bmp-2 Gene-Transferred Skeletal Muscles with Needle-Type Electrodes as Efficient and Reliable Biomaterials for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of bmp-2-Expression Plasmid
2.2. Gene Transfer by Electroporation with Needle-Type Electrodes
2.3. Radiographic Analysis
2.4. Image Analysis
2.5. Histological Analysis
2.6. Statistical Analyses
3. Results
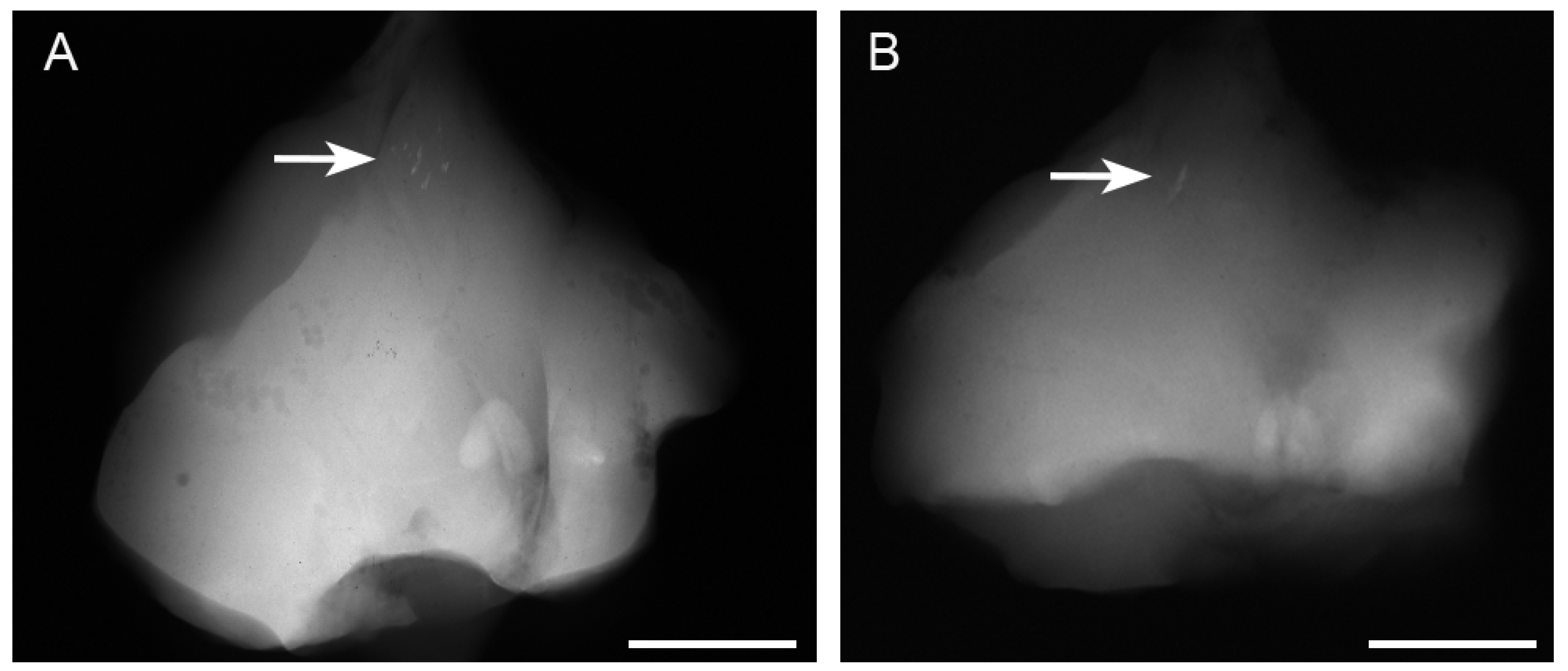
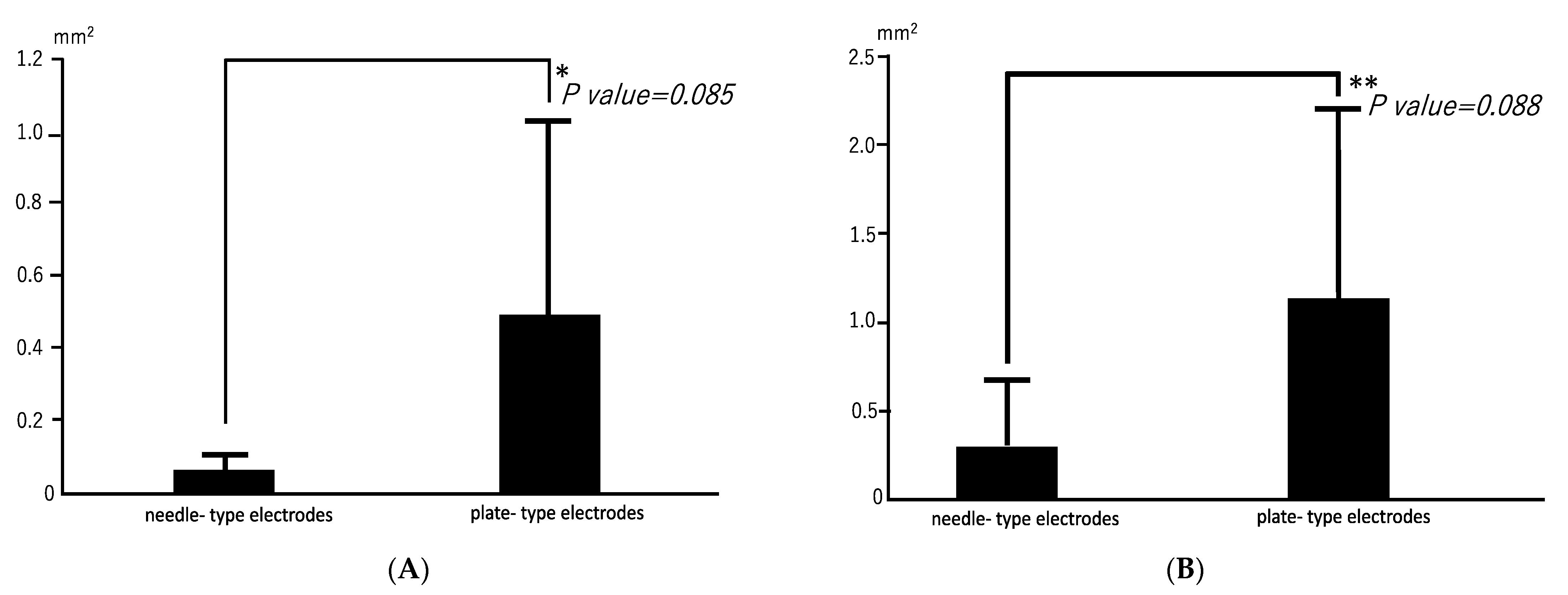
3.1. Radiographic Findings
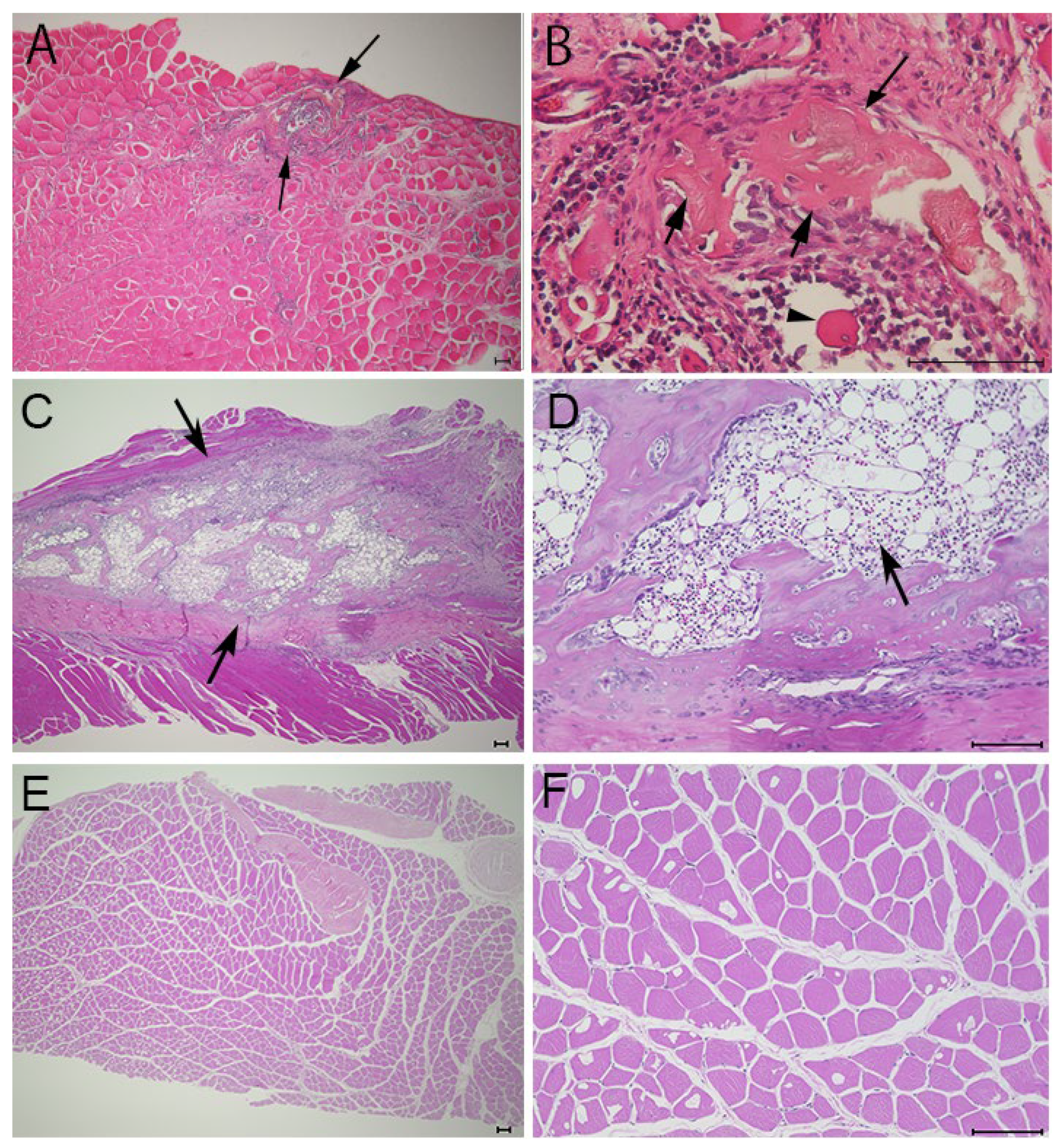
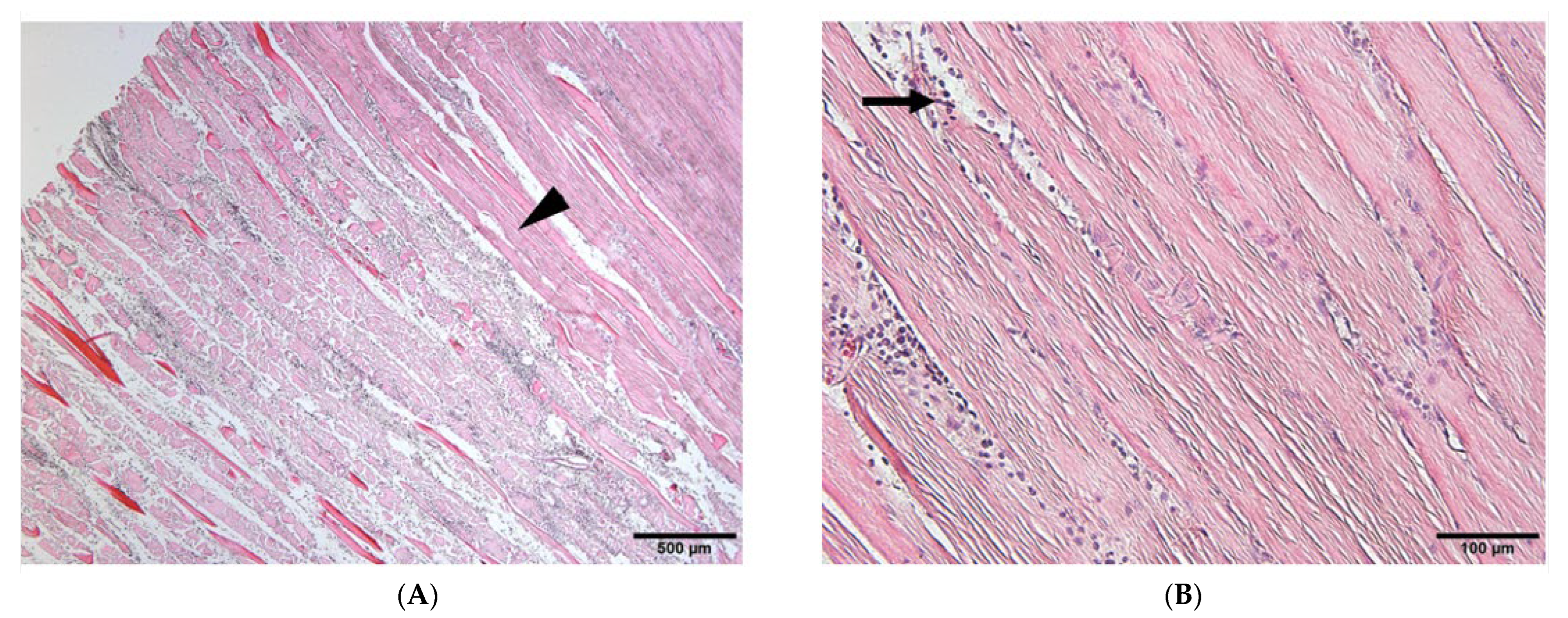
3.2. Histological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomes-Ferrira, P.H.S.; Okamoto, R.; Ferreira, S.; De Oliveira, D.; Momessa, G.A.C.; Faverani, L.P. Scientific evidence on the use of recombinant human bone morphogenetic protein-2 in oral and maxillofacial surgery. Oral Maxillofac. Surg. 2016, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Burkus, J.K.; Gornet, M.F.; Dickman, C.A.; Zdeblick, T.A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 2002, 15, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Harrell, D.B. Translational research: The CD34+ cell is crucial for large-volume bone regeneration from the milieu of bone marrow progenitor cells in craniomandibular reconstruction. Int. J. Oral Maxillofac. Implant. 2014, 29, 201–209. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Kim, J.E.; Park, J.C.; Shin, S.W.; Cho, K.S. Ridge preservation using demineralized bone matrix gel with recombinant human bone morphogenetic protein-2 after tooth extraction: A randomized controlled clinical trial. J. Oral Maxillofac. Surg. 2014, 72, 1281–1290. [Google Scholar] [CrossRef]
- Boyne, P.J.; Marx, R.E.; Nevins, M.; Triplett, G.; Lazaro, E.; Lilly, L.C.; Alder, M.; Nummikoski, P. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 11–25. [Google Scholar]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De novo bone induction by recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.W.; Kubota, A.; Nevins, M.; Fiorellini, J.P. The negative effect of combining rhBMP-2 and Bio-Oss on bone formation for maxillary sinus augmentation. Int. J. Periodontics Restor. Dent. 2012, 32, 61–67. [Google Scholar]
- Sharan, A.; Madjar, D. Maxillary sinus pneumatization following extractions: A radiographic study. Int. J. Oral Maxillofac. Implant. 2008, 23, 48–56. [Google Scholar]
- Bianchi, J.; Fiorellini, J.P.; Howell, T.H.; Sekler, J.; Curtin, H.; Nevins, M.L.; Friedland, B. Measuring the efficacy of rhBMP-2 to regenerate bone: A radiographic study using a commercially available software program. Int. J. Periodontics Restor. Dent. 2004, 24, 579–587. [Google Scholar]
- Fiorellini, J.P.; Howel, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef]
- Jensen, E.D.; Pham, L.; Billington, C.J., Jr.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef]
- Kawai, M.; Bessho, K.; Kaihara, S.; Sonobe, J.; Oda, K.; Iizuka, T. Ectopic bone formation by human bone morphogenetic protein-2 gene transfer to skeletal muscle using transcutaneous electroporation. Hum. Gene Ther. 2003, 14, 1547–1556. [Google Scholar] [CrossRef]
- Kawai, M.; Bessho, K.; Maruyama, H.; Miyazaki, J.; Yamamoto, T. Human BMP-2 gene transfer using transcutaneous in vivo electroporation induced both intramembranous and endochondral ossification. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2005, 287, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Bessho, K.; Maruyama, H.; Miyazaki, J.; Yamamoto, T. Simultaneous gene transfer of bone morphogenetic protein (BMP)-2 and BMP-7 by in vivo electroporation induces rapid bone formation and BMP-4 expression. BMC Musculoskelet. Disord. 2006, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Bessho, K.; Fijimura, K.; Iizuka, T.; Miyatake, S.I. Osteoinduction by bone morphogenetic protein-2 via adenoviral vector under transient. Biochem. Biophys. Res. Commun. 2000, 267, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kaihara, S.; Bessho, K.; Okubo, Y.; Sonobe, J.; Kawai, M.; Iizuka, T. Simple and effective osteoinductive gene therapy by local injection of a bone morphogenetic protein-2-expressing recombinant adenoviral vector and FK506 mixture in rats. Gene Ther. 2004, 11, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Simcikova, M.; Prather, K.L.; Praseres, D.M.; Monteiro, G.A. Towards effective non-viral gene delivery vector. Biotechnol. Genet. Eng. Rev. 2015, 31, 82–107. [Google Scholar] [CrossRef] [PubMed]
- Silvac, I.; Guay, D.; Mangion, M.; Champeil, J.; Gaillet, B. Non-viral nucleic acid delivery methods. Expert Opin. Biol. Ther. 2017, 17, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Midoux, P.; Pigeon, L.; Gonqalves, C.; Pichon, C. Peptides mediating DNA transport on microtubules and their impact on non-viral gene transfer efficiency. Biosci. Rep. 2017, 37, BSR20170995. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, B.; Tang, S.; Guerrero, J.; Higuita-Castro, N.; Salazar-Puerta, A.I.; Croft, A.S.; Gazdhar, A.; Purmessur, D. Non-viral gene delivery methods for bone and joints. Front. Bioeng. Biotechnol. 2020, 8, 598466. [Google Scholar] [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Teslegeev, G.D. Current state of human gene therapy: Approved products and vectors. Pharmaceutials 2023, 16, 1416. [Google Scholar] [CrossRef]
- Xiong, R.; Sauvage, F.; Fraire, J.C.; Huang, C.; De Smedt, S.C.; Braeckmans, K. Photothermal nanomaterial-mediated photoporation. Acc. Chem. Res. 2023, 56, 631–643. [Google Scholar] [CrossRef]
- Rosazza, C.; Meglic, S.H.; Zumbusch, A.; Rols, M.-P.; Miklavcic, D. Gene electrotransfer: A mechanistic perspective. Gene Ther. 2016, 16, 98–129. [Google Scholar] [CrossRef]
- Haberi, S.; Kanduser, M.; Flisar, K.; Hodzic, D.; Bregar, V.B.; Miklavcic, D.; Escoffre, J.-M.; Rols, M.-P.; Pavlin, M. Effect of different parameters used for in vitro gene electrotransfer on gene expression efficiency, cell viability and visualization of plasmid DNA at the membrane level. J. Gene Med. 2013, 15, 169–181. [Google Scholar] [CrossRef]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Pre’at, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Vandermeulen, G.; Vanvarenberg, K.; De Beuckelaer, A.; De Koker, S.; Lambrict, L.; Uyttenhove, C.; Reschner, A.; Vanderplasschen, A.; Grooten, J.; Pre’at, V. The site of administration influences both the type and the magnitude of the immune response induced by DNA vaccine electroporation. Vaccine 2015, 33, 3179–3185. [Google Scholar] [CrossRef]
- Forjanič, T.; Miklavčič, D. Numerical study of gene electrotransfer efficiency based on electroporation volume and electrophoretic movement of plasmid DNA. BioMed. Eng. OnLine 2018, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Moller, P.H.; André, F.; Gehl, J. Electric pulse-mediated gene delivery to various animal tissues. Adv. Genet. 2005, 54, 83–114. [Google Scholar] [PubMed]
- Pankaj, K.M.; Anuj, G.; Suresh, C.G. Results of triple muscle (sartorius, tensor fascia latae and part of gluteus medius) pedicle bone grafting in neglected femoral neck fracture in physiologically active patients. Indian J. Orthopaed. 2014, 48, 470–475. [Google Scholar] [CrossRef]
- Baksi, D.D.; Pal, A.K.; Baksi, D.P. Osteosynthesis of ununited femoral neck fracture by internal fixation combined with iliac crest bone chips and muscle pedicle bone grafting. Indian J. Orthopaed. 2016, 50, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Betz, O.B.; Betz, V.M.; Abdulazim, A.; Penzkofer, R.; Schmitt, B.; Schroder, C.; Augat, P.; Jansson, V.; Muller, P.E. Healing of large segmental bone defects induced by expedited bone morphogenetic protein-2 gene activated, syngeneic muscle grafts. Hum. Gene Ther. 2009, 20, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Betz, O.B.; Betz, V.M.; Schreader, C.; Penzkofer, R.; Goettlinger, M.; Wagner, S.; Augat, P.; Jansson, V.; Mueller, P.E. Repair of large segmental bone defects: BMP-2 gene activated muscle grafts vs. autologous bone grafting. BMC Biotechnol. 2013, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Deutinger, M.; Kuzbari, R.; Patemostro, T.; Todoroff, B.; Becker, M.H. Functional and esthetic assessment of donor site defects following transfer of the gracilis muscle. Handchir. Mikrochir. Plast. Chir. 1995, 27, 90–92. [Google Scholar] [PubMed]
- Chen, H.C.; Sntamaria, E.; Chen, H.H.; Cheng, M.H.; Tang, Y.B. Microvascular vastus lateralis muscle flap for chronic empyema associated with a large cavity. Ann. Thorac. Surg. 1999, 67, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Q.; Yang, A.; Wang, J.; Cheng, W.; Deng, Y.; Zhou, A.; Lu, T.; Xiong, R.; Huang, C. Chitosan enhanced the stability and antibiofilm activity of self-propelled Prussian blue micromotor. Carbohydr. Polym. 2023, 229, 120134. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kawai, M.; Shiotsu, N.; Watanabe, M.; Yoshida, Y.; Suzuki, K.; Maruyama, H.; Miyazaki, J.; Ikegame, M.; Bessho, K.; et al. BMP-2 gene transfer under various conditions with in vivo electroporation and bone induction. J. Oral Maxillofac. Surg. Med. Pathol. 2012, 24, 49–53. [Google Scholar] [CrossRef]
- Taylar, J.; Babbs, C.F.; Alzghoul, M.B.; Olsen, A.; Latour, M.; Pond, A.L.; Hannon, K. Optimization of ectopic gene expression in skeletal muscle through DNA transfer by electroporation. BMC Biotechnol. 2004, 4, 11. [Google Scholar] [CrossRef]
- Mir, L.M. Application of electroporation gene therapy: Past, current, and future. Methods Mol. Biol. 2008, 423, 3–17. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Satterle, A.; Wu, Q.; Wang, J.; Liu, F. Gene transfer to skeletal muscle by site-specific delivery of electroporation and ultrasound. Biochem. Biophys. Res. Commun. 2012, 424, 203–207. [Google Scholar] [CrossRef]
- Kishimoto, K.N.; Watanabe, H.; Nakamura, H.; Kokubun, S. Ectopic bone formation by electroporatic transfer of bone morphogenetic protein-4 gene. Bone 2002, 31, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Benjamin, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Jt. Surg. Am. 2003, 85, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Arosarena, O.; Collins, W. Comparison of BMP-2 and -4 for rat mandibular bone regeneration at various doses. Orthod. Craniofac. Res. 2005, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Yamamura, K.; Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 1991, 108, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Sørensen, T.H.; Nielsen, K.; Raskmark, P.; Nielsen, S.L.; Skovsgaard, T.; Mir, L.M. In vivo electroporation of skeletal muscle: Threshold, efficacy and relation to electric field distribution. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1428, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, A.; Gehl, J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr. Gene Ther. 2010, 10, 287–299. [Google Scholar] [CrossRef]
- Gothelf, A.; Mahmood, F.; Dagnaes-Hansen, F.; Gehl, J. Efficacy of transgene expression in porcine skin as a function of electrode choice. Bioelectrochemistry 2011, 82, 95–102. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawai, M.Y.; Yoshida, T.; Kato, T.; Watanabe, T.; Kashiwagi, M.; Yamanaka, S.; Yamamoto, H.; Nagahiro, S.; Iwamoto, T.; Masud, K.; et al. bmp-2 Gene-Transferred Skeletal Muscles with Needle-Type Electrodes as Efficient and Reliable Biomaterials for Bone Regeneration. Materials 2024, 17, 880. https://doi.org/10.3390/ma17040880
Kawai MY, Yoshida T, Kato T, Watanabe T, Kashiwagi M, Yamanaka S, Yamamoto H, Nagahiro S, Iwamoto T, Masud K, et al. bmp-2 Gene-Transferred Skeletal Muscles with Needle-Type Electrodes as Efficient and Reliable Biomaterials for Bone Regeneration. Materials. 2024; 17(4):880. https://doi.org/10.3390/ma17040880
Chicago/Turabian StyleKawai, Mariko Yamamoto, Takeshi Yoshida, Tomoki Kato, Takuma Watanabe, Marina Kashiwagi, Shigeki Yamanaka, Hiromitsu Yamamoto, Shigeki Nagahiro, Tsutomu Iwamoto, Khan Masud, and et al. 2024. "bmp-2 Gene-Transferred Skeletal Muscles with Needle-Type Electrodes as Efficient and Reliable Biomaterials for Bone Regeneration" Materials 17, no. 4: 880. https://doi.org/10.3390/ma17040880
APA StyleKawai, M. Y., Yoshida, T., Kato, T., Watanabe, T., Kashiwagi, M., Yamanaka, S., Yamamoto, H., Nagahiro, S., Iwamoto, T., Masud, K., Aoki, K., Ohura, K., & Nakao, K. (2024). bmp-2 Gene-Transferred Skeletal Muscles with Needle-Type Electrodes as Efficient and Reliable Biomaterials for Bone Regeneration. Materials, 17(4), 880. https://doi.org/10.3390/ma17040880





