Influence of Exposure to a Wet Atmosphere on the UV-Sensing Characteristics of ZnO Nanorod Arrays
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
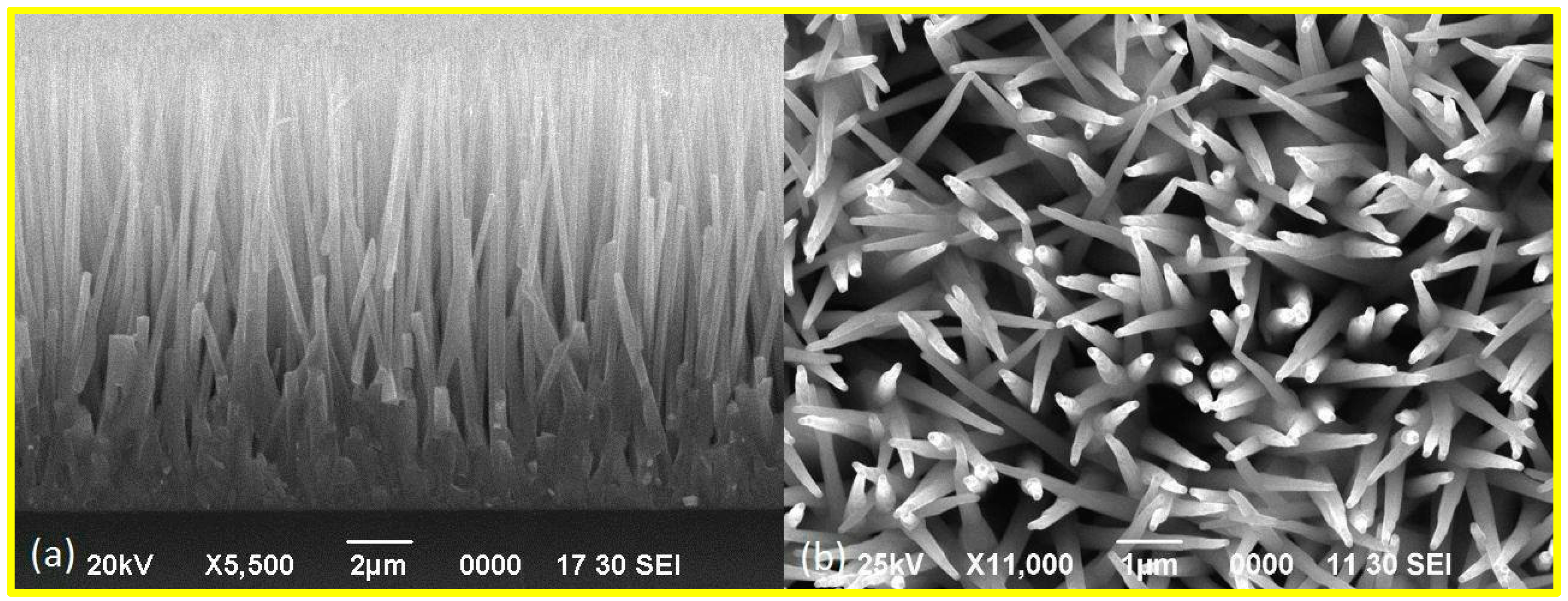
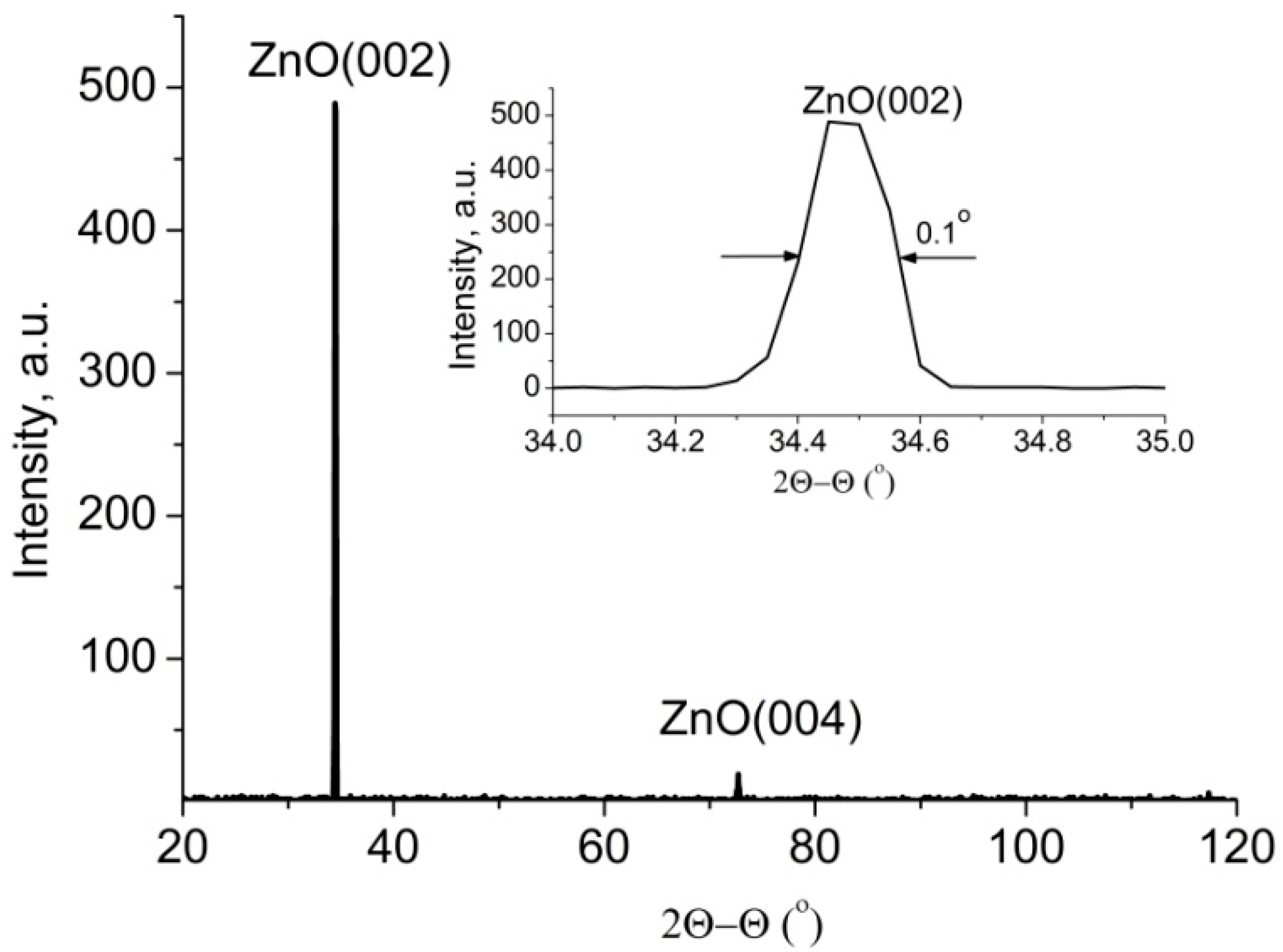
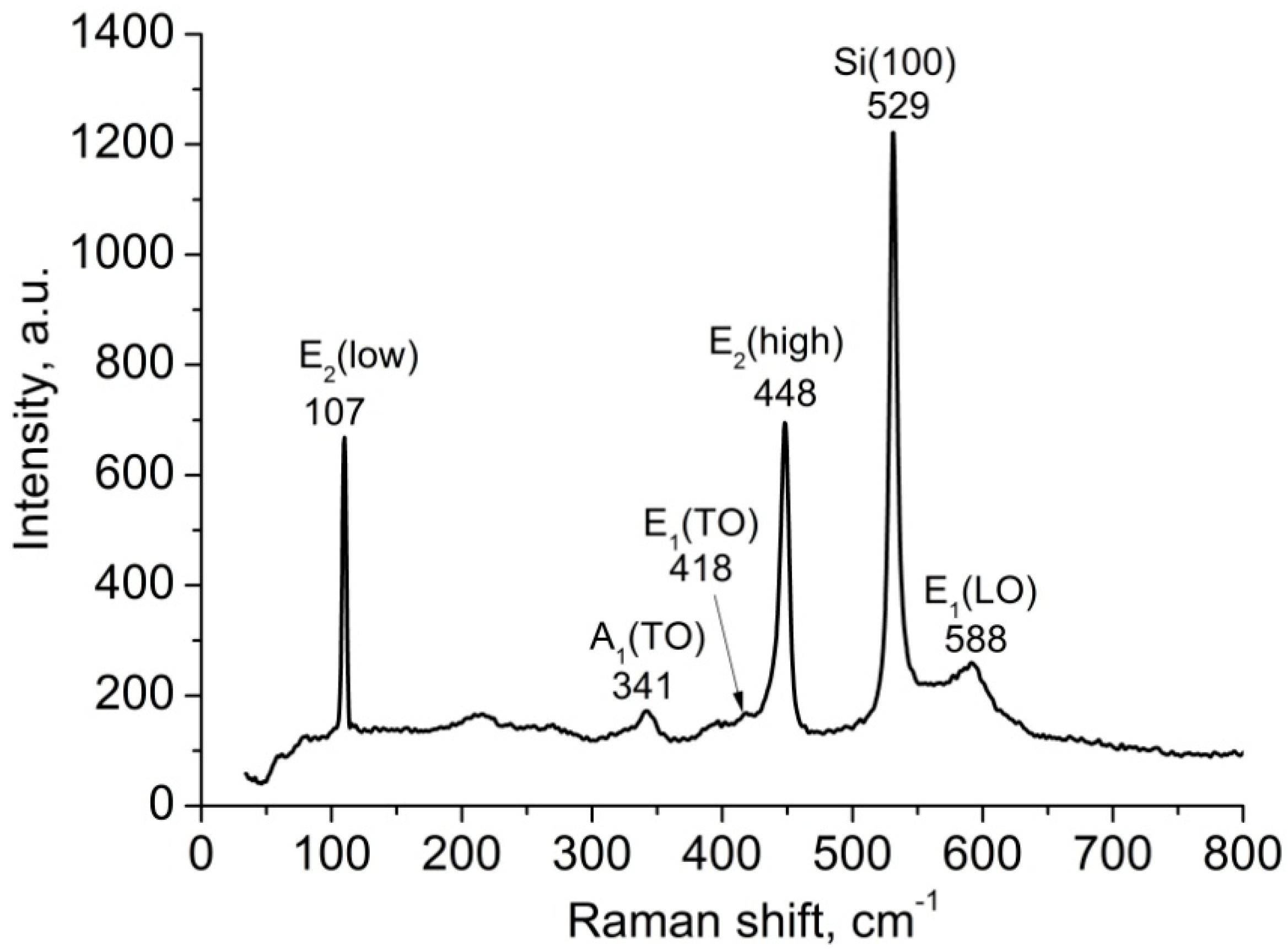
3.1. ZnO Nanorods Arrays Characterization
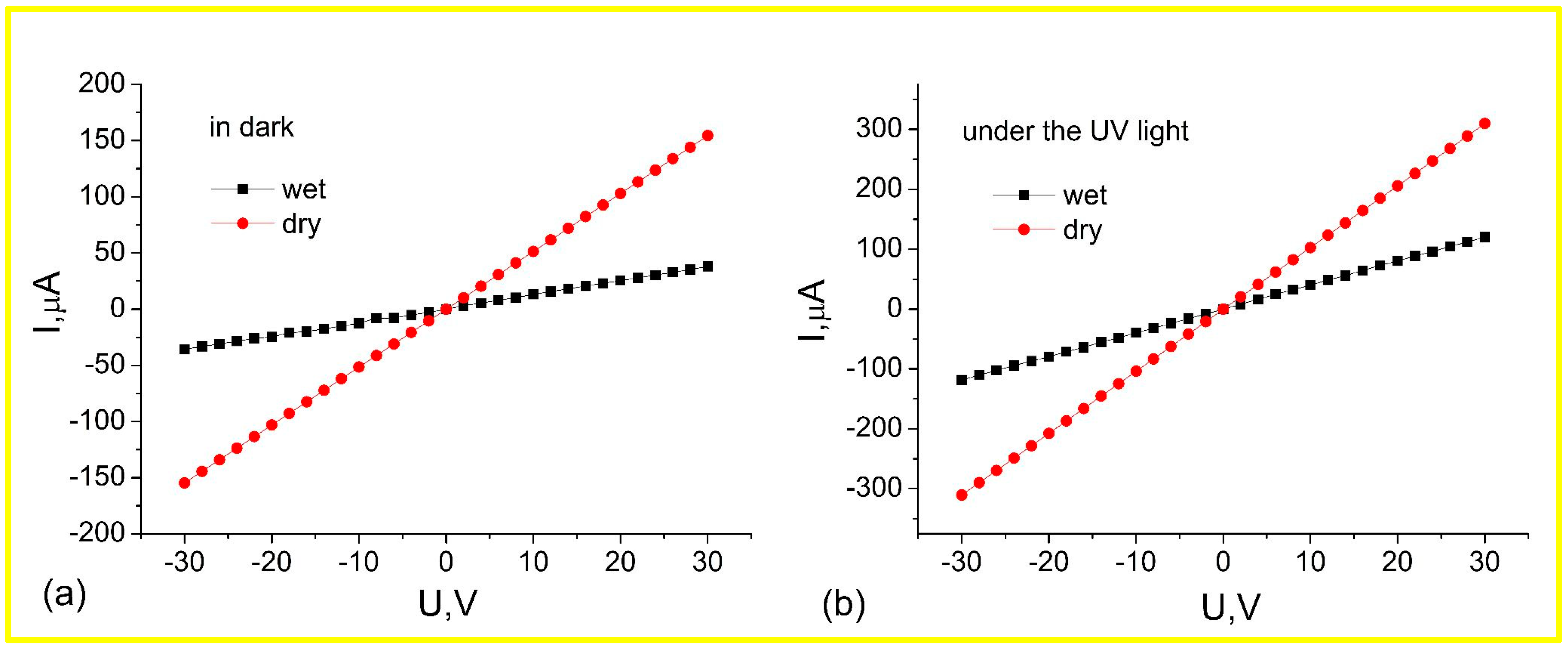
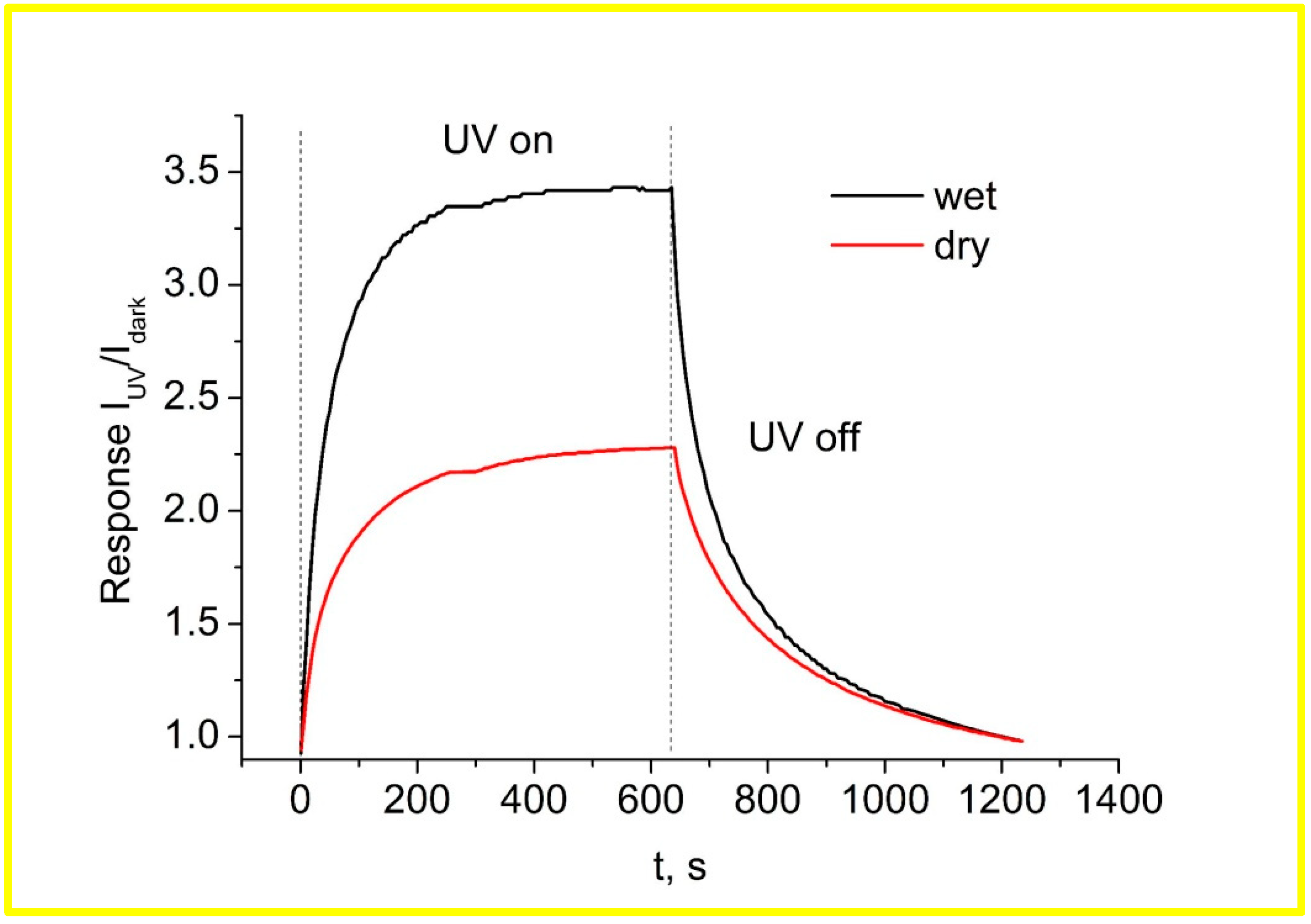
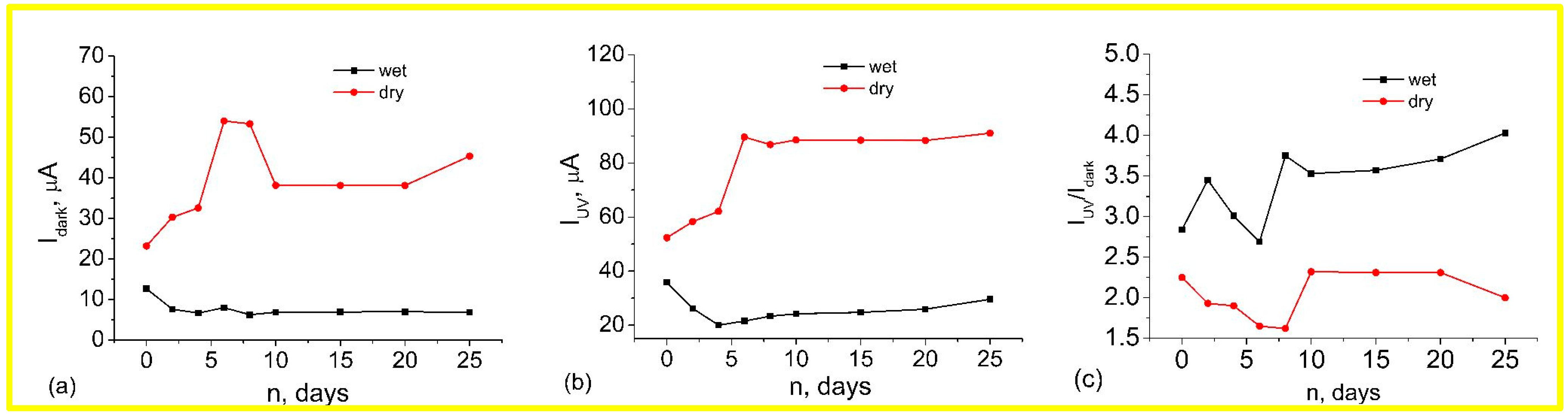
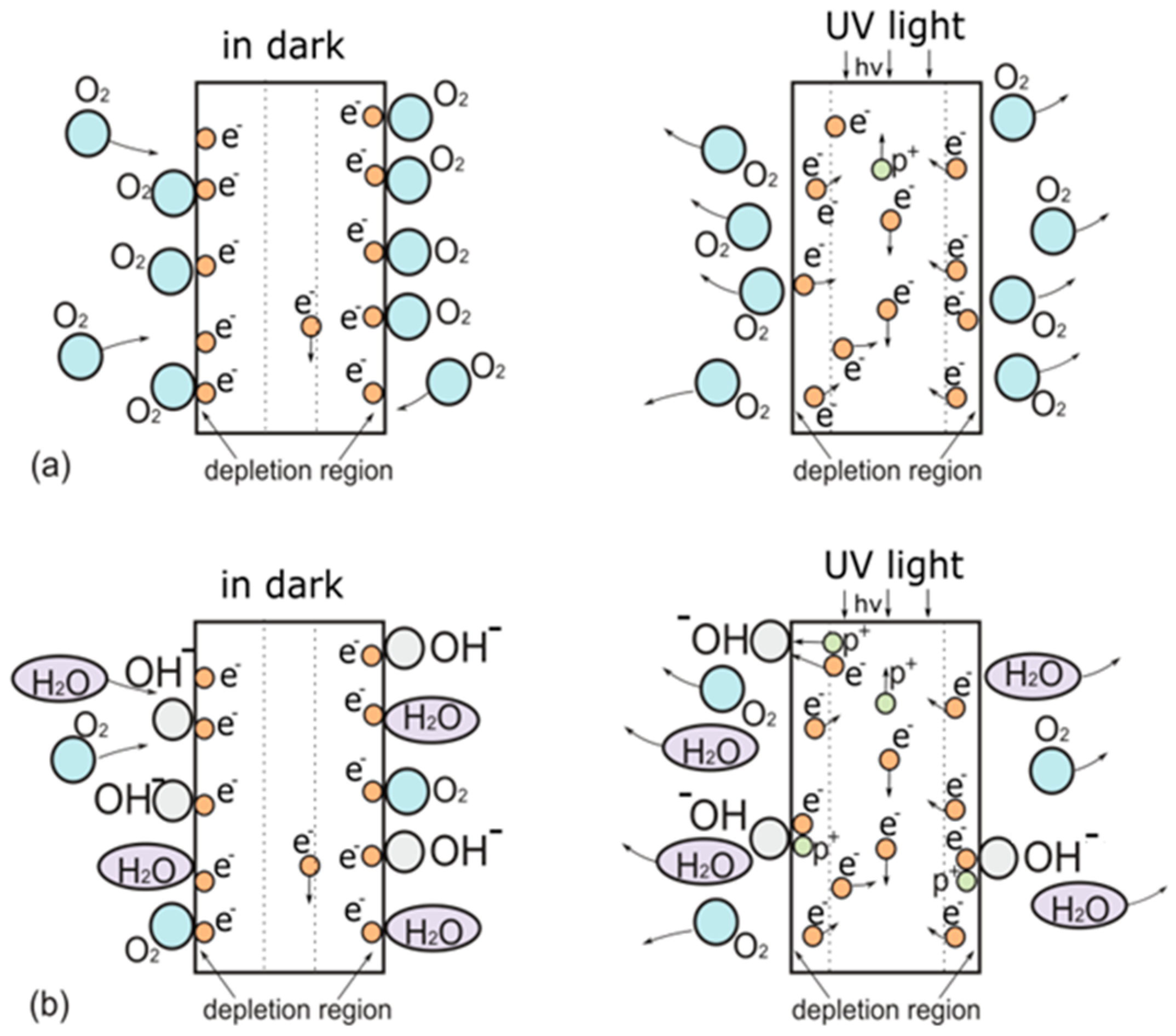
3.2. Effects of Wet Atmosphere on Electrical Characteristics of ZnO Nanorods Arrays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, L.; Xu, C.; Zeng, W.; Nie, C.; Hu, Y. Fabrication and Application of Different Nanostructured ZnO in Ultraviolet Photodetectors: A Review. IEEE Sens. J. 2022, 22, 7451–7462. [Google Scholar] [CrossRef]
- Que, M.; Lin, C.; Sun, J.; Chen, L.; Sun, X.; Sun, Y. Progress in ZnO Nanosensors. Sensors 2021, 21, 5502. [Google Scholar] [CrossRef]
- Hernandez-Como, N.; Rivas-Montes, G.; Hernandez-Cuevas, F.J.; Mejia, I.; Molinar-Solis, J.E.; Aleman, M. Ultraviolet Photodetectors Based on Low Temperature Processed ZnO/PEDOT:PSS Schottky Barrier Diodes. Mater. Sci. Semicond. Process. 2015, 37, 14–18. [Google Scholar] [CrossRef]
- Monroy, E.; Omnès, F.; Calle, F. Wide-Bandgap Semiconductor Ultraviolet Photodetectors. Semicond. Sci. Technol. 2003, 18, R33–R51. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Wang, Z.V. ZnO Nanowire and Nanobelt Platform for Nanotechnology. Mater. Sci. Eng. R 2009, 63, 33–71. [Google Scholar] [CrossRef]
- Prete, P.; Lovergine, N.; Tapfer, L. Nanostructure Size Evolution during Au-Catalysed Growth by Carbo-Thermal Evaporation of Well-Aligned ZnO Nanowires on (100)Si. Appl. Phys. A Mater. Sci. Process. 2007, 88, 21–26. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, M.-C.; Oh, B.-Y.; Lee, W.; Myoung, J.-M. Fabrication of Zn/ZnO Nanocables through Thermal Oxidation of Zn Nanowires Grown by RF Magnetron Sputtering. J. Cryst. Growth 2006, 290, 485–489. [Google Scholar] [CrossRef]
- Wan, H.; Ruda, H.E. A Study of the Growth Mechanism of CVD-Grown ZnO Nanowires. J. Mater. Sci. Mater. Electron. 2010, 21, 1014–1019. [Google Scholar] [CrossRef]
- Mezher, S.J.; Kadhim, B.B.; Dawood, M.O. Synthesis and Characterization of Au–ZnO Nanorods Growth by CVD Method. Opt. Quantum Electron. 2023, 55, 845. [Google Scholar] [CrossRef]
- Sun, Y.; Fuge, G.M.; Ashfold, M.N.R. Growth Mechanisms for ZnO Nanorods Formed by Pulsed Laser Deposition. Superlattices Microstruct. 2006, 39, 33–40. [Google Scholar] [CrossRef]
- Premkumar, T.; Manoravi, P.; Panigrahi, B.K.; Baskar, K. Particulate Assisted Growth of ZnO Nanorods and Microrods by Pulsed Laser Deposition. Appl. Surf. Sci. 2009, 255, 6819–6822. [Google Scholar] [CrossRef]
- Tzou, A.J.; Chien, K.F.; Lai, H.Y.; Ku, J.T.; Lee, L.; Fan, W.C.; Chou, W.C. The Study of Self-Assembled ZnO Nanorods Grown on Si(111) by Plasma-Assisted Molecular Beam Epitaxy. J. Cryst. Growth 2013, 378, 466–469. [Google Scholar] [CrossRef]
- Park, W.I.; Kim, D.H.; Jung, S.-W.; Yi, G.-C. Metalorganic Vapor-Phase Epitaxial Growth of Vertically Well-Aligned ZnO Nanorods. Appl. Phys. Lett. 2002, 80, 4232–4234. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, Y.; Kumar, H.; Vyas, S.; Periasamy, C.; Chakrabarti, P.; Jit, S.; Park, S.-H. A Study of Hydrothermally Grown ZnO Nanorod-Based Metal-Semiconductor-Metal UV Detectors on Glass Substrates. Nanomater. Nanotechnol. 2017, 7, 184798041770214. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Lee, H.S. Temperature Dependence of the Growth of ZnO Nanorod Arrays by Electrochemical Deposition. Electron. Mater. Lett. 2011, 7, 59–62. [Google Scholar] [CrossRef]
- Yi, S.-H.; Choi, S.-K.; Jang, J.-M.; Kim, J.-A.; Jung, W.-G. Low-Temperature Growth of ZnO Nanorods by Chemical Bath Deposition. J. Colloid Interface Sci. 2007, 313, 705–710. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef]
- Klingshirn, C.F.; Waag, A.; Hoffmann, A.; Geurts, J. Zinc Oxide: From Fundamental Properties Towards Novel Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; ISBN 9783642105777. [Google Scholar]
- Kushwaha, A.; Aslam, M. Defect Induced High Photocurrent in Solution Grown Vertically Aligned ZnO Nanowire Array Films. J. Appl. Phys. 2012, 112, 054316. [Google Scholar] [CrossRef]
- Bao, J.; Shalish, I.; Su, Z.; Gurwitz, R.; Capasso, F.; Wang, X.; Ren, Z. Photoinduced Oxygen Release and Persistent Photoconductivity in ZnO Nanowires. Nanoscale Res. Lett. 2011, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Gurwitz, R.; Cohen, R.; Shalish, I. Interaction of Light with the ZnO Surface: Photon Induced Oxygen “breathing,” Oxygen Vacancies, Persistent Photoconductivity, and Persistent Photovoltage. J. Appl. Phys. 2014, 115, 033701. [Google Scholar] [CrossRef]
- Henderson, M.A. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Meyer, B.; Marx, D.; Dulub, O.; Diebold, U.; Kunat, M.; Langenberg, D.; Wöll, C. Partial Dissociation of Water Leads to Stable Superstructures on the Surface of Zinc Oxide. Angew. Chem. Int. Ed Engl. 2004, 43, 6642–6645. [Google Scholar] [CrossRef] [PubMed]
- Woll, C. The Chemistry and Physics of Zinc Oxide Surfaces. Prog. Surf. Sci. 2007, 82, 55–120. [Google Scholar] [CrossRef]
- Newberg, J.T.; Goodwin, C.; Arble, C.; Khalifa, Y.; Boscoboinik, J.A.; Rani, S. ZnO(10) Surface Hydroxylation under Ambient Water Vapor. J. Phys. Chem. B 2018, 122, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, A.; Catellani, A. Water Adsorption on Nonpolar ZnO(10) Surface: A Microscopic Understanding. J. Phys. Chem. C Nanomater. Interfaces 2009, 113, 2896–2902. [Google Scholar] [CrossRef]
- Li, Y.; Della Valle, F.; Simonnet, M.; Yamada, I.; Delaunay, J.-J. High-Performance UV Detector Made of Ultra-Long ZnO Bridging Nanowires. Nanotechnology 2009, 20, 045501. [Google Scholar] [CrossRef]
- Li, Y.; Della Valle, F.; Simonnet, M.; Yamada, I.; Delaunay, J.-J. Competitive Surface Effects of Oxygen and Water on UV Photoresponse of ZnO Nanowires. Appl. Phys. Lett. 2009, 94, 023110. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Pauporté, T.; Hoppe, M.; Adelung, R. UV Nanophotodetectors: A Case Study of Individual Au-Modified ZnO Nanowires. Sens. Actuators A Phys. 2019, 296, 400–408. [Google Scholar] [CrossRef]
- Red’kin, A.N.; Ryzhova, M.V.; Yakimov, E.E.; Gruzintzev, A.N. Aligned Arrays of Zinc Oxide Nanorods on Silicon Substrates. Semiconductors 2013, 47, 252–258. [Google Scholar] [CrossRef]
- Red’kin, A.N.; Makovei, Z.I.; Gruzintzev, A.N.; Dubonos, S.V.; Yakimov, E.E. Vapor Phase Synthesis of Aligned ZnO Nanorod Arrays from Elements. Inorg. Mater. 2006, 43, 301–306. [Google Scholar] [CrossRef]
- Mora-Seró, I.; Fabregat-Santiago, F.; Denier, B.; Bisquert, J.; Tena-Zaera, R.; Elias, J.; Lévy-Clément, C. Determination of Carrier Density of ZnO Nanowires by Electrochemical Techniques. Appl. Phys. Lett. 2006, 89, 203117. [Google Scholar] [CrossRef]
- Hullavarad, S.; Hullavarad, N.; Look, D.; Claflin, B. Persistent Photoconductivity Studies in Nanostructured ZnO UV Sensors. Nanoscale Res. Lett. 2009, 4, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Georgobiani, A.N.; Gruzintsev, A.N.; Volkov, V.T.; Vorob’ev, M.O. Effect of Annealing in Oxygen Radicals on Luminescence and Electrical Conductivity of ZnO:N Films. Semiconductors 2002, 36, 265–269. [Google Scholar] [CrossRef]
- Shasti, M.; Dariani, R.S. Study of Growth Time and Post Annealing Effect on the Performance of ZnO Nanorods Ultraviolet Photodetector. J. Appl. Phys. 2017, 121, 064503. [Google Scholar] [CrossRef]
- Redkin, A.N.; Yakimov, E.E.; Evstafieva, M.V.; Yakimov, E.B. Grown and Characterization of ZnO Aligned Nanorod Arrays for Sensor Applications. Energies 2021, 14, 3750. [Google Scholar] [CrossRef]
- Reddy, N.K.; Ahsanulhaq, Q.; Kim, J.H.; Devika, M.; Hahn, Y.B. Selection of Non-Alloyed Ohmic Contacts for ZnO Nanostructure Based Devices. Nanotechnology 2007, 18, 445710. [Google Scholar] [CrossRef]
- Wei, A.; Pan, L.; Huang, W. Recent Progress in the ZnO Nanostructure-Based Sensors. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2011, 176, 1409–1421. [Google Scholar] [CrossRef]
- Serrano, J.; Manjón, F.J.; Romero, A.H.; Widulle, F.; Lauck, R.; Cardona, M. Dispersive Phonon Linewidths: The E2 Phonons of ZnO. Phys. Rev. Lett. 2003, 90, 055510. [Google Scholar] [CrossRef]
- McCluskey, M.D. Defects in ZnO. In Defects in Advanced Electronic Materials and Novel Low Dimensional Structures; Stehr, J., Buyanova, I., Chen, W., Eds.; Elsevier Ltd.: New York, NY, USA, 2018; pp. 1–25. [Google Scholar]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The Identification of Hydroxyl Groups on ZnO Nanoparticles by Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef]
- Thiel, P.A.; Madey, T.E. The Interaction of Water with Solid Surfaces: Fundamental Aspects. Surf. Sci. Rep. 1987, 7, 211–385. [Google Scholar] [CrossRef]
- Liao, Z.-M.; Liu, K.-J.; Zhang, J.-M.; Xu, J.; Yu, D.-P. Effect of Surface States on Electron Transport in Individual ZnO Nanowires. Phys. Lett. A 2007, 367, 207–210. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evstafieva, M.; Redkin, A.; Roshchupkin, D.; Rudneva, T.; Yakimov, E.E. Influence of Exposure to a Wet Atmosphere on the UV-Sensing Characteristics of ZnO Nanorod Arrays. Materials 2024, 17, 1053. https://doi.org/10.3390/ma17051053
Evstafieva M, Redkin A, Roshchupkin D, Rudneva T, Yakimov EE. Influence of Exposure to a Wet Atmosphere on the UV-Sensing Characteristics of ZnO Nanorod Arrays. Materials. 2024; 17(5):1053. https://doi.org/10.3390/ma17051053
Chicago/Turabian StyleEvstafieva, Maria, Arcady Redkin, Dmitry Roshchupkin, Tatyana Rudneva, and Eugene E. Yakimov. 2024. "Influence of Exposure to a Wet Atmosphere on the UV-Sensing Characteristics of ZnO Nanorod Arrays" Materials 17, no. 5: 1053. https://doi.org/10.3390/ma17051053





