Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy
Abstract
1. Introduction
2. Materials and Methods
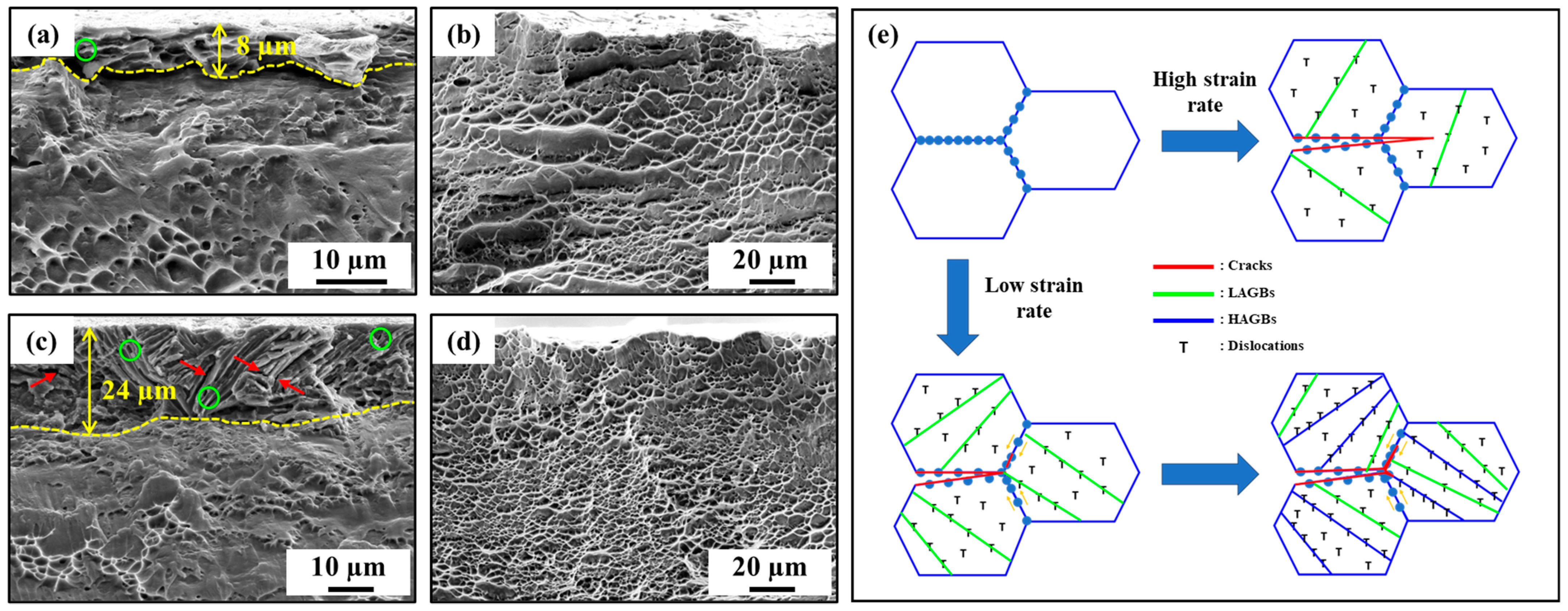
3. Results and Discussion
4. Conclusions
- (1)
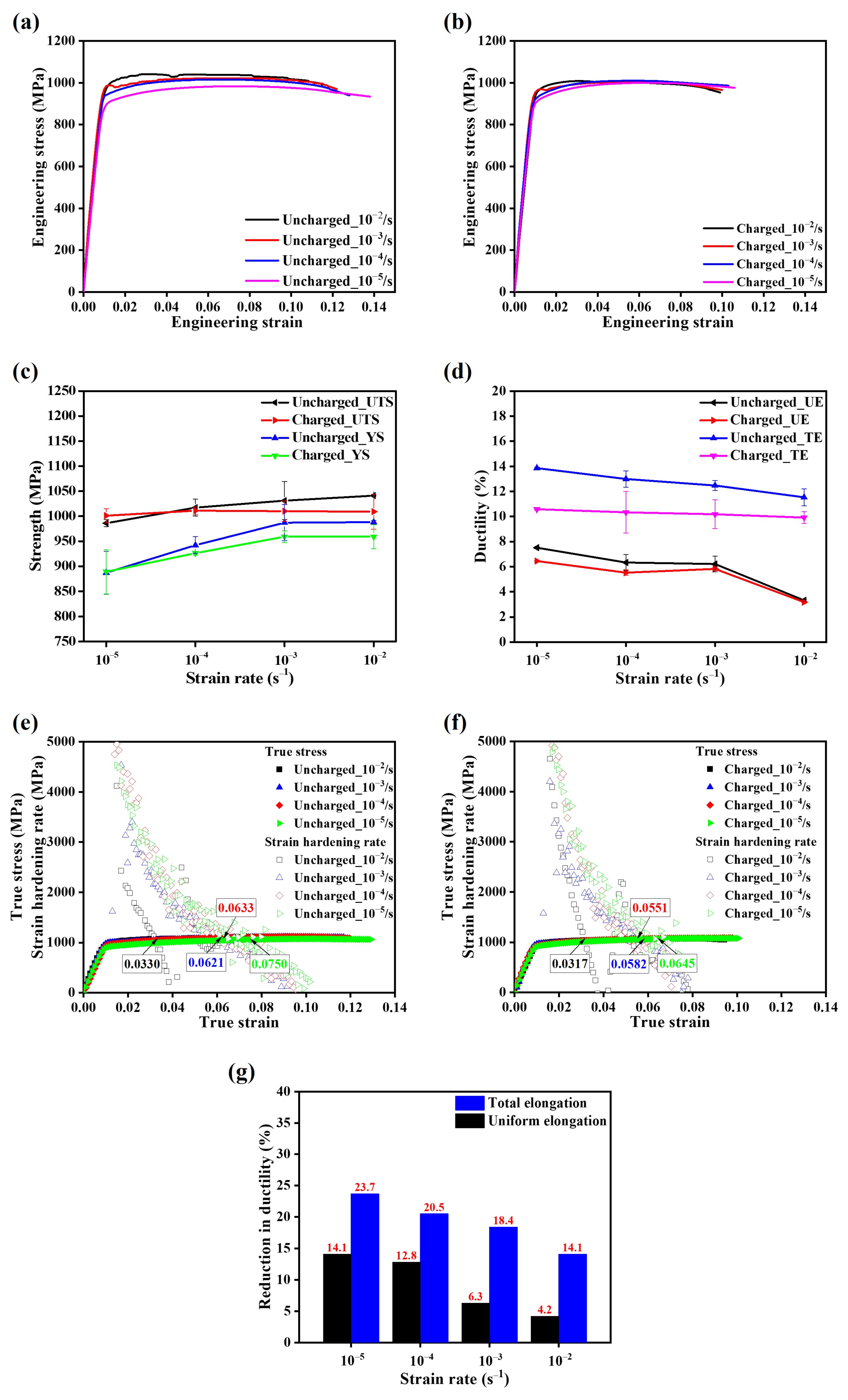
- The presence of solute hydrogen induced embrittlement features, as demonstrated by the formation of nano/micro-voids, micro-cracks, and hydrogen-assisted intergranular cracking behavior. However, the extent of embrittlement varied depending on the strain rate.
- (2)
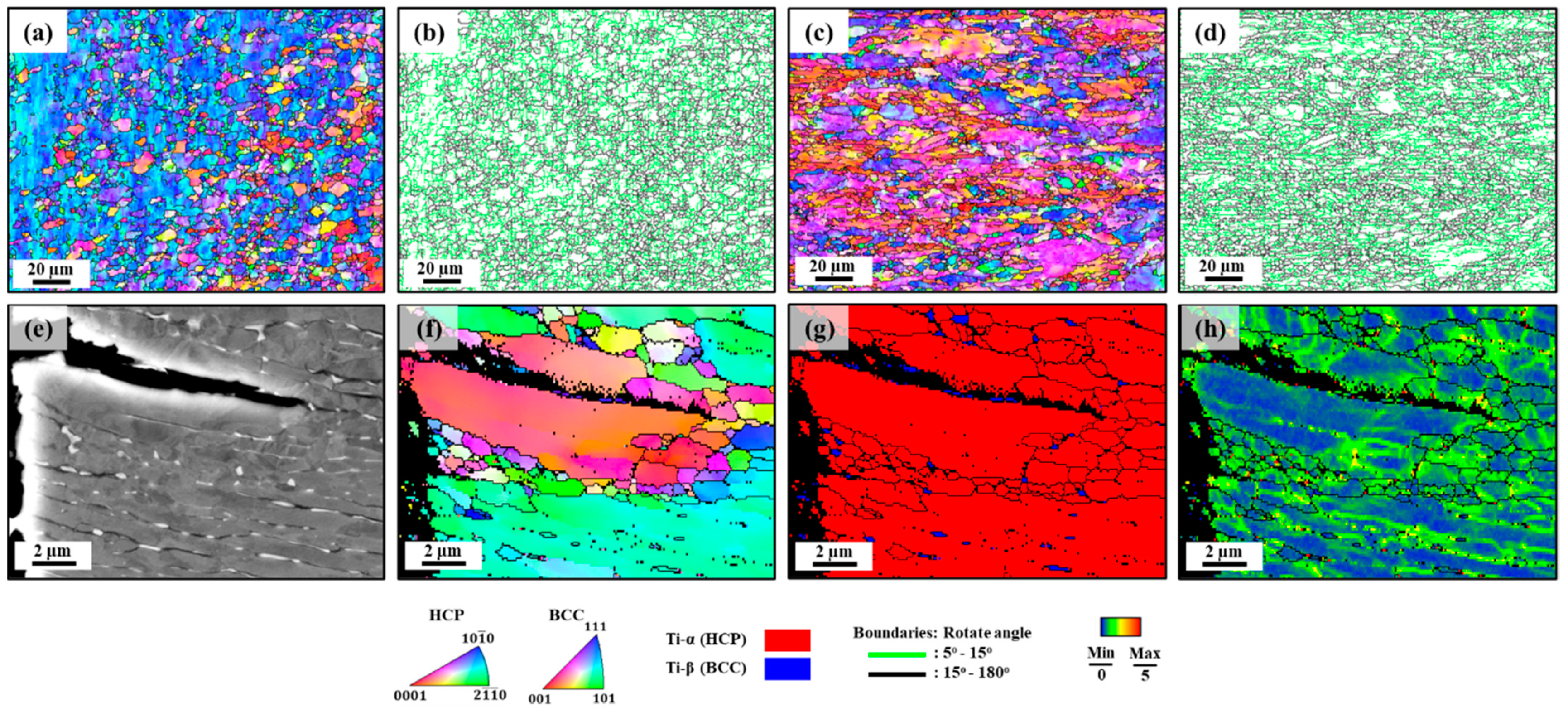
- Strain rates played a crucial role in the microstructural evolution of Ti64. Notable changes in microstructure, particularly a significant increase in the length of high-angle grain boundaries (HAGBs), were observed at low strain rates.
- (3)
- The enlarged, brittle, hydrogen-damaged region at lower strain rates facilitated crack initiation and intergranular crack propagation, resulting in a considerable decrease in ductility of the hydrogen-charged samples.
- (4)
- The hydrogen-charging effect on tensile strength was not substantial, likely due to the insignificant fraction of the hydrogen-affected region compared to the cross-sectional area.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pazhanivel, B.; Sathiya, P.; Sozhan, G. Ultra-Fine Bimodal (α + β) Microstructure Induced Mechanical Strength and Corrosion Resistance of Ti-6Al-4V Alloy Produced via Laser Powder Bed Fusion Process. Opt. Laser Technol. 2020, 125, 106017. [Google Scholar] [CrossRef]
- Cui, C.; Hu, B.M.; Zhao, L.; Liu, S. Titanium Alloy Production Technology, Market Prospects and Industry Development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar] [CrossRef]
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F.H. The Additive Manufacturing (AM) of Titanium Alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Saldaña, L.; Vallés, G.; González-Carrasco, J.L.; González-Cabrero, J.; Martínez, M.E.; Gil-Garay, E.; Munuera, L. In Vitro Corrosion Behaviour and Osteoblast Response of Thermally Oxidised Ti6Al4V Alloy. Biomaterials 2003, 24, 19–26. [Google Scholar] [CrossRef]
- Gorynin, I.V. Titanium Alloys for Marine Application. Mater. Sci. Eng. A 1999, 263, 112–116. [Google Scholar] [CrossRef]
- Lulu-Bitton, N.; Navi, N.U.; Rosen, B.A.; Haroush, S.; Sabatani, E.; Eretz-Kdosha, Y.; Agronov, G.; Eliaz, N. The Influence of Gaseous Hydrogen Charging on the Microstructural and Mechanical Behavior of Electron Beam Melted and Wrought Ti–6Al–4V Alloys Using the Small Punch Test. Int. J. Hydrogen Energy 2023, 48, 34077–34093. [Google Scholar] [CrossRef]
- Metalnikov, P.; Eliezer, D.; Ben-Hamu, G. Hydrogen Trapping in Additive Manufactured Ti–6Al–4V Alloy. Mater. Sci. Eng. A 2021, 811, 141050. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Hydrogen Trapping in 3D-Printed (Additive Manufactured) Ti-6Al-4V. Mater. Charact. 2018, 144, 297–304. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, D.; Zhu, G.; Ni, X.; Zhang, L.; Wu, W.; Man, C.; Zhou, Y.; Dong, C.; Sun, B. Heat Treatment Effects on the Hydrogen Embrittlement of Ti6Al4V Fabricated by Laser Beam Powder Bed Fusion. Addit. Manuf. 2022, 50, 102580. [Google Scholar] [CrossRef]
- Kim, J.; Hall, D.; Yan, H.; Shi, Y.; Joseph, S.; Fearn, S.; Chater, R.J.; Dye, D.; Tasan, C.C. Roughening Improves Hydrogen Embrittlement Resistance of Ti-6Al-4V. Acta Mater. 2021, 220, 117304. [Google Scholar] [CrossRef]
- Yuan, B.G.; Yu, H.P.; Li, C.F.; Sun, D.L. Effect of Hydrogen on Fracture Behavior of Ti-6Al-4V Alloy by in-Situ Tensile Test. Int. J. Hydrogen Energy 2010, 35, 1829–1838. [Google Scholar] [CrossRef]
- Su, Y.; Wang, L.; Luo, L.; Liu, X.; Guo, J.; Fu, H. Investigation of Melt Hydrogenation on the Microstructure and Deformation Behavior of Ti-6Al-4V Alloy. Int. J. Hydrogen Energy 2011, 36, 1027–1036. [Google Scholar] [CrossRef]
- Zhao, J.; Ding, H.; Zhong, Y.; Lee, C.S. Effect of Thermo Hydrogen Treatment on Lattice Defects and Microstructure Refinement of Ti6Al4V Alloy. Int. J. Hydrogen Energy 2010, 35, 6448–6454. [Google Scholar] [CrossRef]
- Turnbull, A. Perspectives on Hydrogen Uptake, Diffusion and Trapping. Int. J. Hydrogen Energy 2015, 40, 16961–16970. [Google Scholar] [CrossRef]
- Wu, W.; He, G.; Huang, J.; Zhang, A.; Liu, X.; Ouyang, Z.; Sun, Z.; Guan, L.; Chu, S.; Li, P.; et al. Influence of Electrochemically Charged Hydrogen on Mechanical Properties of Ti–6Al–4V Alloy Additively Manufactured by Laser Powder-Bed Fusion (L-PBF) Process. Mater. Sci. Eng. A 2023, 866, 144339. [Google Scholar] [CrossRef]
- Deconinck, L.; Villa Vidaller, M.T.; Bernardo Quejido, E.; Jägle, E.A.; Depover, T.; Verbeken, K. In-Situ Hydrogen Embrittlement Evaluation of as-Built and Heat Treated Laser Powder Bed Fused Ti-6Al-4V versus Conventionally Cold Rolled Ti-6Al-4V. Addit. Manuf. 2023, 76, 103768. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of Strain Rate on Hydrogen Embrittlement in Low-Carbon Martensitic Steel. Int. J. Hydrogen Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 1085–1103. [Google Scholar] [CrossRef]
- Martin, M.L.; Somerday, B.P.; Ritchie, R.O.; Sofronis, P.; Robertson, I.M. Hydrogen-Induced Intergranular Failure in Nickel Revisited. Acta Mater. 2012, 60, 2739–2745. [Google Scholar] [CrossRef]
- Deconinck, L.; Depover, T.; Verbeken, K. The Mechanism of Hydride Formation during Electrochemical Hydrogen Charging of Ti–6Al–4V. Mater. Today Sustain. 2023, 22, 100387. [Google Scholar] [CrossRef]
- Nelson, H.G. Hydrogen Embrittlement. Treatise Mater. Sci. Technol. 1983, 25, 275–359. [Google Scholar] [CrossRef]
- Shih, D.S.; Robertson, I.M.; Birnbaum, H.K. Hydrogen Embrittlement of α Titanium: In Situ Tem Studies. Acta Metall. 1988, 36, 111–124. [Google Scholar] [CrossRef]
- Bal, B.; Koyama, M.; Gerstein, G.; Maier, H.J.; Tsuzaki, K. Effect of Strain Rate on Hydrogen Embrittlement Susceptibility of Twinning-Induced Plasticity Steel Pre-Charged with High-Pressure Hydrogen Gas. Int. J. Hydrogen Energy 2016, 41, 15362–15372. [Google Scholar] [CrossRef]
- Hao, C.; Koyama, M.; Ajito, S.; Akiyama, E. Strain Rate Sensitivity of Hydrogen-Assisted ε-Martensitic Transformation and Associated Hydrogen Embrittlement in High-Mn Steel. Int. J. Hydrogen Energy 2021, 46, 27221–27233. [Google Scholar] [CrossRef]
- Kumamoto, T.; Koyama, M.; Sato, K.; Tsuzaki, K. Strain-Rate Sensitivity of Hydrogen-Assisted Damage Evolution and Failure in Dual-Phase Steel: From Vacancy to Micrometer-Scale Void Growth. Eng. Fract. Mech. 2019, 216, 106513. [Google Scholar] [CrossRef]
- Safyari, M.; Moshtaghi, M.; Kuramoto, S. Effect of Strain Rate on Environmental Hydrogen Embrittlement Susceptibility of a Severely Cold-Rolled Al–Cu Alloy. Vacuum 2020, 172, 109057. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.H.; Wu, X.; van der Zwaag, S. Effect of Strain Rate on Stress-Induced Martensitic Formation and the Compressive Properties of Ti-V-(Cr,Fe)-Al Alloys. Mater. Sci. Eng. A 2013, 573, 111–118. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Li, M.; Li, Y.; Wang, Z.; Nie, Z. Effects of Strain Rates on Deformation Twinning Behavior in α-Titanium. Mater. Charact. 2015, 106, 218–225. [Google Scholar] [CrossRef]
- Du, Z.X.; Liu, J.S.; Jiang, S.D.; Xiao, S.L.; Kong, F.T.; Chen, Y.Y. Strain Rate Dependence of Microstructural Evolution in β Titanium Alloy during Subtransus Superplastic Deformation. J. Alloys Compd. 2015, 647, 1–5. [Google Scholar] [CrossRef]
- ASTM B265-15; Standard Specification for Titanium and Titanium Alloy Strip, Sheet, and Plate. American Society for Testing and Materials—ASTM International: West Conshohocken, PA, USA, 2015.
- Crank, J. The Mathematics of Diffusion, 2nd ed.; IOP Publishing Ltd.: Bristol, UK, 1979. [Google Scholar] [CrossRef]
- Demetriou, V.; Robson, J.D.; Preuss, M.; Morana, R. Effect of Hydrogen on the Mechanical Properties of Alloy 945X (UNS N09945) and Influence of Microstructural Features. Mater. Sci. Eng. A 2017, 684, 423–434. [Google Scholar] [CrossRef]
- Lee, W.S.; Lin, C.F. Plastic Deformation and Fracture Behaviour of Ti-6Al-4V Alloy Loaded with High Strain Rate under Various Temperatures. Mater. Sci. Eng. A 1998, 241, 48–59. [Google Scholar] [CrossRef]
- Li, P.H.; Guo, W.G.; Huang, W.D.; Su, Y.; Lin, X.; Yuan, K.B. Thermomechanical Response of 3D Laser-Deposited Ti-6Al-4V Alloy over a Wide Range of Strain Rates and Temperatures. Mater. Sci. Eng. A 2015, 647, 34–42. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, D.; Law, C.C. Brittle-to-Ductile Transition Temperature and Its Strain Rate Sensitivity in a Two-Phase Titanium Aluminide with near Lamellar Microstructure. J. Mater. Sci. 1999, 34, 3155–3159. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y. Hydrogen Effect against Hydrogen Embrittlement. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2010, 41, 2548–2562. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-Enhanced Localized Plasticity-a Mechanism for Hydrogen-Related Fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D.; Boellinghaus, T. Investigation of Hydrogen-Deformation Interactions in β-21S Titanium Alloy Using Thermal Desorption Spectroscopy. J. Alloys Compd. 2007, 440, 204–209. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, C.; Zheng, Y.; Zhang, L.; Zheng, J.; Chen, X. Dependence of Strain Rate on Hydrogen-Induced Hardening of Austenitic Stainless Steel Investigated by Nanoindentation. Int. J. Hydrogen Energy 2019, 44, 14055–14063. [Google Scholar] [CrossRef]
- Lee, D.H.; Jung, J.Y.; Lee, K.H.; Lee, S.Y.; Zhao, Y.; Lau, K.B.; Wang, P.; Ramamurty, U. Distinct Effects of In-Situ and Ex-Situ Hydrogen Charging Methods on the Mechanical Behavior of CoCrFeNi High-Entropy Alloy Fabricated by Laser-Powder Bed Fusion. J. Alloys Compd. 2023, 940, 168858. [Google Scholar] [CrossRef]
- Lee, D.H.; Zhao, Y.; Lee, S.Y.; Ponge, D.; Jägle, E.A. Hydrogen-Assisted Failure in Inconel 718 Fabricated by Laser Powder Bed Fusion: The Role of Solidification Substructure in the Embrittlement. Scr. Mater. 2022, 207, 114308. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, L.; Xiong, Z.; Yang, J.; Tan, Y.; Liu, Y.; Zhang, Y.; Tan, Q.; Hao, C.; Cao, L.; et al. The Response of Dislocations, Low Angle Grain Boundaries and High Angle Grain Boundaries at High Strain Rates. Mater. Sci. Eng. A 2021, 822, 141704. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, L. Structure and Nucleation Mechanisms of Misfit Dislocations in Epitaxial FCC Thin Films with Positive and Negative Mismatches. Mater. Chem. Phys. 2006, 100, 168–173. [Google Scholar] [CrossRef]
- Olmsted, D.L.; Holm, E.A.; Foiles, S.M. Survey of Computed Grain Boundary Properties in Face-Centered Cubic Metals-II: Grain Boundary Mobility. Acta Mater. 2009, 57, 3704–3713. [Google Scholar] [CrossRef]
- Bechtle, S.; Kumar, M.; Somerday, B.P.; Launey, M.E.; Ritchie, R.O. Grain-Boundary Engineering Markedly Reduces Susceptibility to Intergranular Hydrogen Embrittlement in Metallic Materials. Acta Mater. 2009, 57, 4148–4157. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Loder, B.; Safyari, M.; Willidal, T.; Hojo, T.; Mori, G. Hydrogen Trapping and Desorption Affected by Ferrite Grain Boundary Types in Shielded Metal and Flux-Cored Arc Weldments with Ni Addition. Int. J. Hydrogen Energy 2022, 47, 20676–20683. [Google Scholar] [CrossRef]
- Pichler, S.; Bendo, A.; Mori, G.; Safyari, M.; Moshtaghi, M. Inhibition of Grain Growth by Pearlite Improves Hydrogen Embrittlement Susceptibility of the Ultra-Low Carbon Ferritic Steel: The Influence of H-Assisted Crack Initiation and Propagation Mechanisms. J. Mater. Sci. 2023, 58, 13460–13475. [Google Scholar] [CrossRef]
- Luo, L.; Su, Y.; Guo, J.; Fu, H. Formation of Titanium Hydride in Ti-6Al-4V Alloy. J. Alloys Compd. 2006, 425, 140–144. [Google Scholar] [CrossRef]
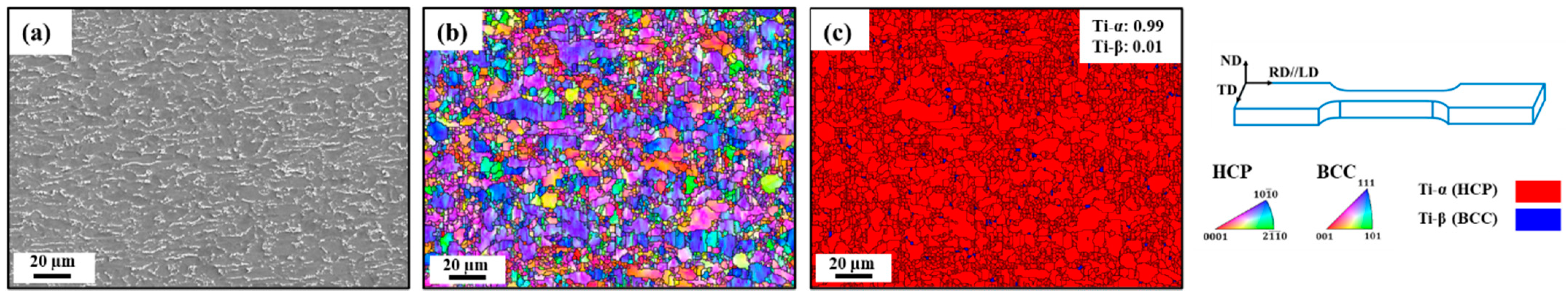
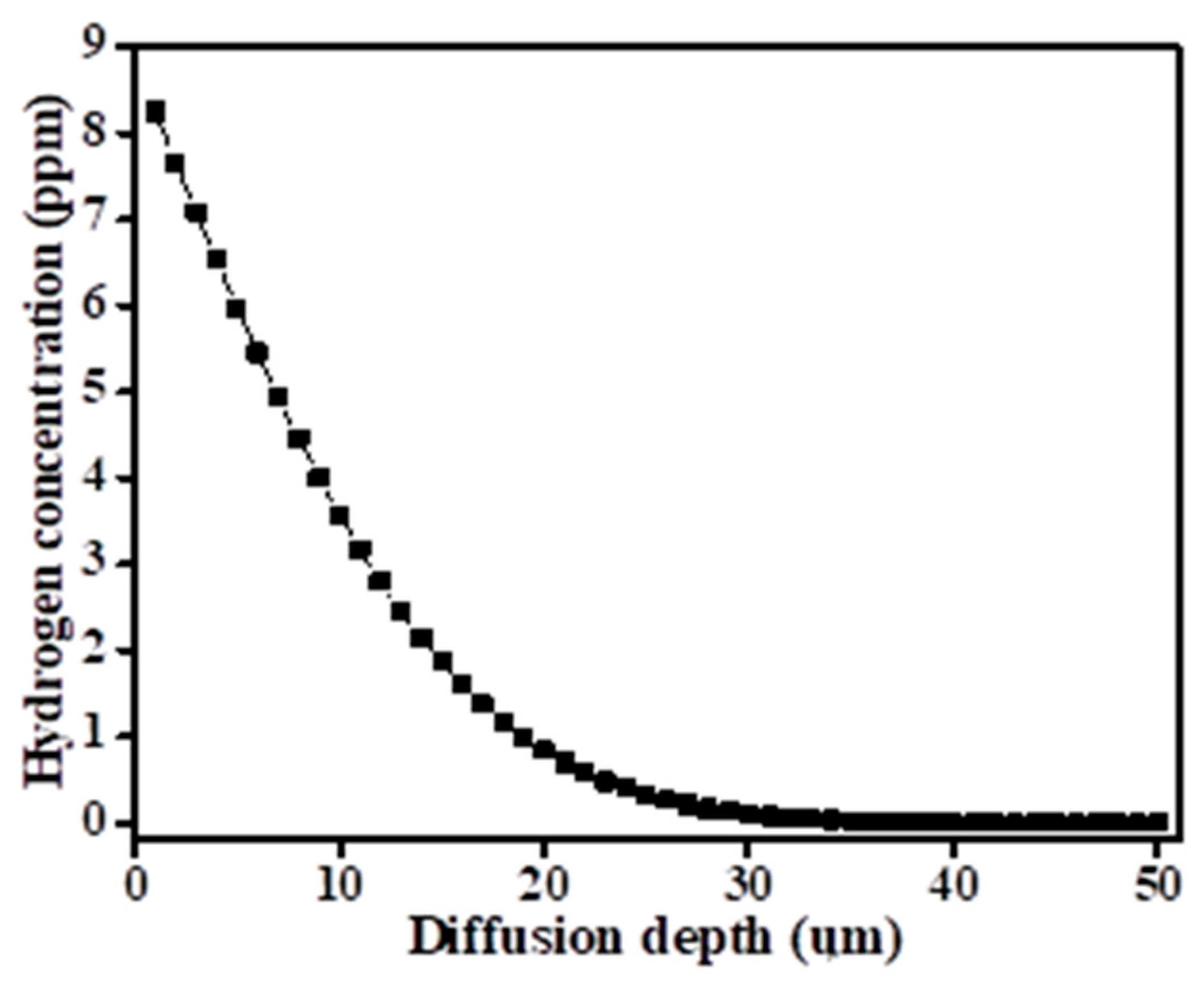
| Ti6Al4V | Al | V | Fe | O | C | N | H | Ti |
|---|---|---|---|---|---|---|---|---|
| wt% | 5.76 | 3.93 | 0.087 | 0.13 | 0.0068 | 0.011 | 0.0019 | Balance |
| Samples | Boundary | Total Boundary Length/Area (µm−1) |
|---|---|---|
| HSR-tested sample | LAGBs | 0.47 |
| HAGBs | 0.48 | |
| LSR-tested sample | LAGBs | 0.46 |
| HAGBs | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-D.; Ansari, N.; Lee, K.H.; Lee, D.-H.; Han, J.H.; Lee, S.Y. Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy. Materials 2024, 17, 1100. https://doi.org/10.3390/ma17051100
Nguyen T-D, Ansari N, Lee KH, Lee D-H, Han JH, Lee SY. Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy. Materials. 2024; 17(5):1100. https://doi.org/10.3390/ma17051100
Chicago/Turabian StyleNguyen, Tien-Dung, Nooruddin Ansari, Keun Hyung Lee, Dong-Hyun Lee, Jun Hyun Han, and Soo Yeol Lee. 2024. "Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy" Materials 17, no. 5: 1100. https://doi.org/10.3390/ma17051100
APA StyleNguyen, T.-D., Ansari, N., Lee, K. H., Lee, D.-H., Han, J. H., & Lee, S. Y. (2024). Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy. Materials, 17(5), 1100. https://doi.org/10.3390/ma17051100








