Bubble-Mediated Production of Few-Layer Graphene via Vapor–Liquid Reaction between Carbon Dioxide and Magnesium Melt
Abstract
:1. Introduction
2. Experimental Details
2.1. Methods
2.2. Materials Characterizations
3. Results and Discuss
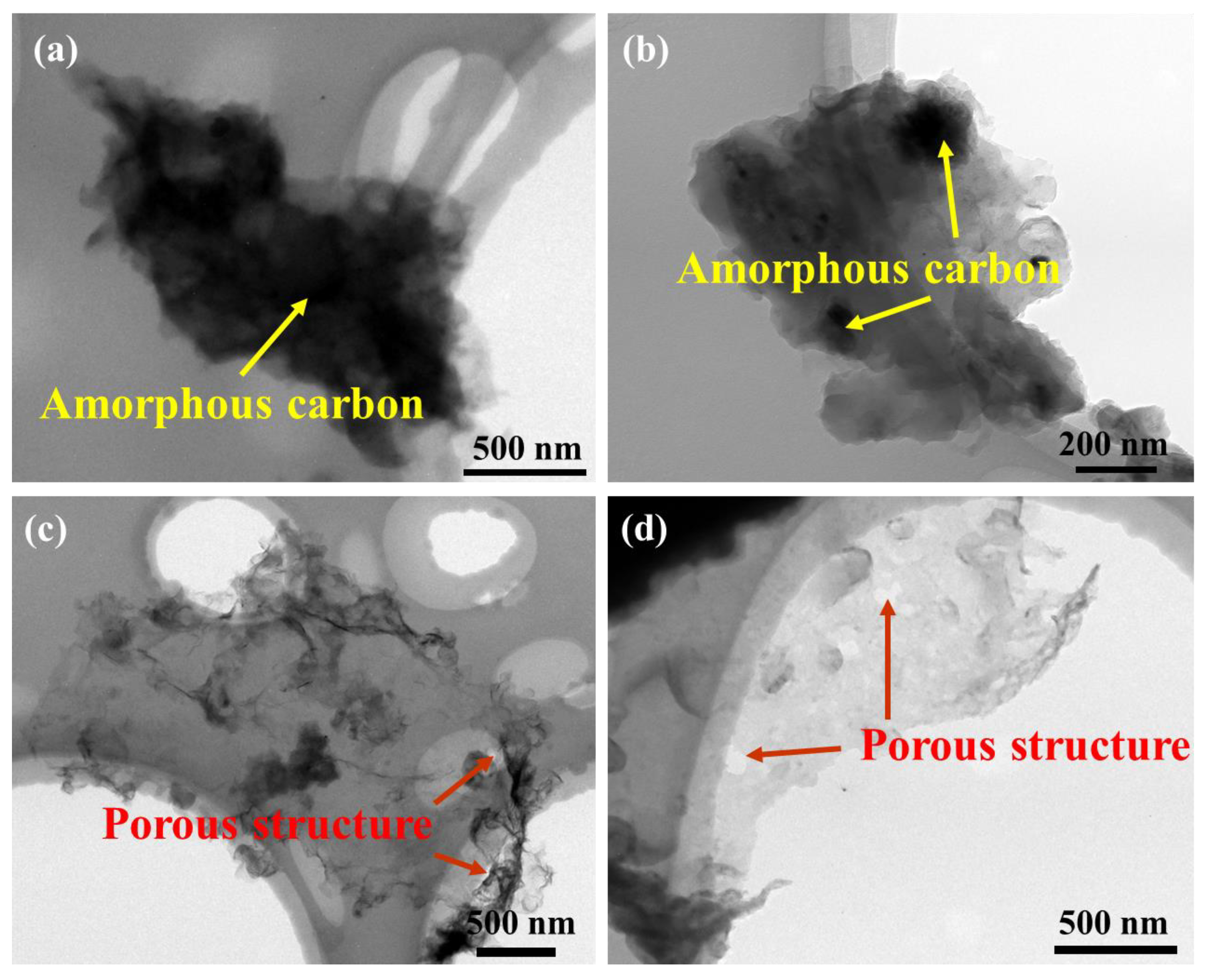
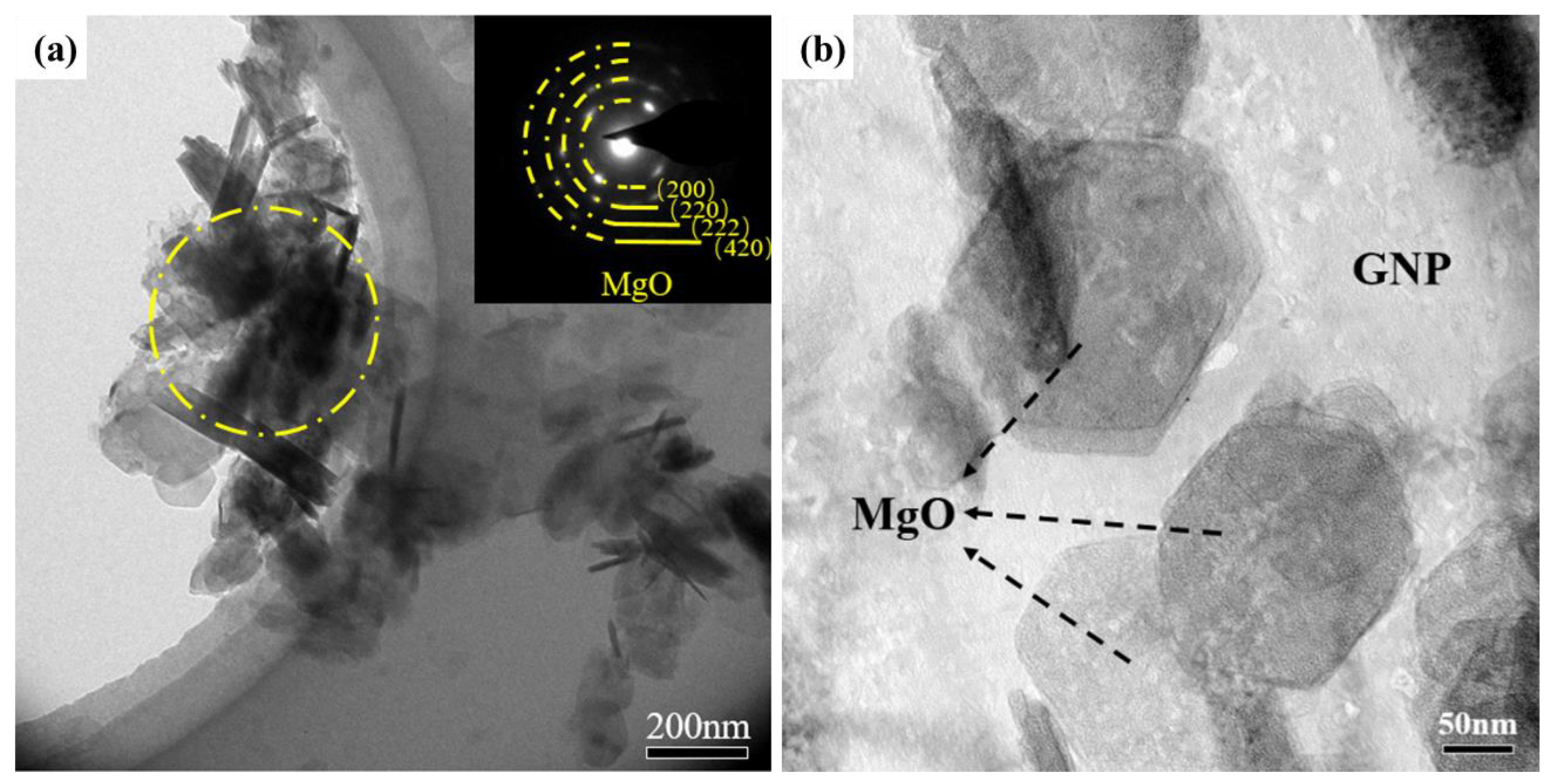
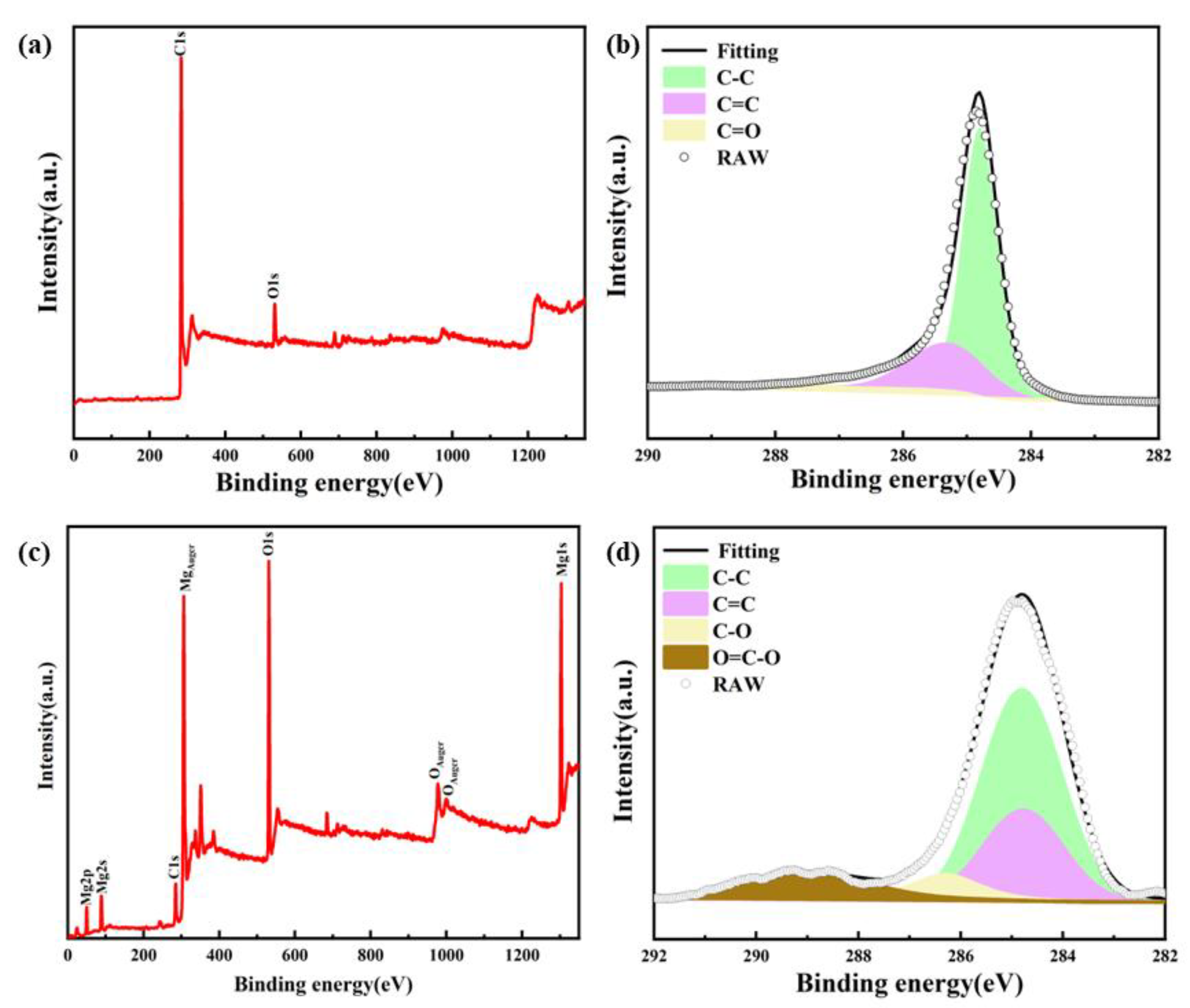
3.1. Characterizations of As-Fabricated Carbon Product
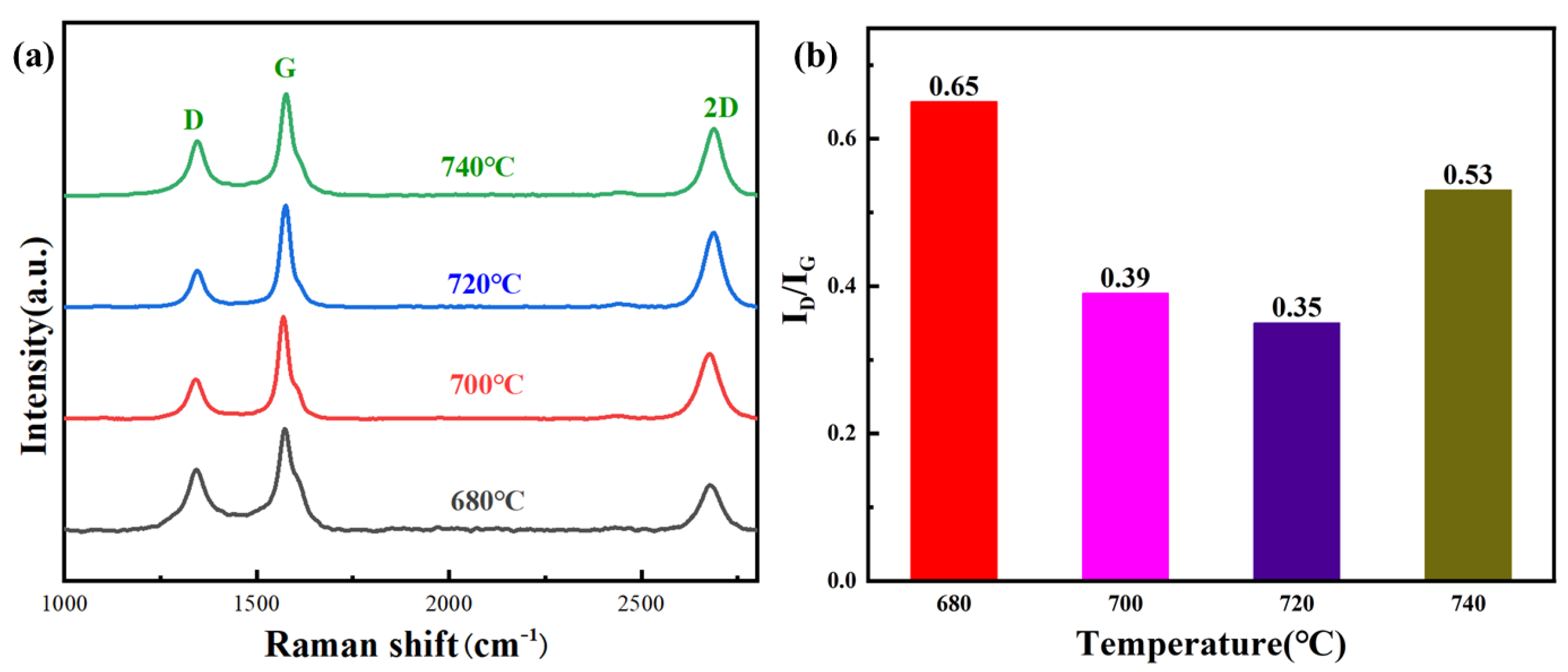
3.2. Defect Density of As-Fabricated Few-Layer Graphene
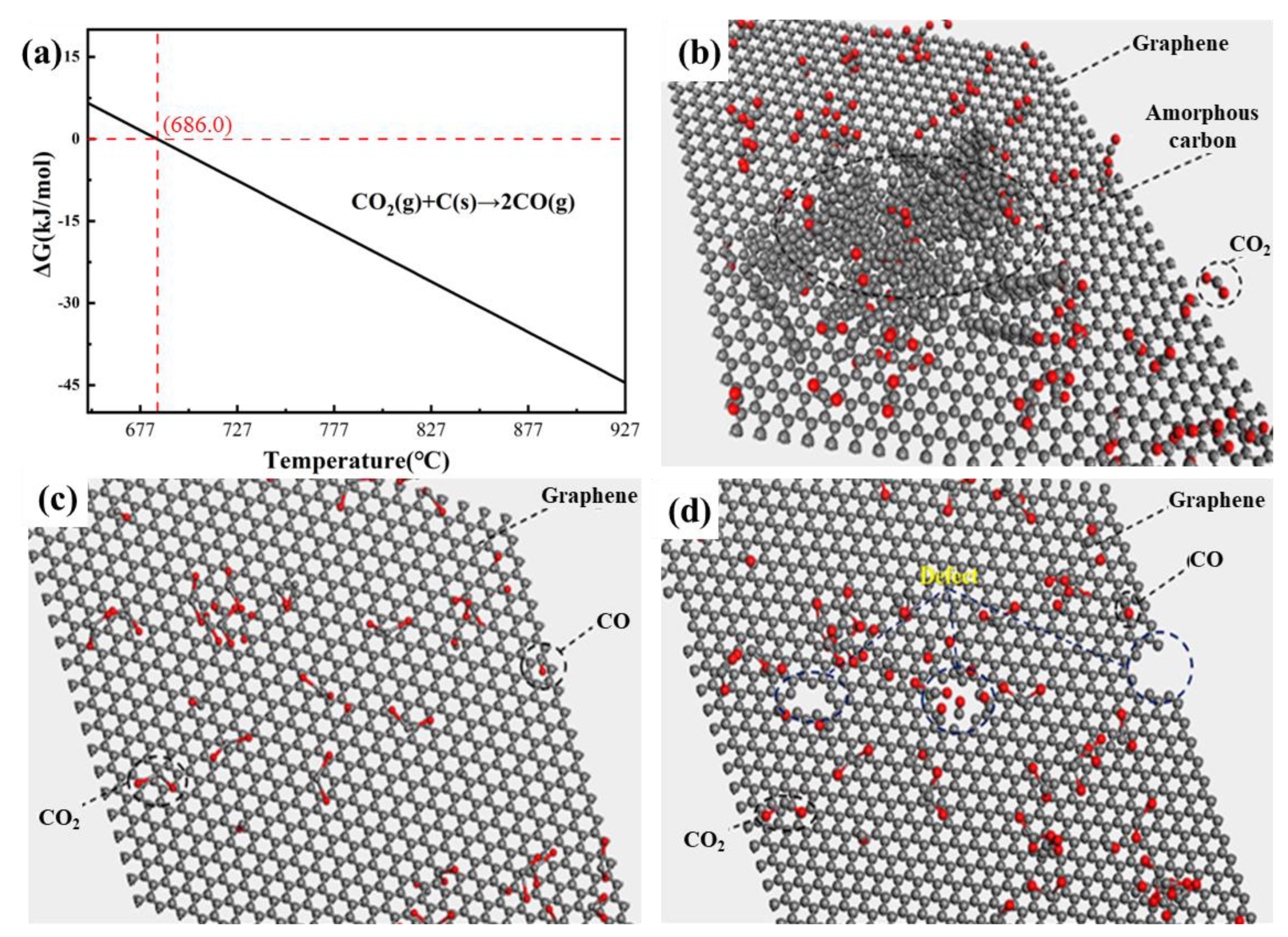
3.3. Analysis of the Growth Mechanism of Few-Layer Graphene
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonchio, M.; Bonin, J.; Ishitani, O.; Lu, T.B.; Morikawa, T.; Morris, A.J.; Robert, M. Best practices for experiments and reporting in photocatalytic CO2 reduction. Nat. Catal. 2023, 6, 657–665. [Google Scholar] [CrossRef]
- Albitar, K.; Borgi, H.; Khan, M.; Zahra, A. Business environmental innovation and CO2 emissions: The moderating role of environmental governance. Bus. Strategy Environ. 2023, 32, 1996–2007. [Google Scholar] [CrossRef]
- Karakurt, I.; Aydin, G. Development of regression models to forecast the CO2 emissions from fossil fuels in the BRICS and MINT countries. Energy 2023, 263, 125650. [Google Scholar] [CrossRef]
- He, R.; Wang, Z.; Jin, X. Preparation of graphitic carbon nanosheets by reaction between CO2 and CaC2 for lithium-ion batteries. Carbon 2017, 116, 246–254. [Google Scholar] [CrossRef]
- Moreno, V.; Murtada, K.; Zougagh, M.; Rios, A. Analytical control of Rhodamine B by SERS using reduced graphene decorated with copper selenide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117302. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Chen, Y.; Wu, M.; Wang, K.; Zhang, W.; Gan, Y.; Huang, H.; Chen, J.; Yang, Y.; Zhang, J.; et al. Green synthesis of graphite from CO2 without graphitization process of amorphous carbon. Nat. Commun. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Wang, K.; Su, F.; Chen, C.M.; Liu, F.; Wu, Z.S.; Ma, Y.W. Recent advances in carbon nanostructures prepared from carbon dioxide for high-performance supercapacitors. J. Energy Chem. 2021, 54, 352–367. [Google Scholar] [CrossRef]
- Chang, L.; Wei, W.; Sun, K.; Hu, Y.H. 3D flower-structured graphene from CO2 for supercapacitors with ultrahigh areal capacitance at high current density. J. Mater. Chem. A 2015, 3, 10183–10187. [Google Scholar] [CrossRef]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.S.; Zhang, N.; Chong, S.K.; Li, D.; Chen, Y.; Sun, C.G. Three-dimensional porous graphene-like sheets synthesized from biocarbon via low-temperature graphitization for a supercapacitor. Green Chem. 2018, 20, 694–700. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Su, X.Z.; Ma, Y.W. Magnesiothermic sequestration of CO2 into carbon nanomaterials for electrochemical energy storage: A mini review. Electrochem. Commun. 2021, 130, 107109. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Lu, J.; Skrabutenas, J.C.; Hosmane, N. Conversion of carbon dioxide to few-layer graphene. J. Mater. Chem. 2011, 21, 9491–9493. [Google Scholar] [CrossRef]
- Zhang, H.T.; Zang, X.; Suun, X.Z.; Ma, Y.W. Shape-controlled synthesis of nanocarbons through direct conversion of carbon dioxide. Sci. Rep. 2013, 3, 3534. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wang, B.; Gao, W.; Pan, C.; Halsted, J.K.; Chong, E.S.; Lu, J.; Wang, X.F.; Luo, W.; Chang, C.H.; et al. Reducing CO2 to dense nanoporous graphene by Mg/Zn for high power electrochemical capacitors. Nano Energy 2015, 11, 600–610. [Google Scholar] [CrossRef]
- Bahri, M.; Gebre, S.H.; Elaguech, M.A.; Dajan, F.T.; Sendeku, M.G.; Tlili, C.; Wang, D. Recent advances in chemical vapour deposition techniques for graphene-based nanoarchitectures: From synthesis to contemporary applications. Coord. Chem. Rev. 2023, 475, 214910. [Google Scholar] [CrossRef]
- Arya, A.K.; Raman, R.S.; Parmar, R.; Amati, M.; Gregoratti, L.; Saxena, S. Spectroscopic investigation of improved corrosion resistance of nickel due to multilayer graphene coating developed with suitably tilted substrate during CVD. Carbon 2022, 200, 215–226. [Google Scholar] [CrossRef]
- Shi, Z.; He, P.; Wang, N.; Liu, Y.; Chen, X.; Li, Y.; Ding, G.Q.; Yu, Q.K.; Xie, X.M. Bubble-Mediated Mass Production of Graphene: A Review. Adv. Funct. Mater. 2022, 32, 2203124. [Google Scholar] [CrossRef]
- Di Bari, S.; Robinson, A.J. Experimental study of gas injected bubble growth from submerged orifices. Exp. Therm. Fluid Sci. 2013, 44, 124–137. [Google Scholar] [CrossRef]
- Ghazivini, M.; Hafez, M.; Ratanpara, A.; Kim, M. A review on correlations of bubble growth mechanisms and bubble dynamics parameters in nucleate boiling. J. Therm. Anal. Calorim. 2022, 147, 1–37. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, P.; Wang, S.; Liu, Z.; Yu, Q.K. Continuous Production of Graphite Nanosheets by Bubbling Chemical Vapor Deposition Using Molten Copper. Chem. Mater. 2017, 29, 8404–8411. [Google Scholar] [CrossRef]
- Li, X.J.; Shi, H.L.; Wang, X.J.; Hu, X.S.; Xu, C.; Shao, W.Z. Direct synthesis and modification of graphene in Mg melt by converting CO2: A novel route to achieve high strength and stiffness in graphene/Mg composites. Carbon 2022, 186, 632–643. [Google Scholar] [CrossRef]
- Li, X.J.; Shi, H.L.; Wang, X.J.; Hu, X.S.; Xu, C.; Shao, W.Z. Achieving high strength and ductility in GNSs/Mg nanocomposites fabricated by in-situ liquid metallurgy combined with hot extrusion. Compos. Part A Appl. Sci. Manuf. 2022, 161, 107079. [Google Scholar] [CrossRef]
- Wei, S.H.; Shi, H.L.; Li, X.J.; Hu, X.S.; Xu, C.; Wang, X.J. A green and efficient method for preparing graphene using CO2@Mg in-situ reaction and its application in high-performance lithium-ion batteries. J. Alloys Compd. 2022, 902, 163700. [Google Scholar] [CrossRef]
- Solís-Fernández, P.; Ago, H. Machine learning determination of the twist angle of bilayer graphene by Raman spectroscopy: Implications for van der Waals heterostructures. ACS Appl. Nano Mater. 2022, 5, 1356–1366. [Google Scholar] [CrossRef]
- Won, K.; Lee, C.; Jung, J.; Kwon, S.; Gebredingle, Y.; Lim, J.G.; Lee, C. Raman scattering measurement of suspended graphene under extreme strain induced by nanoindentation. Adv. Mater. 2022, 34, 2200946. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, D.; Jiang, K.S.; Novoselov, S.R.; Geim, A.K. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1416. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, K.; Lin, L.; Zhao, W.; Quang, H.; Sun, L.Z.; Li, T.R.; Li, Z.Z.; Liu, X.T.; Zheng, L.M.; et al. Large-area synthesis of superclean graphene via selective etching of amorphous carbon with carbon dioxide. Angew. Chem. Int. Ed. 2019, 58, 14446–14451. [Google Scholar] [CrossRef]
- Zhu, X.; Ning, G.; Fan, Z.; Gao, J.; Xu, C.; Qian, W.; Fei, W. One-step synthesis of a graphene-carbon nanotube hybrid decorated by magnetic nanoparticles. Carbon 2012, 50, 2764–2771. [Google Scholar] [CrossRef]
- Miao, Q.; Wang, L.; Liu, Z.; Wei, B.; Xu, F.; Fei, W. Magnetic properties of N-doped graphene with high Curie temperature. Sci. Rep. 2016, 6, 21832. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.Z.; Xu, Y.; Su, F.Y.; Chen, C.M.; Liu, F.Y.; Wu, Z.S.; Ma, Y.W. Nitrogen-enriched graphene framework from a large-scale magnesiothermic conversion of CO2 with synergistic kinetics for high-power lithium-ion capacitors. NPG Asia Mater. 2021, 13, 59. [Google Scholar] [CrossRef]
- Karaman, C.; Karaman, O.; Show, P.L.; Orooji, Y.; Karimi-Maleh, H. Utilization of a double-cross-linked amino-functionalized three-dimensional graphene networks as a monolithic adsorbent for methyl orange removal: Equilibrium, kinetics, thermodynamics and artificial neural network modeling. Environ. Res. 2022, 207, 112156. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, X.; Shi, H.; Jin, Y.; Hu, X.; Xu, C.; Tang, L.; Ma, M.; Lu, L. Bubble-Mediated Production of Few-Layer Graphene via Vapor–Liquid Reaction between Carbon Dioxide and Magnesium Melt. Materials 2024, 17, 897. https://doi.org/10.3390/ma17040897
Li X, Wang X, Shi H, Jin Y, Hu X, Xu C, Tang L, Ma M, Lu L. Bubble-Mediated Production of Few-Layer Graphene via Vapor–Liquid Reaction between Carbon Dioxide and Magnesium Melt. Materials. 2024; 17(4):897. https://doi.org/10.3390/ma17040897
Chicago/Turabian StyleLi, Xuejian, Xiaojun Wang, Hailong Shi, Yuchao Jin, Xiaoshi Hu, Chao Xu, Lunyuan Tang, Min Ma, and Liwei Lu. 2024. "Bubble-Mediated Production of Few-Layer Graphene via Vapor–Liquid Reaction between Carbon Dioxide and Magnesium Melt" Materials 17, no. 4: 897. https://doi.org/10.3390/ma17040897
APA StyleLi, X., Wang, X., Shi, H., Jin, Y., Hu, X., Xu, C., Tang, L., Ma, M., & Lu, L. (2024). Bubble-Mediated Production of Few-Layer Graphene via Vapor–Liquid Reaction between Carbon Dioxide and Magnesium Melt. Materials, 17(4), 897. https://doi.org/10.3390/ma17040897








