Research on the Formation Conditions and Preventive Measures of Uranium Precipitates during the Service Process of Medical Isotope Production Reactors
Abstract
1. Introduction
2. Research Methods
3. Model Establishment and Experimental Process
3.1. Modeling of Precipitation Formation Conditions
- Preliminary Analysis: This stage involves a detailed examination of the target species and the equilibrium constants of the fundamental reactions for the ancillary species utilized in formulating these reactions. Subsequently, the researched equilibrium constant K, as delineated in Table 1, is integrated into the thermodynamic database.
- Configuration in Act2 Interface: At this stage, it is necessary to set the main species for computation within the Act2 interface (in this instance, UO22+). Additionally, this stage involves the adjustment of two principal variables that influence the morphology of the key species—specifically, the pH value and hydrogen peroxide activity for this process. This step culminates with the definition of the overall solution system, including establishing the initial concentration of other species within the system.
- Computational Process in Act2: At this stage, Act2 computes the initial equilibrium state of the reaction. Subsequently, it employs the Gibbs free energy minimization method to deduce the unique convergent equilibrium state of the system for each specified pH value and hydrogen peroxide activity. The outcome is the simulation of a phase diagram for the key species, which vividly illustrates the diverse forms of the key species (ions, complexes, precipitates, gases, etc.) under the collective influence of multiple variables.
3.2. Experimental Process
3.2.1. Preparation of Reaction Mixtures
3.2.2. Isolation of Precipitates
3.3. Characterization of Precipitates
3.3.1. Field Emission Scanning Electron Microscope
3.3.2. Powder X-ray Diffraction (PXRD)
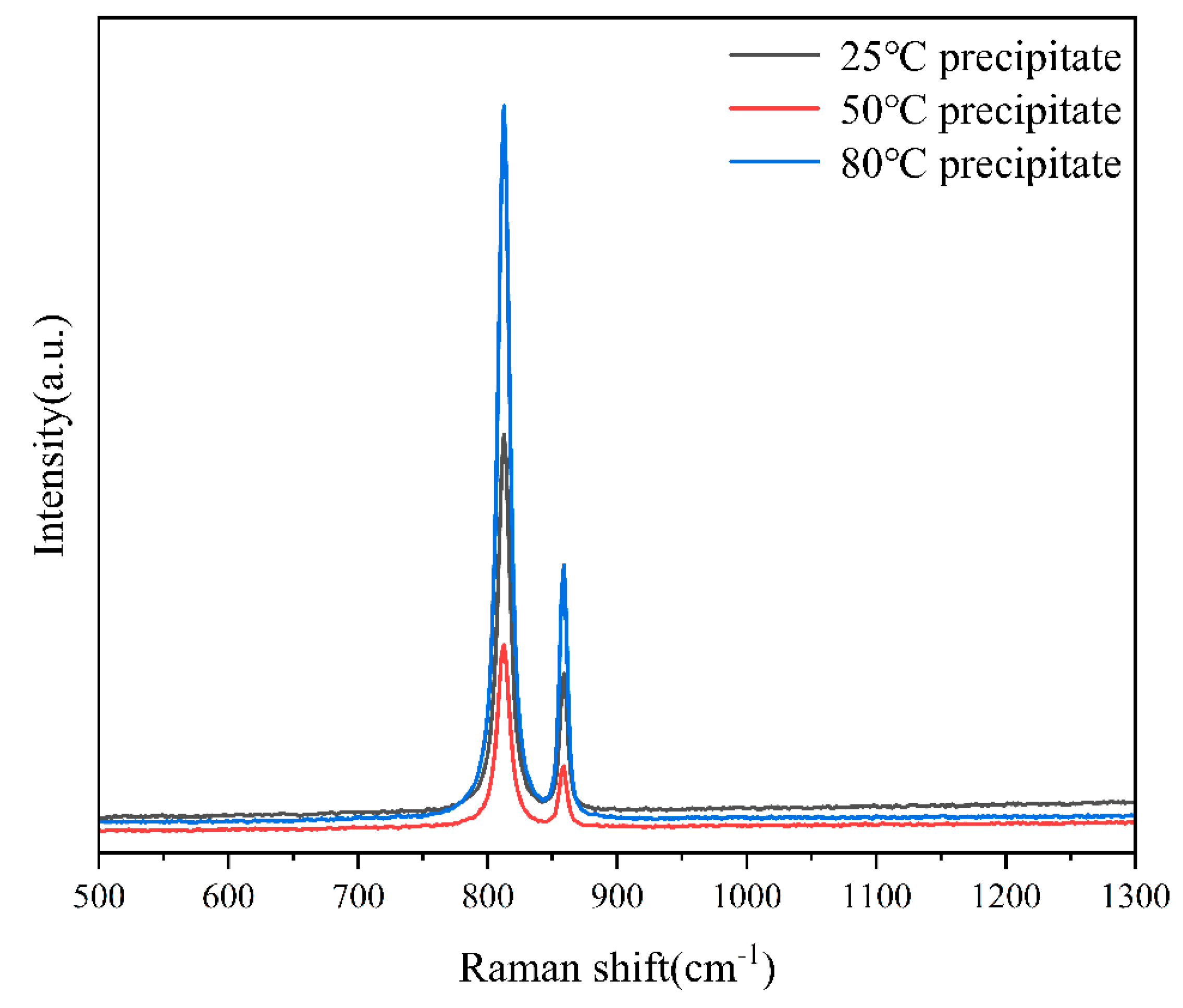
3.3.3. Raman Spectroscopy
4. Results and Discussion
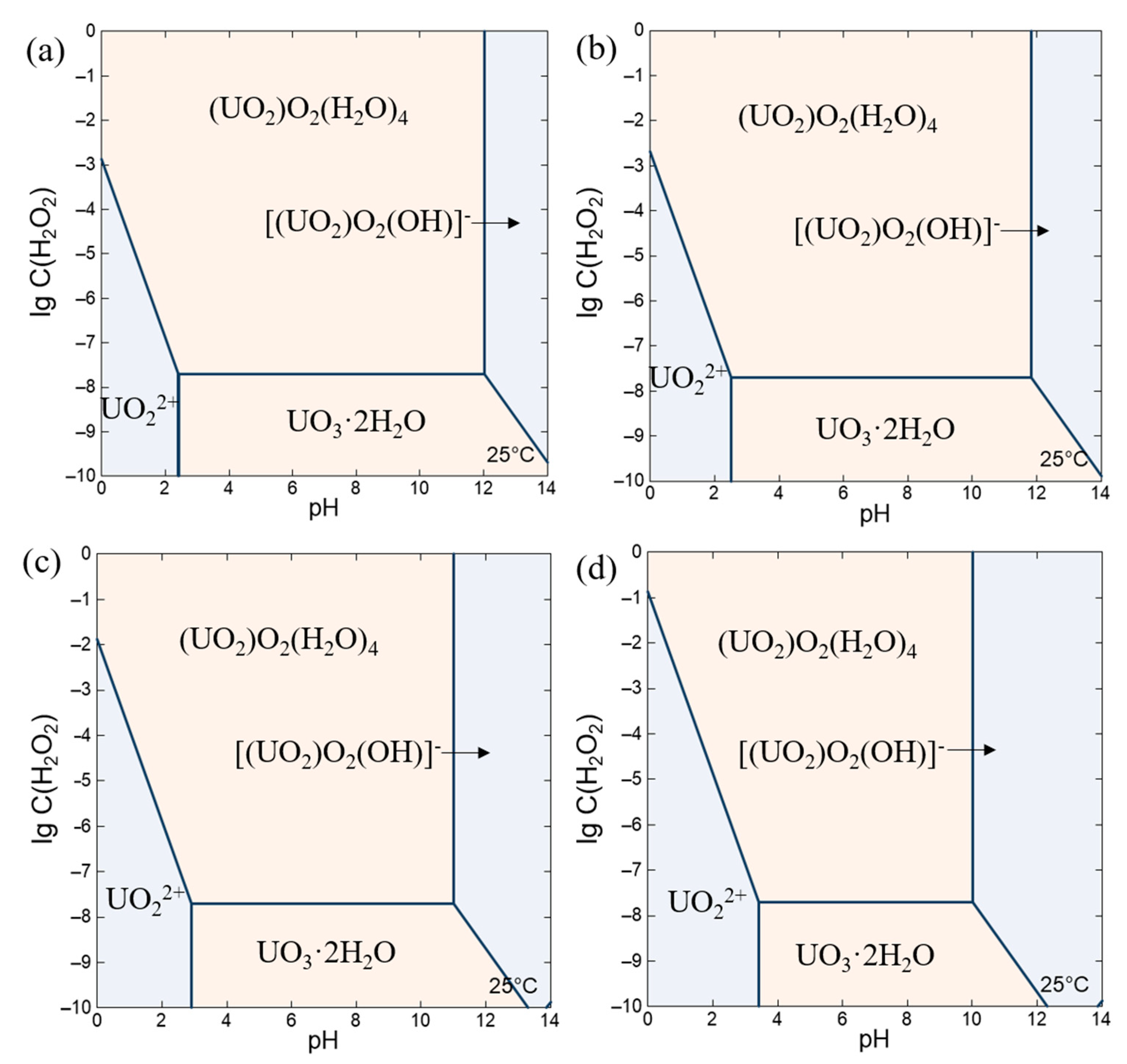
4.1. Simulation Calculation of Sedimentation Boundary Conditions
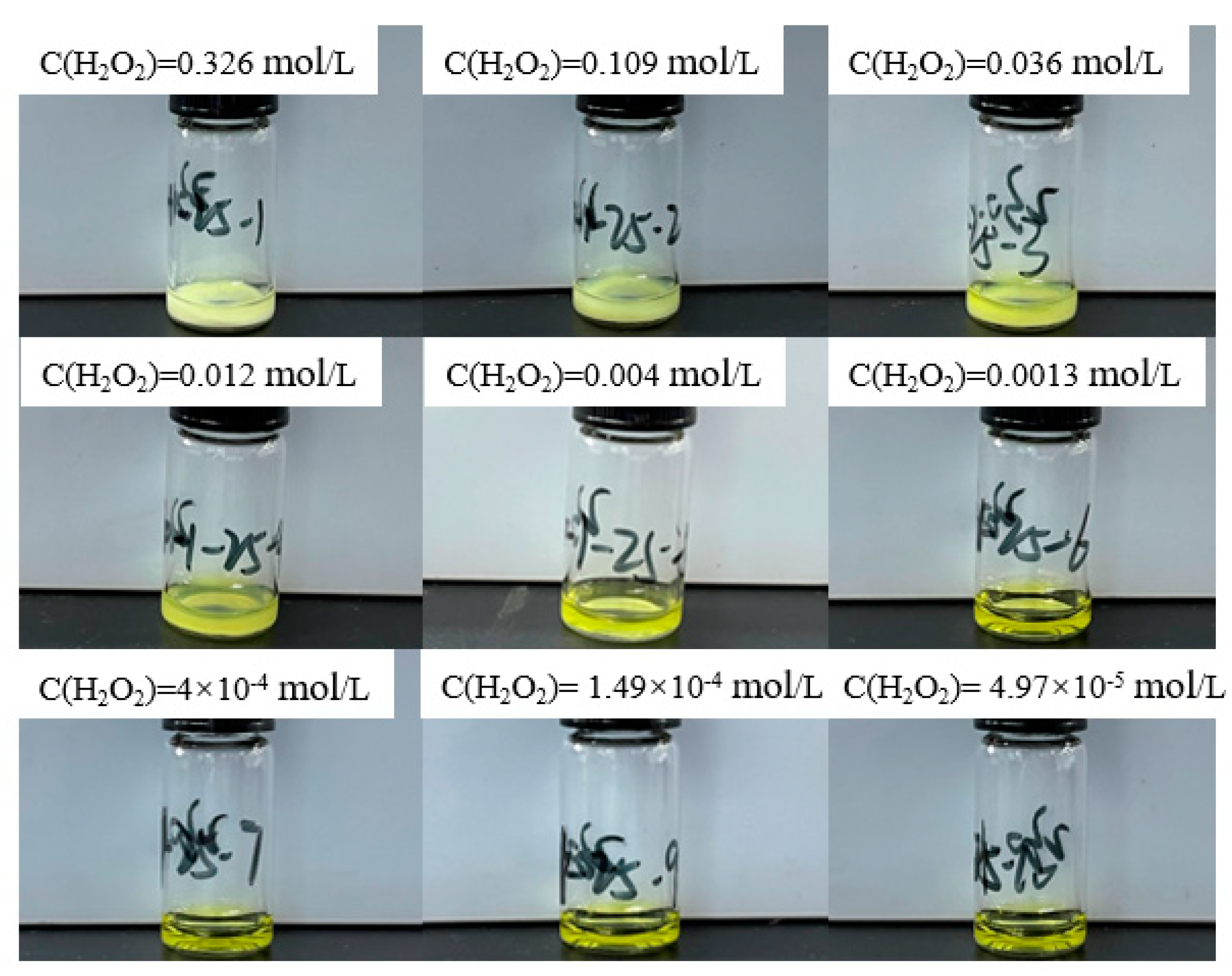
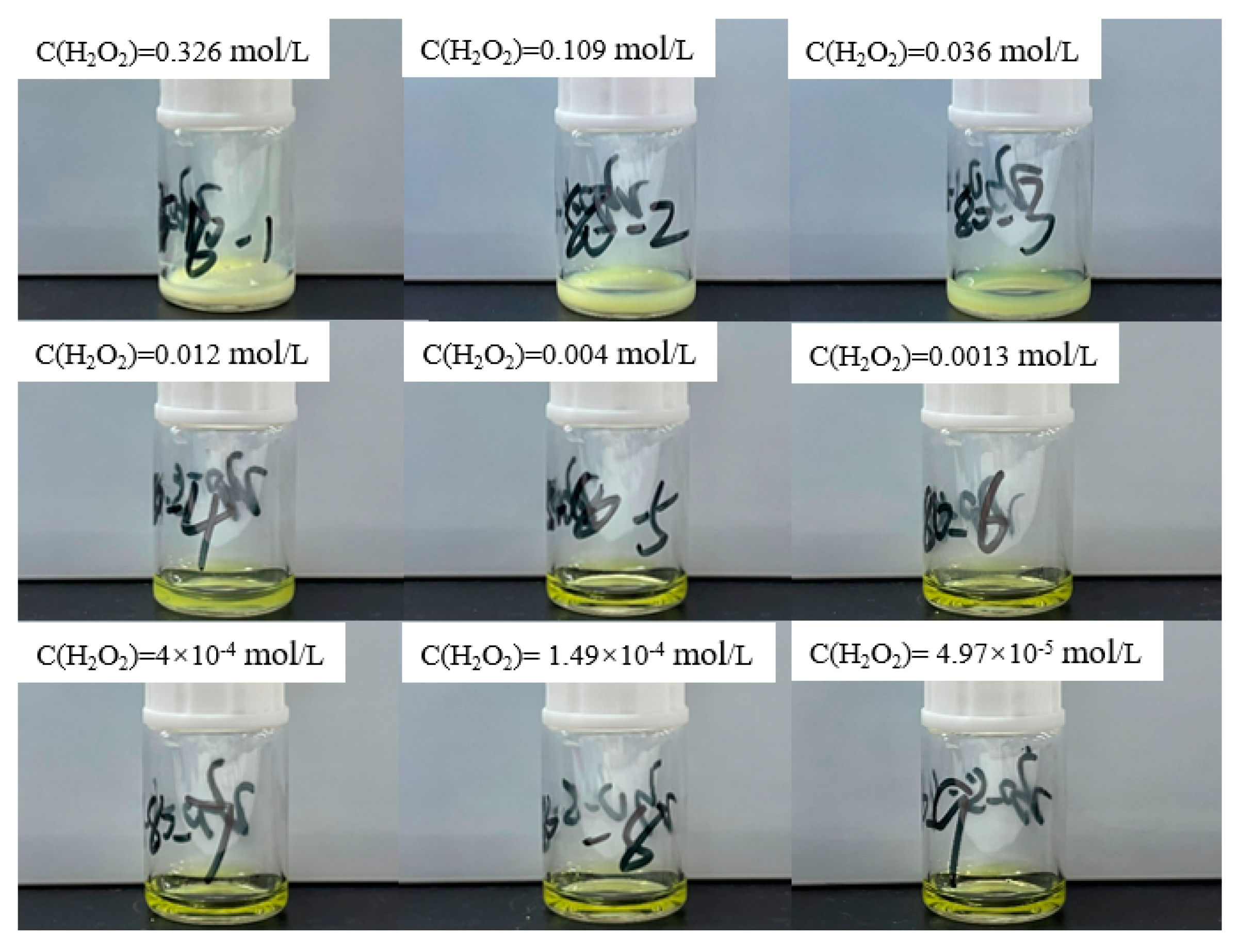
4.2. Verification Experiment of Precipitationlimit Conditions
4.3. Experimental Study on the Influence of Temperature on Precipitation Formation Conditions
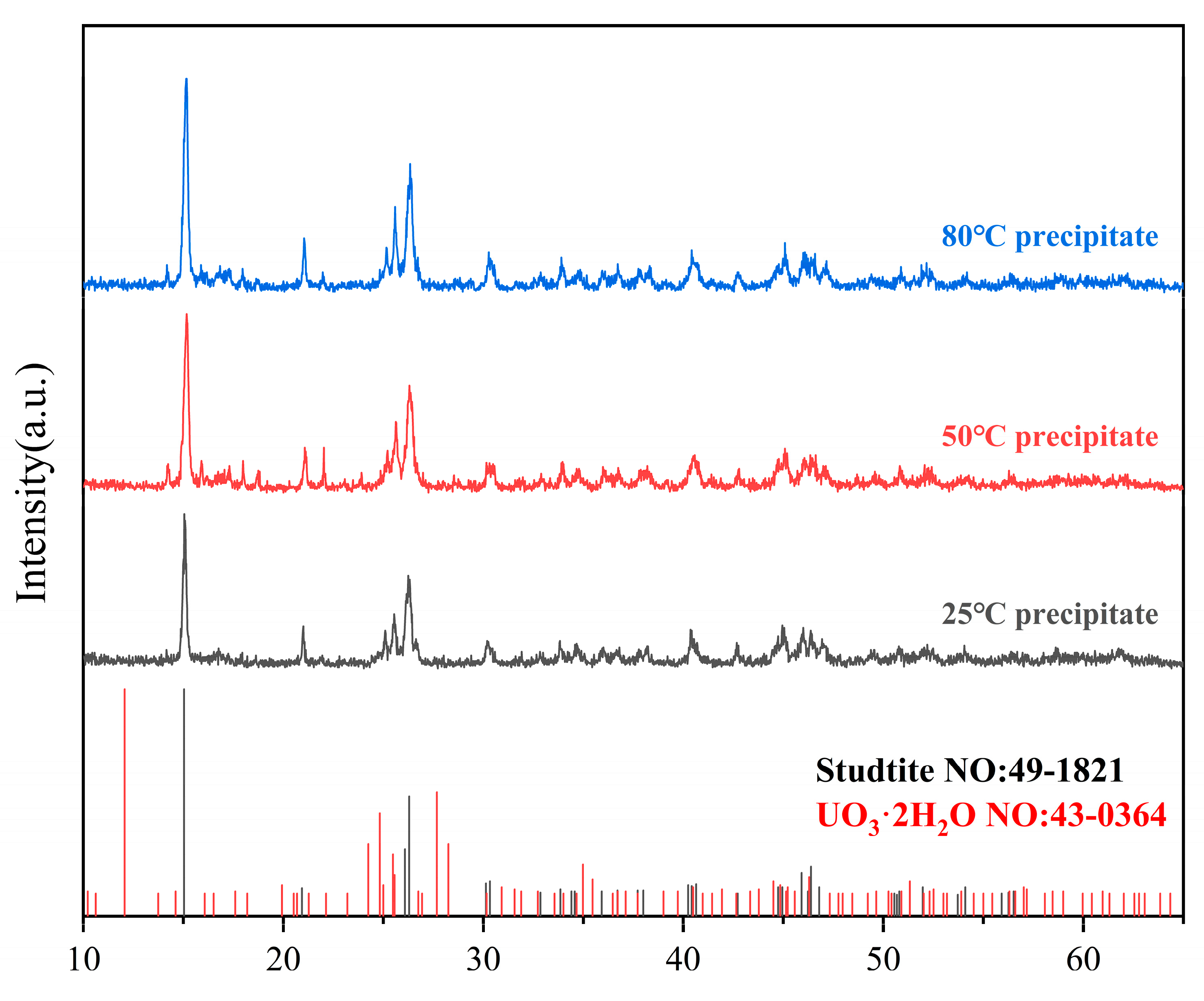
4.4. Characterization of Precipitated Species
4.5. Precipitation Prevention Mechanism
5. Conclusions
- (1)
- Simulation Analysis with GWB**: Employing GWB software, the study simulates the evolution of species in a uranyl nitrate solution at 25 °C, with a uranyl ion concentration of 1 mol/L, 0.64 mol/L, 0.1 mol/L and 0.01 mol/L across different pH values and hydrogen peroxide concentrations. This model meticulously considers the fundamental reactions of the species and their equilibrium constants. The simulation results are corroborated by similar findings reported in the scientific literature.
- (2)
- Model Insights and pH Management**: According to the model outcomes, for a uranyl ion concentration of 0.64 mol/L, it is feasible to prevent the formation of hydrolysis-related precipitates by maintaining the pH of the solution below 2.514. Additionally, when the pH is below 2.514, to avert the precipitation of uranium peroxide in the solution, the concentration of hydrogen peroxide must be controlled to remain below the critical concentration limit corresponding to the specific pH value.
- (3)
- Experimental Verification and Precipitate Characterization**: Through experimental approaches involving the addition of varied concentrations of hydrogen peroxide to a uranyl nitrate solution, the boundary conditions for the precipitation of uranyl ions as studtite were validated. Advanced analytical techniques, including Raman spectroscopy, SEM-EDS, and PXRD, were utilized to confirm that the precipitates are indeed composed of pure phase studtite.
- (4)
- Proposed Measures to Prevent Precipitation**: Building on the understanding of the type of precipitation and its formation conditions, the study proposes specific measures to inhibit the formation of uranium precipitates. To suppress the formation and accumulation of UO3·2H2O, it is necessary to maintain high solution acidity, specifically controlling the pH to be less than 2.514 for a 0.64 mol/L uranyl nitrate solution. To prevent the formation and accumulation of studtite, controlling the pH and increasing the operating temperature are recommended.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Guo, Z.; Wang, L.; Li, J.; Jiao, L.; Gao, X.; Weng, H.; Lin, M. Development Status and Prospects of Electron Accelerator Production of Medical Isotope Molybdenum-99. Isotopes 2022, 35, 114–127. [Google Scholar]
- Knapp, F.F.; Dash, A. Introduction: Radiopharmaceuticals Play an Important Role in Both Diagnostic and Therapeutic Nuclear Medicine; Springer: New Delhi, India, 2016. [Google Scholar]
- Li, Z.; Han, Y.; Wang, X.; Zhang, J.; Wang, Y.; Huang, Q. Production status and prospects of medical radioactive isotopes 99Mo/99mTc. Rev. Nucl. Phys. 2019, 36, 170–183. [Google Scholar]
- Ball, R.M. Characteristic of Nuclear Reactors Used for the Production 99Mo; Ball Systems, Inc.: Westfield, IN, USA, 1997. [Google Scholar]
- Lyra, M.; Charalambatou, P.; Roussou, E.; Fytros, S.; Baka, J. Material Alternative production methods to face global molybdenum-99 supply short term. Hell. J. Nucl. Med. 2011, 14, 49–55. [Google Scholar] [PubMed]
- Gao, F.; Lin, L.; Liu, Y.; Ma, X. The current status and technological prospects of medical isotope production. Isotopes 2016, 29, 116–120. [Google Scholar]
- Wu, H.; Zhao, H. Medical isotope supply faces transportation and distribution challenges due to the epidemic. Foreign Nuclear News, 21 April 2020; 12. [Google Scholar]
- International Atomic Energy Agency. Homogeneous Aqueous Solution Nuclear Reactors for the Production of Mo-99 and other Short Live Radiologists; IAEA-TECDOC-1601; IAEA: Vienna, Austria, 2008. [Google Scholar]
- Peng, H.L.; Tran, H.H.; Sembiring, T.M.; Arbie, B. Conceptual design of a new homologous reactor for medical radiomotope Mo-99/Tc-99m production. AIP Conf. Proc. 2014, 2014, 37–39. [Google Scholar]
- BALL R M. Medical Isotope Production Reactor. U.S. Patent 5596611 [P/OL], 11 October 1997. [Google Scholar]
- Luo, Q.; Liu, S. A water solution reactor for producing 99Mo, 131I, and 89Sr medical isotopes. Guangdong Trace Elements Sci. 2006, 13, 7. [Google Scholar]
- Lane, J.A.; Macpherson, H.O.; Maslan, F. Fluid Fuel Reactors; Addison Wesley Publishing Co. Inc.: Boston, MA, USA, 1958. [Google Scholar]
- Silverman, L.; Sallach, R.; Seitz, R.; Bradshaw, W. Catalyst Decomposition of Hydrogen Peroxide and Uranyl Peroxide. Ind. Eng. Chem. 1958, 50, 1785–1786. [Google Scholar] [CrossRef]
- Knope, E.K.; Soderholm, L. Solution and Solid State Structural Chemistry of Actinide Hydrates and Their Hydrolysis and Condensation Products. Chem. Rev. 2013, 113, 944–994. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.C.; Hughes, K.A. Studtite, [(UO2)O2(H2O)2](H2O)2: The first structure of a peroxide mineral. Am. Mineral 2003, 88, 1165–1168. [Google Scholar] [CrossRef]
- Forbes, T.Z.; Horan, P.; Devine, T.; Mcinnis, D.; Burns, P.C. Alteration of dehydrated Schoepite and sludge to study, [(UO2)O2(H2O)2](H2O)2. Am. Mineral 2011, 96, 202–206. [Google Scholar] [CrossRef]
- Kubatko, K.A.H.; Helean, K.B.; Navrotsky, A.; Burns, P.C. Stability of peroxide containing uranyl minerals. Science 2003, 302, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Jerden, J.; Kropf, J.; Bakel, A.; Vandergrift, G. Specification and Concentration of Metals in a Homogeneous Reactor Fuel Solution; Argonne National Laboratory: Lemont, IL, USA, 2009. [Google Scholar]
- Zanonato, P.L.; Di Bernardo, P.; Grenthe, I. Chemical equilibria in the binary and ternary uranyl(VI)-hydroxide- peroxide systems. Dalton Trans. 2012, 41, 380–3386. [Google Scholar] [CrossRef] [PubMed]
- Zanonato, P.L.; Di Bernardo, P.; Grenthe, I. A calorimetric study of the hydrolysis and peroxide complex formation of the uranyl(VI ) ion. Dalton Trans. 2014, 43, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Torrents, A.; Meca, S.; Baumann, N.; Martí, V.; Giménez, J.; de Pablo, J.; Casas, I. Uranium speciation studies at alkaline pH and in the presence of hydrogen peroxide using time-resolved laser-induced fluorescence spectroscopy. Polyhedron 2013, 55, 92–101. [Google Scholar] [CrossRef]
- Meca, S.; Martínez-Torrents, A.; Martí, V.; Giménez, J.; Casas, I.; de Pablo, J. Determination of the equilibrium formation constants of two U(VI)-peroxide complexes at alkaline pH. Dalton Trans. 2011, 40, 7976–7982. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, F.; Bonales, L.J.; Cobos, J.; Timón, V. Study of the thermal stability of studtite by in situ Raman spectroscopy and DFT calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 174, 245–253. [Google Scholar] [CrossRef] [PubMed]


| Reaction Equation | |
|---|---|
| −(2.340 ± 0.070) | |
| 7.990 ± 0.160 | |
| 28.7 ± 0.4 | |
| 36.8 ± 0.2 | |
| −2.87 |
| Elements | Weight % | Atomic % | |
|---|---|---|---|
| 25 °C precipitate | O | 18.75 | 77.45 |
| U | 81.25 | 22.55 | |
| 50 °C precipitate | O | 13.93 | 70.66 |
| U | 86.07 | 29.34 | |
| 80 °C precipitate | O | 23.47 | 82.03 |
| U | 76.53 | 17.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Gao, Y.; Li, X.; Le, Y.; Zhang, Y.; Qiu, J.; Xin, Y. Research on the Formation Conditions and Preventive Measures of Uranium Precipitates during the Service Process of Medical Isotope Production Reactors. Materials 2024, 17, 945. https://doi.org/10.3390/ma17040945
Zhao Y, Gao Y, Li X, Le Y, Zhang Y, Qiu J, Xin Y. Research on the Formation Conditions and Preventive Measures of Uranium Precipitates during the Service Process of Medical Isotope Production Reactors. Materials. 2024; 17(4):945. https://doi.org/10.3390/ma17040945
Chicago/Turabian StyleZhao, Yanli, Yuan Gao, Xinyue Li, Yi Le, Yang Zhang, Jie Qiu, and Yong Xin. 2024. "Research on the Formation Conditions and Preventive Measures of Uranium Precipitates during the Service Process of Medical Isotope Production Reactors" Materials 17, no. 4: 945. https://doi.org/10.3390/ma17040945
APA StyleZhao, Y., Gao, Y., Li, X., Le, Y., Zhang, Y., Qiu, J., & Xin, Y. (2024). Research on the Formation Conditions and Preventive Measures of Uranium Precipitates during the Service Process of Medical Isotope Production Reactors. Materials, 17(4), 945. https://doi.org/10.3390/ma17040945





