Abstract
In order to enhance the degree of binding reaction of TiO2 in titanium-containing ceramic glazes and prevent the reaction of its transformation into rutile to eliminate the yellowing phenomenon of the glaze surface, an apatite–TiO2 composite opacifier (ATO) was prepared through the mechanical grinding of hydroxyapatite and anatase TiO2. The properties, opacification mechanism, and yellowing inhibition of the prepared ceramic glazes were studied. The results show that the ATO is characterized by a uniform coating of TiO2 on the surface of the apatite and the formation of close chemical bonding between the apatite and TiO2. The ceramic glaze surface when using an ATO has a white appearance and excellent opacification performance. When an ATO was used, the L*, a*, and b* values of the glaze were 89.99, −0.85, and 3.37, respectively, which were comparable to those of a ZrSiO4 glaze (L*, a*, and b* were 88.24, −0.02, and 2.29, respectively). The opacification of the glaze was slightly lower than that of the TiO2 glaze (L* value was 92.13), but the appearance changed from yellow to the white of the TiO2 glaze (b* value was 9.18). The ceramic glaze layer when using an ATO mainly consists of titanite, glass phase, and a small amount of quartz, and the opacification mechanism is the crystallization of the generated titanite. ATOs can play an active role in solving the critical problem that arises when TiO2 replaces ZrSiO4 as an opacifier.
1. Introduction
An opacifier is an inorganic material that generates opacified substances under high-temperature calcination conditions. A ceramic glaze added with an opacifier can show a strong opacifying effect after firing to cover the ceramic body, improve the function of the ceramic product, and provide a good appearance and aesthetic effect [1]. Common ceramic opacifiers include ZrSiO4 [2], SnO2 [3], ZnO [4], TiO2 [5], and phosphates [6,7]. Among them, ZrSiO4 has developed a stable application technology and production process for long-term use because of its stable opacification ability and the fact that it is not affected by a sintered atmosphere. It has become the most widely used opacifier in the ceramic industry [8,9,10,11]. However, in the processes of its production and application, zirconium silicate opacifiers have also exhibited some problems, such as the fact that the hardness of zircon sand as a raw material is very high (its Mohs hardness is 8~9) and the production costs of crushing and grinding are high. At the same time, zircon sand contains trace amounts of radioactive elements Th and U, resulting in certain radioactivity in zirconium silicate and ceramic products with zirconium silicate as an opacifier, which causes a threat to human health [12].
TiO2 is considered an alternative to ZrSiO4 as a ceramic opacifier due to its extremely high hiding power, refractive index, and non-radioactivity [13]. When TiO2 is used as an opacifier, TiO2 reacts with CaO and SiO2 during a high-temperature calcination process to form titanite (CaTiSiO5), which scatters visible light [14]. However, when TiO2 is singularly added to a glaze as an opacifier, TiO2 is often not fully involved in the formation of titanite. These free TiO2 particles are easily transformed into a rutile phase and cause the yellowing of the glaze, which has a negative effect on the appearance of ceramic products and is the main reason limiting the application of TiO2 as an opacifier. Using the solid-phase method, Li et al. [15] used anatase, calcium carbonate, and white carbon to prepare synthetic titanite powder. Preparing TiO2 in advance as a stable compound effectively inhibited the formation of rutile. Zhang [16] et al. synthesized perovskite by mixing a deactivated selective catalytic reduction (SCR) catalyst (mainly composed of TiO2) with CaCO3, and then perovskite was used as a titanium opacifier. The method of synthesizing perovskite through the pre-synthesis of TiO2 avoids TiO2 dissociating and a high-temperature phase transition to the rutile phase during ceramic sintering. Sun [14] et al. prepared a SiO2-CaCO3-TiO2 powder opacifier by wet grinding. Using the interfacial bonding between SiO2, CaCO3, and TiO2 can induce and accelerate the transformation of TiO2 to opaque titanite during the glaze sintering process, thus inhibiting the phase transition of anatase. These studies have shown that the pre-combination or pre-reaction between TiO2 and CaO or CaCO3 or CaO and SiO2 before the sintering of a glaze makes the synthesis of titanite a priority reaction in the sintering process, which is the key to improving the conversion degree of TiO2 to opacified titanite and inhibiting its conversion to rutile.
Phosphorus compounds are an important kind of chemical mineral raw material. In the ceramic industry, bone ceramics and bioactive glass ceramics are often produced by adding phosphorus compounds such as calcium phosphate [17], apatite [18], and bone ash [19] to enhance the appearance of ceramic products. Among them, apatite is divided into fluorapatite, chlorapatite, and hydroxyapatite according to its different binding anions [20]. The main components are CaO and P2O5, which play an essential role in the sintering of ceramic glaze. When apatite provides a CaO component, the precipitated P2O5 component can catalyze phase separation crystallization, making the crystal form faster, thereby improving the reflectivity of the glaze surface and making the glaze surface glossier. At the same time, a spherical glass differential phase is formed in the glaze, and the tiny glass phase separation close to the light wavelength leads to light scattering, thus having an opacifying effect [21,22].
Therefore, to improve and enhance the performance of TiO2 as a ceramic opacifier, solve the problem of glaze yellowing in its application process, and play the role of phosphorus compounds, in this study, hydroxyapatite was used as the carrier, and composite particles (ATO) coated with TiO2 on the surface of hydroxyapatite were prepared by a mechanical grinding method. In order to verify the opacifiers prepared from different sources of apatite, hydroxyapatite was replaced with concentrated apatite to prepare the opacifier (PTO). Different kinds of apatite were used as components to provide phosphorus compounds and were combined with TiO2 in advance to induce the reaction of CaO in TiO2 combined with hydroxyapatite and SiO2 in the glaze to form CaTiSiO5 so that TiO2 could form titanite without the formation of a rutile phase, to prevent the yellowing of the glaze induced by rutile in the glaze layer.
2. Materials and Methods
2.1. Materials
The raw materials used in this experiment are TiO2 and apatite. Among them, the TiO2 raw material is an anatase titanium dioxide product produced by Henan Lomon Billions Group Co., Ltd. (Jiaozuo, China). The XRD spectrum (in Section 3.1.2) shows that the characteristic peak of the anatase phase is obvious, and there are no other miscellaneous peaks. The D50 (median diameter) of the TiO2 raw material is 9.42 μm, and the D90 is 27.35 μm, indicating a serious agglomeration phenomenon. The apatite raw materials include reagent hydroxyapatite (HAP) and concentrate apatite (CAP). HAP was provided by Xi’an Haoyuan Biotechnology Co., Ltd. (Xi’an, China), and its chemical composition was analyzed using the XRF test (Table 1). CaO and P2O5 were the main components of apatite, accounting for 98.72%, indicating its high purity. CAP is obtained from the flotation of phosphate rock (Fanshan, China). From the XRF quantitative analysis in Table 1, CAP is mainly composed of apatite (86%), calcite (8%), and mica (6%). The D50 and D90 of HAP were 61.84 μm and 140.86 μm, respectively. The D50 and D90 of CAP were 98.50 μm and 289.43 μm, respectively. Both had large particle sizes, and further treatment was needed before compositing. The other reagents used in the test were mainly deionized water (self-made in the laboratory) and ethanol (Aladdin Reagent (Shanghai) Co., Ltd. (Shanghai, China)).

Table 1.
XRF results of HAP and CAP composites.
2.2. Preparation of ATO and Sanitary Ceramic Glaze
The preparation process for the ATO composite opacifier was as follows: (1) HAP was ground and disaggregated in a grinding machine (GSDM-S3, Beijing Gosdel & Technology Co., Ltd., Beijing, China. The grinding medium is a zirconia ball with a diameter of 1–3 mm; the grinding jar is made of polytetrapluroethylene material) to make its particle size meet the requirements of the ATO. The grinding conditions were as follows: the rotational speed of the grinding machine was 1000 r/min, the ball-to-powder ratio was 4:1, grinding was carried out for 60 min, and the solid content was 30%. After grinding, the D50 of HAP decreased to 1.92 μm, the D90 decreased to 4.49 μm, and the grinding effect was remarkable. (2) The TiO2 raw material was placed in the above grinding machine to grind and disaggregate. The grinding conditions were as follows: the rotational speed of the grinding machine was 1000 r/min, the ball-to-powder ratio was 4:1, grinding was carried out for 40 min, and the solid content was 30%. After grinding, the D50 of TiO2 decreased to 1.03 μm, the D90 decreased to 2.01 μm, and HAP and TiO2 reached the best particle size ratio of the particle composite. (3) The ground HAP and TiO2 were mixed in proportion and then mechanically ground to initiate the reaction between the two at the phase interface to obtain the apatite –TiO2 composite opacifier (ATO). The grinding conditions were as follows: mill speed was 1000 r/min, ball-to-material ratio was 4:1, grinding was carried out for 60 min, and the solid content was 30%. The samples with different TiO2 composite ratios were recorded as ATO-B, where B is the mass fraction of TiO2.
Ceramic samples with an ATO were prepared in an industrial roller kiln in a sanitary ceramics factory (Tangshan Zhongtao Sanitary Bath Manufacturing Co., Ltd., Tangshan, China), and the highest firing temperature was 1180 °C. The formula and proportion of the glaze are as follows (weight, %): potash feldspar, 28; quartz, 34; Suzhou kaolin, 4.5; alumina, 3; calcite, 11; dolomite, 5.5; zinc oxide, 2.5; frit, 2; methyl-rich cellulose, 0.15; CA-100, 0.07; opacifier, 8.5. (All raw materials in the glaze formula are from Tangshan Zhongtao Sanitary Bath Manufacturing Co., Ltd., Tangshan, China.). According to the above formula for an opacified glaze, the components of the basic glaze and the opacifier (with a total mass of 500 g) were weighed and placed in a ball mill, and then a 1000 g zirconium silicate grinding ball and 240 g of water were added to grind for 45 min. The grinding ball was separated by screening to obtain a glaze slurry, and the iron impurities were removed by stirring the glaze slurry continuously for 5~10 min with a magnet rod. Then, the standard porcelain body was glazed using the splashing glaze method, and the glaze thickness was about 0.8 mm. Finally, the glazed ceramic blocks were placed in an industrial roller kiln. To verify the opacifiers prepared from different sources of apatite, a PTO was prepared using the above grinding method with CAP and TiO2 as the raw materials, and the corresponding ceramic samples were sintered in a factory. For comparison, ceramic samples with pure TiO2 and ZrSiO4 as opacifiers were prepared using the same formula and sintering process.
2.3. Characterization
To characterize the opacifying effect of the opacifier, the Lab color values (L*, a*, and b*) and glossiness, which represent the glaze color, were tested. The color values (L*, a*, and b*) of the ceramic glazes were measured by an integrating sphere spectrophotometer (SP-60, X-Rite, Grand Rapids, MI, USA). The Lab color model is composed of brightness (L*), red–green (a*), and yellow–blue (b*) values. The L* value can be used to reflect the opacity performance of the glaze surface. The bigger the L* value of the glaze, the better the opacity performance. The a* and b* values can be used to calibrate the hue color of the glaze surface, where the b* value is positive, and the larger the glaze surface, the yellower it is. A gloss meter (WGG60-Y4, KSJ Photoelectrical Instruments Co., Ltd. (Shenzhen China) was used to measure the glossiness of the ceramic glaze. Glossiness is used to represent the degree of similarity of the surface of an object to a mirror surface. The measurement and evaluation of glossiness also depend on the angle between the test light source and the sample; in this paper, 60° is used for testing.
Characterization tests such as SEM, EDS, XRD, XRF, XPS, and FT-IR were used to observe the phase and microstructure and to study the bonding mechanism between HAP and TiO2. A scanning electron microscope (SEM, S-3500N, Hitachi Electron Microscope Co., Ltd., Tokyo, Japan) was used to observe the microstructure of ATO, the raw materials used, and the glaze layer of the ceramic samples. The observed glaze layer was first etched with 10 % HF to remove the glass phase. X-ray powder diffraction (XRD, D/MAX2000, Rigaku Corporation, Tokyo, Japan) was used to analyze and identify the phases in the samples. HAP and TiO2 opacifiers and an ATO were tested with an infrared spectrometer (Spectrum100, Shanghai Platinum Elmer Instrument Co., Ltd. (Shanghai, China)) and an X-ray photoelectron spectrometer (ESCAlab250, Thermo Scientific, Waltham, MA, USA) to explore the binding mechanism of HAP and TiO2.
3. Results
3.1. Morphology and Structure of ATO
3.1.1. Effect of the TiO2 Ratio on the Morphology of ATO
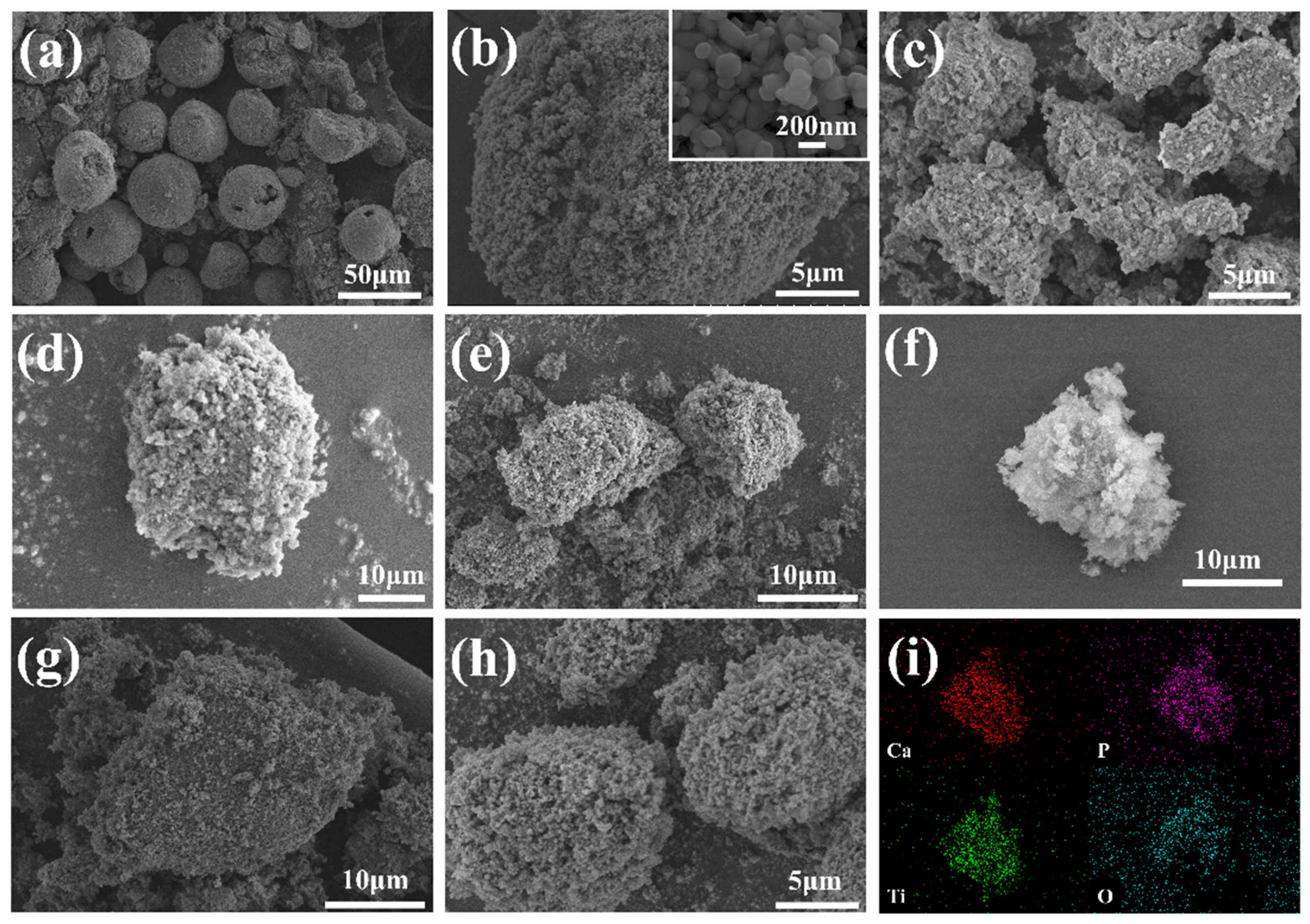
Figure 1 shows the SEM images of each sample. It can be seen from Figure 1a that the HAP raw materials are spherically distributed, the particles are large (about 50–60 μm), and the agglomeration is serious. The agglomeration of HAP after grinding (Figure 1c) is significantly improved, and the particle size is reduced to about 2–5 μm, which is more beneficial for its combination with TiO2. The morphology of the TiO2 raw material particles (Figure 1b) is spherical, and the particle size is between 200 and 300 nm, which is in the scale range of TiO2 pigment [23], but agglomeration occurs due to the small particle size. The HAP and TiO2 particles in the ATO composite particles are combined by mechanical force, which is characterized by a coating of TiO2 on the surface of the apatite to improve and reduce the agglomeration problem between TiO2 particles (Figure 1d–h). Among them, the ATO-50 sample has a better composite effect than the other samples. Figure 1i is the EDS scan of the ATO-50 sample. The Ca, P, O, and Ti elements are evenly distributed within the contour of the particles, indicating that TiO2 particles are more evenly distributed on the surface of the apatite and that the coating is more complete. To avoid the existence of free TiO2 in the ATO, ATO-50 was finally selected as the optimized composite condition.

Figure 1.
(a) HAP; (b) TiO2; (c) HAP grinding product; (d–h) ATO (30–40-50–60-70); and (i) EDS surface scan of ATO-50.
3.1.2. Phase Analysis of ATO
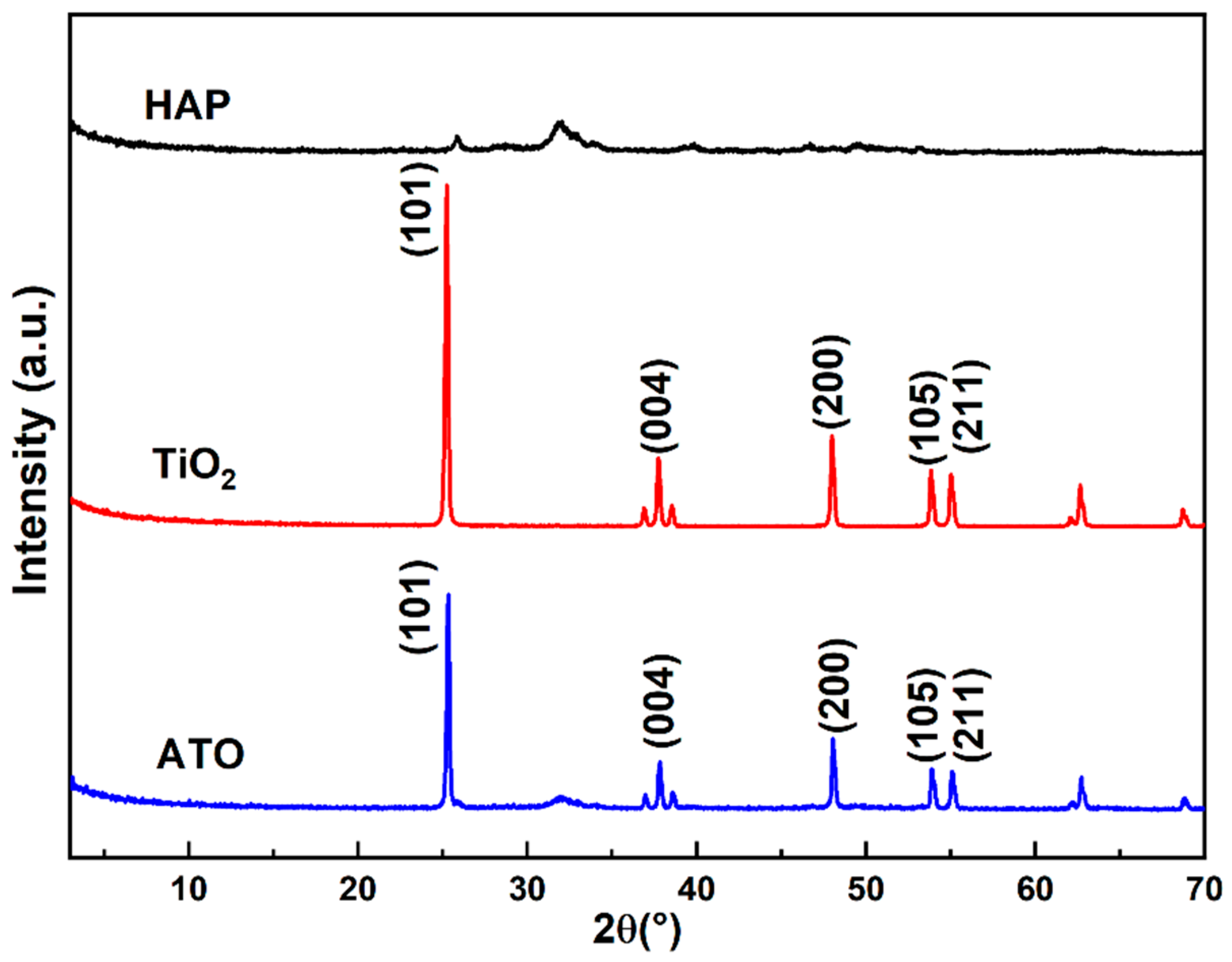
The phases of HAP, the TiO2 raw materials, and the ATO composite opacifier were analyzed using the XRD test method. From Figure 2, it is observed that the XRD spectrum of the raw hydroxyapatite has an approximately symmetrical and flat diffraction peak between diffraction angles (2θ) of 30° and 35°, and the main phase is HA. The five characteristic peaks in the XRD spectrum of TiO2 are located at 25.2°, 37.8°, 47.9°, 53.9°, and 55.1°, corresponding to the (101), (004), (200), (105), and (211) crystal planes of anatase (JCPDS 21-1272), respectively. The diffraction peaks are sharp, and there are no other peaks, indicating that the purity of the raw materials is very high. The XRD spectrum of the ATO has both the characteristic peaks of anatase and the characteristic peaks of apatite, and no new phase appears, demonstrating that the phase did not change during the composite process and that the correlation only occurs at the phase interface.
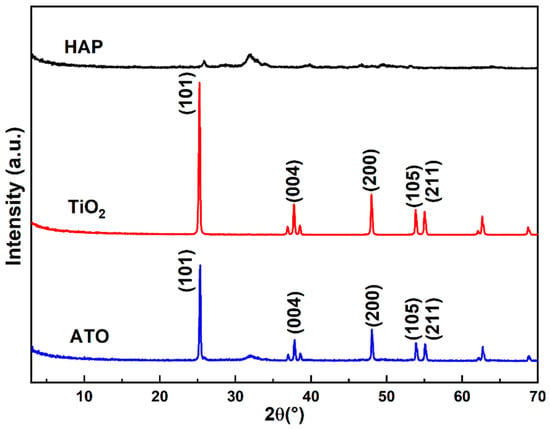
Figure 2.
XRD spectrum of HAP, TiO2, and ATO composite opacifiers.
3.1.3. Binding Properties between Apatite and TiO2
To explore and analyze the binding properties and mechanisms of HAP and TiO2 in the ATO composite opacifier, the Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) of HAP, the TiO2 raw materials, and the ATO composite opacifier were tested. The results are shown in Figure 3.
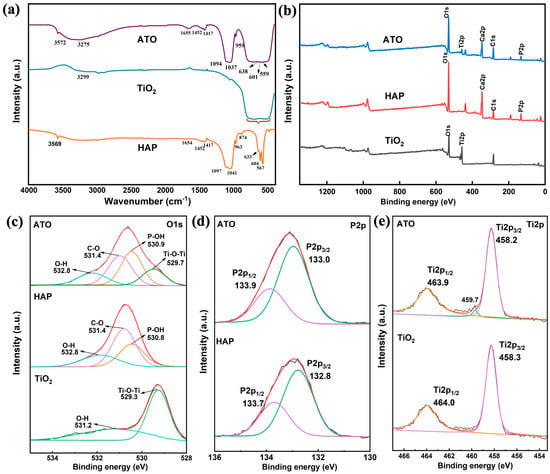
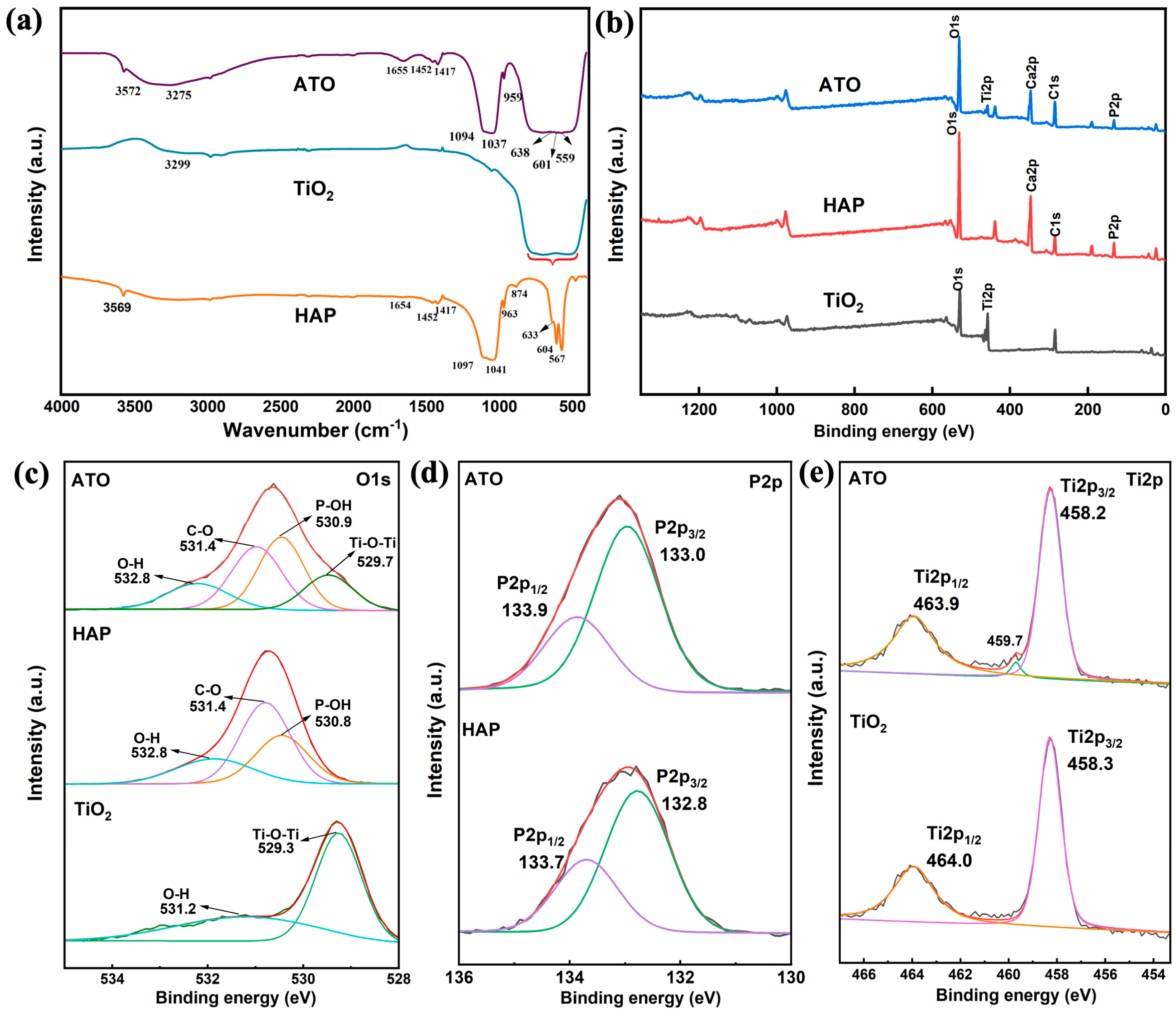
Figure 3.
(a) FT-IR spectra of HAP, TiO2, and ATO composite opacifier and (b–e) XPS spectra.
In the infrared spectrum of TiO2 in Figure 3a, the characteristic peak of the surface hydroxyl group appears at 3299 cm−1 [13], and the wide absorption band generated by the bending vibration of Ti-O-Ti is located in the range of 500–800 cm−1 [14]. In the infrared spectrum of HAP, the characteristic peaks at 567, 607, 963, 1041, and 1097 cm−1 belong to the vibration of the hydroxyapatite PO43− tetrahedron [24,25,26,27,28]. The absorption peak at 3569 cm−1 is related to -OH in HAP, and the absorption peak at 1649 cm−1 is related to the adsorbed H2O [29]. After the HAP and TiO2 composite, the wide absorption band of the Ti-O-Ti bond of the ATO sample appears at 500–1000 cm−1. At the same time, the characteristic peaks of PO43− shift to 559, 601, 959, 1037, and 1094 cm−1, respectively. The characteristic peak of the hydroxyl group on the surface of TiO2 moves to the low band and broadens. This indicates that the vibration mode of the P-O bond changed significantly after compounding with TiO2, indicating that HAP and TiO2 undergo chemical bonding on the surface. Through the obvious change in the hydroxyl position of HAP and TiO2, it is speculated that this process is completed by the hydroxyl action of the two. The detailed correspondence between the absorption peak and the functional group is shown in Table 2.

Table 2.
The corresponding relationship between infrared absorption peaks and functional groups.
Figure 3b shows the characteristic peaks of O1s, Ti2p, Ca2p, C1s, and P2p that appeared on the XPS broad-spectrum scan of the ATO, which proved the existence of the five elements O, Ti, Ca, C, and P, reflecting its component characteristics. In the narrow scanning spectrum of O1s in Figure 3c, the binding energies of O1s in HAP are 532.8 eV, 531.4 eV, and 530.8 eV, which are related to the adsorption of water, CO2, and O atoms in P-OH, respectively [30,31]. From the narrow O1s scanning spectrum of ATO, the O atoms of P-OH, H-O-H, and C=O correspond to a binding energy of 530.9 eV, 532.8 eV, and 531.4 eV, respectively. Compared with the raw HAP, the binding energy of P-OH is reduced by 0.1 eV. In addition, according to Figure 3d, the binding energy of P2p in the ATO also changed greatly compared with the HAP, indicating that the chemical environment around the P atoms has changed. In the Ti2p scanning spectrum of the ATO, a peak different from TiO2 appeared at a binding energy of 459.7 eV [14], indicating that the binding energy of Ti2p1/2 and Ti2p3/2 derived from TiO2 also changed. It is speculated that HAP and TiO2 are combined by surface hydroxyl groups, resulting in changes in the binding energy of each element.
According to the analysis of the infrared and XPS spectra, combined with the surface properties of hydroxyapatite, the mechanism of HAP and TiO2 surface bonding can be analyzed. The P-OH group on the surface of HAP is the surface-OH group produced by the surface protonation of PO43− ions on the surface of HAP particles to maintain the balance of surface electrical properties [32,33]. Through the shift of the P-OH absorption peak in the FT-IR test results, the change in the P-OH binding energy in the XPS analysis, and the change in the chemical environment of the P and Ti atoms, it is speculated that there should be a combination of a P-OH group on the surface of the HAP particles and a hydroxyl group (Ti-OH) on the surface of TiO2 to form P-O-Ti so that the two are closely combined.
3.2. The Glaze Performance of Sanitary Ceramics with an ATO
3.2.1. Effect of TiO2 Content in ATO on the Glaze Properties of Sanitary Ceramics
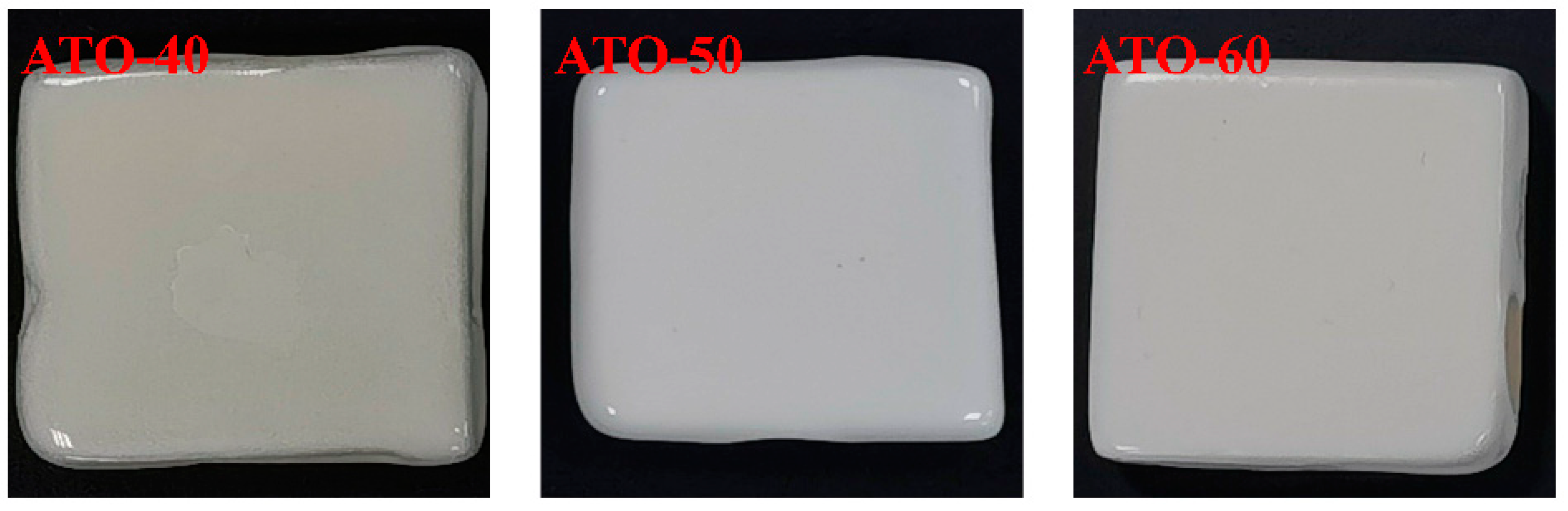
ATO-40, ATO-50, and ATO-60 were added to the ceramic base glaze as opacifiers to make a glaze slurry and sintered into a ceramic sample in a roller kiln. The Lab value, glossiness, and melting length of the glaze surface were tested. The test results are shown in Figure 4 and Table 3. As seen in Figure 4, when the opacifier is ATO-40, the ceramic glaze surface exposes the original color of the body due to insufficient covering ability (the L* value is only 74.10). When the content of TiO2 increases, the opacification performance of the glaze is also significantly improved, and a delicate, hard, and smooth white glaze is formed. When the content of TiO2 is 50%, the opacification performance of the glaze is the best, and the L* value is 89.99. When the content of TiO2 is too low, the precipitation of the opacifying phase is too small, the opacifying performance of the glaze is less, the glaze surface is affected by the body itself, and the color is dark. Compared with the sample with 50% TiO2 content, the appearance of the ceramic sample with 60% TiO2 content is yellowish, the L* value is 84.41, and the opacification performance is relatively poor. It was found that the b* value of the ceramic glaze with 60% TiO2 was 3.85, and the b* value of the glaze with 50% TiO2 was 3.37. Therefore, considering the appearance characteristics, opacification performance, and color of ceramic samples with ATOs, the ATO with a TiO2 content of 50% should be selected as the best opacifier.

Figure 4.
Digital photos of ceramic samples with ATO-40, ATO-50, and ATO-60 as opacifiers.

Table 3.
Glaze properties of ATO-40, ATO-50, ATO-60 ceramic samples as opacifiers.
3.2.2. Comparison of Properties between Ceramic Glazes with ATO, PTO, ZrSiO4, and TiO2 as Opacifiers
Figure 5 and Table 4 are digital photos of ATO, PTO, ZrSiO4, and TiO2 as opacifier ceramic samples and data comparing the chromaticity, color, and characteristics of the glazes. The results show that the L* values of the ceramic samples with an ATO and PTO are 89.99 and 88.10, respectively, which are close to the L* value (88.24) of the ceramic samples with ZrSiO4 as an opacifier, indicating that the opacification properties of the ceramic samples using an ATO and PTO are comparable to those of ZrSiO4 opacifiers. The b* values of the two glazes are 3.37 and 3.10, respectively, which are similar to those of the glaze using ZrSiO4 (the b* value is 2.29), and the appearance of the three is white, indicating that using an ATO and PTO can achieve a similar effect as using an ZrSiO4 opacifier. In contrast, the b* value of the ceramic sample with pure TiO2 as the opacifier is much higher (the b* value is 9.18), and the color of the glaze is yellow. This result shows that an ATO or PTO can solve the problem of directly using TiO2 as an opacifier. This is attributed to the intense interfacial interaction between TiO2 and apatite or phosphate concentrate through mechanical force, which improves the degree of the TiO2 chemical reaction and inhibits the transformation of anatase to rutile during high-temperature sintering.

Figure 5.
Digital photos of ATO, PTO, ZrSiO4, and TiO2 as opacifiers in ceramic samples.

Table 4.
Glaze properties and characteristics of ceramic samples with different opacifiers (ATO, PTO, ZrSiO4, and TiO2) added.
3.3. Mechanism of ATO
3.3.1. Phase Analysis
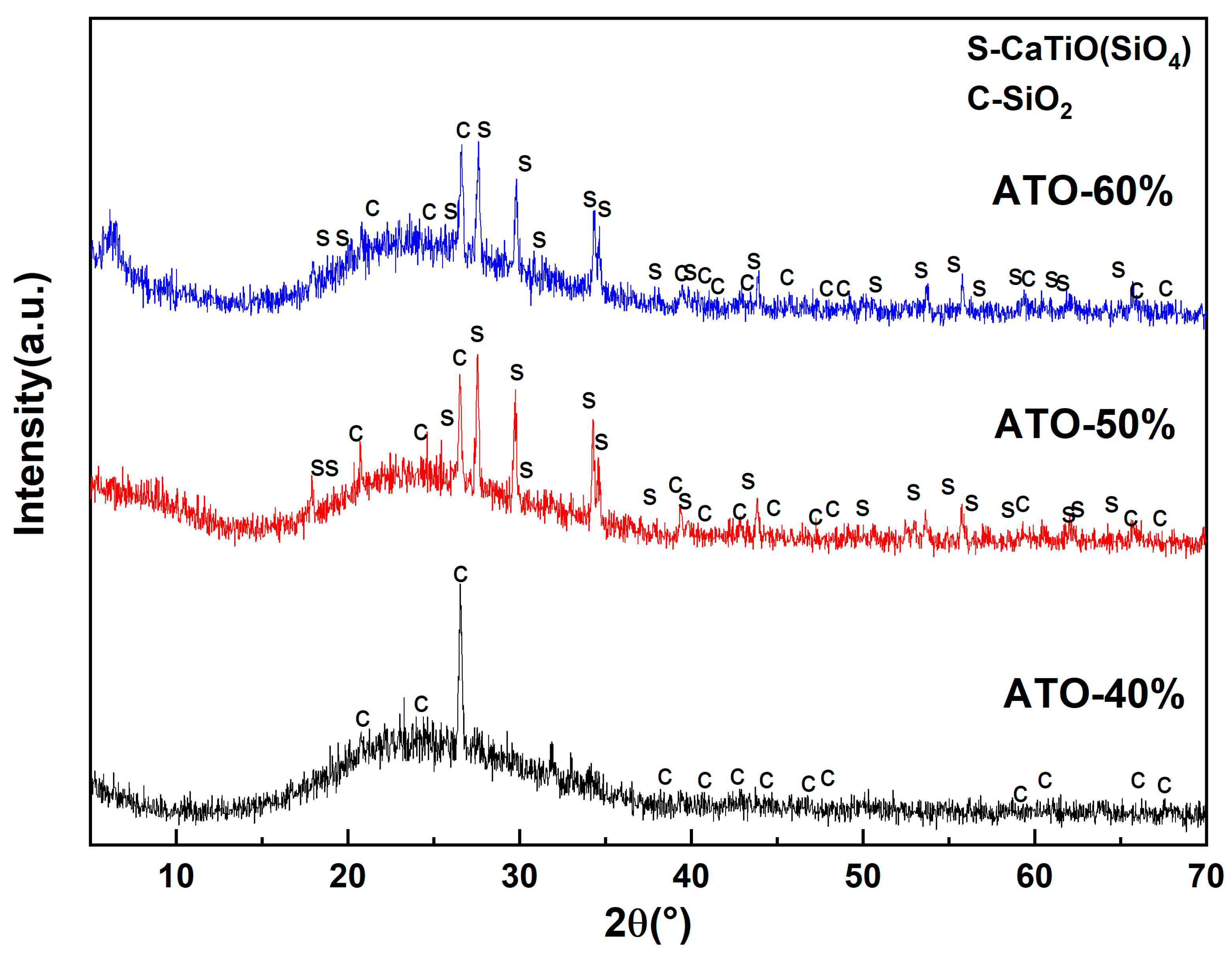
To further explore the opacification mechanism of the ATO, the composition of the glaze layers of the ceramic samples with ATO-40, ATO-50, and ATO-60 were analyzed using an XRD test. The test resulted in Figure 6. Amorphous peaks appeared in the XRD spectra of the above three samples, indicating that there were many glass phases. In addition to sphene, there were obvious quartz peaks (C, SiO2) in the ATO-40 spectrum. Quartz and sphene phases (S, CaTiSiO5) were also found in the ATO-50 and ATO-60 spectra, and the amount of sphene phase in the ATO-50 spectrum was greater than that of the quartz phase, while no sphene phase appeared in the ATO-40 spectrum. According to the results of the glaze chromaticity values of ATO-40, ATO-50, and ATO-60, the content of titanite is closely related to the opacification performance of the glaze. The ATO-40 glaze does not generate titanite, so the opacification performance of the ATO-40 glaze is poor, while the high content of titanite in the ATO-50 glaze makes its glaze performance better. Therefore, it is considered that the formation of titanite during sintering is the reason for the opacification effect of the ATO, and quartz has an auxiliary opacification effect.
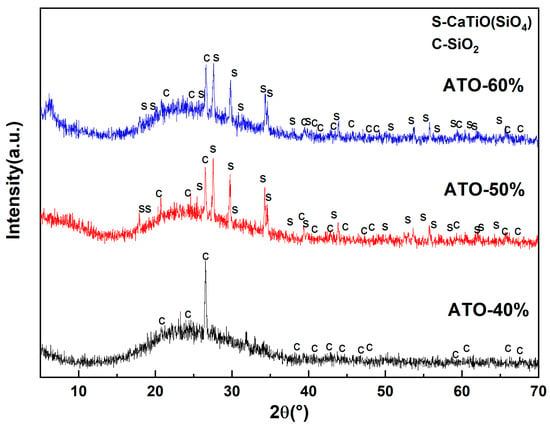
Figure 6.
XRD spectrum of glazes with ATO-40, ATO-50, and ATO-60 as opacifiers.
3.3.2. Morphology Analysis
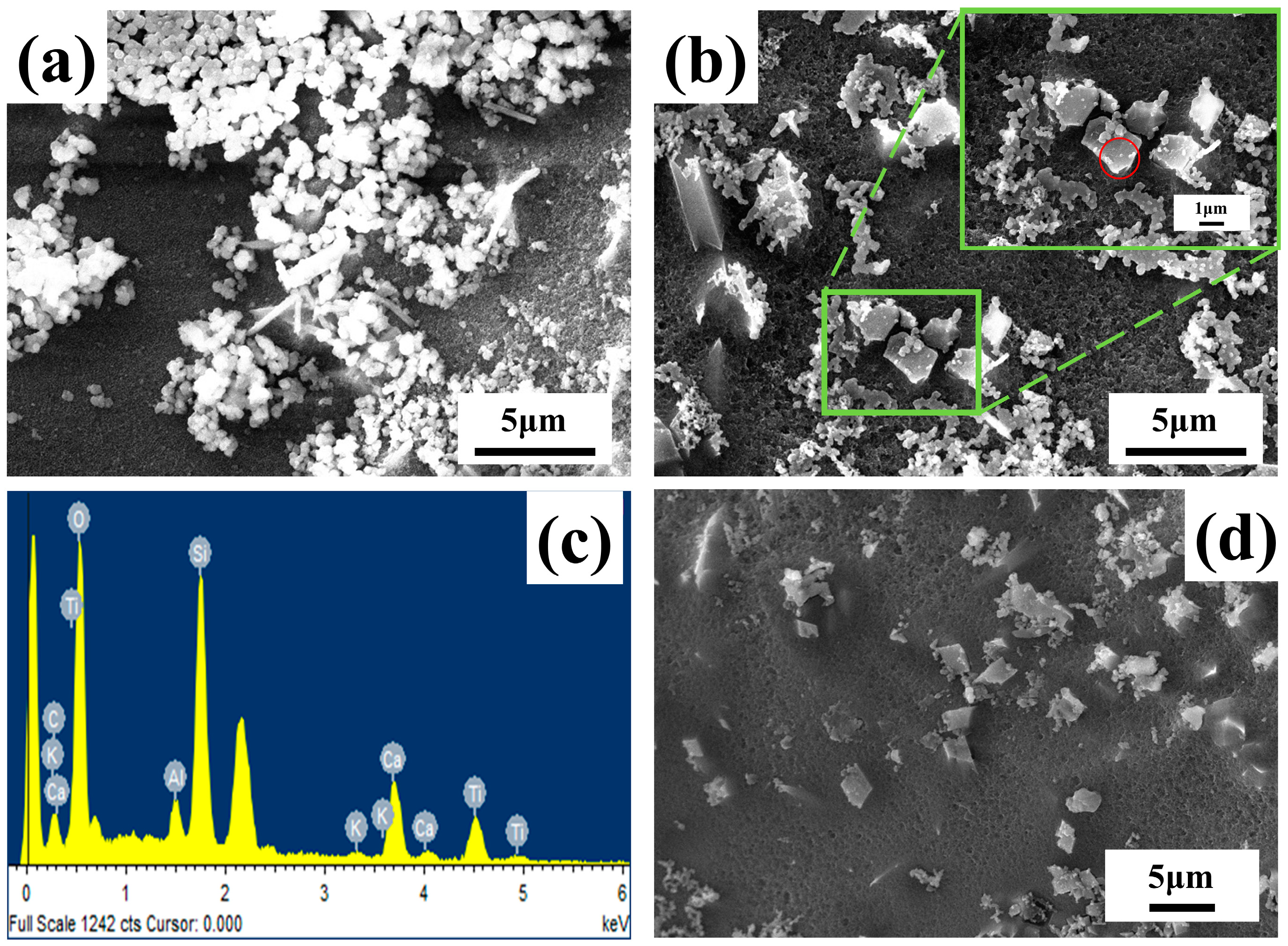
Figure 7 shows the analysis and characterization of the microstructure and morphology of the ATO composite opacifier glaze after HF acid corrosion by SEM. There are many spherical particles with a particle size of about 300 nm in the ATO-40 glaze layer, while the spherical particles in the ATO-50 and ATO-60 glaze layers become less, and wedge-shaped crystals with a particle size of about 2 μm appear. The EDS (Figure 7c) characterization shows that the wedge-shaped particles in Figure 7b are composed of Ti, Si, Ca, and O, and the atomic percentage of Ti, Si, Ca, and O is close to 1:1:1:5 (Table 5), which is the same as the chemical composition of titanite. Combined with the XRD test results and the characteristics of the titanite, it can be preliminarily inferred that the wedge crystal is titanite [16]. The spherical particles are amorphous, opaque particles composed of oxides such as Ca, Al, and Si. It should be that the phosphide generated by the decomposition of the apatite will induce the formation of a phase independent of the glass phase. According to the glaze performance data, the glaze covering ability of the ATO-50 and ATO-60 samples with titanite crystals is significantly stronger than that of the ATO-40 sample, which indicates that titanite is the component of the ATO agent with strong opacifying ability, while amorphous opacifying particles and quartz are auxiliary opacifying components.
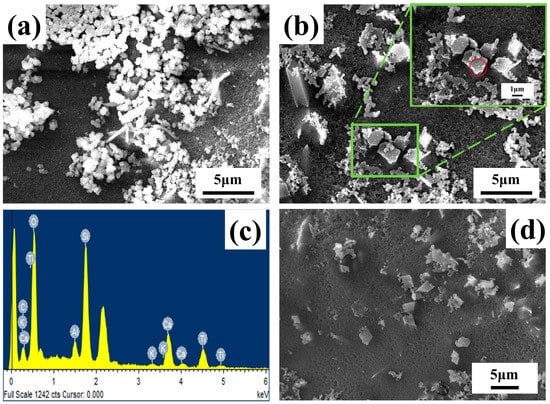
Figure 7.
SEM images of (a) ATO-40, (b)ATO-50, (d) ATO-60 glaze surfaces after HF acid corrosion and (c) EDS element scanning in the red circle area in (b).

Table 5.
EDS analysis results of the specified area of the glaze layer (ATO-50).
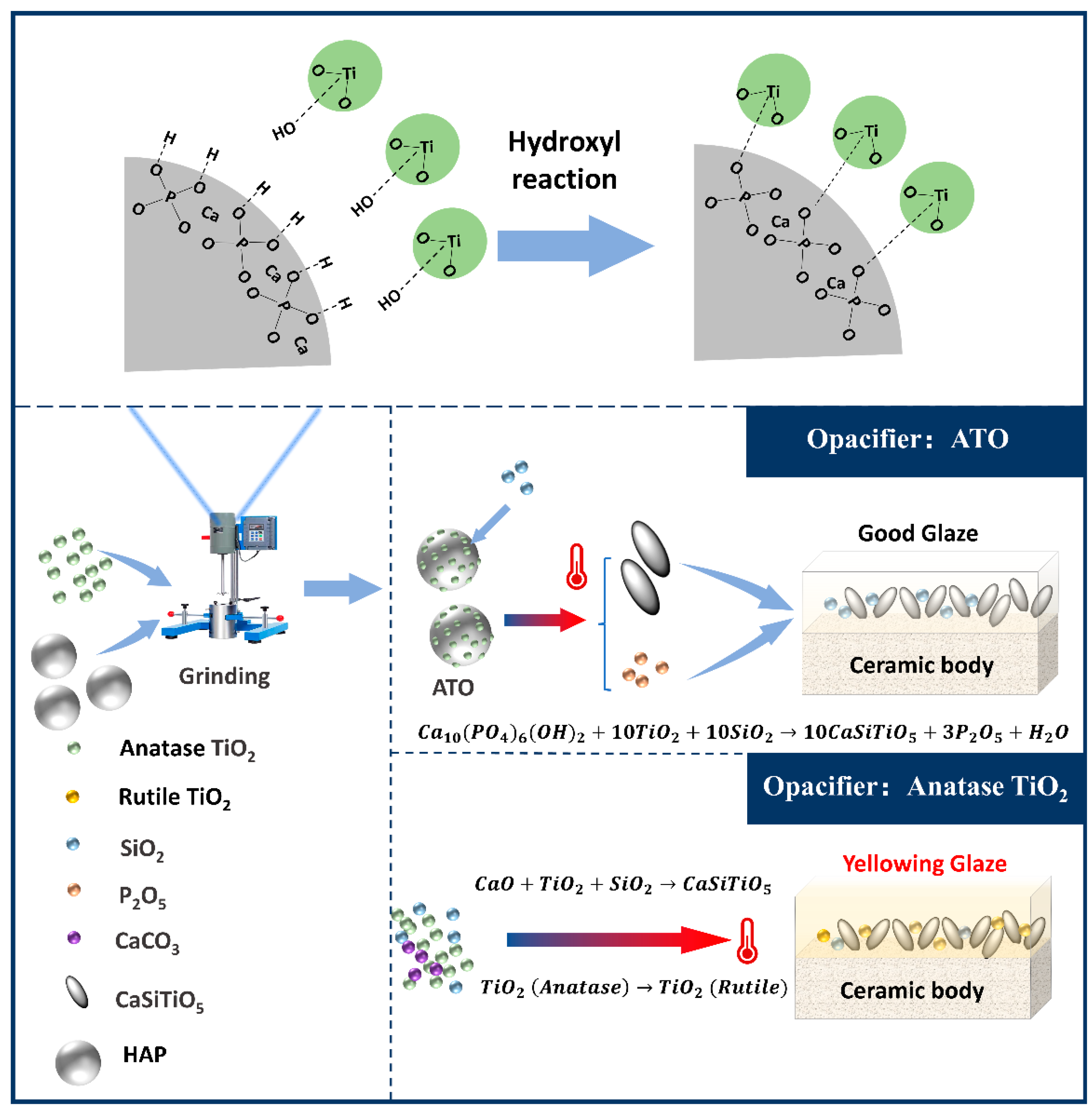
Based on this, we can analyze the unique advantages of using an ATO instead of TiO2 as a ceramic opacifier. (1) The ordered combination of the interface between hydroxyapatite and TiO2 through the dehydration condensation of hydroxyl groups in advance creates the conditions for a preferential chemical reaction of TiO2 combined with CaO during the sintering process so that TiO2 can be fully incorporated into titanite, reducing the conversion of free TiO2 into the rutile phase during high-temperature firing and avoiding glaze yellowing. (2) After the decomposition of hydroxyapatite, the generated phosphorus oxide will induce the formation of a spherical glass differential phase in the glaze. These phases will lead to light scattering, which is more conducive to the opacification effect. The binding mechanism of HAP and TiO2 in the ATO and the mechanism of avoiding the yellowing of the titanium glaze with the ATO are shown in Figure 8.
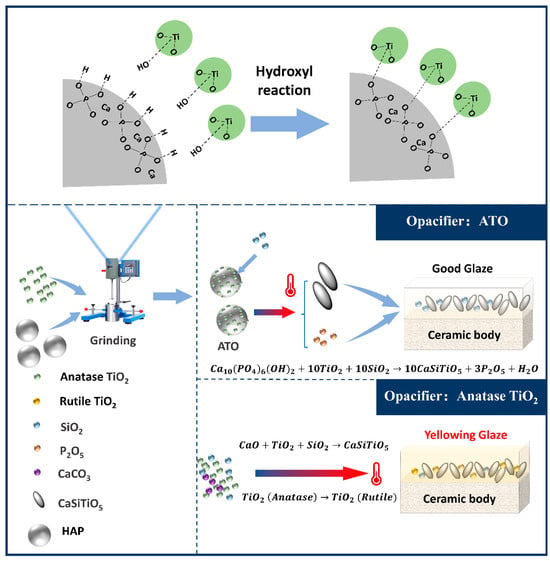
Figure 8.
The mechanism of the composite ATO and the mechanism for the prevention of glaze yellowing.
4. Conclusions
The apatite–TiO2 composite opacifier (ATO) was prepared by mechanical grinding using hydroxyapatite (HAP) and anatase TiO2 as raw materials. The ATO is characterized by the uniform adhesion of the TiO2 on the surface of the apatite, and there is a close chemical bond between the HAP and the TiO2. White-glazed sanitary ceramics were prepared by using the ATO. The appearance of the ceramic glaze was white, and the opacification was excellent. The L*, a*, and b* values of the glaze were 89.99, −0.85, and 3.37, respectively. The opacification and whiteness of the glaze were similar to those of the ZrSiO4 glaze. The opacification of the glaze was slightly lower than that of the TiO2 glaze but whiter than TiO2. The composition of the glaze is a glass phase, titanite, and a small amount of quartz, in which titanite is in an opacified phase. The opacification mechanism is the crystallization opacification of the titanite phase, and the spherical glass formed at the same time is relatively beneficial to improving the opacification of the glaze surface.
The advantage of this study is that when an ATO is sintered at a high temperature, the interfacial ordered bonding between hydroxyapatite and TiO2 induces the binding reaction of TiO2 with CaO and further with SiO2 at high temperatures so that TiO2 can enter the sphene without rutile phase formation, thus inhibiting the yellowing of the glaze. The use of an ATO can play an active role in solving the key problem that occurs when TiO2 replaces ZrSiO4 as an opacifier.
Author Contributions
Conceptualization, X.B., L.C. and M.B.; methodology, Y.L. and J.Z.; software, X.B.; validation, X.B., H.Z. and Y.T.; formal analysis, H.Z.; investigation, H.D.; resources, H.D.; data curation, X.B., H.Z. and S.S.; writing—original draft preparation, H.Z.; writing—review and editing, X.B.; visualization, X.B.; supervision, S.S.; project administration, H.D.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Jianmeng Zhang was employed by the company Beijing Building Materials Academy of Sciences Research Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Casasola, R.; Rincón, J.M.; Romero, M. Glass-ceramic glazes for ceramic tiles: A review. J. Mater. Sci. 2012, 47, 553–582. [Google Scholar] [CrossRef]
- Tang, H.D.; Hu, Q.; Jiang, F.; Jiang, W.H.; Liu, J.M.; Chen, T.; Feng, G.; Wang, T.; Luo, W. Size control of C@ZrSiO4 pigments via soft mechano-chemistry assisted non-aqueous sol-gel method and their application in ceramic glaze. Ceram. Int. 2019, 45, 10756–10764. [Google Scholar] [CrossRef]
- Molera, J.; Pradell, T.; Salvadó, N.; Vendrell-Saz, M. Evidence of tin oxide recrystallization in opacified lead glazes. J. Am. Ceram. Soc. 1999, 82, 2871–2875. [Google Scholar] [CrossRef]
- Noble, B.D.; Parker, J.M.; James, P.F.; Hand, R.J.; Smith, A.; Booth, J. Opacification of ZnO-B2O3-ZrO2 glasses. Glass Technol. Part A 2004, 45, 101–104. [Google Scholar]
- Shimanskaya, A.N.; Levitskii, I.A. Formation Particularities of Titanium-Containing Glaze Coatings for Floor Tiles. Glass Ceram. 2016, 73, 94–99. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Tian, S.; Xu, L.; Xie, J.; Zhong, T. Study on Yellow Opaque Glass with Mixed Coloring Agents CeO2 and TiO2. J. Wuhan Univ. Technol. 2013, 35, 34–37. [Google Scholar]
- Colomban, P.; Gironda, M.; Vangu, D.; Kirmizi, B.; Zhao, B.; Cochet, V. The Technology Transfer from Europe to China in the 17th–18th Centuries: Non-Invasive On-Site XRF and Raman Analyses of Chinese Qing Dynasty Enameled Masterpieces Made Using European Ingredients/Recipes. Materials 2021, 14, 7434. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.K.; Liu, K.; Liu, J.L.; Wang, Y.Q.; Yang, Y.L.; Chang, Q.B. Synthesis, characterization and application of submicron ZrSiO4 powder via sol-gel-microemulsion-hydrothermal method. J. Alloys Compd. 2020, 828, 154332. [Google Scholar] [CrossRef]
- Yu, Y.G.; Su, H.Z.; Peng, C.; Wu, J.G. Submicro-zirconia crystal-intergrown zircon opaque glaze. J. Eur. Ceram. Soc. 2019, 39, 652–659. [Google Scholar] [CrossRef]
- Snyders, E.; Potgieter, J.H.; Nel, J. The upgrading of an inferior grade zircon to superior opacifier for sanitary ware and glazes. J. South. Afr. Inst. Min. Metall. 2005, 105, 459–463. [Google Scholar]
- Eldefrawi, S.A.; Serry, M.A.; Elfattah, W.I.A.; Weisweiler, W. Microchemistry and Microstructure of Some Opaque Glaze Tile Interfaces in Relation to their Physical-Properties. Ceram. Int. 1995, 21, 69–75. [Google Scholar] [CrossRef]
- Tarity, T.D.; Vigdorchik, J.M.; Westrich, G.H.; Gonzalez Della Valle, A.; Cerrito, P.; Baral, E.C.; Bromage, T.G.; Bauer, T.W. Adaptive Immune Response Associated with a Zirconium-Containing, Cemented, Total Knee Arthroplasty: A Case Report. JBJS Case Connect. 2021, 11, e21. [Google Scholar] [CrossRef]
- Teixeira, S.; Bernardin, A.M. Development of TiO2 white glazes for ceramic tiles. Dyes Pigments 2009, 80, 292–296. [Google Scholar] [CrossRef]
- Sun, S.; Ding, H.; Ao, W.; Zhang, B.; Liu, Y.; Zhang, J.; Zhang, H.; Li, M. A zirconium-free glaze system for sanitary ceramics with SiO2-CaCO3-TiO2 composite opacifier containing anatase: Effect of interface combination among SiO2, CaCO3 and TiO2. J. Eur. Ceram. Soc. 2022, 42, 2523–2534. [Google Scholar] [CrossRef]
- Li, Y.Z.; Zhang, T.; Ding, H.; Sun, S.J.; Ao, W.H.; Zhang, J.M.; Zhang, H. Synthesis of sphene by TiO2 combined with CaCO3 and SiO2 in solid phase and its application as ceramic opacifier. Chem. Phys. 2023, 575, 2076. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.M.; Ding, H.; Li, Y.Z.; Sun, S.J.; Ao, W.H.; Liang, Y. Solid-phase synthesis of perovskite using spent SCR catalyst and calcium carbonate and its application as ceramic opacifier. J. Ind. Eng. Chem. 2022, 114, 499–507. [Google Scholar] [CrossRef]
- Krut’ko, V.K.; Maslova, L.Y.; Musskaya, O.N.; Safronova, T.V.; Kulak, A.I. Calcium Phosphate Ceramic Foam Obtained by Firing a Hydroxyapatite—Monocalcium Phosphate Monohydrate Powder Mixture. Glass Ceram. 2022, 78, 476–480. [Google Scholar] [CrossRef]
- Teramoto, H.; Kawai, A.; Sugihara, S.; Yoshida, A.; Inoue, H. Resorption of apatite-wollastonite containing glass-ceramic and β-tricalcium phosphate in vivo. Acta Med. Okayama 2005, 59, 201–207. [Google Scholar] [PubMed]
- Bih, N.L.; Mahamat, A.A.; Chinweze, C.; Ayeni, O.; Bidossèssi, H.J.; Onwualu, P.A.; Boakye, E.E. The Effect of Bone Ash on the Physio-Chemical and Mechanical Properties of Clay Ceramic Bricks. Buildings 2022, 12, 336. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Cheng, J.S.; Qiu, H.G.; Li, H.; Xie, J. Effects of phosphorus and fluorine on the crystallization and structure of CaO-Al2O3-SiO2 system glass-ceramic. In High-Performance Ceramics V; Chinese Ceram Soc: Changsha, China, 2008; Volume 368–372, Parts 1 and 2; pp. 1412–1414. [Google Scholar]
- London, D.; Morgan, G.B.; Babb, H.A.; Loomis, J.L. Behavior and effects of phosphorus in the system Na2O-K2O-Al2O3-SiO2-P2O5-H2O at 200 MPA(H2O). Contrib. Mineral. Petrol. 1993, 113, 450–465. [Google Scholar] [CrossRef]
- Sun, S.J.; Ding, H.; Hou, X.F.; Chen, D.M.; Yu, S.R.; Zhou, H.; Chen, Y. Effects of organic modifiers on the properties of TiO2-coated CaCO3 composite pigments prepared by the hydrophobic aggregation of particles. Appl. Surf. Sci. 2018, 456, 923–931. [Google Scholar] [CrossRef]
- Deisinger, U.; Stenzel, F.; Ziegler, G. Hydroxyapatite ceramics with tailored pore structure. In Euro Ceramics VIII; Mandal, H., Ovecoglu, L., Eds.; Turkish Ceram Soc and European Ceram Soc: Istanbul, Turkey, 2004; Volume 264–268, Parts 1–3; pp. 2047–2050. [Google Scholar]
- Figueroa-Rosales, E.X.; Martinez-Juarez, J.; Garcia-Diaz, E.; Hernandez-Cruz, D.; Sabinas-Hernandez, S.A.; Robles-aguila, M.J. Photoluminescent Properties of Hydroxyapatite and Hydroxyapatite/Multi-Walled Carbon Nanotube Composites. Crystals 2021, 11, 832. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Kimber, J.A.; Borkowski, L.; Pawlowska, M.; Polkowska, I.; Kalisz, G.; Belcarz, A.; Jozwiak, K.; Ginalska, G.; Kazarian, S.G. The biocompatibility of carbon hydroxyapatite/β-glucan composite for bone tissue engineering studied with Raman and FTIR spectroscopic imaging. Anal. Bioanal. Chem. 2015, 407, 7775–7785. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Altomare, A.; Mesto, E.; Lacalamita, M.; Dida, B.; Mele, A.; Bauer, E.M.; Puzone, M.; Tempesta, E.; Capelli, D.; et al. Structural Characterization of Low-Sr-Doped Hydroxyapatite Obtained by Solid-State Synthesis. Crystals 2023, 13, 117. [Google Scholar] [CrossRef]
- Skwarek, E.; Janusz, W.; Gun’ko, V.M.; Pakhlov, E.M.; Zarko, V.I.; Gdula, K. Characteristics of surface and electrochemical properties of composites with fumed metal oxides and hydroxyapatite. Adsorption 2016, 22, 725–734. [Google Scholar] [CrossRef][Green Version]
- Noviyanti, A.R.; Asyiah, E.N.; Permana, M.D.; Dwiyanti, D.; Suryana; Eddy, D.R. Preparation of Hydroxyapatite-Titanium Dioxide Composite from Eggshell by Hydrothermal Method: Characterization and Antibacterial Activity. Crystals 2022, 12, 1599. [Google Scholar] [CrossRef]
- Negrila, C.C.; Predoi, M.V.; Iconaru, S.L.; Predoi, D. Development of Zinc-Doped Hydroxyapatite by Sol-Gel Method for Medical Applications. Molecules 2018, 23, 2986. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, X.; Song, W.; Wang, C.; Zhang, H.; Huang, X. Removal of benzohydroxamic acid from aqueous solutions using multi-walled carbon nanotubes/iron-doped hydroxyapatite composites: Synthesis, adsorption performance, and characteristics. J. Environ. Chem. Eng. 2023, 11, 111259. [Google Scholar] [CrossRef]
- Zhou, S.; Zheng, X.; Yu, X.; Wang, J.; Weng, J.; Li, X.; Feng, B.; Yin, M. Hydrogen bonding interaction of poly(D,L-lactide)/hydroxyapatite nanocomposites. Chem. Mater. 2007, 19, 247–253. [Google Scholar] [CrossRef]
- Hu, A.M.; Lei, T.; Li, M.; Chang, C.K.; Ling, H.Q.; Mao, D.L. Preparation of nanocrystals hydroxyapatite/TiO2 compound by hydrothermal treatment. Appl. Catal. B Environ. 2006, 63, 41–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).