Nanocrystals Incorporated with Mordenite Zeolite Composites with Enhanced Upconversion Emission for Cu2+ Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Desilication of Mordenite Zeolite
2.3. Synthesis of NaREF4:Yb/Er (RE = Y, Gd) @DSixMOR Composites
2.4. Detection of Cu2+
2.4.1. Surface Modification of NaYF4:Yb/Er@DSi1.0MOR-HT Composite
2.4.2. Detection of Cu2+ by Using the NaYF4:Yb/Er@DSi1.0MOR-HT/BPEI Composite
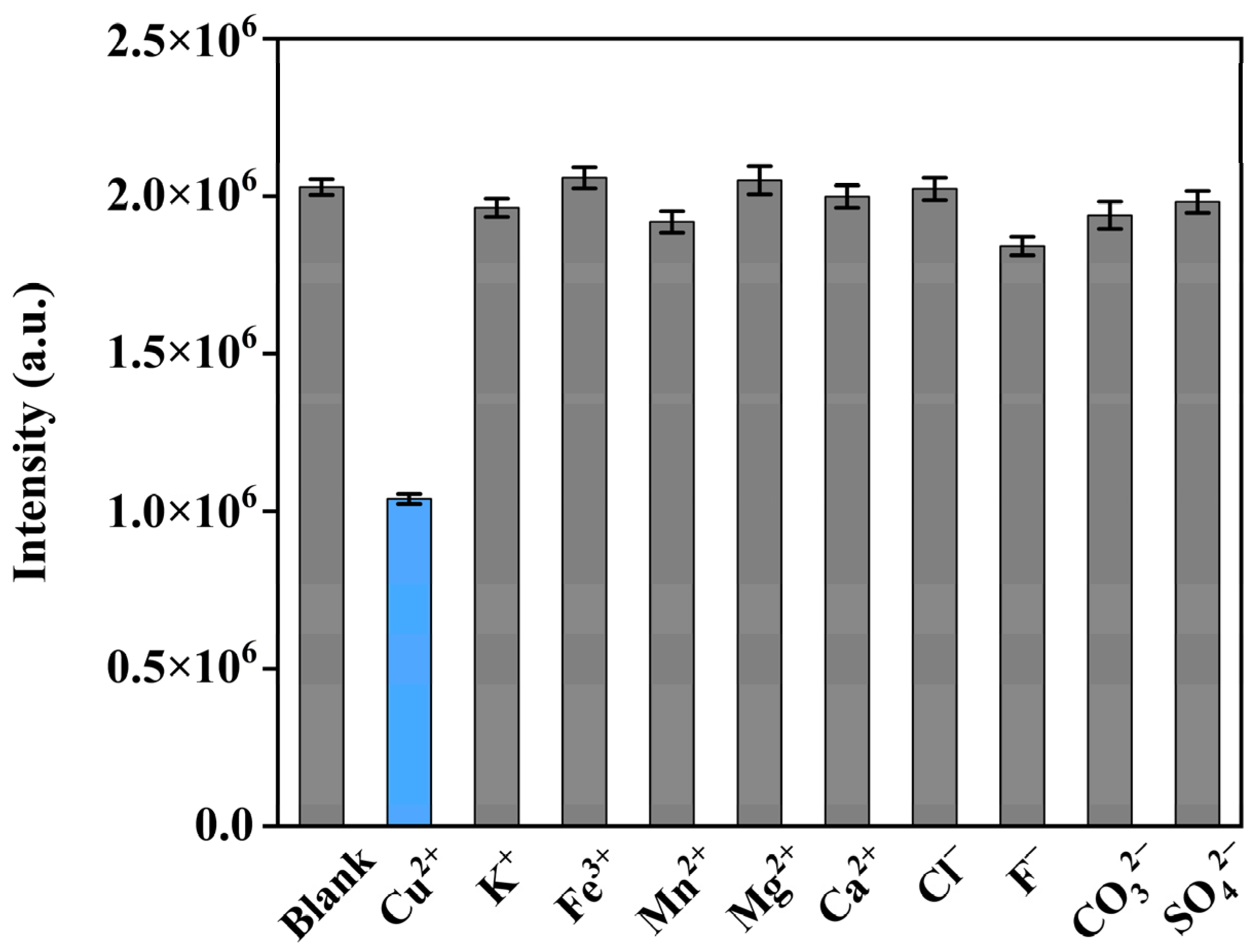
2.4.3. Anti-Interference Test
2.5. Characterization
3. Results and Discussion
3.1. Structure and Morphology
3.2. Upconversion Properties
3.3. Detection of Cu2+ via Upconversion Emission
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huy, B.T.; Kumar, A.P.; Thuy, T.T.; Nghia, N.N.; Lee, Y.-I. Recent advances in fluorescent upconversion nanomaterials: Novel strategies for enhancing optical and magnetic properties to biochemical sensing and imaging applications. Appl. Spectrosc. Rev. 2020, 57, 265–299. [Google Scholar] [CrossRef]
- Hlavacek, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horak, D.; Skladal, P.; Foret, F.; Gorris, H.H. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Fekar, D.; Mahilang, M. Up-conversion phosphors: A short review. Ferroelectrics 2023, 614, 112–118. [Google Scholar] [CrossRef]
- Richards, B.S.; Hudry, D.; Busko, D.; Turshatov, A.; Howard, I.A. Photon Upconversion for Photovoltaics and Photocatalysis: A Critical Review. Chem. Rev. 2021, 121, 9165–9195. [Google Scholar] [CrossRef] [PubMed]
- Mandl, G.A.; Cooper, D.R.; Hirsch, T.; Seuntjens, J.; Capobianco, J.A. Perspective: Lanthanide-doped upconverting nanoparticles. Methods Appl. Fluoresc. 2019, 7, 012004. [Google Scholar] [CrossRef]
- Cooper, D.R.; Capobianco, J.A.; Seuntjens, J. Radioluminescence studies of colloidal oleate-capped β-Na(Gd,Lu)F4:Ln3+ nanoparticles (Ln = Ce, Eu, Tb). Nanoscale 2018, 10, 7821–7832. [Google Scholar] [CrossRef] [PubMed]
- Naccache, R.; Yu, Q.; Capobianco, J.A. The Fluoride Host: Nucleation, Growth, and Upconversion of Lanthanide-Doped Nanoparticles. Adv. Opt. Mater. 2015, 3, 482–509. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Liu, S.; Li, Q.; Zhou, B. Controllable synthesis of ultrasmall core-shell hexagonal upconversion nanoparticles towards full-color output. Optik 2020, 207, 164398. [Google Scholar] [CrossRef]
- Sun, C.; Gradzielski, M. Advances in fluorescence sensing enabled by lanthanide-doped upconversion nanophosphors. Adv. Colloid Interface Sci. 2022, 300, 102579. [Google Scholar] [CrossRef]
- Banerjee, A.; Shwetabh, K.; Kumar, K.; Poddar, R. Exploring the Effect of Size Variability on Efficiency of Upconversion Nanoparticles as Optical Contrast Agents. J. Fluoresc. 2023. [Google Scholar] [CrossRef] [PubMed]
- Hudry, D.; Howard, I.A.; Popescu, R.; Gerthsen, D.; Richards, B.S. Structure-Property Relationships in Lanthanide-Doped Upconverting Nanocrystals: Recent Advances in Understanding Core-Shell Structures. Adv. Mater. 2019, 31, e1900623. [Google Scholar] [CrossRef]
- Hasegawa, M.; Ishii, A. Thin-film formation for promoting the potential of luminescent lanthanide coordination complexes. Coord. Chem. Rev. 2020, 421, 213458. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Li, L.-D.; Si, R.; Liu, R.; Yan, C.-H. Selective Cation Exchange Enabled Growth of Lanthanide Core/Shell Nanoparticles with Dissimilar Structure. J. Am. Chem. Soc. 2017, 139, 18492–18495. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Lin, P.; Pan, L.; Wang, D. In situ growth of lanthanides-doped nanoparticles inside zeolites with enhanced upconversion emission for gallic acid detection. J. Colloid Interface Sci. 2023, 652, 1297–1307. [Google Scholar] [CrossRef]
- Du, J.; Jia, T.; Zhang, J.; Chen, G. Heterostructures Combining Upconversion Nanoparticles and Metal–Organic Framework: Fundamental, Classification, and Theranostic Applications. Adv. Opt. Mater. 2023, 11, 2202122. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, S.; Huang, G.; Zhan, S.; Nie, G.; Su, X.; Cheng, D.; Liu, Y. Flexible composite film with enhanced upconversion emission for ultrasensitive gas detection. Ceram. Int. 2024, 50, 3843–3851. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C.; Kooh, M.R.R. Zeolite Application in Wastewater Treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Na, K. Synthesis and Application of Zeolite Catalysts. Catalysts 2021, 11, 685. [Google Scholar] [CrossRef]
- Matusiak, J.; Przekora, A.; Franus, W. Zeolites and zeolite imidazolate frameworks on a quest to obtain the ideal biomaterial for biomedical applications: A review. Mater. Today 2023, 67, 495–517. [Google Scholar] [CrossRef]
- Pan, L.; Ye, S.; Xv, X.; Lin, P.; Huang, R.; Wang, D. Zeolite-Encaged Luminescent Silver Nanoclusters. Materials 2023, 16, 3736. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Sun, F.; Ouyang, J.; Su, R.; Meng, F.; Luo, Y.; Xu, C.; Zhang, W.; Zhang, S.; et al. Incorporating metal nanoparticles in porous materials via selective heating effect using microwave. Nano Res. 2023, 1–5. [Google Scholar] [CrossRef]
- Jiang, Y.; Tong, X.; E, Y.; Wei, P.; Fang, F.; Chen, P.; Qian, K. TiO2-doped LTA zeolite as a sensitive non-enzymatic electrochemical sensor toward hydrogen peroxide detection. J. Alloys Compd. 2023, 968, 171866. [Google Scholar] [CrossRef]
- Groen, J.C.; Zhu, W.; Brouwer, S.; Huynink, S.J.; Kapteijn, F.; Moulijn, J.A.; Pérez-Ramírez, J. Direct Demonstration of Enhanced Diffusion in Mesoporous ZSM-5 Zeolite Obtained via Controlled Desilication. J. Am. Chem. Soc. 2006, 129, 355–360. [Google Scholar] [CrossRef]
- Shahid, A.; Inayat, A.; Avadhut, Y.; Hartmann, M.; Schwieger, W. A comparative study of the desilication of channel- and cage-like zeolites. Microporous Mesoporous Mater. 2022, 341, 111903. [Google Scholar] [CrossRef]
- Gackowski, M.; Tarach, K.; Kuterasiński, Ł.; Podobiński, J.; Jarczewski, S.; Kuśtrowski, P.; Datka, J. Hierarchical zeolites Y obtained by desilication: Porosity, acidity and catalytic properties. Microporous Mesoporous Mater. 2018, 263, 282–288. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Sauer, J.; Raybaud, P. Dealumination mechanisms of zeolites and extra-framework aluminum confinement. J. Catal. 2016, 339, 242–255. [Google Scholar] [CrossRef]
- Roy, N.; Neerugatti, K.R.E.; Sinha, A.; Mukherjee, A. Ceria-decorated zeolite nanocomposite for synergistic adsorption and photocatalytic degradation of caffeine. Surf. Interfaces 2023, 43, 103515. [Google Scholar] [CrossRef]
- Liao, H.; Ye, S.; Ding, J.; Yu, J.; Xv, X.; Pan, L.; Lin, P.; Wang, D. Ship-in-a-bottle growth of NaYF4: Yb3+/Tm3+ upconversion nanocrystals in desilicated ZSM-5 zeolite for drug release monitoring. Mater. Res. Bull. 2022, 154, 111926. [Google Scholar] [CrossRef]
- Kostiv, U.; Engstova, H.; Krajnik, B.; Slouf, M.; Proks, V.; Podhorodecki, A.; Jezek, P.; Horak, D. Monodisperse Core-Shell NaYF4:Yb3+/Er3+@NaYF4:Nd3+-PEG-GGGRGDSGGGY-NH2 Nanoparticles Excitable at 808 and 980 nm: Design, Surface Engineering, and Application in Life Sciences. Front. Chem. 2020, 8, 497. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Li, X.; Zhang, J.; Sun, J.; Tong, L.; Zhong, H.; Xia, H.; Hua, R.; Chen, B. Improved LRET-based detection characters of Cu2+ using sandwich structured NaYF4@NaYF4:Er3+/Yb3+@NaYF4 nanoparticles as energy donor. Sens. Actuators B Chem. 2018, 257, 829–838. [Google Scholar] [CrossRef]
- Shao, H.; Li, F.; Li, D.; Ma, Q.; Yu, W.; Liu, G.; Dong, X. Up-/Downconversion Fluorescence Dual-Channel Probe Based on NaYF4: Yb/Er/Eu Nanoparticles for the Determination of Cu(II). ACS Appl. Nano Mater. 2022, 5, 3333–3341. [Google Scholar] [CrossRef]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef] [PubMed]
- Weissenberger, T.; Machoke, A.G.F.; Reiprich, B.; Schwieger, W. Preparation and Potential Catalytic Applications of Hierarchically Structured Zeolites with Macropores. Adv. Mater. Interfaces 2021, 8, 2001653. [Google Scholar] [CrossRef]
- Gruenert, W.; Muhler, M.; Schroeder, K.-P.; Sauer, J.; Schloegl, R. Investigations of Zeolites by Photoelectron and Ion Scattering Spectroscopy. 2. A New Interpretation of XPS Binding Energy Shifts in Zeolites. J. Phys. Chem. 1994, 98, 10920–10929. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Xv, X.; Ye, S.; Pan, L.; Lin, P.; Liao, H.; Wang, D. Tailoring the Luminescence Properties of Silver Clusters Confined in Faujasite Zeolite through Framework Modification. Materials 2022, 15, 7431. [Google Scholar] [CrossRef]
- Remy, M.J.; Genet, M.J.; Poncelet, G.; Lardinois, P.F.; Notte, P.P. Investigation of dealuminated mordenites by X-ray photoelectron spectroscopy. J. Phys. Chem. 2002, 96, 2614–2617. [Google Scholar] [CrossRef]
- Kavand, A.; Serra, C.A.; Blanck, C.; Lenertz, M.; Anton, N.; Vandamme, T.F.; Mély, Y.; Przybilla, F.; Chan-Seng, D. Controlled Synthesis of NaYF4:Yb,Er Upconversion Nanocrystals as Potential Probe for Bioimaging: A Focus on Heat Treatment. ACS Appl. Nano Mater. 2021, 4, 5319–5329. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Plackowski, C.A.; Nguyen, A.V. Sulfuric acid dissolution of 4A and Na-Y synthetic zeolites and effects on Na-Y surface and particle properties. Appl. Surf. Sci. 2016, 367, 281–290. [Google Scholar] [CrossRef]
- Choi, J.; Jung, R. In-situ XPS Study of Core-levels of ZnO Thin Films at the Interface with Graphene/Cu. J. Korean Phys. Soc. 2018, 73, 1546–1549. [Google Scholar] [CrossRef]
- Haslam, G.E.; Sato, K.; Mizokawa, T.; Chin, X.Y.; Burstein, G.T. Charge transfer effects in electrocatalytic Ni-C revealed by X-ray photoelectron spectroscopy. Appl. Phys. Lett. 2012, 100, 231601. [Google Scholar] [CrossRef]
- Nannuri, S.H.; Singh, S.; Misra, S.K.; C, S.; George, S.D. Microwave-assisted synthesis and upconversion luminescence of NaYF4:Yb, Gd, Er and NaYF4:Yb, Gd, Tm nanorods. Methods Appl. Fluoresc. 2022, 10, 024004. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.C.; Bastos, A.R.N.; Ferreira, R.A.S.; Wang, X.; Chen, G.; Carlos, L.D. Primary Luminescent Nanothermometers for Temperature Measurements Reliability Assessment. Adv. Photonics Res. 2021, 2, 2000169. [Google Scholar] [CrossRef]
- Tegze, B.; Tolnai, G.; Hessz, D.; Kubinyi, M.; Madarász, J.; Sáfrán, G.; Hórvölgyi, Z. Effect of heat treatment temperature on the morphology and upconversion properties of LaF3:Yb,Er nanoparticles. J. Therm. Anal. Calorim. 2023, 148, 10795–10802. [Google Scholar] [CrossRef]
- Shao, H.; Xu, D.; Ding, Y.; Hong, X.; Liu, Y. An “off-on” colorimetric and fluorometric assay for Cu(II) based on the use of NaYF4:Yb(III),Er(III) upconversion nanoparticles functionalized with branched polyethylenimine. Microchim. Acta 2018, 185, 211. [Google Scholar] [CrossRef]
- Lal, B.; Kumar, S.; Tittal, R.K.; Singh, G.; Singh, J.; Ghule, V.D.; Mathpati, R.S.; Sabane, J.K. 4-aminoantipyrine linked bis-1,2,3-triazole based probes for Cu(II) sensing. J. Mol. Struct. 2024, 1297, 136995. [Google Scholar] [CrossRef]
- Liu, M.; Wang, K.; Wang, H.; Lu, J.; Xu, S.; Zhao, L.; Wang, X.; Du, J. Simple and sensitive colorimetric sensors for the selective detection of Cu(ii). RSC Adv. 2021, 11, 11732–11738. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, T.; He, Q.; Hu, Y.; Zuo, Z.; Cao, Z.; Ke, B.; Zhang, W.; Qi, Q. A selective and sensitive near-infrared fluorescent probe for real-time detection of Cu(i). RSC Adv. 2021, 11, 14824–14828. [Google Scholar] [CrossRef]
- Pei, M.; Kong, H.; Tian, A.; Liu, X.; Zheng, K.; Ren, Z.; Wang, L. Novel benzotriazole-based probes for the selective detection of Cu(II). J. Mol. Struct. 2022, 1250, 131806. [Google Scholar] [CrossRef]
- Keller, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Copper(i) and silver(i) complexes of 9,9-dimethyl-4,5-bis(di-tert-butylphosphino)xanthene: Photophysical properties and structural rigidity under pressure. Photochem. Photobiol. Sci. 2018, 17, 375–385. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Kumar, A.; Kumar, A.; Kumar, S. Synthesis and optical properties of copper(II) and nickel(II) complexes of a highly fluorescent morpholine-derivative. Polyhedron 2019, 171, 559–570. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Zhang, L.; Jiang, H. An optical sensor for Cu(II) detection with upconverting luminescent nanoparticles as an excitation source. Chem. Commun. (Camb.) 2012, 48, 4860–4862. [Google Scholar] [CrossRef]

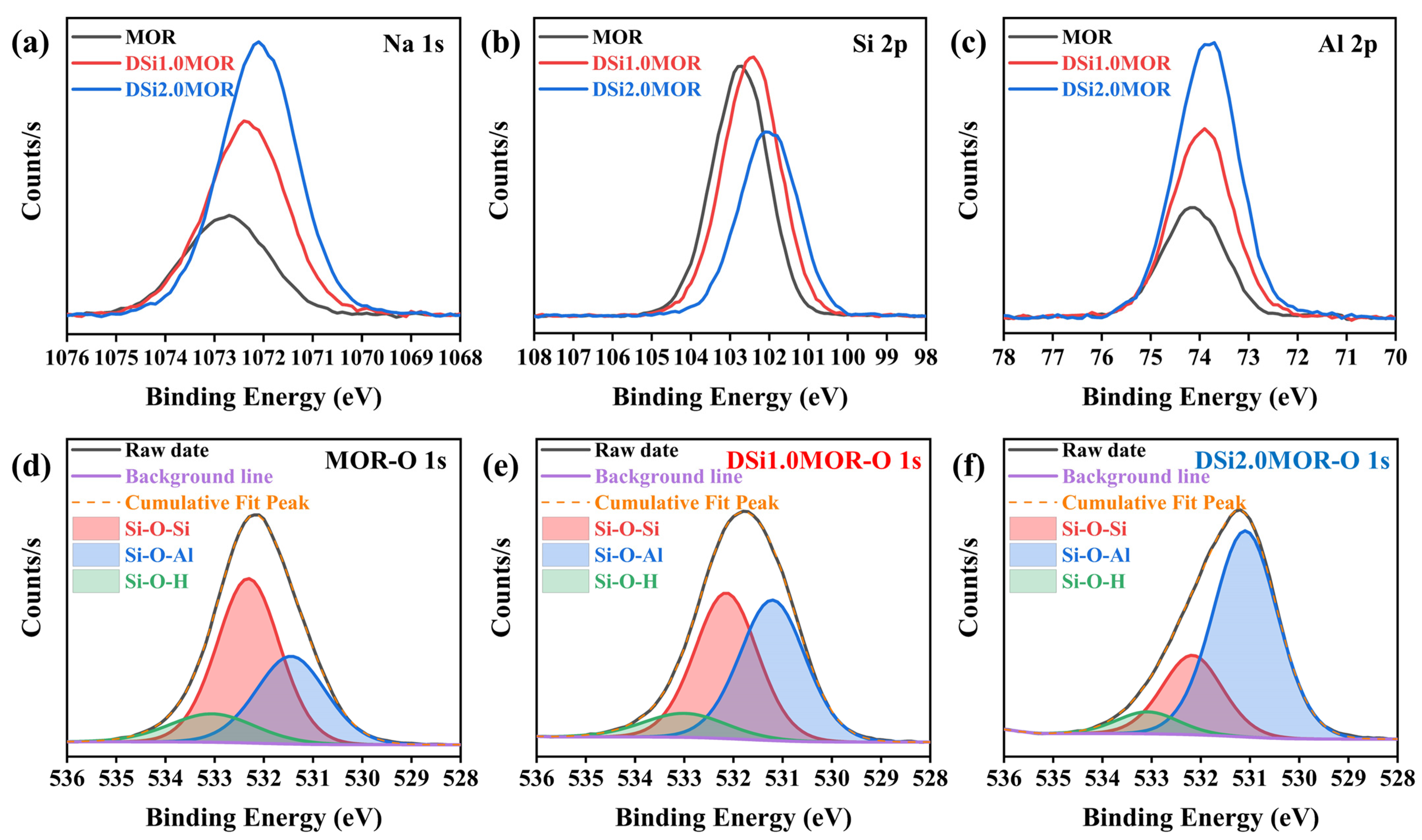

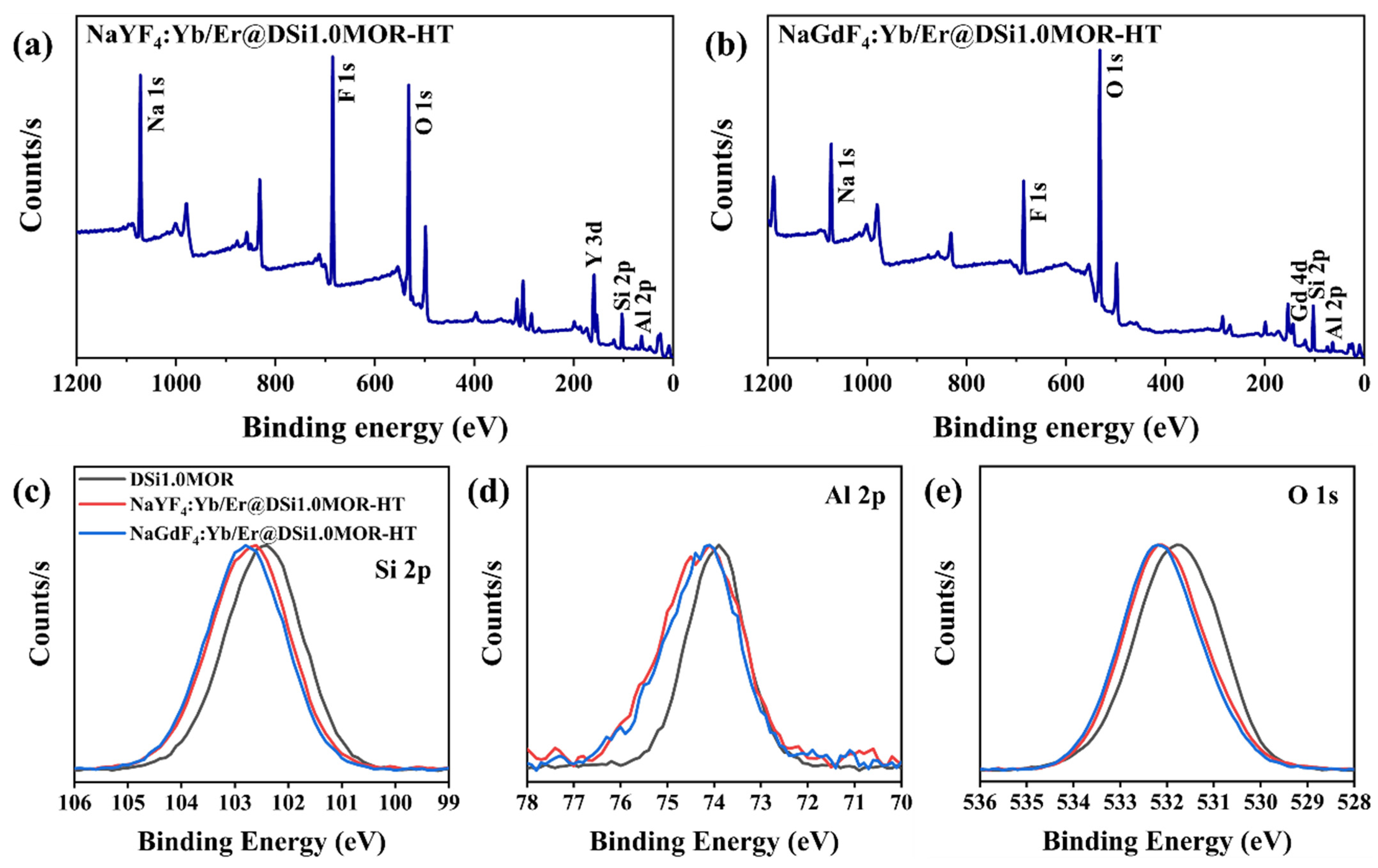
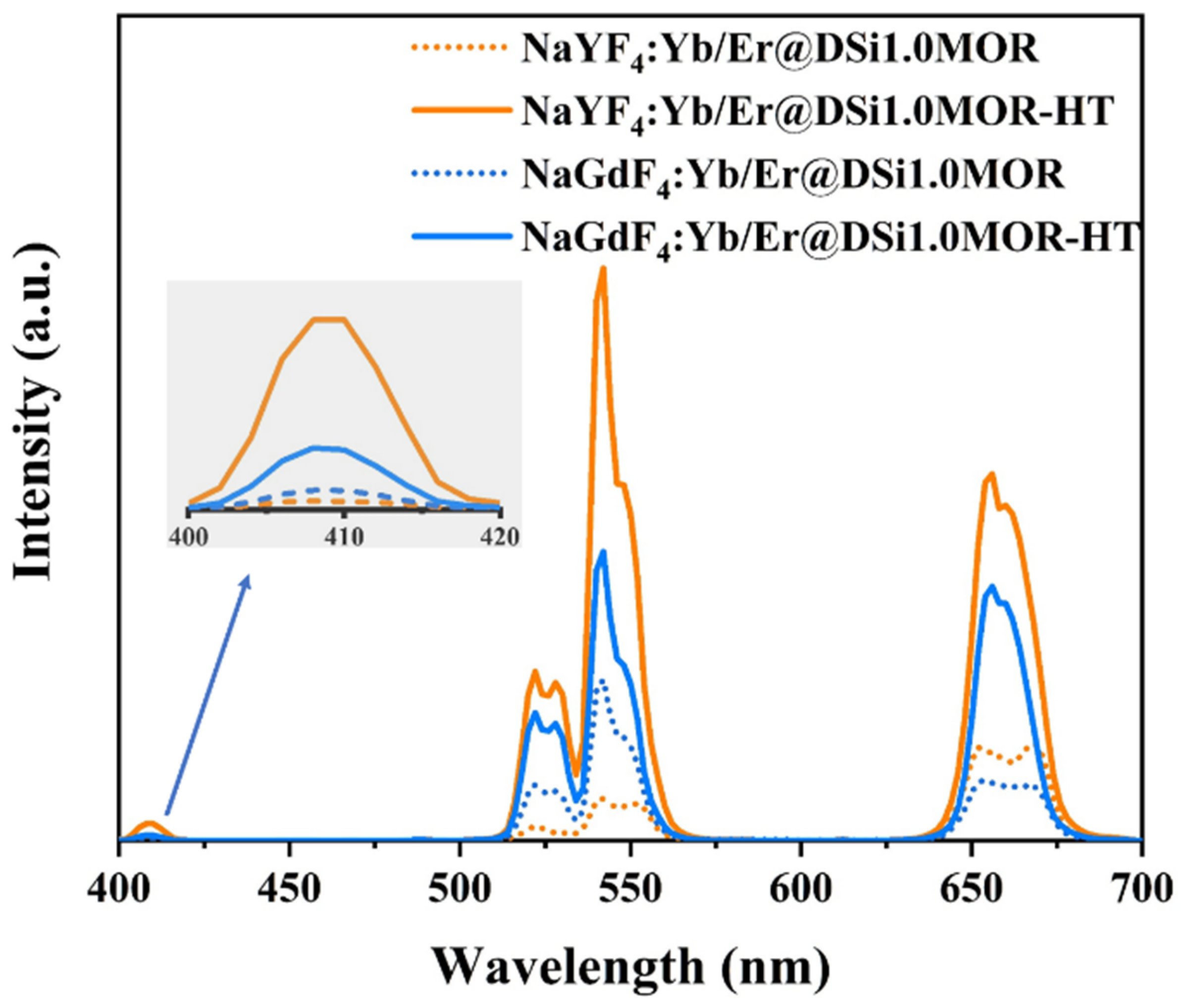
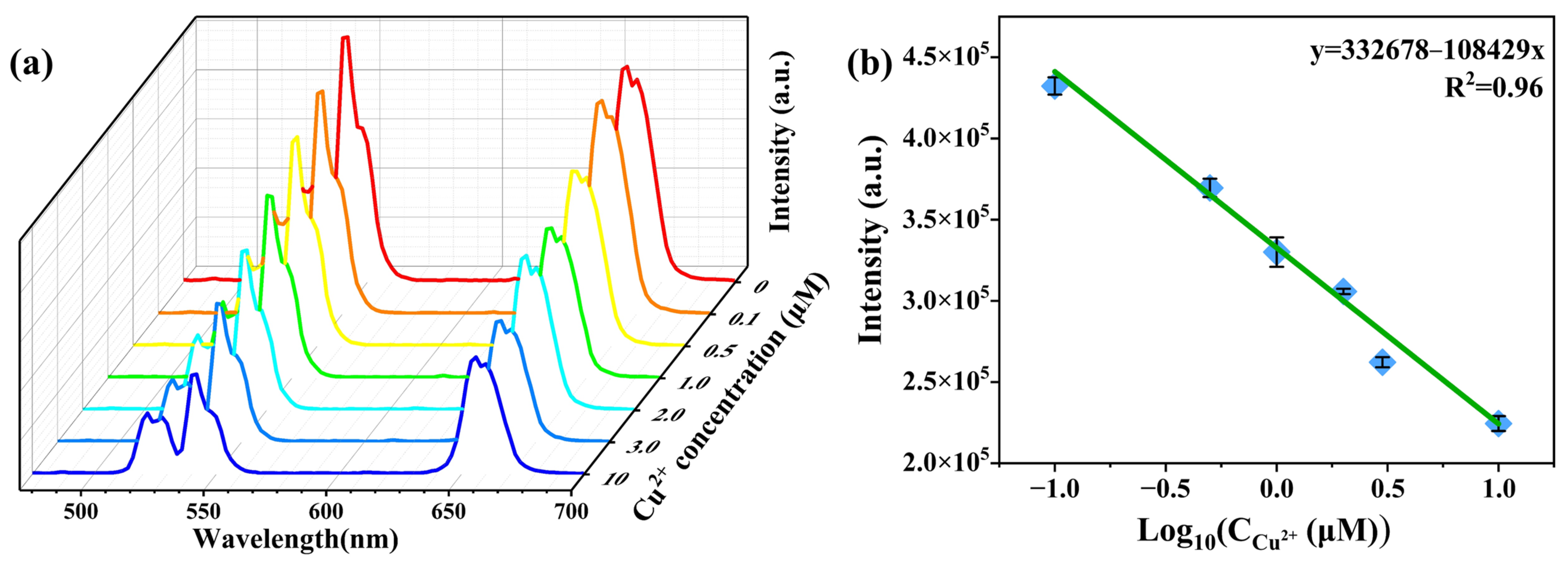
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Meso | Micro | BET | Meso | Micro | Total | Meso | Micro | BET | |
| MOR | 1 | 320 | 321 | 0.020 | 0.147 | 0.167 | 5.98 | 0.51 | 2.08 |
| DSi1.0MOR | 20 | 73 | 93 | 0.061 | 0.034 | 0.095 | 8.05 | 0.99 | 4.06 |
| Sample | XPS Atomic (%) | Si/Al | |||
|---|---|---|---|---|---|
| Si | Al | Na | O | ||
| MOR | 25.88 | 5.11 | 5.41 | 63.61 | 5.06 |
| DSi1.0MOR | 23.45 | 6.73 | 8.67 | 61.14 | 3.48 |
| DSi2.0MOR | 17.26 | 9.88 | 12.08 | 60.78 | 1.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Ye, S.; Pan, L.; Huang, R.; Zhang, H.; Wang, D. Nanocrystals Incorporated with Mordenite Zeolite Composites with Enhanced Upconversion Emission for Cu2+ Detection. Materials 2024, 17, 854. https://doi.org/10.3390/ma17040854
Lin P, Ye S, Pan L, Huang R, Zhang H, Wang D. Nanocrystals Incorporated with Mordenite Zeolite Composites with Enhanced Upconversion Emission for Cu2+ Detection. Materials. 2024; 17(4):854. https://doi.org/10.3390/ma17040854
Chicago/Turabian StyleLin, Peixuan, Song Ye, Ling Pan, Ruihao Huang, Haoran Zhang, and Deping Wang. 2024. "Nanocrystals Incorporated with Mordenite Zeolite Composites with Enhanced Upconversion Emission for Cu2+ Detection" Materials 17, no. 4: 854. https://doi.org/10.3390/ma17040854
APA StyleLin, P., Ye, S., Pan, L., Huang, R., Zhang, H., & Wang, D. (2024). Nanocrystals Incorporated with Mordenite Zeolite Composites with Enhanced Upconversion Emission for Cu2+ Detection. Materials, 17(4), 854. https://doi.org/10.3390/ma17040854






