Ni/WS2/WC Composite Nanosheets as an Efficient Catalyst for Photoelectrochemical Hydrogen Peroxide Sensing and Hydrogen Evolution
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Preparation of Ni/WS2/WC Composite Nanosheets
2.3. Material Characterizations and PEC Measurement
3. Results and Discussion
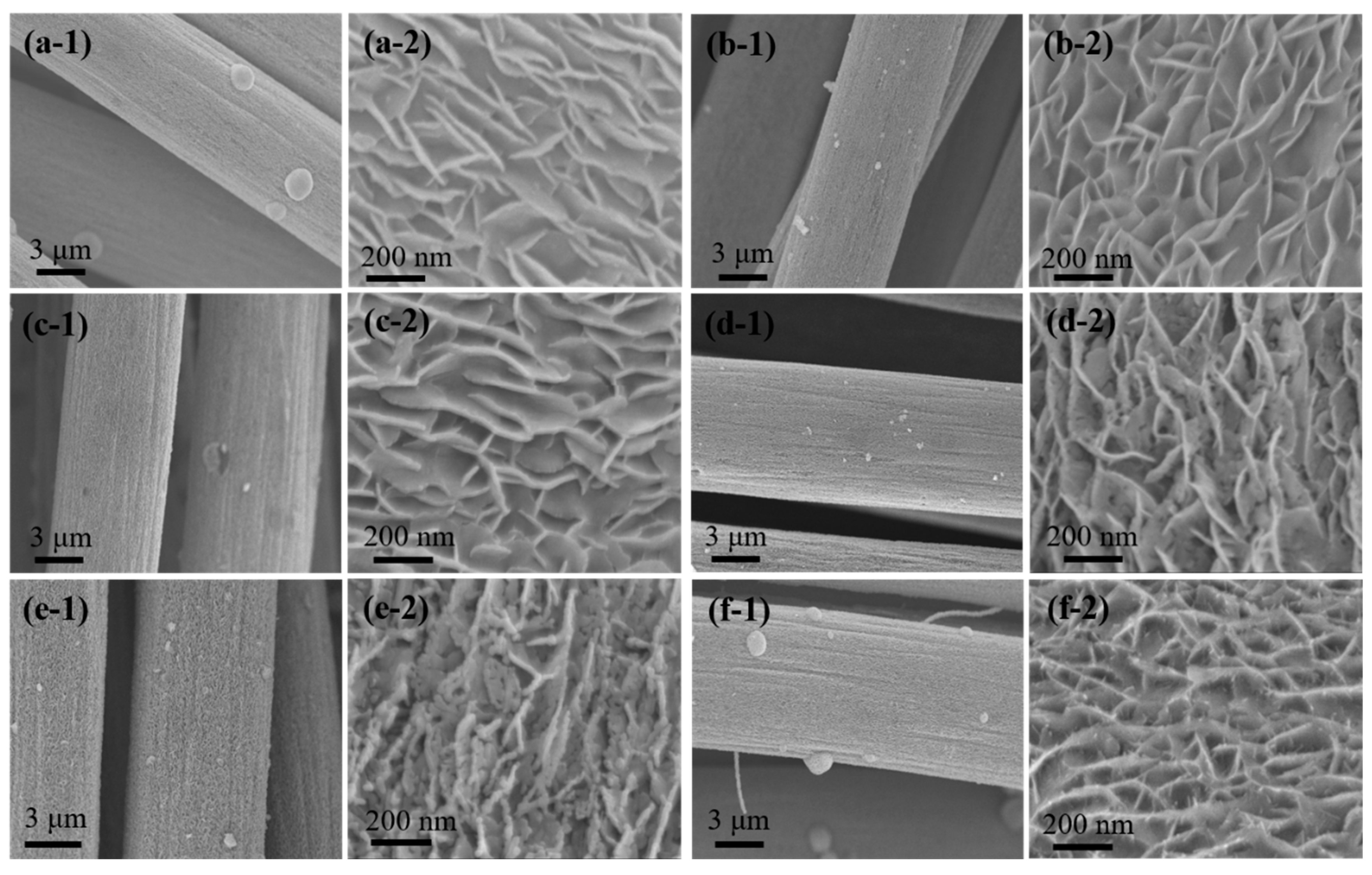
3.1. Morphology Research
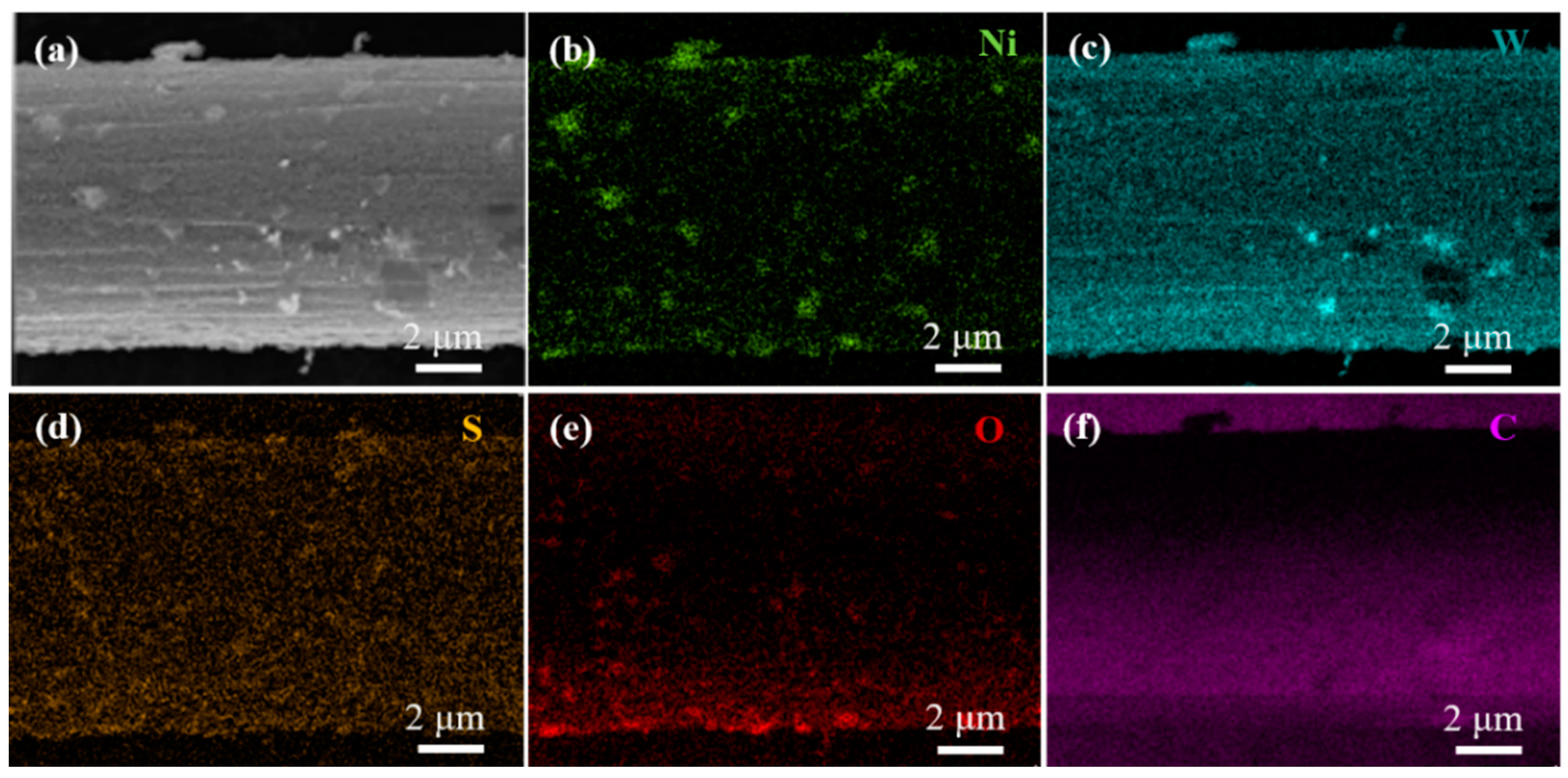
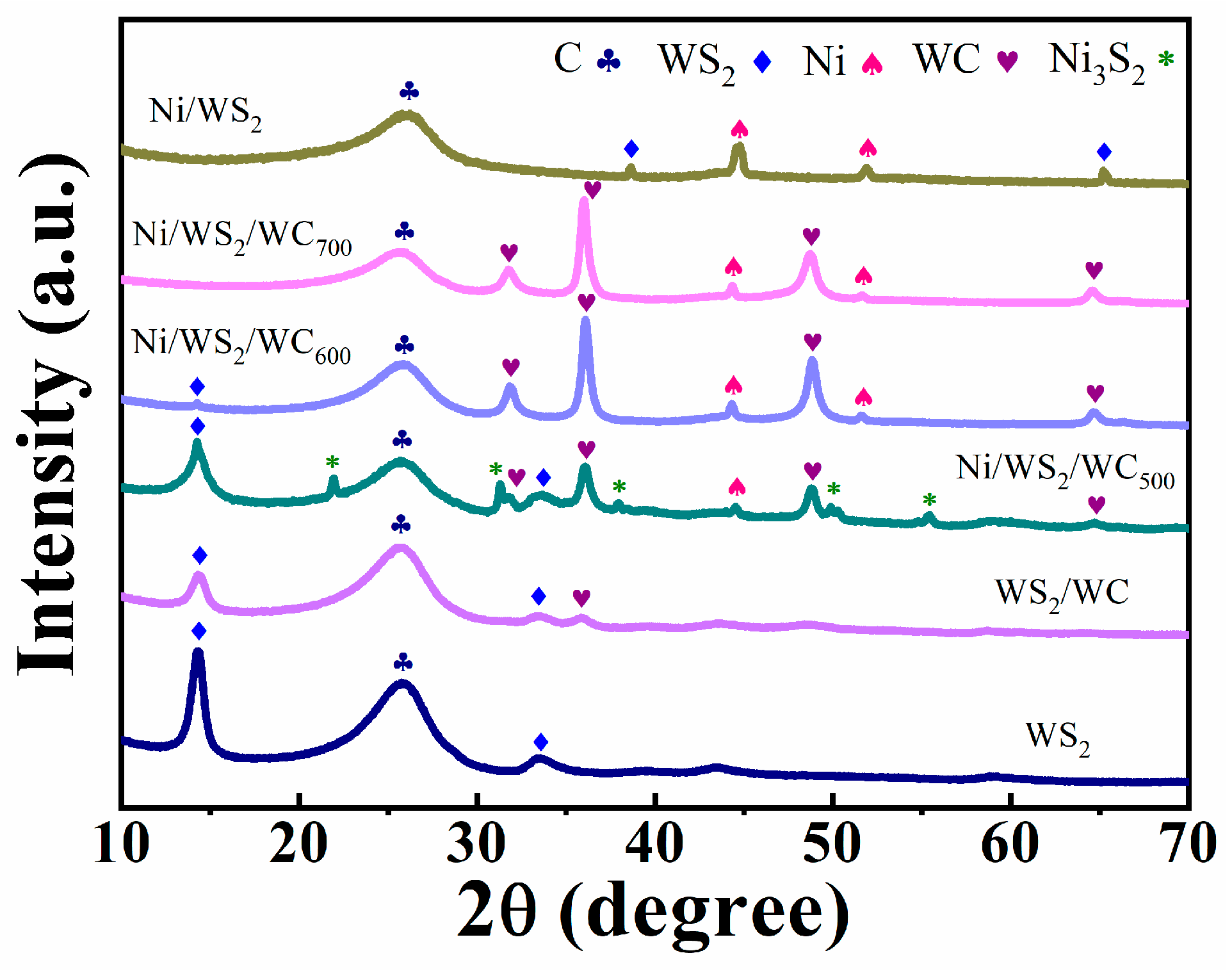
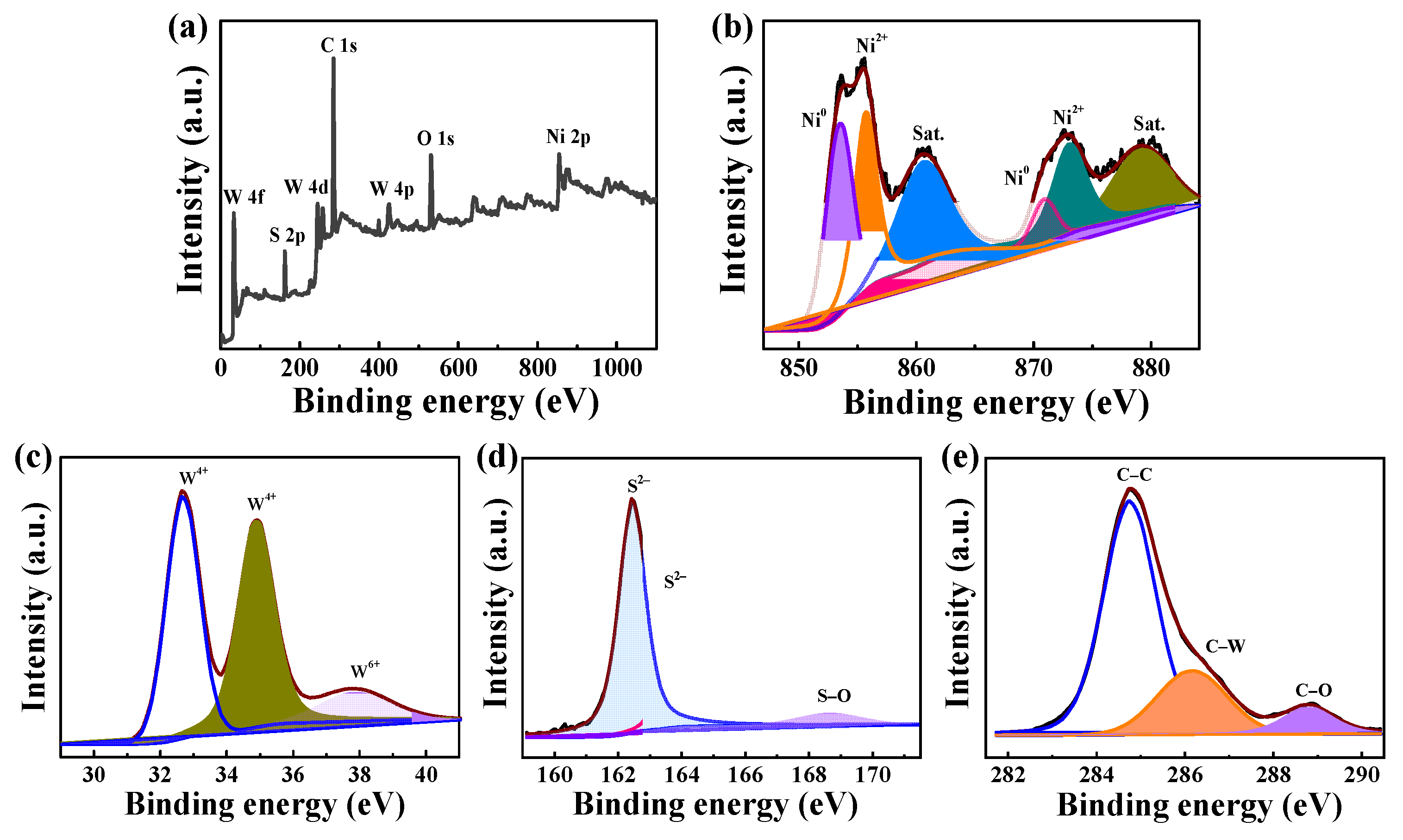
3.2. Composition Research
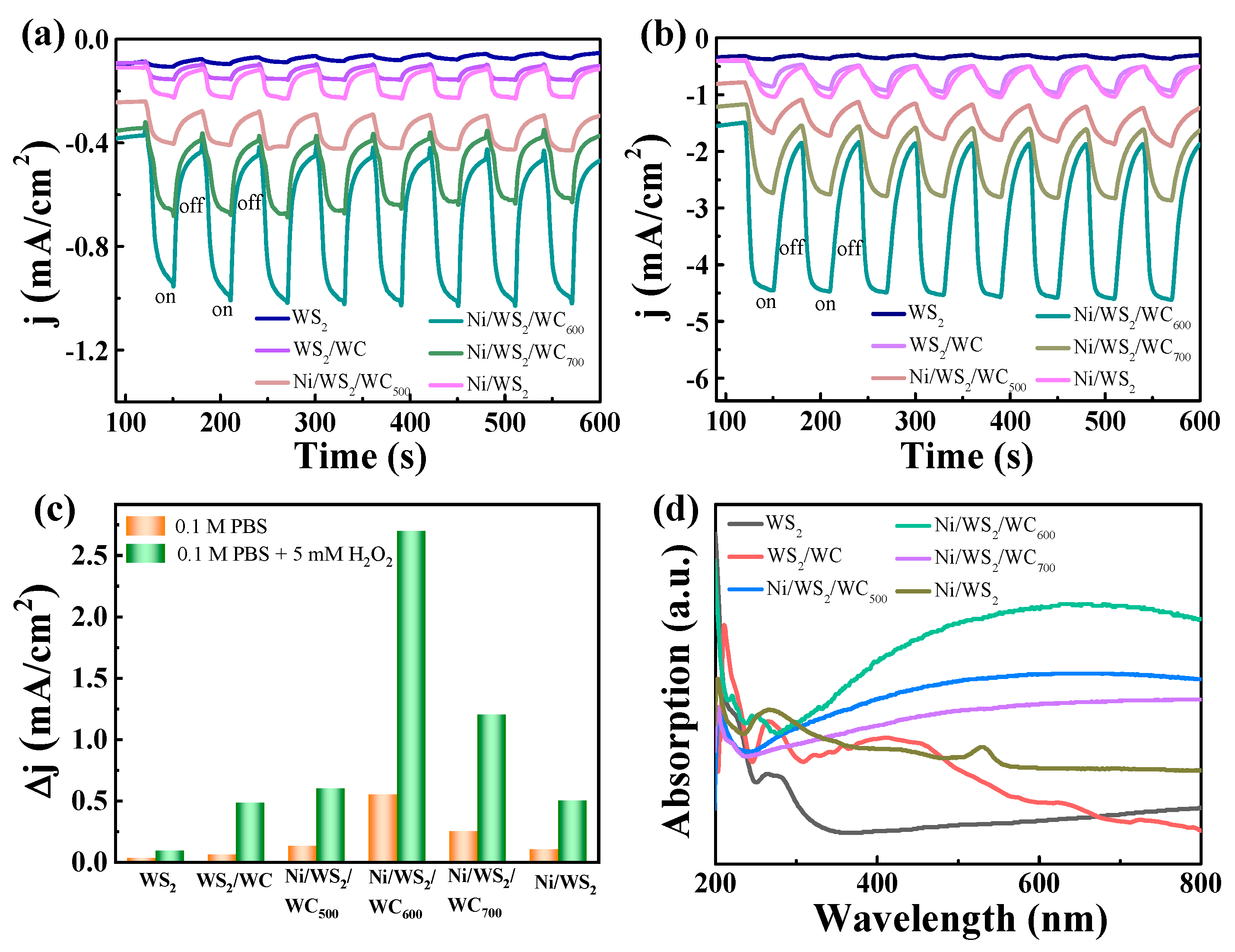
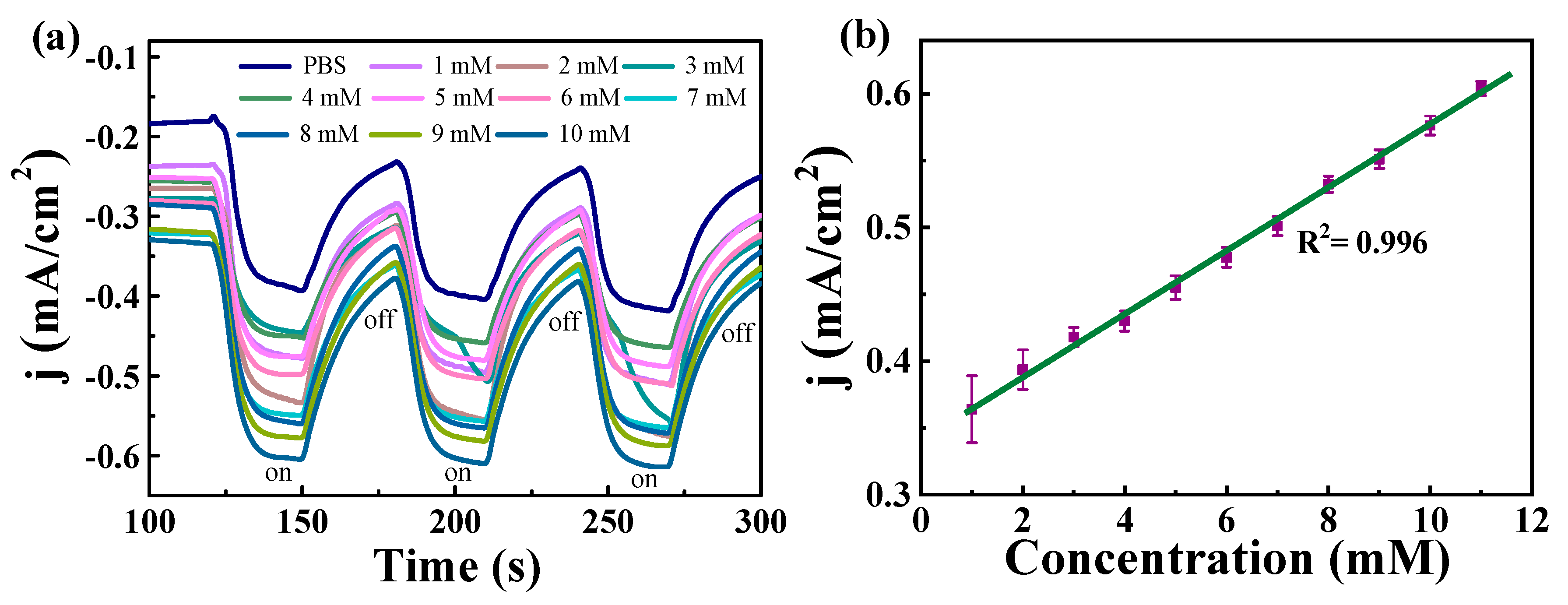
3.3. H2O2 Sensing Performance
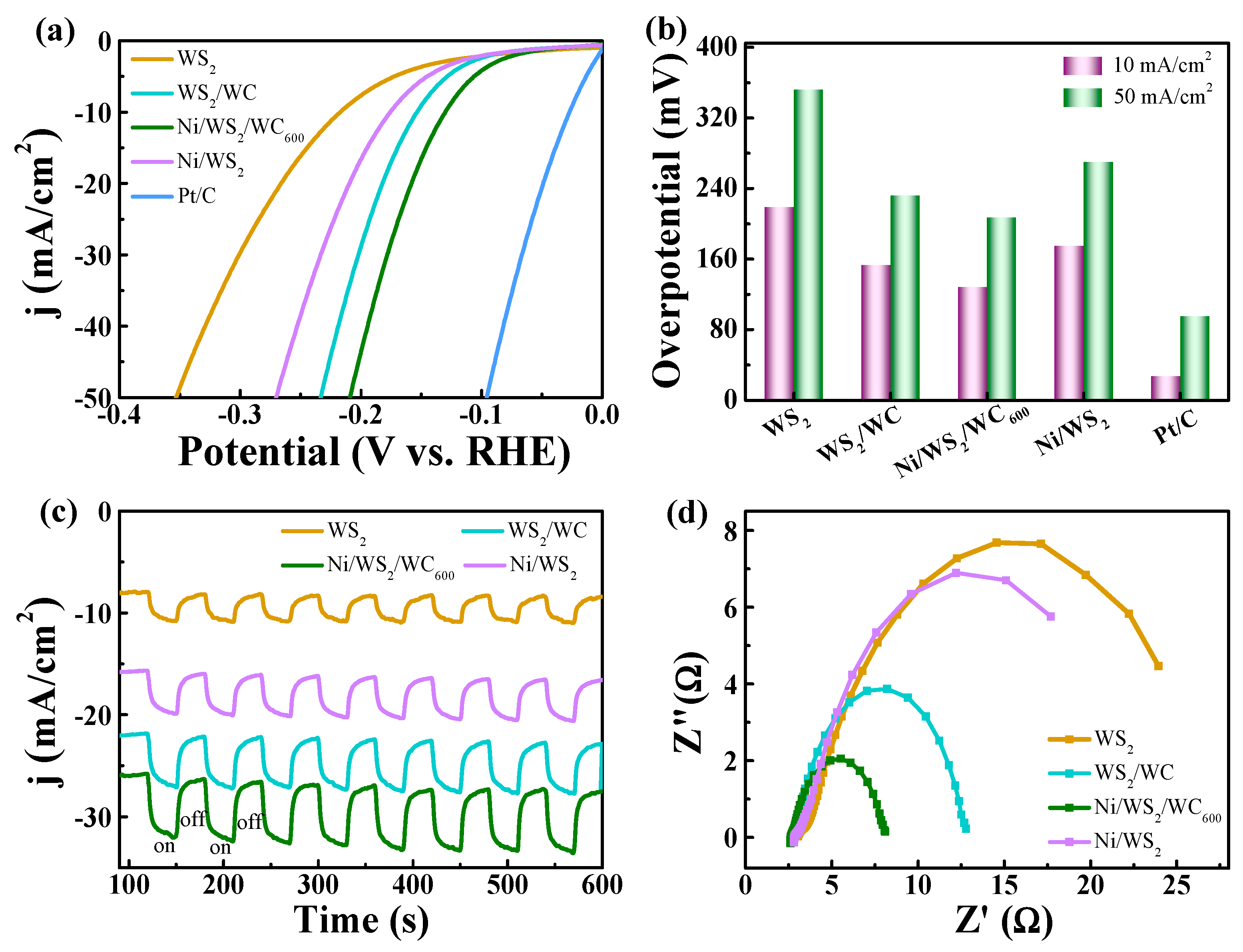
3.4. HER Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitte, S.A.; Gao, W.; Zholudov, Y.T.; Qi, L.; Nsabimana, A.; Liu, Z.; Xu, G. Stainless steel electrode for sensitive luminol electrochemiluminescent detection of H2O2, glucose, and glucose oxidase activity. Anal. Chem. 2017, 89, 9864–9869. [Google Scholar] [CrossRef]
- Riaz, M.A.; Chen, Y. Electrodes and electrocatalysts for electrochemical hydrogen peroxide sensors: A review of design strategies. Nanoscale Horiz. 2022, 7, 463–479. [Google Scholar] [CrossRef]
- Wang, D.; Dang, X.; Tan, B.; Zhang, Q.; Zhao, H. 3D V2O5-MoS2/rGO nanocomposites with enhanced peroxidase mimicking activity for sensitive colorimetric determination of H2O2 and glucose. Spectrochim. Acta A 2022, 269, 120750. [Google Scholar] [CrossRef]
- Sobahi, N.; Imran, M.; Khan, M.E.; Mohammad, A.; Alam, M.M.; Yoon, T.; Mehedi, I.M.; Hussain, M.A.; Abdulaal, M.J.; Jiman, A.A. Electrochemical sensing of H2O2 by employing a flexible Fe3O4/graphene/carbon cloth as working electrode. Materials 2023, 16, 2770. [Google Scholar] [CrossRef]
- Shi, Q.; Song, Y.; Zhu, C.; Yang, H.; Du, D.; Lin, Y. Mesoporous Pt nanotubes as a novel sensitive detection platform for intracellular hydrogen peroxide. ACS Appl. Mater. Interfaces 2015, 7, 24288–24295. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Wang, J.; Wang, J. Dual-mode sensing of biomarkers based on nano 3D Cu-Flo.@AuNPs-electrocatalyzed oxidation of glucose inducing in-situ H2O2-generation system. Biosens. Bioelectron. 2022, 198, 113820. [Google Scholar] [CrossRef]
- Atchudan, R.; Muthuchamy, N.; Edison, T.; Perumal, S.; Vinodh, R.; Park, K.H.; Lee, Y.R. An ultrasensitive photoelectrochemical biosensor for glucose based on bio-derived nitrogen-doped carbon sheets wrapped titanium dioxide nanoparticles. Biosens. Bioelectron. 2019, 126, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.I.; Muthuchamy, N.; Lee, K.P. A novel bismuth oxychloride-graphene hybrid nanosheets based non-enzymatic photoelectrochemical glucose sensing platform for high performances. Biosens. Bioelectron. 2017, 89, 352–360. [Google Scholar] [CrossRef]
- Robatjazi, H.; Bahauddin, S.M.; Doiron, C.; Thomann, I. Direct plasmon-driven photoelectrocatalysis. Nano Lett. 2015, 15, 6155–6161. [Google Scholar] [CrossRef]
- Wang, C.; Nie, X.G.; Shi, Y.; Zhou, Y.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Direct plasmon-accelerated electrochemical reaction on gold nanoparticles. ACS Nano 2017, 11, 5897–5905. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, W.; Lu, W.; Qin, X.; Xu, X. Surface plasmon aided high sensitive non-enzymatic glucose sensor using Au/NiAu multilayered nanowire arrays. Biosens. Bioelectron. 2018, 111, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Zhu, W.; Wu, H.; Wang, F.; Xu, X. A photoelectrochemical sensor for highly sensitive detection of glucose based on Au-NiO1–x hybrid nanowires. Sensor. Actuat. B-Chem. 2020, 304, 127330. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.; Wu, D.; Wei, Q. Preparation of PbS NPs/RGO/NiO nanosheet arrays heterostructure: Function-switchable self-powered photoelectrochemical biosensor for H2O2 and glucose monitoring. Biosens. Bioelectron. 2021, 173, 112803. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, K.; Zhang, J. Dual non-enzymatic glucose sensing on Ni(OH)2/TiO2 photoanode under visible light illumination. Electrochim. Acta 2017, 228, 28–35. [Google Scholar] [CrossRef]
- Kang, Z.; Yan, X.; Wang, Y.; Zhao, Y.; Bai, Z.; Liu, Y.; Zhao, K.; Cao, S.; Zhang, Y. Self-powered photoelectrochemical biosensing platform based on Au NPs@ZnO nanorods array. Nano Res. 2015, 9, 344–352. [Google Scholar] [CrossRef]
- Yu, Y.; Han, Y.; Xu, M.; Zhang, L.; Dong, S. Automatic illumination compensation device based on a photoelectrochemical biofuel cell driven by visible light. Nanoscale 2016, 8, 9004–9008. [Google Scholar] [CrossRef]
- Gu, T.T.; Wu, X.M.; Dong, Y.M.; Wang, G.L. Novel photoelectrochemical hydrogen peroxide sensor based on hemin sensitized nanoporous NiO based photocathode. J. Electroanal. Chem. 2015, 759, 27–31. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Chen, M.; Xu, X. W2N/WC composite nanofibers as an efficient electrocatalyst for photoelectrochemical hydrogen evolution. RSC Adv. 2021, 11, 20285–20291. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Fu, J.; Cao, Y.; Ding, R.; Xu, X. Vertically aligned W(Mo)S2/N-W(Mo)C-based light-assisted electrocatalysis for hydrogen evolution in acidic solutions. Rare Metals 2023, 42, 1535–1544. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Liu, X.; Yin, Z.; Ma, X.; Zhou, Y.; Chen, W.; Li, J.; Xu, L. Rough Ni@MoN corals for the hydrogen evolution reaction in acidic and alkaline media. N. J. Chem. 2023, 47, 12678–12687. [Google Scholar] [CrossRef]
- Habibi, M.M.; Mousavi, M.; Shadman, Z.; Ghasemi, J.B. Preparation of a nonenzymatic electrochemical sensor based on a g-C3N4/MWO4 (M: Cu, Mn, Co, Ni) composite for the determination of H2O2. N. J. Chem. 2022, 46, 3766–3776. [Google Scholar] [CrossRef]
- Huang, L.; He, L.; Ni, J.; Liu, H.; Xu, Z.; Gong, C.; Zhang, Q.; Zhang, B. WO3/Mo:BiVO4 heterojunction structured photoelectrochemical sensor for enhancing hydrogen peroxide monitoring and mechanism investigation. Electrochim. Acta 2023, 439, 141641. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, L.; Cai, H.; Yang, Y.; Zeng, J.; Ma, D.; Gao, Q. Hierarchical Mo2C@MoS2 nanorods as electrochemical sensors for highly sensitive detection of hydrogen peroxide and cancer cells. Sensor. Actuat. B-Chem. 2020, 311, 127863. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Baek, M.; Kim, S.; Kim, W.; Yong, K. An antenna/spacer/reflector based Au/BiVO4/WO3/Au nanopatterned photoanode for plasmon-enhanced photoelectrochemical water splitting. Appl. Catal. B Environ. 2018, 237, 763–771. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhang, H.; Zhang, J. Interface designing over WS2/W2C for enhanced hydrogen evolution catalysis. ACS Appl. Energy Mater. 2018, 1, 3377–3384. [Google Scholar] [CrossRef]
- Huang, J.; He, Y.; Jin, J.; Li, Y.; Dong, Z.; Li, R. A novel glucose sensor based on MoS2 nanosheet functionalized with Ni nanoparticles. Electrochim. Acta 2014, 136, 41–46. [Google Scholar] [CrossRef]
- Chen, D.; Peng, L.; Yuan, Y.; Zhu, Y.; Fang, Z.; Yan, C.; Chen, G.; Shahbazian-Yassar, R.; Lu, J.; Amine, K.; et al. Two-dimensional holey Co3O4 nanosheets for high-rate alkali-ion batteries: From rational synthesis to in situ probing. Nano Lett. 2017, 17, 3907–3913. [Google Scholar] [CrossRef]
- Chung, C.; Chen, Y.; Juang, F.; Kao, K.; Lee, E. Preparation of MoS2 nanospheres using a hydrothermal method and their application as ammonia gas sensors based on delay line surface acoustic wave devices. Materials 2023, 16, 4703. [Google Scholar] [CrossRef]
- Levshakova, A.; Kaneva, M.; Borisov, E.; Panov, M.; Shmalko, A.; Nedelko, N.; Mereshchenko, A.S.; Skripkin, M.; Manshina, A.; Khairullina, E. Simultaneous catechol and hydroquinone detection with laser fabricated MOF-derived Cu-CuO@C composite electrochemical sensor. Materials 2023, 16, 7225. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent advances in chitosan-based applications—A review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Pligovka, A. Reflectant photonic crystals produced via porous-alumina-assisted-anodizing of Al/Nb and Al/Ta systems. Surf. Rev. Lett. 2021, 28, 2150055. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Zakhlebayeva, A. Electro-physical properties of niobia columnlike nanostructures via the anodizing of Al/Nb layers. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; pp. 1–5. [Google Scholar]
- Wu, T.; Liu, F.; Lyu, X.; Wu, F.; Zhao, H.; Xin, Y.; Li, L.; Fan, G.; Zhu, X.; Liu, Q.; et al. Reusable nickel foam supported 3D hierarchical Co-Fe-Ni mixed metal oxides with peroxidase-like activity as biosensors for the colorimetric detection of H2O2. N. J. Chem. 2023, 47, 7575–7582. [Google Scholar] [CrossRef]
- Lei, Z.; Bai, J.; Li, Y.; Wang, Z.; Zhao, C. Fabrication of nanoporous nickel-iron hydroxylphosphate composite as bifunctional and reversible catalyst for highly efficient intermittent water splitting. ACS Appl. Mater. Interfaces 2017, 9, 35837–35846. [Google Scholar] [CrossRef]
- Han, K.; Kreuger, T.; Mei, B.; Mul, G. Transient behavior of Ni@NiOx functionalized SrTiO3 in overall water splitting. ACS Catal. 2017, 7, 1610–1614. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, Y.; Chen, L.; Li, Y.; Wang, L. Ni/WS2/WC Composite Nanosheets as an Efficient Catalyst for Photoelectrochemical Hydrogen Peroxide Sensing and Hydrogen Evolution. Materials 2024, 17, 1037. https://doi.org/10.3390/ma17051037
Liu Y, Zhu Y, Chen L, Li Y, Wang L. Ni/WS2/WC Composite Nanosheets as an Efficient Catalyst for Photoelectrochemical Hydrogen Peroxide Sensing and Hydrogen Evolution. Materials. 2024; 17(5):1037. https://doi.org/10.3390/ma17051037
Chicago/Turabian StyleLiu, Yanping, Yixin Zhu, Leqin Chen, Yujia Li, and Lanfang Wang. 2024. "Ni/WS2/WC Composite Nanosheets as an Efficient Catalyst for Photoelectrochemical Hydrogen Peroxide Sensing and Hydrogen Evolution" Materials 17, no. 5: 1037. https://doi.org/10.3390/ma17051037





