The Influence of the Chemical Composition of Flax and Hemp Fibers on the Value of Surface Free Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Flax Fibers Preparation
2.1.2. Hemp Fibers Preparation
- dew retting in the field for a period of 7 weeks until they were fully retted,
- water retting under laboratory conditions (process duration: 120 h, water temperature: 34 °C).
2.2. Method
2.2.1. Chemical Analyses
- Waxes and fats content (%), was measured according to the Polish Standard no. BN-86/7501-10 [37]. The percentage content of wax and fat substances was determined by extracting them with an organic solvent (petroleum ether) in a Soxhlet extractor and weighing the residues after vaporization of the solvent.
- Lignin content (%) was determined according to the Polish Standard BN-86/7501-11 [38]. The lignin content was measured by dissolving cellulose, hemicellulose and pectin with a mixture of concentrated sulphuric and ortho phosphoric acids, followed by draining off the remaining insoluble lignin.
- Pectin content (%) tests were conducted by a gravimetric method according to a method developed at the Institute of Natural Fibres and Medicinal Plants National Research Institute. The percent share of pectin was determined by dissolving them in ammonium citrate and then precipitating from the solution with calcium chloride and by measuring the weight of the calcium pectinate precipitated from the solution.
- Hemicellulose content (%) in the flax end hemp fiber was determined according to the Polish Standard BN-77/7529-02 [39]. The hemicellulose content was measured by dissolving the hemicellulose present in the fiber with a 1% solution of sodium hydroxide, filtering off the residue after dissolution, drying it and weighing it. Then the hemicelluloses were calculated from the mass loss of the sample.
- Cellulose content (%) in flax and hemp fiber was measured according to the Polish Standard no. PN-92/50092 [40]. The cellulose content was measured by dissolving lignin and other substances present in the fiber with a mixture of acetylacetone and dioxane, acidified with hydrochloric acid.
- Fourier transform infrared spectroscopy (FTIR) with an ATR (Attenuated Total Reflectance) attachment was performed in TA Instruments the iS10 model with Smart iTX ZnSe cristal. The spectrum of the fibers contained 16 scans per second at a resolution of 4 cm−1 within the range from 600 to 4000 cm−1.
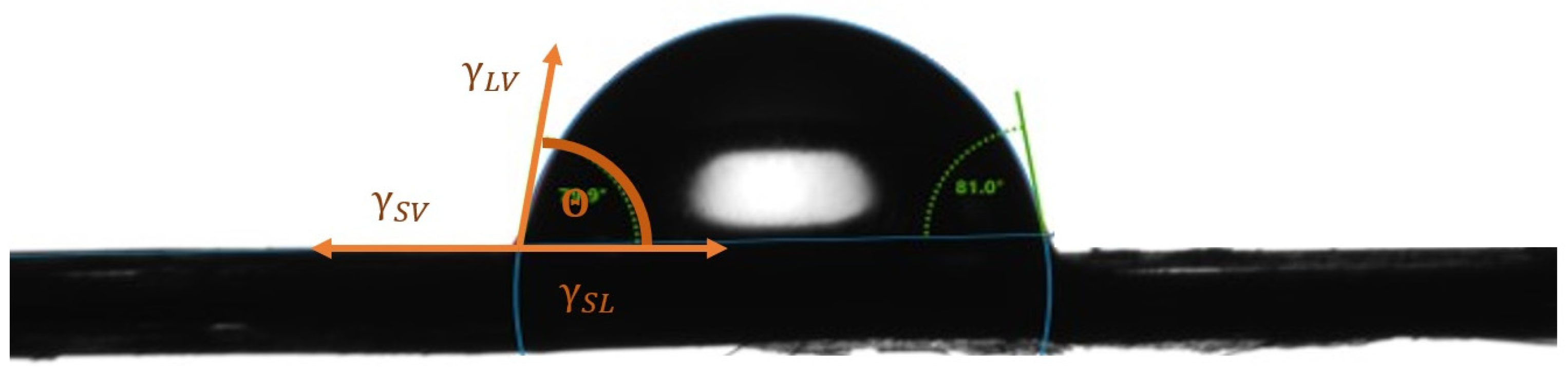
2.2.2. Surface Free Energy Test
- —surface free energy,
- —geometric mean of dispersive components,
- —geometric mean of polar components [42].
- —surface free energy of solid,
- —surface free energy of solid in contact with the measuring liquid,
- —surface free energy of the measuring liquid [43].
2.2.3. Statistical Analyses
3. Results and Discussion
3.1. Chemical Analyses
3.1.1. FLAX Fiber Chemical Composition in Relationship of Plant Variety and Subsequent Stages of the Applied Technological Chain
3.1.2. HEMP Fiber Chemical Composition in Relationship of Plant Variety
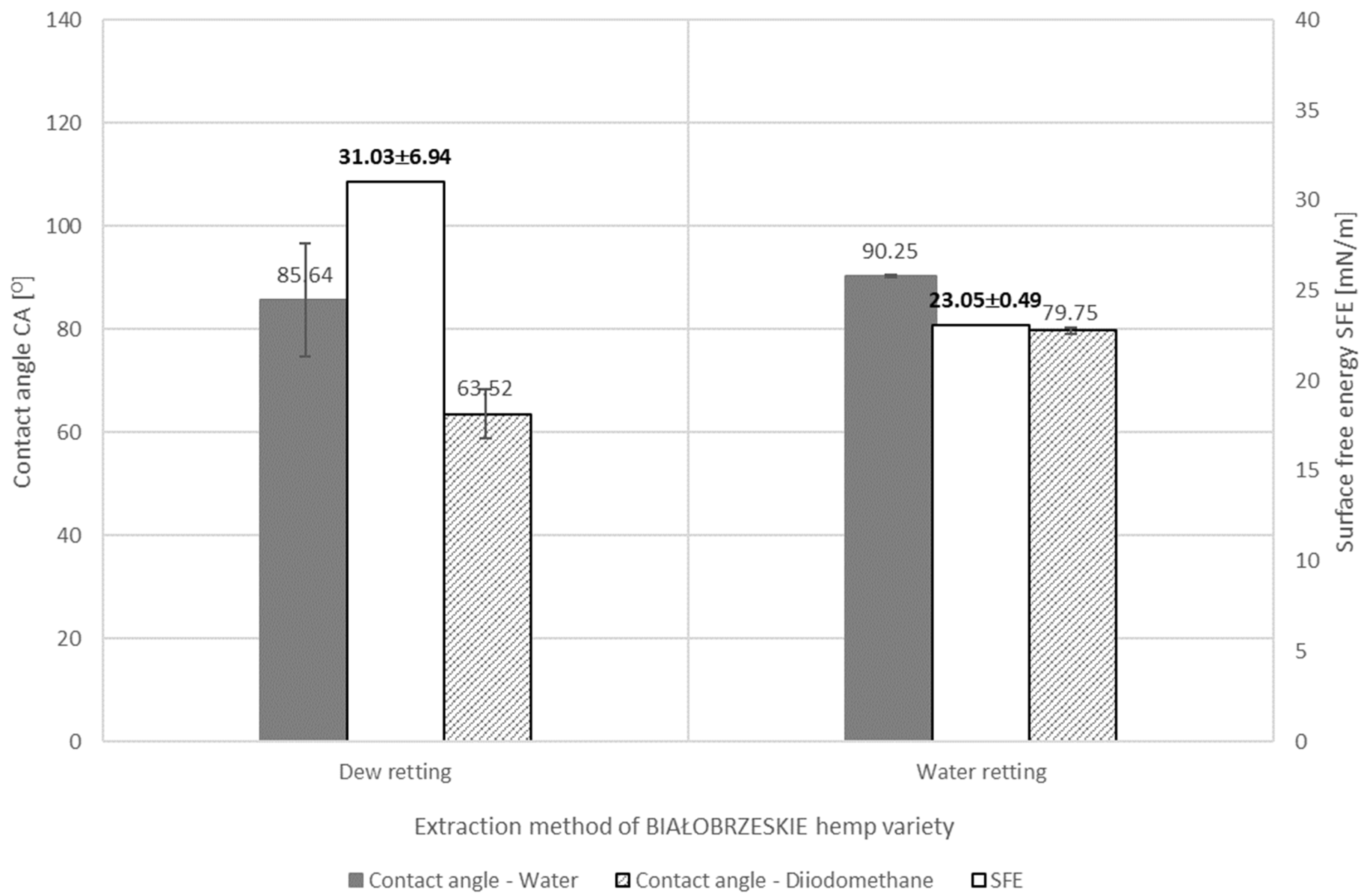
3.1.3. HEMP Fiber Chemical Composition in Relationship of Applied Retting Method
3.2. Surface Free Energy Test
3.3. Statistical Analyses
4. Conclusions
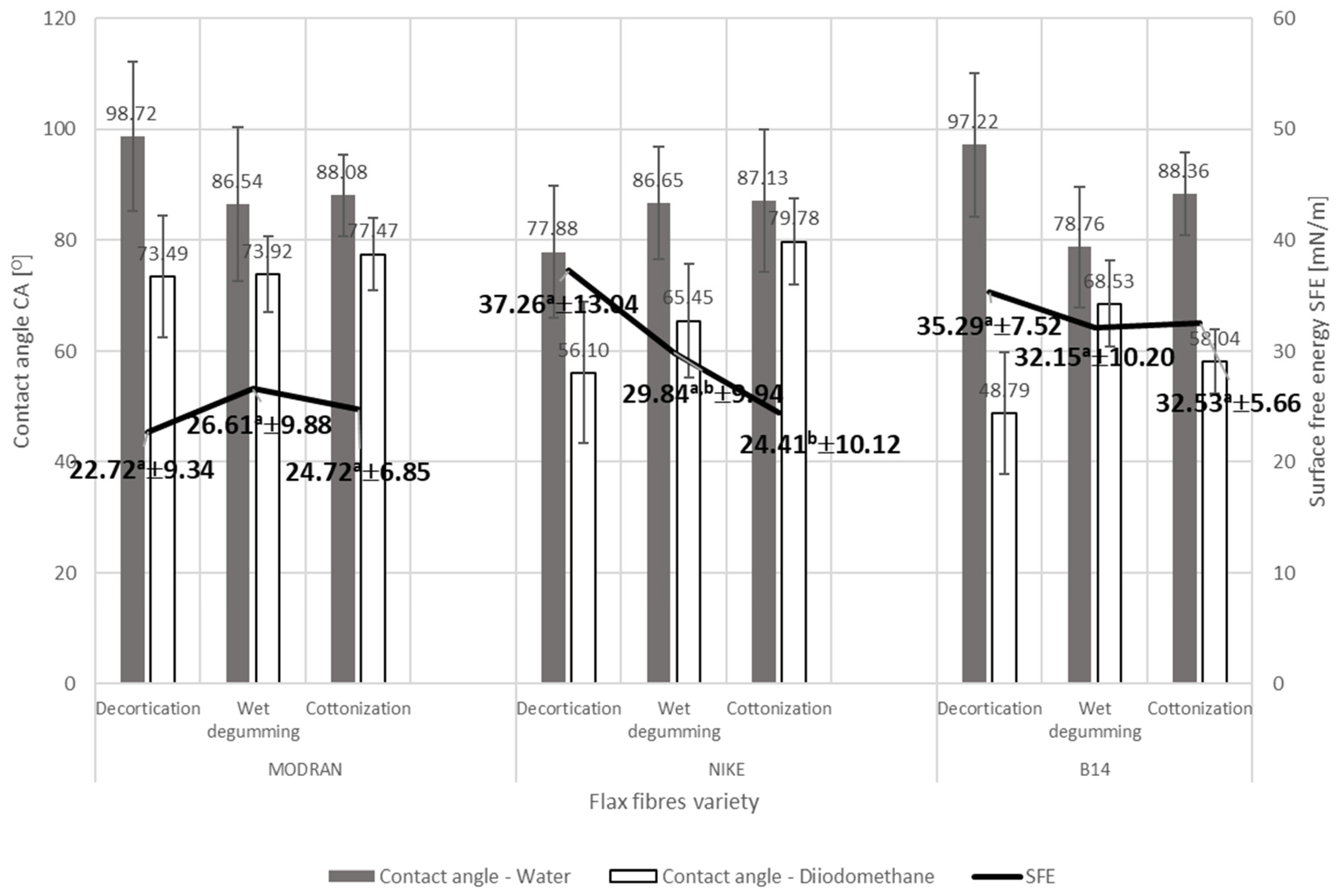
- The surface free energy depends on the plant variety, which determines fibers chemical composition—The surface free energy of the decorticated flax fibers ranges from 22.72 mN/m (MODRAN) to 37.26 mN/m (NIKE) and depends on the plant variety.
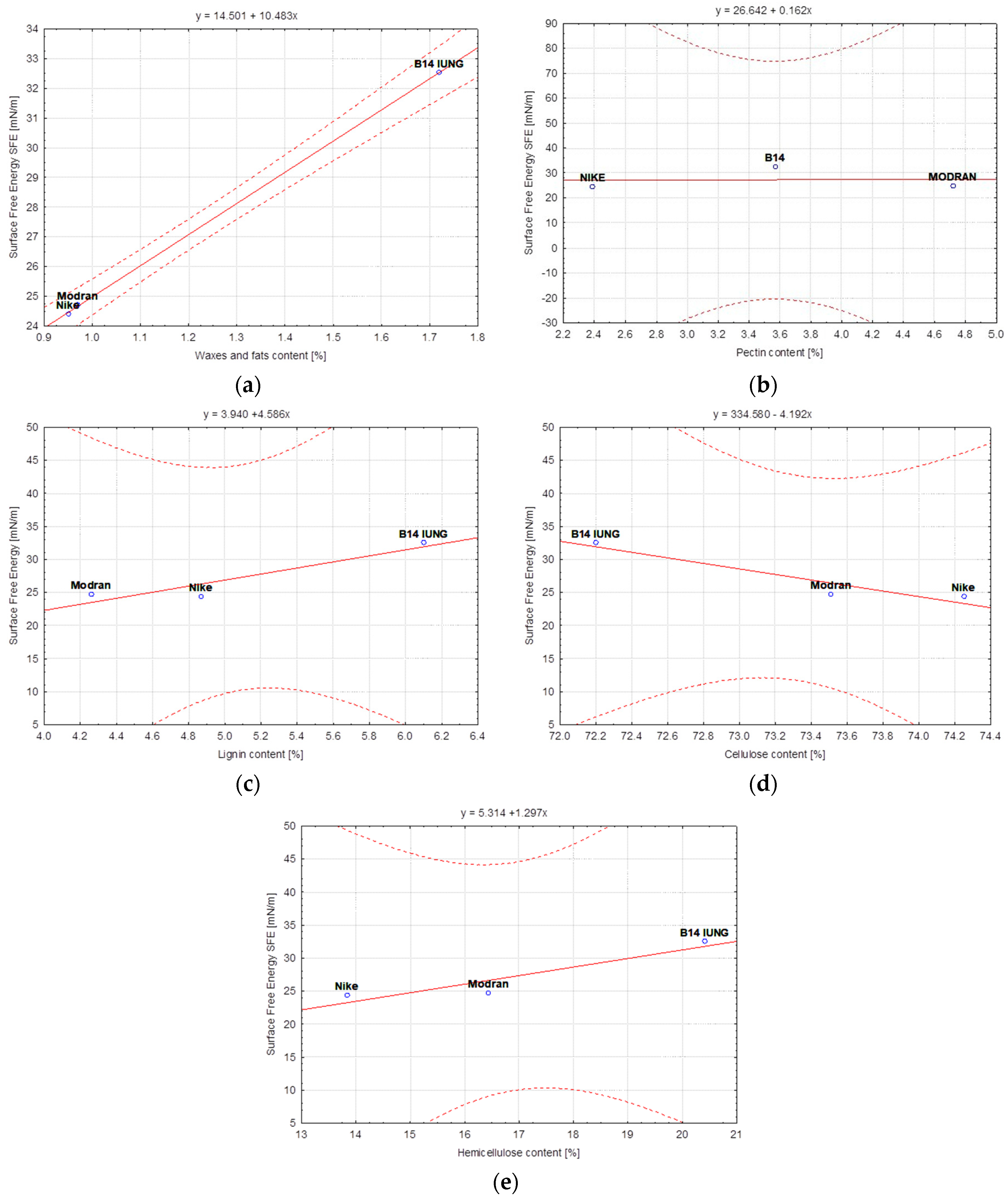
- The consecutive stages of the flax fiber refining process, i.e., hydrodynamic degumming of decorticated fibers and then cottonization, lead to the reorientation of the polar and dispersion character of the fiber and thus cause a change in the surface free energy of the fibers. In the case of NIKE on each consecutive stage, the differences between the polar and dispersion components of the fiber became smaller and led to a decrease in the value of surface free energy. In the case of the MODRAN fiber, the differences between the polar components were also reduced in the case of fibers obtained in the last stage of the technology, but to a lesser extent than in the case of NIKE, hence the surface free energy is approximately at the same level for the MODRAN fiber after each processing stage. The B14 fiber is not analogous to other flax fibers, the differences between the polar and dispersion components were more differentiated and the surface free energy was slightly reduced in the subsequent stages of the process. However, the results of the research on flax fibers allowed for the conclusion that the subsequent stages of the technological process do not have a statistically significant impact on the level of surface free energy of the processed fibers. Finally, the fibers after the final refinement stage, i.e., cottonization, are characterized by a surface free energy from 24.72 mN/m (MODRAN) to 32.53 mN/m (B14).
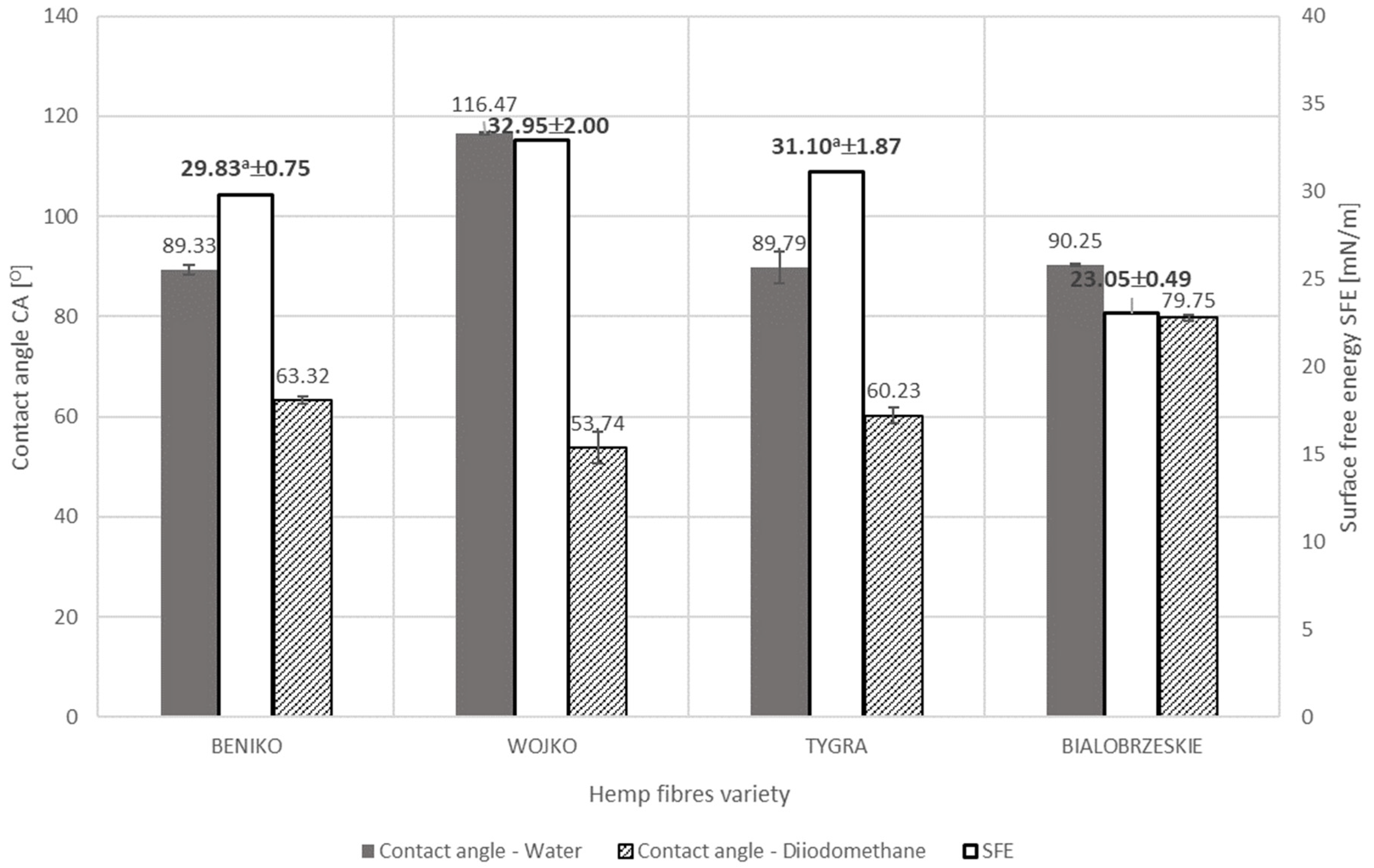
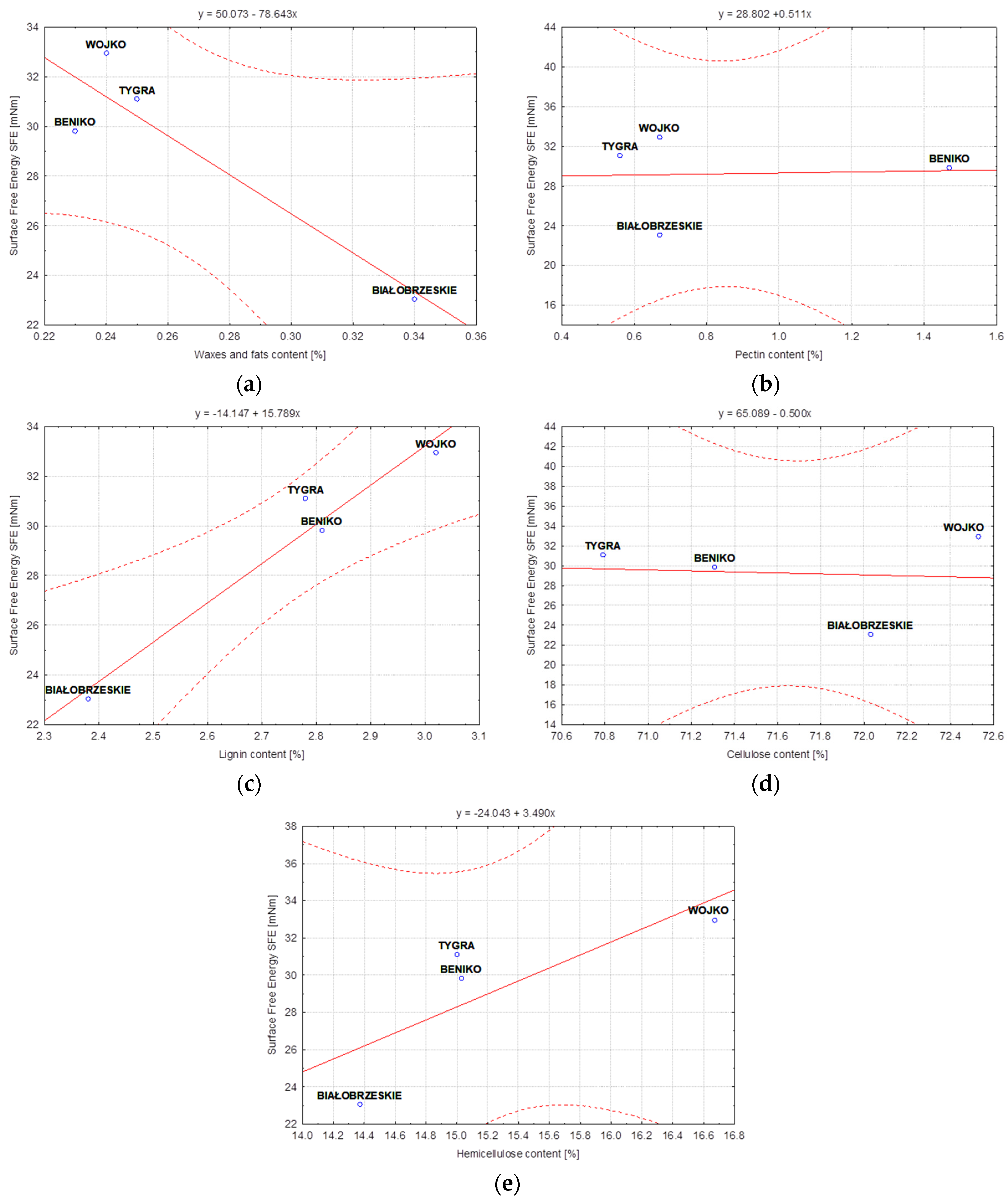
- The values of fiber surface free energy tests showed the dependence of this parameter on the content of individual chemical components of the fiber, both in the case of flax and hemp. From the point of view of wettability, the most important aspects for bast fibers are the amount of cellulose and hemicellulose contained in the fiber. However, the hydrophobic ingredients such as lignin, waxes and fats in the fibers cannot be overlooked. The diversified proportion of hydrophilic and hydrophobic components in flax or hemp fibers is responsible for the differences in the surface free energy between fibers of different fibrous plant varieties. In the case of hemp fibers extracted by water retting, the WOJKO fiber had the highest surface free energy (32.95 mN/m). WOJKO fiber was characterized by a high content of hydrophobic components and the highest content of hydrophilic components as well, hence the difference between the polar and dispersion components was the highest of all the hemp fibers considered. The BIAŁOBRZESKIE fibers had the lowest content of hydrophobic components resulting in the lowest difference between the polar and non-polar components and thus the surface free energy of the BIAŁOBRZESKIE variety is the lowest (23.05 mN/m) among all the tested hemp fibers.
- The surface free energy depends on the use of the defined method of retting—In the case of hemp fiber, the BIAŁOBRZESKIE variety influenced the wettability of the fiber. Water molecules bind to the hydrophilic groups of the fiber during water retting, causing changes in its polarity. Hemp fiber of the BIAŁOBRZESKIE variety extracted using water retting was therefore characterized by a lower surface free energy (23.05 mN/m) than the same fiber extracted with the use of dew retting of the straw (31.03 mN/m).
- Statistical analysis confirmed that the surface free energy was strongly correlated with the content of individual components in the fibers. However, the intensity of the correlation between the fibers and the values of surface free energy, shown in the statistical analysis, proved that it can be weakened or strengthened as a result of subsequent processes in the technological chain and at the stage of straw retting.
- Identification of the relationship of moisture management in textiles and values of surface free energy of the fibers,
- Selection of flax and hemp fibers in terms of their free surface energy value in order to improve moisture transport in the clothing,
- Selection of the appropriate variety and method of extraction of flax and hemp fibers for the production of biocomposites in terms of their surface free energy value and their ability to bond with the matrix,
- Carrying out chemical modification of flax and hemp fibers in order to increase the adhesion of fibers with the polymer matrix in biocomposites by increasing/decreasing the value of surface free energy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhi, C.; Shi, S.; Zhang, S.; Si, Y.; Yang, J.; Meng, S.; Fei, B.; Hu, J. Bioinspired All-Fibrous Directional Moisture-Wicking Electronic Skins for Biomechanical Energy Harvesting and All-Range Health Sensing. Nano-Micro Lett. 2023, 15, 60. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, S.; Liu, Y.; Lei, R.; Guo, T.; Yao, Y.; Gao, S.; Ding, J.; Zhang, Z. The effect of surface-free energy and microstructure on the condensation mechanism of water vapor. Prog. Nat. Sci. Mater. Int. 2023, 33, 37–46. [Google Scholar] [CrossRef]
- Gondaliya, A.; Alipoormazandarani, N.; Kleiman, M.; Foster, E.J. Sustainable compressed biocomposite: Review on development and novel approaches. Mater. Today Commun. 2023, 35, 105846. [Google Scholar] [CrossRef]
- Kicińska-Jakubowska, A.; Bogacz, E.; Zimniewska, M. Review of Natural Fibers. Part I Vegetable Fibers. J. Nat. Fibers 2012, 9, 150–167. [Google Scholar] [CrossRef]
- Kim, Y.K. Ultraviolet protection finishes for textiles. In Functional Finishes for Textiles; Roshan, P., Ed.; Woodhead Publishing Limited: Sawston, UK, 2015; Chapter 15; pp. 463–485. ISBN 978-0-85709-839-9. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Bontempo Freitas, M.; Esposito, D. Cellular and Molecular Mechanisms of Oxidative Stress in Wound Healing. Oxidative Med. Cell. Longev. 2022, 2022, 9785094. [Google Scholar] [CrossRef] [PubMed]
- Zimniewska, M.; Różańska, W.; Gryszczyńska, A.; Romanowska, B.; Kicińska-Jakubowska, A. Antioxidant Potential of Hemp and Flax Fibers Depending on Their Chemical Composition. Molecules 2018, 23, 1993. [Google Scholar] [CrossRef]
- Zimniewska, M.; Krucińska, I. The Role of Apparels Based on Natural Fibres: Flax in Human Physiology and Health. In Natural Fibers: Properties, Mechanical Behavior, Functionalization and Applications; Kozłowski, R., Muzyczek, M., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2017; Chapter 10; pp. 193–212. ISBN 978-1-53612-071-4. [Google Scholar]
- Zimniewska, M.; Pawlaczyk, M.; Krucińska, I.; Frydrych, I.; Mikołajczak, P.; Schmidt-Przewoźna, K.; Cichocka, A.; Urbaniak, M.; Herczyńska, L.; Kowalska, S.; et al. The influence of natural functional clothing on some biophysical parameters of the skin. Text. Res. J. 2019, 89, 1381–1393. [Google Scholar] [CrossRef]
- Gassan, J.; Gutowski, V.; Bledzki, A. About the surface characteristics of natural fibres. Macromol. Mater. Eng. 2000, 283, 132–139. [Google Scholar] [CrossRef]
- Navarro, M.; Pais, V.; Silva, P.; Guise, C.; Martins, R.; Ferreira, F.; Fangueiro, R. Influence of the addition of nettle and hemp fibres to cotton based yarns on the moisture management properties. In Book of Abstract, Proceedings of the 4th International Conference on Natural Fibers—Smart Sustainable Solutions, Porto, Portugal, 1–3 July 2019; Fangueiro, R., Ed.; Tecminho—Associação Universidade-Empresa Para O Desenvolvimento: Guimarães, Portugal, 2019; pp. 208–209. [Google Scholar]
- Morison, W.H., III.; Archilad, D.D.; Sharma, H.S.S.; Akin, D.E. Chemical and physical characterization of water- and dew- retted flax fibers. Ind. Crops Prod. 2000, 12, 39–46. [Google Scholar] [CrossRef]
- Zimniewska, M.; Romanowska, B. Bast Fiber Textiles Addressed Improvement of Human Life. In Natural Fiber; Jeon, H.-Y., Ed.; IntechOpen: London, UK, 2022; Chapter 3; pp. 35–61. ISBN 978-1-80355-213-2. [Google Scholar] [CrossRef]
- Preisner, M.; Wojtasik, W.; Szopa, J.; Kulma, A. The development, differentiation and composition of flax fiber cells. Postępy Biochem. 2015, 61, 416–429. (In Polish) [Google Scholar]
- Khazraji, A.C.; Robert, S. Self-Assembly and Intermolecular Forces When Cellulose and Water Interact Using Molecular Modeling. J. Nanomater. 2013, 2013, 745979. [Google Scholar] [CrossRef]
- Urbańczyk, G. Nauka o Włóknie; Wydawnictwa Naukowo-Techniczne: Warsaw, Poland, 1985; ISBN 83-204-0632-3. (In Polish) [Google Scholar]
- Petridis, L.; Smith, J.C. Conformations of low-molecular weight lignin polymers in water. ChemSusChem 2016, 9, 289–295. [Google Scholar] [CrossRef]
- Akin, D.E. Linen Most Useful: Perspectives on Structure, Chemistry and Enzymes for Retting Flax. ISRN Biotechnol. 2013, 2013, 186534. [Google Scholar] [CrossRef]
- Lusas, E.W. Animal and Vegetable Fats, Oils, and Waxes. In Riegel’s Handbook of Industrial Chemistry, 10th ed.; Kent, J.A., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003; Chapter 8; pp. 243–328. ISBN 978-0-387-23816-6. [Google Scholar]
- Krawiec, H. Celuloza. In Podstawy Chemii Polimerów; Akademia Górniczo-Hutnicza: Cracow, Poland, 2022; pp. 119–120. (In Polish) [Google Scholar]
- Kaith, B.S.; Mittal, H.; Jindal, R.; Maiti, M.; Kali, S. Environment Benevolent Biodegradable Polymers: Synthesis, Biodegradability and Applications. In Cellulose Fibers: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B.S., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 16; pp. 425–451. [Google Scholar]
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/PL/pl/product/aldrich/374741 (accessed on 23 December 2022).
- PolarTREC. Available online: https://www.polartrec.com/expeditions/carbon-in-the-arctic/journals/2019-05-25 (accessed on 25 May 2019).
- Wikipedia. Available online: https://pl.wikipedia.org/wiki/Triacyloglicerole (accessed on 16 January 2023).
- Heng, J.; Pearse, D.; Thielmann, F.; Lampke, T.; Bismarck, A. Methods to determine surface energies of natural fibres: A review. Compos. Interface 2007, 14, 581–604. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Wettability of natural fibres used as reinforcement for composites. Die Angew. Makromol. Chem. 2000, 272, 87–93. [Google Scholar] [CrossRef]
- Pucci, M.F.; Liotier, P.J.; Drapier, S. Capillary effects on flax fibers—Modification and characterization of the wetting dynamics. Compos. Part A 2015, 77, 257–265. [Google Scholar] [CrossRef]
- Baley, C.; Busnel, F.; Grohens, Y.; Sire, O. Influence of chemical treatments on surface properties and adhesion of flax fibre–polyester resin. Compos. Part A 2006, 37, 1626–1637. [Google Scholar] [CrossRef]
- Aranberri-Askargorta, I.; Lampke, T.; Bismarck, A. Wetting behavior of flax fibers as reinforcement for polypropylene. J. Colloid Interface Sci. 2003, 263, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S. Mechanical and Surface Properties of Technical and Single Flax Fiber in Micro and Nano Scale. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2017. [Google Scholar]
- Shahzad, A. A Study in Physical and Mechanical Properties of Hemp Fibres. Adv. Mater. Sci. Eng. 2013, 2013, 325085. [Google Scholar] [CrossRef]
- Gulati, D.; Sain, M. Surface Characteristics of Untreated and Modified Hemp Fibers. Polym. Eng. Sci. 2006, 46, 269–273. [Google Scholar] [CrossRef]
- Schellbach, S.; Monteiro, S.; Drelich, J. A novel method for contact angle measurements on natural fibers. Mater. Lett. 2016, 164, 599–604. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zhang, X.; Jing, G.; Yu, D.; Wang, S. Effects on surface properties of natural bamboo fibers treated with atmospheric pressure argon plasma. Surf. Interface Anal. 2006, 38, 1211–1217. [Google Scholar] [CrossRef]
- Zimniewska, M.; Zbrowski, A.; Konczewicz, W.; Majcher, A.; Przybylski, J.; Matecki, K.; Wiśniewski, M.; Kicińska-Jakubowska, A.; Mańkowski, J. Cottonization of decorticated flax fibres. Fibres Text. East. Eur. 2017, 25, 26–33. [Google Scholar] [CrossRef]
- Jóźwik, W.; Kozioł, S.; Matecki, K.; Neska, M.; Przybylski, J.; Wiśniewski, M.; Zbrowski, A.; Zimniewska, M.; Konczewicz, W.; Kicińska-Jakubowska, A.; et al. A prototype of a technological line for processing decorticated flax fibre. J. Mach. Constr. Maint. Maintenence Probl. 2017, 1, 73–79. [Google Scholar]
- BN-86/7501-10; Determination of Waxes and Fats Content. Methods of Testing Textile Products. Flax and Hemp Fiber. Wydawnictwo Normalizacyjne “Alfa”: Warsaw, Poland, 1986. (In Polish)
- BN-86/7501-11; Determination of Lignin Content. Methods of Textile Products Testing. Flax and Hemp Fiber. Wydawnictwo Normalizacyjne “Alfa”: Warsaw, Poland, 1986. (In Polish)
- BN-77/7529-02; Determination of Hemicellulose Content. Methods of Testing Textile Products. Flax and Hemp Fiber. Semi-Products and Textiles. Wydawnictwo Normalizacyjne “Alfa”: Warsaw, Poland, 1977. (In Polish)
- PN-P-50092:1992; Determination of Cellulose Content. Raw Materials for the Paper Industry. Wood. Chemical Analysis. Polski Komitet Normalizacyjny (PKN): Warsaw, Poland, 1992. (In Polish)
- Beketov, G.V.; Shynkarenko, O.V. Surface Wetting and Contact Angle: Basics and Characterization. Him. Fiz. Ta Tehnol. Poverhni 2022, 13, 3–35. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Liber-Kneć, A.; Łagan, S. The Use of Contact Angle and the Surface Free Energy as the Surface Characteristics of the Polymers Used in Medicine. Polym. Med. 2014, 44, 29–37. [Google Scholar]
- Kozłowski, R.; Konczewicz, W.; Kaniewski, R.; Dochia, M.; Pernevan, M.S. The Methods of Degumming Bast Fibrous Plants. Sci. Bull. ESCORENA 2011, 4, 17–31. [Google Scholar]
- Montreuil, A.; Mertz, G.; Bardon, J.; Guillot, J.; Grysan, P.; Addiego, F. Flax fiber treatment by an alkali solution and poly(dopamine) coating: Effects on the fiber physico-chemistry and flax/Elium® composite interfacial properties. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107963. [Google Scholar] [CrossRef]
- Nayak, S.Y.; Heckadka, S.S.; Seth, A.; Prabhu, S.; Sharma, R.; Shenoy, K.R. Effect of chemical treatment on the physical and mechanical properties of flax fibers: A comparative assessment. Mater. Proc. 2021, 38, 2406–2410. [Google Scholar] [CrossRef]
- Ding, J.; Liang, L.; Meng, X.; Yang, F.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G.; Yu, C. The physiochemical alteration of flax fibers structuring components after different scouring and bleaching treatments. Ind. Crops Prod. 2021, 160, 113112. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2016, 47, 2153–2183. [Google Scholar] [CrossRef]
- Soppie, A.G.; Betené, A.D.O.; Noah, P.M.A.; Njom, A.E.; Ebanda, F.B.; Ateba, A.; Mewoli, A.; Efeze, D.N.; Moukené, R. Chemical extraction and its effect on the properties of cordleaf burbark (Triumfetta cordifolia A. rich) fibres for the manufacture of textile yarns. Heliyon 2023, 9, e17581. [Google Scholar] [CrossRef] [PubMed]
- Elfaleh, I.; Abbassi, F.; Habibi, M.; Ahmad, F.; Guedri, M.; Nasri, M.; Garnier, C. A comprehensive review of natural fibers and their composites: An eco-friendly alternative to conventional materials. Results Eng. 2023, 19, 101271. [Google Scholar] [CrossRef]
- Moradkhani, G.; Profili, J.; Robert, M.; Laroche, G.; Elkoun, S. Effects of Wet and Dry Treatments on Surface Functional Groups and Mechanical Properties of Flax Fiber Composites. Coatings 2023, 13, 1036. [Google Scholar] [CrossRef]
- Bismarck, A.; Aranberri-Askargorta, I.; Springer, J. Surface Characterization of Flax, Hemp and Cellulose Fibers; Surface Properties and the Water Uptake Behavior. Polym. Compos. 2002, 23, 872–894. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.K.; Charlet, K.; Béakou, A. The effect of lignin on the reactivity of natural fibres towards molecular fluorine. Mater. Des. 2017, 120, 66–74. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.-X.; Sun, R.C.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Li, B.; Li, C.; Li, D.; Zhang, L.; Zhang, S.; Wang, D.; Leng, C.; Wang, Y.; Xiang, J.; Hu, X. Impacts of partial removal of lignin on development of pore structures in activation of Chinese parasol. Fuel 2024, 363, 131017. [Google Scholar] [CrossRef]
- Palanikumar, K.; Elango Natarajan, E.; Markandan, K.; Ang, C.K.; Franz, G. Targeted Pre-Treatment of Hemp Fibers and the Effect on Mechanical Properties of Polymer Composites. Fibers 2023, 11, 43. [Google Scholar] [CrossRef]
- Jankauskiene, Z.; Butkute, B.; Gruzdeviene, E.; Ceseviciene, J.; Fernando, A.L. Chemical composition and physical properties of dew- and water-retted hemp fibers. Ind. Crops Prod. 2015, 75, 206–211. [Google Scholar] [CrossRef]
- Poonsawat, T.; Sae-bae, P.; Bunphami, M.; Wetchaiyo, T.; Jatamaneerat, P. Effect of Water and Chemical Retting on Properties of Hemp Fibre and Hybrid Hemp/Cotton Spun Yarn. J. Eng. Appl. Sci. 2016, 11, 1991–1995. [Google Scholar] [CrossRef]
- Réquilé, S.; Mazian, B.; Grégoire, M.; Musio, S.; Gautreau, M.; Nuez, L.; Day, A.; Thiébeau, P.; Philippe, F.; Chabbert, B.; et al. Exploring the dew retting feasibility of hemp in very contrasting European environments: Influence on the tensile mechanical properties of fibres and composites. Ind. Crops Prod. 2021, 164, 113337. [Google Scholar] [CrossRef]
- Różańska, W.; Romanowska, B.; Rojewski, S. The Quantity and Quality of Flax and Hemp Fibers Obtained Using the Osmotic, Water-, and Dew-Retting Processes. Materials 2023, 16, 7436. [Google Scholar] [CrossRef] [PubMed]
- Pinsard, L.; Revol, N.; Pomikal, H.; De Luycker, E.; Ouagne, P. Production of Long Hemp Fibers Using the Flax Value Chain. Fibers 2023, 11, 38. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Van Sumere, C.F. The Biology and Processing of Flax; M Publications: Belfast, UK, 1992; ISBN 0 9519963 04. [Google Scholar]
- Madhav, S.; Ahamad, A.; Singh, P.; Mishra, P.K. A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ. Qual. Manag. 2018, 27, 31–41. [Google Scholar] [CrossRef]
- Holding, A.J. Cellulose: Structure, Morphology and Chemistry. In Ionic Liquids and Electrolytes for Cellulose Dissolution; University of Helsinki: Helsinki, Finland, 2016; pp. 18–23. ISBN 978-951-51-2641-2. [Google Scholar] [CrossRef]
- Akin, D.E. Chemistry of Plant Fibres. In Industrial Applications of Natural Fibres: Structure, Properties and Technical Applications; Mussig, J., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2010; pp. 13–22. ISBN 978-0-470-69501-1. [Google Scholar]
- Ma, R.; Pekarovicova, A.; Fleming, P.D., III; Husovska, V. Preparation and Characterization of Hemicellulose-Based Printable Films. Cellul. Chem. Technol. 2017, 51, 939–948. [Google Scholar]
- Muchlisyam, B.; Silalahi, J.; Harahap, U. Hemicellulose: Isolation and Its Application in Pharmacy. In Handbook of Sustainable Polymers: Processing and Applications; Thakur, V.K., Thakur, M.K., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016; Chapter 8; pp. 307–339. ISBN 978-981-4613-53-8. [Google Scholar]
- Day, A.; Ruel, K.; Neutelings, G.; Cronier, D.; David, H.; Hawkins, S.; Chabbert, B. Lignification in the flax stem: Evidence for an unusual lignin in bast fibers. Planta Int. J. Plant Biol. 2005, 222, 234–245. [Google Scholar] [CrossRef]
- Vanitha, T.; Khan, M. Role of Pectin in Food Processing and Food Packaging. In Pectins—Extraction, Purification, Characterization and Applications; Masuelli, M., Blumenberg, M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]



| Fibers | Waxes and Fats | Pectin | Lignin | Cellulose | Hemicellulose | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| [%] | SD | [%] | SD | [%] | SD | [%] | SD | [%] | SD | |
| MODRAN | ||||||||||
| Decortication | 1.26 | 0.00 | 4.62 a | 0.16 | 4.00 a | 0.16 | 68.89 | 1.91 | 29.35 | 0.16 |
| Wet degumming | 0.69 | 0.07 | 4.41 a | 0.50 | 4.20 a | 0.16 | 75.54 a | 1.18 | 19.62 | 0.15 |
| Cottonization | 0.97 | 0.10 | 4.72 a | 0.39 | 4.26 a | 0.15 | 73.51 a | 0.98 | 16.44 | 0.23 |
| NIKE | ||||||||||
| Decortication | 1.47 | 0.07 | 4.11 | 0.38 | 8.60 | 0.30 | 64.57 | 0.85 | 29.38 | 0.08 |
| Wet degumming | 0.76 | 0.00 | 3.56 | 0.27 | 4.46 a | 0.48 | 77.44 | 1.58 | 16.43 | 0.25 |
| Cottonization | 0.95 | 0.05 | 2.39 | 0.22 | 4.87 a | 0.51 | 74.25 | 0.20 | 13.84 | 0.06 |
| B14 | ||||||||||
| Decortication | 0.88 | 0.01 | 4.16 | 0.12 | 5.25 | 0.03 | 68.21 | 1.06 | 31.02 | 0.25 |
| Wet degumming | 1.33 | 0.01 | 5.43 | 0.28 | 6.69 a | 0.48 | 75.04 | 0.46 | 23.92 | 0.02 |
| Cottonization | 1.72 | 0.09 | 3.57 | 0.23 | 6.10 a | 0.01 | 72.20 | 0.47 | 20.41 | 0.09 |
| Fibers | Waxes and Fats | Pectin | Lignin | Cellulose | Hemicellulose | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| [%] | SD | [%] | SD | [%] | SD | [%] | SD | [%] | SD | |
| Water retting | ||||||||||
| BENIKO | 0.23 a | 0.01 | 1.47 | 0.09 | 2.81 a | 0.29 | 71.31 a | 1.32 | 15.03 a | 0.02 |
| WOJKO | 0.24 a | 0.04 | 0.67 a | 0.02 | 3.02 a | 0.31 | 72.53 a | 0.11 | 16.67 | 0.24 |
| TYGRA | 0.25 a | 0.04 | 0.56 | 0.00 | 2.78 a | 0.28 | 70.79 a | 0.13 | 15.00 a | 0.28 |
| BIAŁOBRZESKIE | 0.34 | 0.02 | 0.67 a | 0.02 | 2.38 a | 0.22 | 72.03 a | 0.22 | 14.37 | 0.29 |
| Fibers | Waxes and Fats | Pectin | Lignin | Cellulose | Hemicellulose | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| [%] | SD | [%] | SD | [%] | SD | [%] | SD | [%] | SD | |
| BIAŁOBRZESKIE | ||||||||||
| Dew retting | 0.56 a | 0.14 | 3.68 | 0.19 | 4.31 | 0.04 | 66.16 | 0.48 | 21.72 | 0.12 |
| Water retting | 0.34 a | 0.02 | 0.67 | 0.02 | 2.38 | 0.22 | 72.03 | 0.22 | 14.37 | 0.29 |
| Bond | Vibration Type | Wavenumber [cm−1] | Remarks |
|---|---|---|---|
| O-H | Stretching | 3100–3600 | Cellulose, hemicellulose, lignin, pectin |
| C-H3 | Stretching | 2954–2970 | Lignin |
| C-H, C-H2 | Stretching | 2915–2923; 2895–2897; 2841–2848 | Cellulose, hemicellulose, lignin, pectin, waxes and fats |
| C=O | Stretching | 1730–1736 | Carboxylic acids, aldehydes, esters (pectin, lignin, waxes and fats) |
| O-H | Stretching | 1615–1645 | Adsorbed water |
| C=C Aromatic | Symmetrical Stretching | 1593–1595; 1507–1508 | Peaks characteristic of lignin |
| O-H and C-H3 and C-H2 | Bending and Deforming | 1461–1463 and 1461–1463; 1472–1473 | Adsorbed water and Lignin and cellulose, hemicellulose, pectin, waxes and fats |
| COO | Stretching | 1418–1420; 1424–1426 | Acids (pectin) |
| C-H3 | Symmetrical Deformation | 1370–1373 | Lignin |
| O-H | Bending | 1332–1338 | Cellulose, hemicellulose, lignin, pectin |
| CH2 | Scissoring (bending) | 1312–1314 | Cellulose, hemicellulose |
| C-H | Bending | 1271–1278 | Peak characteristic for lignin |
| C-O | Stretching | 1244–1246 | Hemicellulose, pectin |
| C-H | Bending | 1201–1204 | Flax, hemp |
| C-O-C | Bending | 1156–1161; 1051; 1020–1028 | Cellulose, hemicellulose, pectin |
| C-O | Stretching | 910–1125 | Cellulose, hemicellulose, pectin |
| Β-Glycosidic bond | Stretching | 893–897 | Cellulose, hemicellulose, pectin |
| Waxes and Fats Content [%] | Pectin Content [%] | Lignin Content [%] | Cellulose Content [%] | Hemicellulose Content [%] | |
|---|---|---|---|---|---|
| Flax fibers tested after each stage of processing | |||||
| SFE of flax fibers after decortication process [mN/m] | −0.04 | −1.00 | 0.79 | −0.71 | 0.40 |
| SFE of flax fibers after wet degumming process [mN/m] | 0.87 | 0.46 | 0.87 | −0.10 | 0.49 |
| SFE of flax fibers after cottonization process [mN/m] | 1.00 | 0.04 | 0.93 | −0.95 | 0.93 |
| Hemp fibers extracted with the use of water retting | |||||
| SFE of hemp fibers after water-retting process [mN/m] | −0.92 | 0.05 | 0.98 | −0.09 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowska, B.; Różańska, W.; Zimniewska, M. The Influence of the Chemical Composition of Flax and Hemp Fibers on the Value of Surface Free Energy. Materials 2024, 17, 1104. https://doi.org/10.3390/ma17051104
Romanowska B, Różańska W, Zimniewska M. The Influence of the Chemical Composition of Flax and Hemp Fibers on the Value of Surface Free Energy. Materials. 2024; 17(5):1104. https://doi.org/10.3390/ma17051104
Chicago/Turabian StyleRomanowska, Barbara, Wanda Różańska, and Małgorzata Zimniewska. 2024. "The Influence of the Chemical Composition of Flax and Hemp Fibers on the Value of Surface Free Energy" Materials 17, no. 5: 1104. https://doi.org/10.3390/ma17051104
APA StyleRomanowska, B., Różańska, W., & Zimniewska, M. (2024). The Influence of the Chemical Composition of Flax and Hemp Fibers on the Value of Surface Free Energy. Materials, 17(5), 1104. https://doi.org/10.3390/ma17051104







