu aspersum Eobania vermiculata | Normally aragonite; calcite in repaired parts Aragonite packed together by conchiolin | 97% [26] 95–99% [27] Close to 100% [28] |
| Columnar stacked aragonite | |||
| Chicken eggshell | Gallus gallus | Calcite, yet also aragonite and vaterite | Over 95% [29] |
| Quail eggshell | Coturnix coturnix | Almost pure calcite | Around 96% [30] |
| Clam shell | Chamelea gallina | Calcite (10 wt.%) and aragonite (90 wt.%) | Over 99% [31] |
| Mussel shell | Mitilus galloprovincialis | Calcite (40 ± 4 wt%) and aragonite (60 ± 6 wt%) | Around 98.5% [32] |
| Oyster shell | Ostrea edulis | Almost pure calcite | Close to 100% [33] |
| Scallop shells | Pecten maximus | Almost pure calcite | 98–99% [34] |
| Cuttlefish bone | Sepia officinalis | Pure aragonite | 95% [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, S.; Salathia, S.; Guzzini, A.; Zovi, A.; Jackson, S.; Smirnov, A.; Fragassa, C.; Santulli, C. Biomimetic Use of Food-Waste Sources of Calcium Carbonate and Phosphate for Sustainable Materials—A Review. Materials 2024, 17, 843. https://doi.org/10.3390/ma17040843
Piras S, Salathia S, Guzzini A, Zovi A, Jackson S, Smirnov A, Fragassa C, Santulli C. Biomimetic Use of Food-Waste Sources of Calcium Carbonate and Phosphate for Sustainable Materials—A Review. Materials. 2024; 17(4):843. https://doi.org/10.3390/ma17040843
Chicago/Turabian StylePiras, Sara, Saniya Salathia, Alessandro Guzzini, Andrea Zovi, Stefan Jackson, Aleksei Smirnov, Cristiano Fragassa, and Carlo Santulli. 2024. "Biomimetic Use of Food-Waste Sources of Calcium Carbonate and Phosphate for Sustainable Materials—A Review" Materials 17, no. 4: 843. https://doi.org/10.3390/ma17040843
APA StylePiras, S., Salathia, S., Guzzini, A., Zovi, A., Jackson, S., Smirnov, A., Fragassa, C., & Santulli, C. (2024). Biomimetic Use of Food-Waste Sources of Calcium Carbonate and Phosphate for Sustainable Materials—A Review. Materials, 17(4), 843. https://doi.org/10.3390/ma17040843










