Fabrication and Characterization of Silicon-Based Antimonene Thin Film via Electron Beam Evaporation
Abstract
1. Introduction
2. Experiment, Results and Discussion
2.1. Selection of Substrate
2.2. Experimental Preparation and Result Discussion of Antimonen
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Giovanni, P.; Marco, G.; Elias, D.; Nicola, M.; Giuseppe, I.; Gianluca, F. Performance of arsenene and antimonene double-gate MOSFETs from first principles. Nat. Commun. 2016, 7, 12585. [Google Scholar]
- Zhang, S.; Yan, Z.; Li, Y.; Chen, Z.; Zeng, H. Atomically thin arsenene and antimonene: Semimetal–semiconductor and indirect–direct band-gap transitions. Angew. Chem. 2015, 127, 3155–3158. [Google Scholar] [CrossRef]
- Ji, J.; Song, X.; Liu, J.; Yan, Z.; Huo, C.; Zhang, S.; Su, M.; Liao, L.; Wang, W.; Ni, Z.; et al. Two-dimensional antimonene single crystals grown by van der Waals epitaxy. Nat. Commun. 2016, 7, 13352. [Google Scholar] [CrossRef] [PubMed]
- Carrasco Andrés, J.A.; Congost Escoin, P.; Assebban, M.; Abellán Sáez, G. Antimonene: A tuneable post-graphene material for advanced applications in optoelectronics, catalysis, energy and biomedicine. Chem. Soc. Rev. 2023, 52, 1288–1330. [Google Scholar] [CrossRef] [PubMed]
- Ares, P.; Pakdel, S.; Palacio, I.; Paz, W.S.; Rassekh, M.; Rodríguez-San Miguel, D.; Aballe, L.; Foerster, M.; del Árbol, N.R.; Martín-Gago, J.Á.; et al. Few-layer antimonene electrical properties. Appl. Mater. Today 2021, 24, 101132. [Google Scholar] [CrossRef]
- Fortin-Deschênes, M.; Waller, O.; Mentes, T.O.; Locatelli, A.; Mukherjee, S.; Genuzio, F.; Levesque, P.L.; Hébert, A.; Martel, R.; Moutanabbir, O. Synthesis of antimonene on germanium. Nano Lett. 2017, 17, 4970–4975. [Google Scholar] [CrossRef] [PubMed]
- Sheverdyaeva, P.M.; Hogan, C.; Bihlmayer, G.; Fujii, J.; Vobornik, I.; Jugovac, M.; Kundu, A.K.; Gardonio, S.; Benher, Z.R.; Santo, G.D.; et al. Giant and Tunable Out-of-Plane Spin Polarization of Topological Antimonene. Nano Lett. 2023, 23, 6277–6283. [Google Scholar] [CrossRef] [PubMed]
- Lugovskoi, A.V.; Katsnelson, M.I.; Rudenko, A.N. Electron-phonon properties, structural stability, and superconductivity of doped antimonene. Phys. Rev. B 2019, 99, 064513. [Google Scholar] [CrossRef]
- Cheung, C.H.; Fuh, H.R.; Hsu, M.C.; Lin, Y.C.; Chang, C.R. Spin orbit coupling gap and indirect gap in strain-tuned topological insulator-antimonene. Nanoscale Res. Lett. 2016, 11, 459. [Google Scholar] [CrossRef]
- Kripalani, D.R.; Kistanov, A.A.; Cai, Y.; Xue, M.; Zhou, K. Strain engineering of antimonene by a first-principles study: Mechanical and electronic properties. Phys. Rev. B 2018, 98, 085410. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, X.; Li, L. Strain-driven band inversion and topological aspects in Antimonene. Sci. Rep. 2015, 5, 16108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhang, Y.; Dong, S. Protective layer enhanced the stability and superconductivity of tailored antimonene bilayer. Phys. Rev. Mater. 2018, 2, 126004. [Google Scholar] [CrossRef]
- Yu, Y.; Meng, Q.; Liu, T. Multifunctional and durable graphene-based composite sponge doped with antimonene nanosheets. J. Mater. Res. Technol. 2022, 17, 2466–2479. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Down, M.P.; Gibaja, C.; Lorenzo, E.; Zamora, F.; Banks, C.E. Antimonene: A novel 2D nanomaterial for supercapacitor applications. Adv. Energy Mater. 2018, 8, 1702606. [Google Scholar] [CrossRef]
- Ismail, M.M.; Vigneshwaran, J.; Arunbalaji, S.; Mani, D.; Arivanandhan, M.; Jose, S.; Jayavel, R. Antimonene nanosheets with enhanced electrochemical performance for energy storage applications. Dalton Trans. 2020, 49, 13717–13725. [Google Scholar] [CrossRef]
- Khaled, A.; Arun, U.; Gaurav, S.; Arjuna, M.; Meshari, A.; Ammar, A. A theoretical analysis of refractive index sensor with improved sensitivity using titanium dioxide, graphene, and antimonene grating: Pseudomonas bacteria detection. Measurement 2023, 216, 112957. [Google Scholar]
- Raikwar, S.; Srivastava, D.K.; Saini, J.P.; Raikwar, Y.K. 2D-antimonene-based surface plasmon resonance sensor for improvement of sensitivity. Appl. Phys. A 2021, 127, 92. [Google Scholar] [CrossRef]
- Xue, T.; Liang, W.; Li, Y.; Sun, Y.; Xiang, Y.; Zhang, Y.; Dai, Z.; Duo, Y.; Wu, L.; Qi, K.; et al. Ultrasensitive detection of miRNA with an antimonene-based surface plasmon resonance sensor. Nat. Commun. 2019, 10, 28. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, S.K.; Sonvane, Y.; Lukačević, I. Antimonene: A monolayer material for ultraviolet optical nanodevices. J. Mater. Chem. C 2016, 4, 6386–6390. [Google Scholar] [CrossRef]
- Hai, T.; Xie, G.; Ma, J.; Shao, H.; Qiao, Z.; Qin, Z.; Sun, Y.; Wang, F.; Yuan, P.; Ma, J.; et al. Pushing optical switch into deep mid-infrared region: Band theory, characterization, and performance of topological semimetal antimonene. ACS Nano 2021, 15, 7430–7438. [Google Scholar] [CrossRef]
- Harith, A.; Atikah, N.A.; Zharif, S.; Aisyah, S.R.; Norazriena, Y. Solution-processed antimonene integrated arc-shaped fiber for mode-locked pulse laser generation at 1.9 μm spectral region. Opt. Mater. 2022, 131, 112635. [Google Scholar]
- Ahmad, H.; Nizamani, B.; Samion, M.Z.; Yusoff, N.; Ismail, M.F. Antimonene-based saturable absorber for a soliton mode-locked and -switched fiber laser in the 2 μm wavelength region. Chin. Phys. B 2023, 32, 064205. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, G.; Xin, Y.; Xing, F.; Han, K.; Zhang, W.; Zhang, F.; Fu, S. Passively Q-switched modulation based on antimonene in erbium-doped fiber laser with a long term stability. Opt. Mater. 2021, 118, 111256. [Google Scholar] [CrossRef]
- Kumar, A.; Sachdeva, G.; Pandey, R.; Karna, P.S. Optical absorbance in multilayer two-dimensional materials: Graphene and antimonene. Appl. Phys. Lett. 2020, 116, 263102. [Google Scholar] [CrossRef]
- Wang, G.; Pandey, R.; Karna, S.P. Atomically thin group V elemental films: Theoretical investigations of antimonene allotropes. ACS Appl. Mater. Interfaces 2015, 7, 11490–11496. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Sonvane, Y.; Wang, G.; Pandey, R. Size and edge roughness effects on thermal conductivity of pristine antimoneneallotropes. Chem. Phys. Lett. 2015, 641, 169–172. [Google Scholar] [CrossRef]
- Ares, P.; Aguilar-Galindo, F.; Rodríguez-San-Miguel, D.; Aldave, D.A.; Diaz-Tendero, S.; Alcamí, M.; Martín, F.; Gómez-Herrero, J.; Zamora, F. Mechanical isolation of highly stable antimonene under ambient conditions. Adv. Mater. 2016, 28, 6332–6336. [Google Scholar] [CrossRef]
- Gibaja, C.; Rodriguez-San-Miguel, D.; Ares, P.; Gómez-Herrero, J.; Varela, M.; Gillen, R.; Maultzsch, J.; Hauke, F.; Hirsch, A.; Abellán, G.; et al. Few-layer antimonene by liquid-phase exfoliation. Angew. Chem. 2016, 128, 14557–14561. [Google Scholar] [CrossRef]
- Gibaja, C.; Assebban, M.; Torres, I.; Fickert, M.; Sanchis-Gual, R.; Brotons, I.; Paz, W.S.; Palacios, J.J.; Michel, E.G.; Abellán, G.; et al. Liquid phase exfoliation of antimonene: Systematic optimization, characterization and electrocatalytic properties. J. Mater. Chem. A 2019, 7, 22475–22486. [Google Scholar] [CrossRef]
- Kim, S.H.; Jin, K.H.; Park, J.; Kim, J.S.; Jhi, S.H.; Yeom, H.W. Topological phase transition and quantum spin Hall edge states of antimony few layers. Sci. Rep. 2016, 6, 33193. [Google Scholar] [CrossRef]
- Fortin-Deschênes, M.; Jacobberger, R.M.; Deslauriers, C.A.; Waller, O.; Bouthillier, É.; Arnold, M.S.; Moutanabbir, O. Dynamics of antimonene–graphene van der Waals growth. Adv. Mater. 2019, 31, 1900569. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Zeng, L.; Li, Z.; Sun, L.; Qiao, Z.; Qu, Y.; Liu, G.; Li, L. Research Progress and Applications of 2D Antimonene. Appl. Sci. 2022, 13, 35. [Google Scholar] [CrossRef]
- Kuriakose, S.; Jain, K.S.; Tawfik, A.S.; Spencer, S.J.M.; Murdoch, J.B.; Singh, M.; Rahman, F.; Mayes, H.L.E.; Taha, Y.M.; Ako, T.R.; et al. Monocrystalline Antimonene Nanosheets via Physical Vapor Deposition. Adv. Mater. Interfaces 2020, 7, 2001678. [Google Scholar] [CrossRef]
- Sander, M. Diffusion-limited aggregation: A kinetic critical phenomenon. Contemp. Phys. 2000, 41, 203–218. [Google Scholar] [CrossRef]
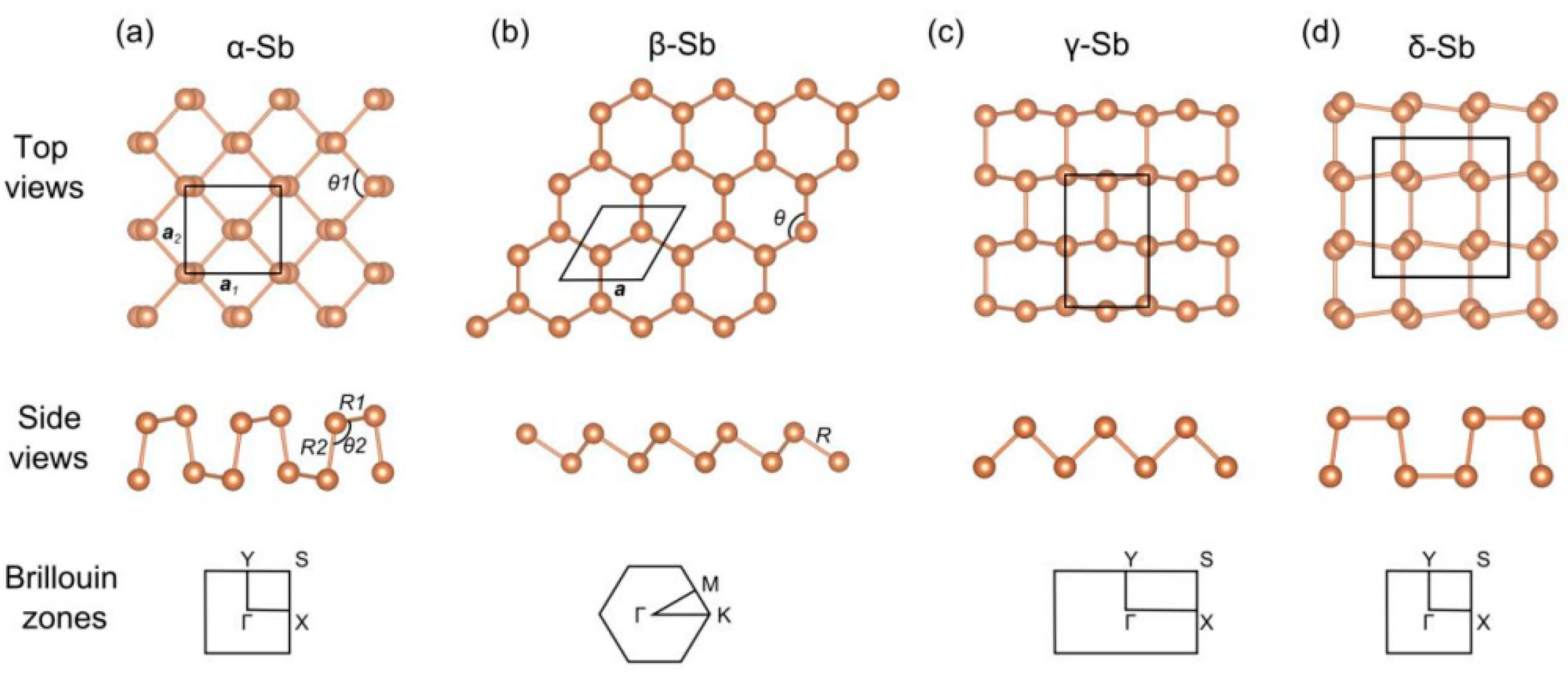




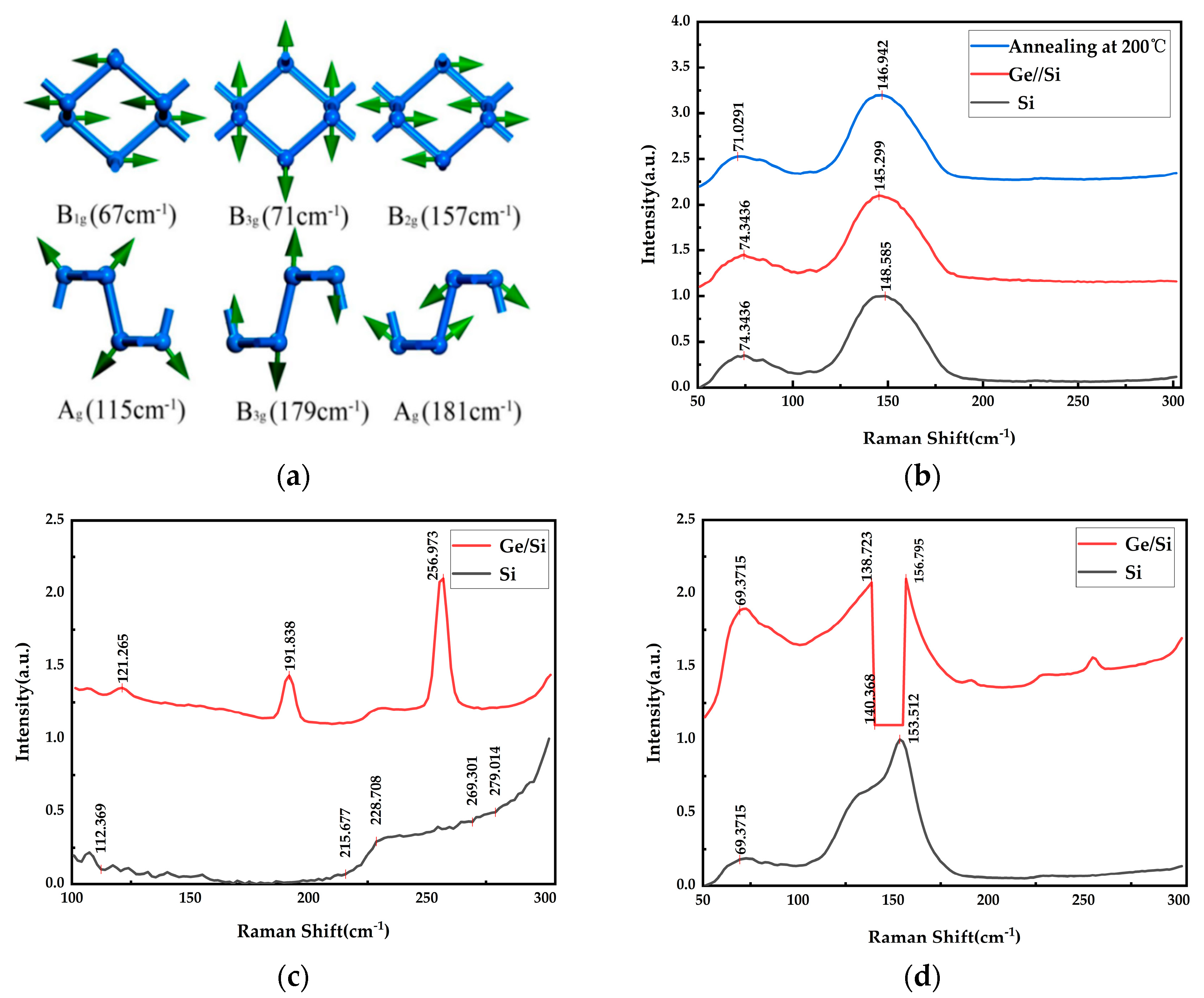


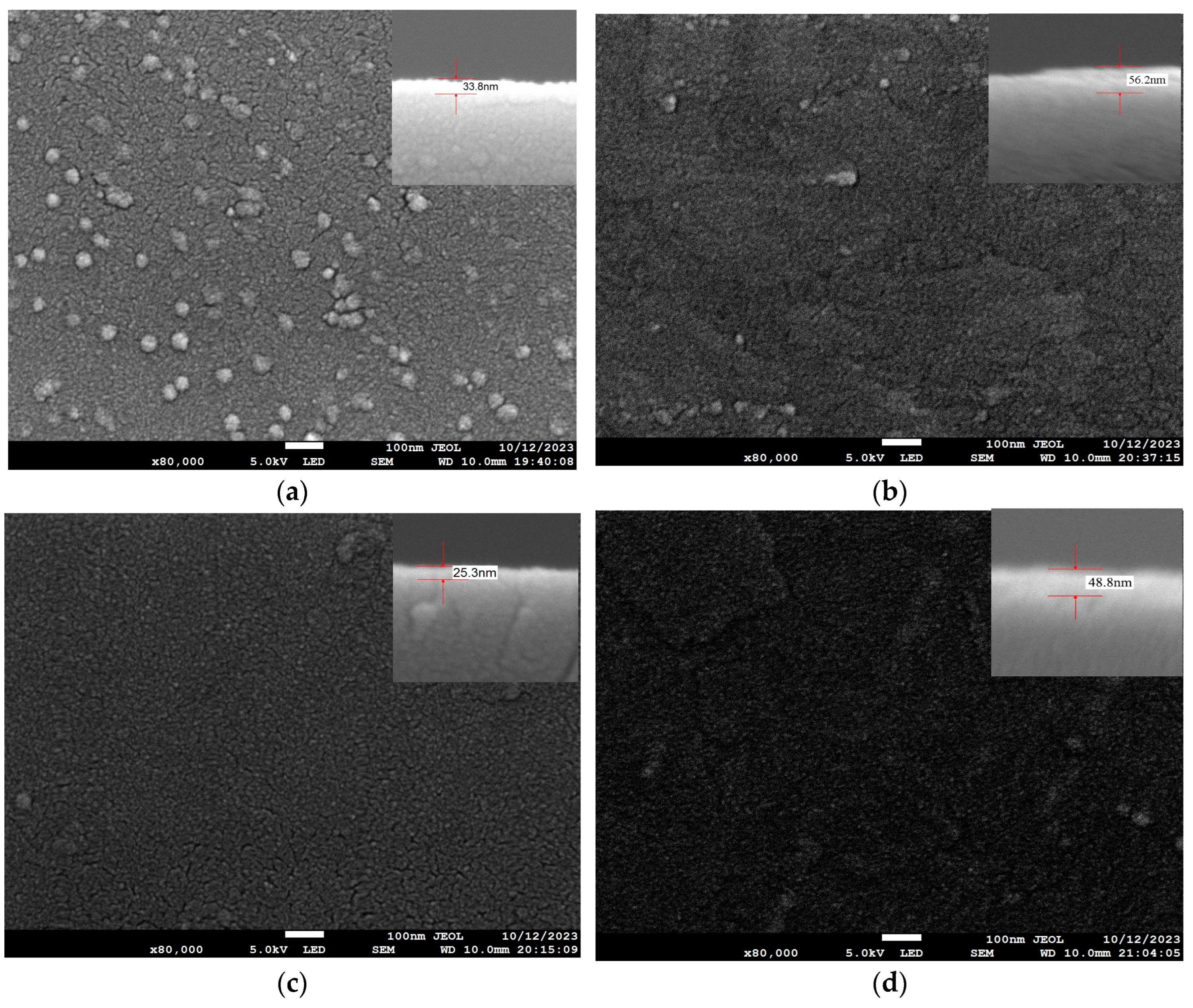
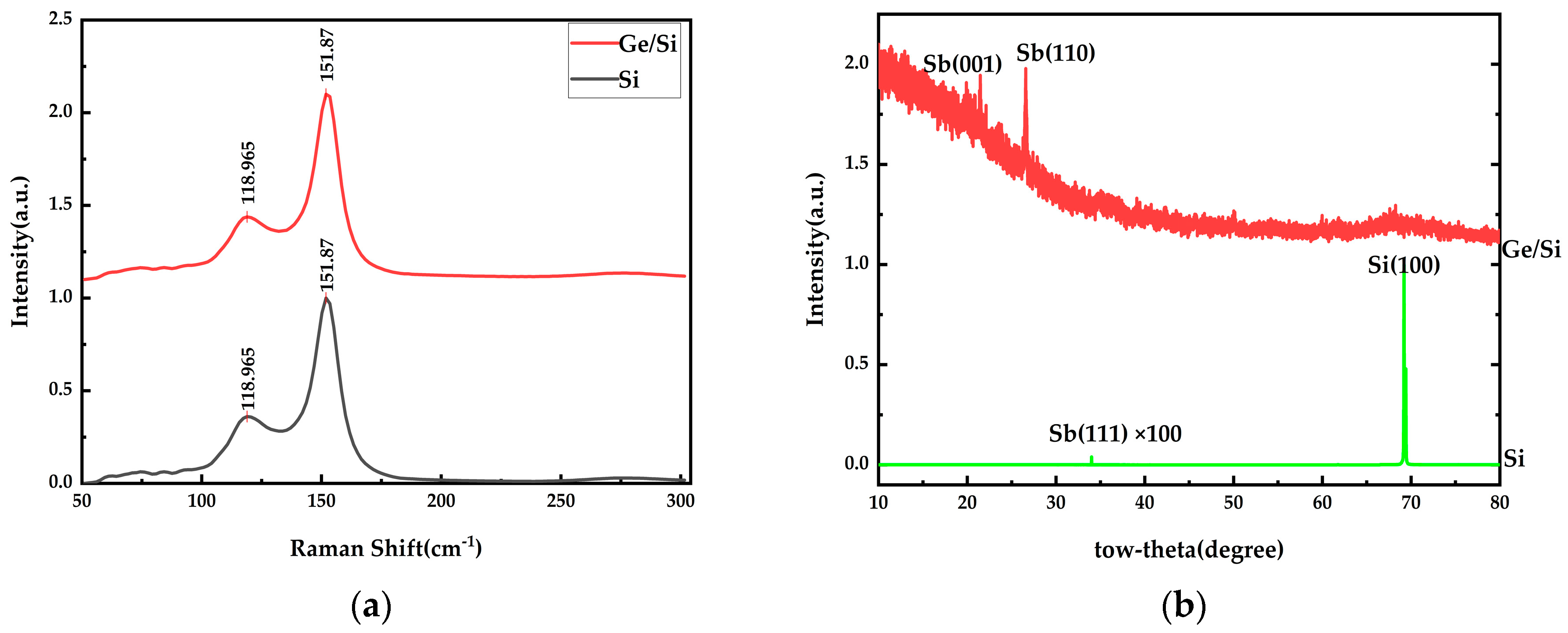
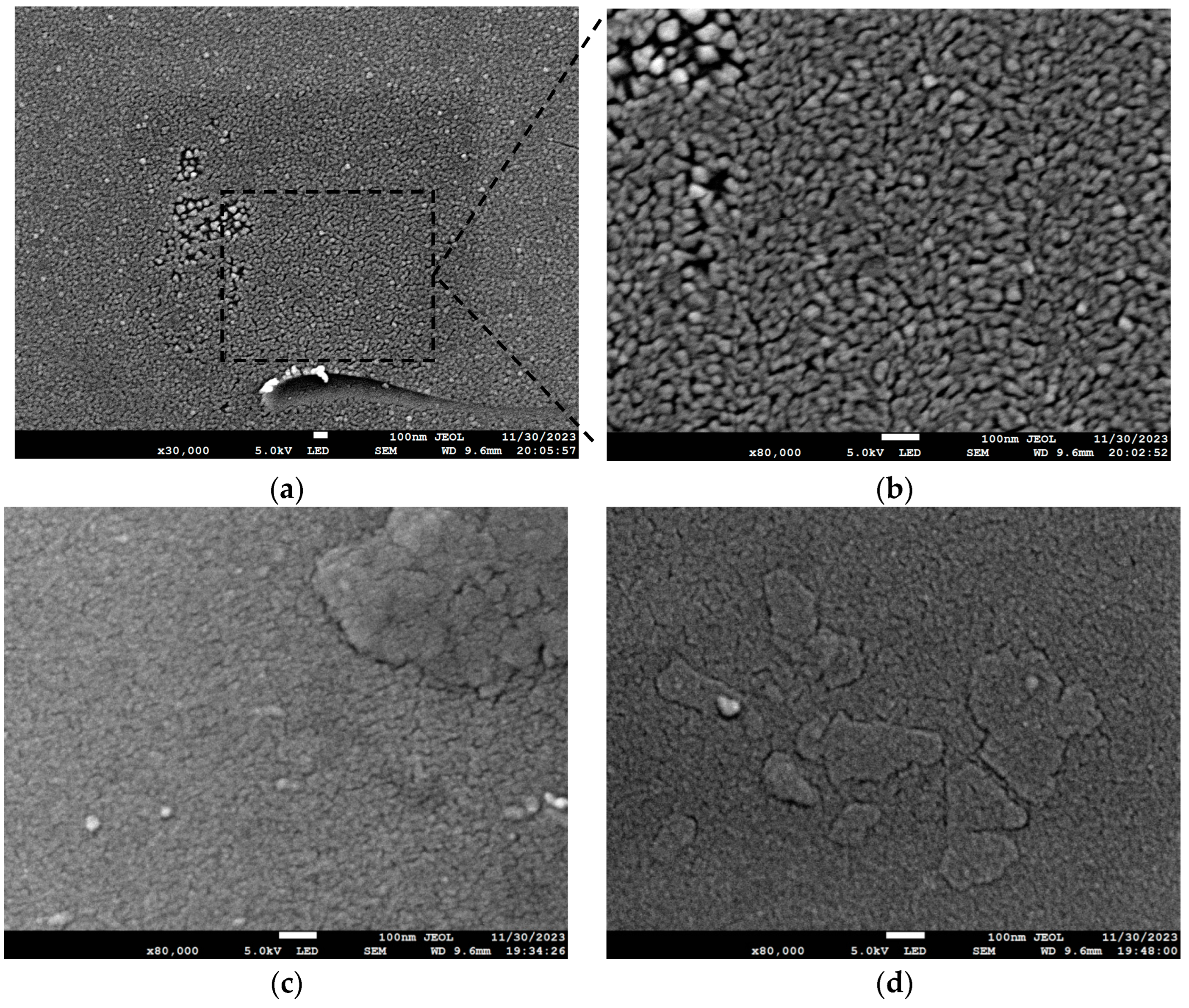
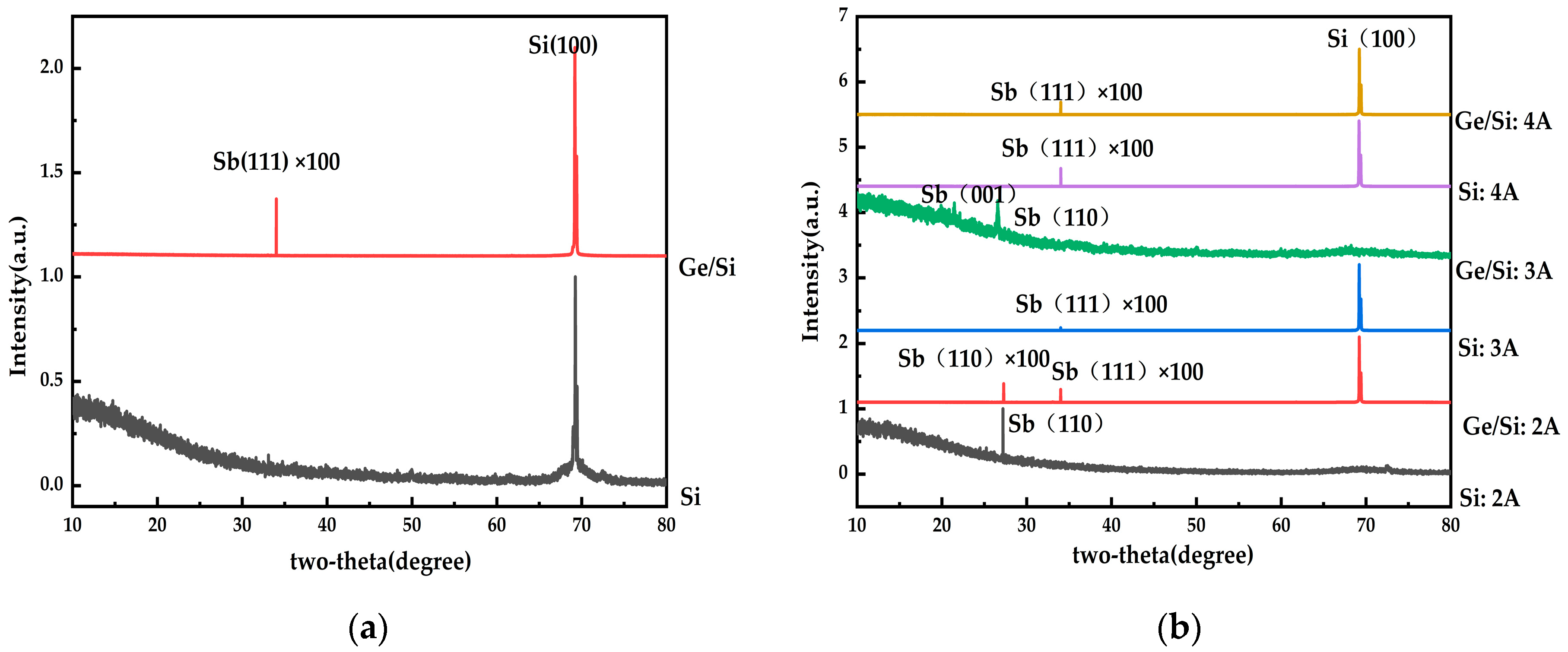
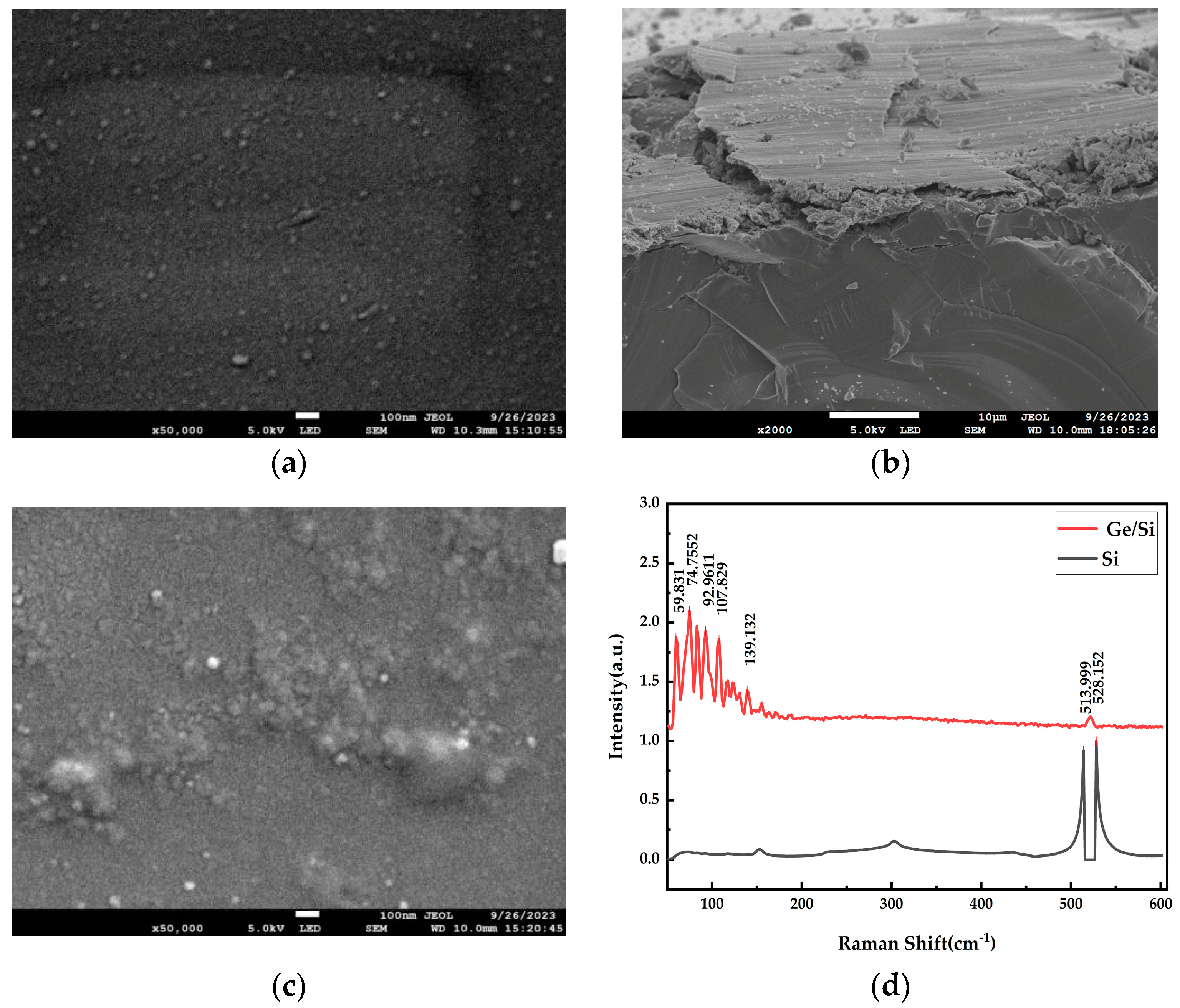
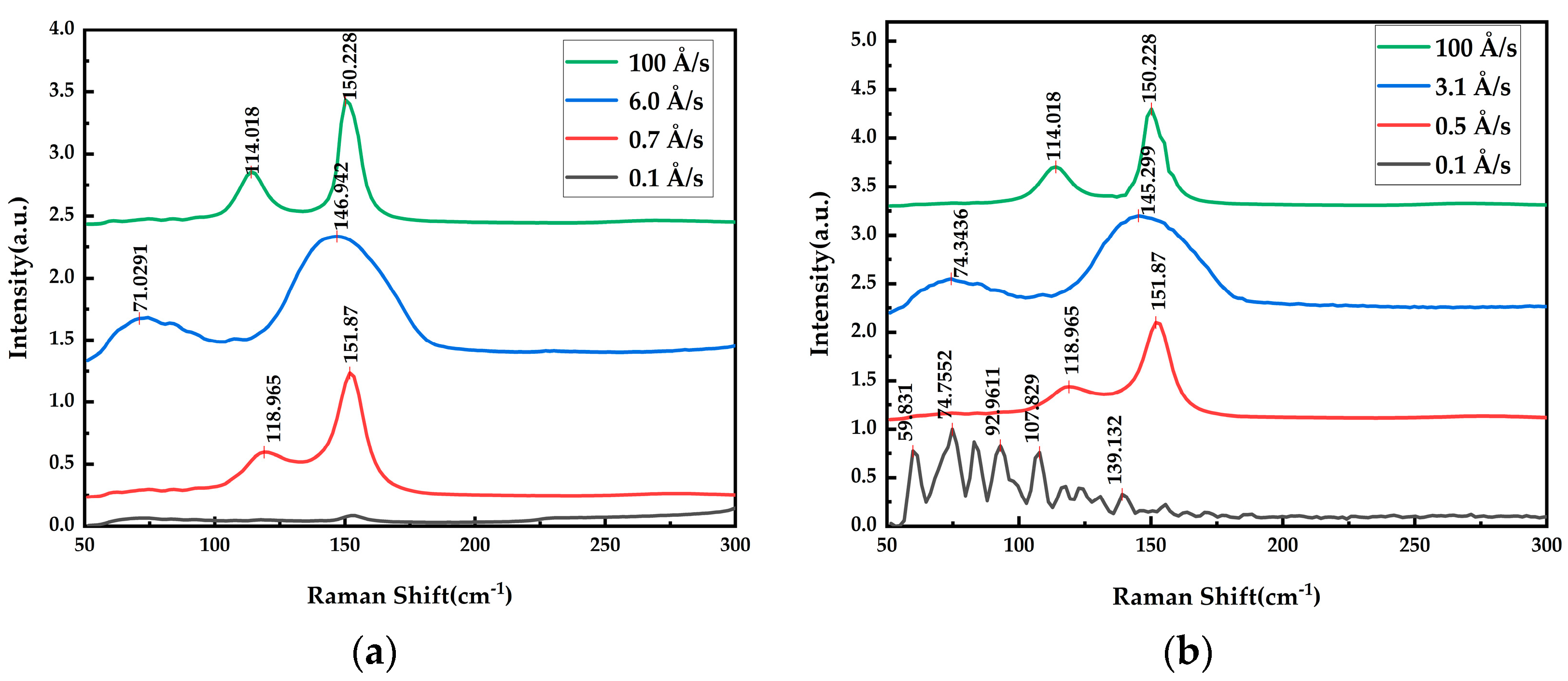
| Substrate | Deposition Rate (Å/s) | Time (s) | Thickness (nm) | |
|---|---|---|---|---|
| A | Si | 6.0 | 84 | 50 |
| B | Ge/Si | 3.1 | 84 | 26 |
| Substrate | Annealing Temperature (°C) | Annealing Time (s) | |
|---|---|---|---|
| A1 | Si | 200 | 1800 |
| A2 | Si | 300 | 1800 |
| A3 | Si | 400 | 1800 |
| B1 | Ge/Si | 200 | 1800 |
| B2 | Ge/Si | 300 | 1800 |
| B3 | Ge/Si | 400 | 1800 |
| Substrate | Deposition Rate (Å/s) | Time (s) | Thickness (nm) | |
|---|---|---|---|---|
| C | Si | 0.7 | 228 | 16 |
| D | Ge/Si | 0.5 | 228 | 11 |
| E | Si | 0.7 | 400 | 28 |
| F | Ge/Si | 0.5 | 400 | 20 |
| G | Si | 0.7 | 815 | 57 |
| H | Ge/Si | 0.5 | 815 | 41 |
| Substrate | Electron Gun (W) | Cavity Temperature (°C) | Ion Beam Current (A) | Time (s) | Thickness (nm) | |
|---|---|---|---|---|---|---|
| I1 | Si | 1.8 | 200 | 3 | 362 | 25 |
| I2 | Ge/Si | 1.8 | 200 | 3 | 362 | 18 |
| J1 | Si | 1.8 | 100 | 4 | 374 | 26 |
| J2 | Ge/Si | 1.8 | 100 | 4 | 374 | 19 |
| K1 | Si | 1.8 | 100 | 2 | 366 | 26 |
| K2 | Ge/Si | 1.8 | 100 | 2 | 366 | 18 |
| L | Si | 1.6 | 100 | 3 | 2000 | 20 |
| M | Ge/Si | 1.6 | 100 | 3 | 2000 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, T.; Zeng, L.; Yang, J.; Shu, Y.; Sun, L.; Li, Z.; Chen, H.; Liu, G.; Qiao, Z.; Qu, Y.; et al. Fabrication and Characterization of Silicon-Based Antimonene Thin Film via Electron Beam Evaporation. Materials 2024, 17, 1090. https://doi.org/10.3390/ma17051090
Zhong T, Zeng L, Yang J, Shu Y, Sun L, Li Z, Chen H, Liu G, Qiao Z, Qu Y, et al. Fabrication and Characterization of Silicon-Based Antimonene Thin Film via Electron Beam Evaporation. Materials. 2024; 17(5):1090. https://doi.org/10.3390/ma17051090
Chicago/Turabian StyleZhong, Tingting, Lina Zeng, Junfeng Yang, Yichao Shu, Li Sun, Zaijin Li, Hao Chen, Guojun Liu, Zhongliang Qiao, Yi Qu, and et al. 2024. "Fabrication and Characterization of Silicon-Based Antimonene Thin Film via Electron Beam Evaporation" Materials 17, no. 5: 1090. https://doi.org/10.3390/ma17051090
APA StyleZhong, T., Zeng, L., Yang, J., Shu, Y., Sun, L., Li, Z., Chen, H., Liu, G., Qiao, Z., Qu, Y., Xu, D., Li, L., & Li, L. (2024). Fabrication and Characterization of Silicon-Based Antimonene Thin Film via Electron Beam Evaporation. Materials, 17(5), 1090. https://doi.org/10.3390/ma17051090






