Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Effect of Current Density on the Formation of Solute Hydrogen and Hydride Phase
3.2. Comparison of Nano-Mechanical Behavior by Nanoindentation
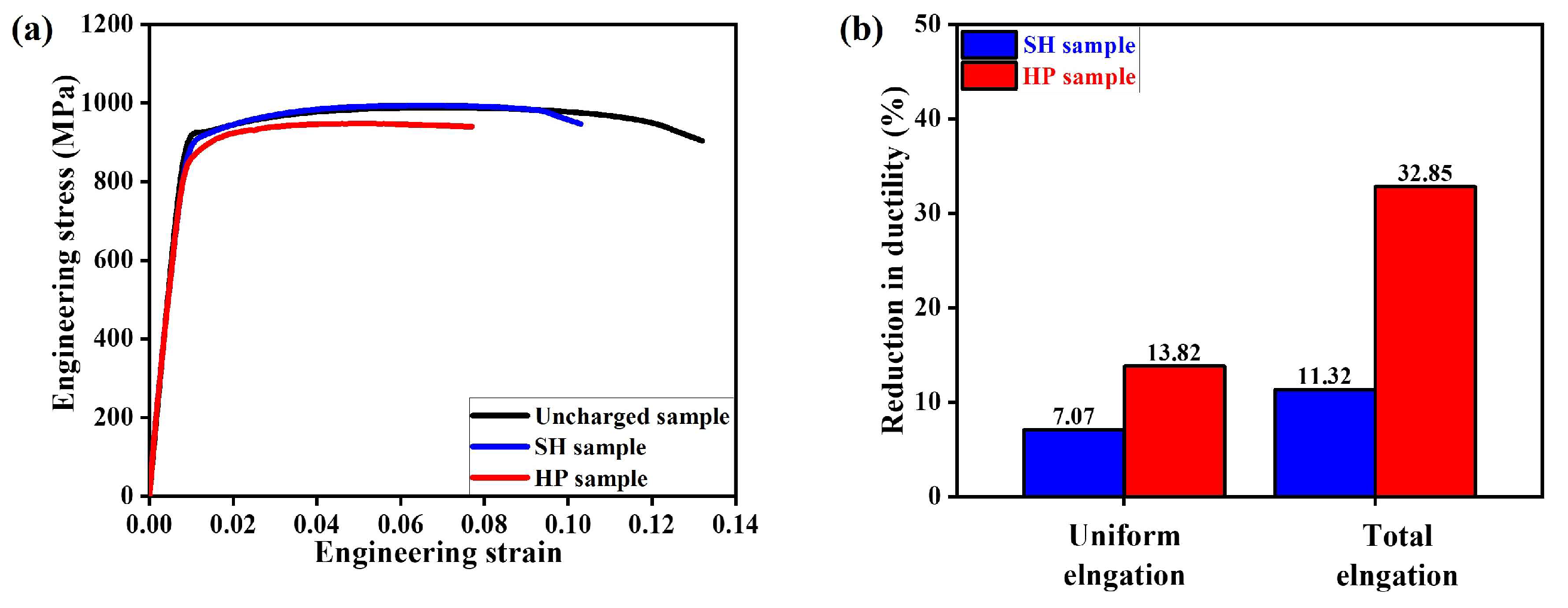
3.3. Comparison of Micro-Mechanical Behavior by Tensile Test
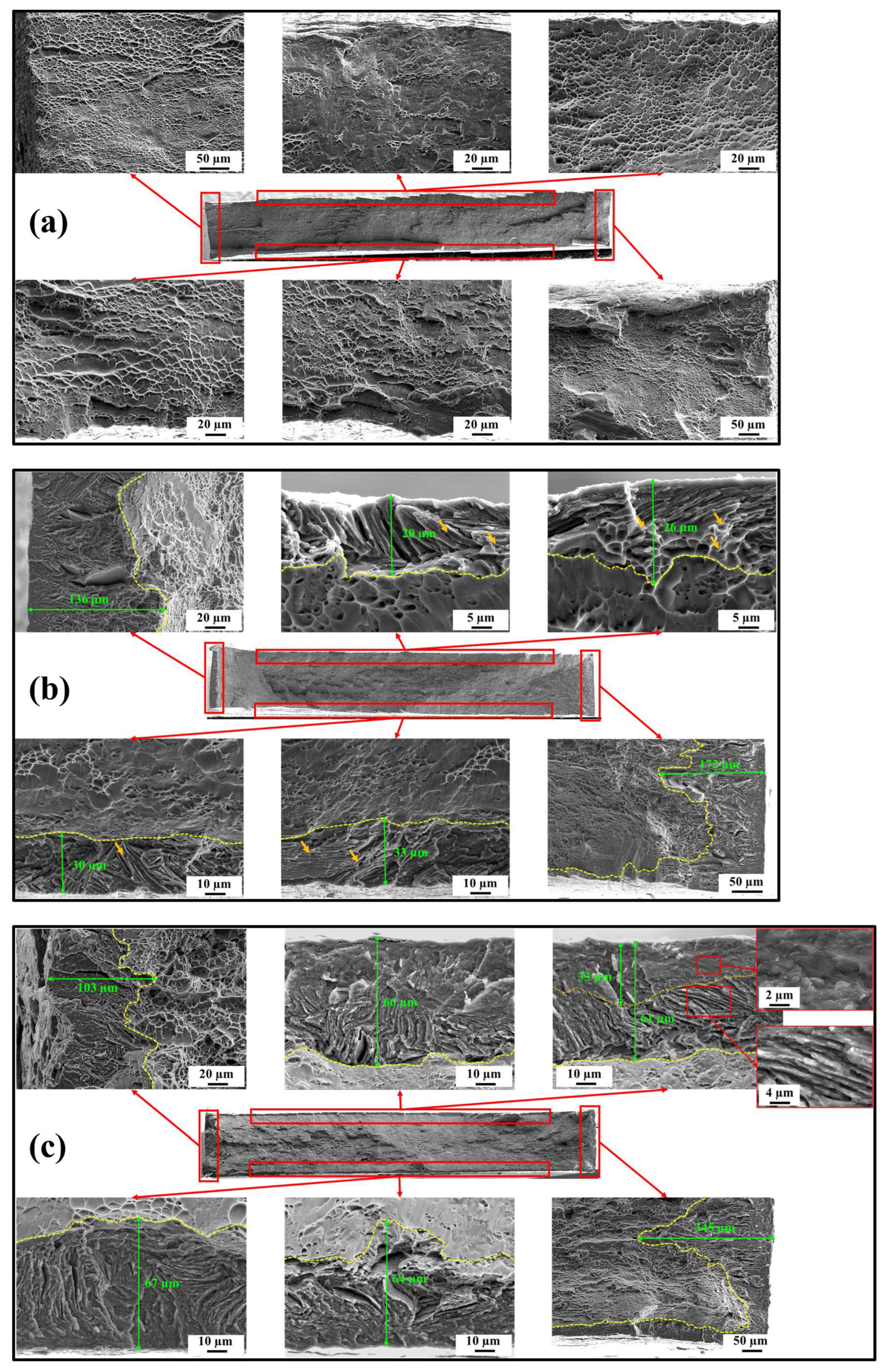
3.4. Fractography
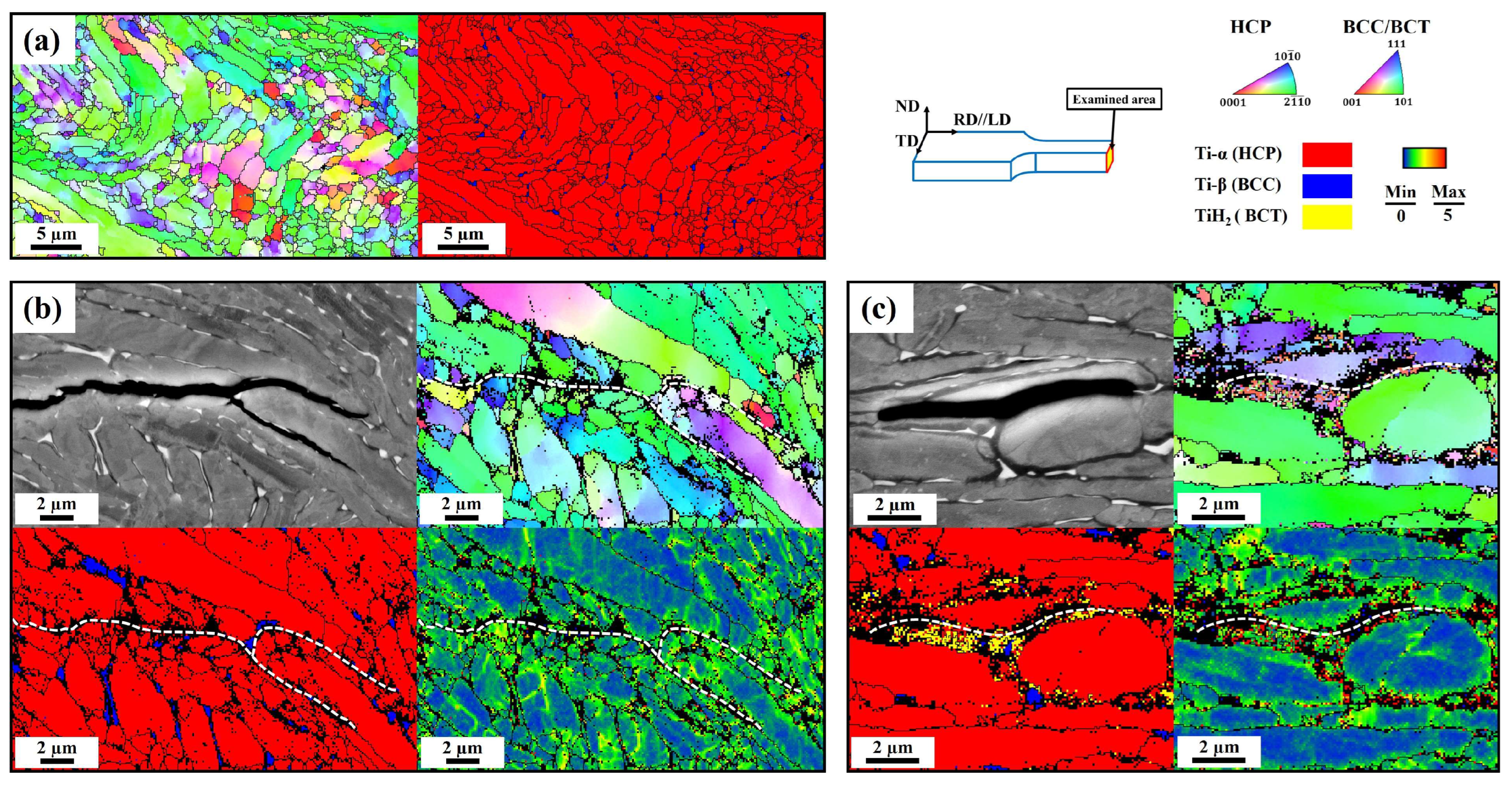
3.5. Hydrogen-Assisted Cracking Behavior
4. Conclusions
- (1)
- Solute hydrogen and the hydride phase were successfully induced by different electrochemically hydrogen-charging processes conducted at low (1 mA/cm2) and high (50 mA/cm2) current densities, respectively;
- (2)
- Hydrogen-charged samples generally exhibited brittle fracture features. On the microscopic scale, the impact of different hydrogen-charging conditions was not significant as evidenced by the relatively consistent results of nanoindentation measurements. However, macroscopic fracture behavior strongly depended on the diffused hydrogen concentration. With a low diffused hydrogen concentration indicating the presence of solute hydrogen, elongation was primarily reduced by 7.07% in the UE and 11.32% in the TE, respectively, but the strength was negligible. Conversely, when the hydrogen concentration was sufficient to form the hydride phase, a substantial reduction by 13.82% in the UE and 32.85% in the TE was found;
- (3)
- Both solute hydrogen and the hydride phase give rise to hydrogen embrittlement. Solute hydrogen enhanced intergranular cracking behavior by forming nano/micro-voids at the grain boundaries. Conversely, the occurrence of cleavage or intergranular fracture modes depended on the distribution of the hydride phase. The increased volume within the hydride phase could act as sites for crack initiation and propagation, leading to more severe hydrogen embrittlement.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Williams, J.C.; Boyer, R.R. Opportunities and Issues in the Application of Titanium Alloys for Aerospace Components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F.H. (Sam) The Additive Manufacturing (AM) of Titanium Alloys. Metal Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Saldaña, L.; Vallés, G.; González-Carrasco, J.L.; González-Cabrero, J.; Martínez, M.E.; Gil-Garay, E.; Munuera, L. In Vitro Corrosion Behaviour and Osteoblast Response of Thermally Oxidised Ti6Al4V Alloy. Biomaterials 2003, 24, 19–26. [Google Scholar] [CrossRef]
- Pazhanivel, B.; Sathiya, P.; Sozhan, G. Ultra-Fine Bimodal (α + β) Microstructure Induced Mechanical Strength and Corrosion Resistance of Ti-6Al-4V Alloy Produced via Laser Powder Bed Fusion Process. Opt. Laser Technol. 2020, 125, 106017. [Google Scholar] [CrossRef]
- Silverstein, R.; Eliezer, D. Hydrogen Trapping in 3D-Printed (Additive Manufactured) Ti-6Al-4V. Mater. Charact. 2018, 144, 297–304. [Google Scholar] [CrossRef]
- Kong, D.; Zhao, D.; Zhu, G.; Ni, X.; Zhang, L.; Wu, W.; Man, C.; Zhou, Y.; Dong, C.; Sun, B. Heat Treatment Effects on the Hydrogen Embrittlement of Ti6Al4V Fabricated by Laser Beam Powder Bed Fusion. Addit. Manuf. 2022, 50, 102580. [Google Scholar] [CrossRef]
- Deconinck, L.; Depover, T.; Verbeken, K. The Mechanism of Hydride Formation during Electrochemical Hydrogen Charging of Ti-6Al-4V. Mater. Today Sustain. 2023, 22, 100387. [Google Scholar] [CrossRef]
- Kim, J.; Hall, D.; Yan, H.; Shi, Y.; Joseph, S.; Fearn, S.; Chater, R.J.; Dye, D.; Tasan, C.C. Roughening Improves Hydrogen Embrittlement Resistance of Ti-6Al-4V. Acta Mater. 2021, 220, 117304. [Google Scholar] [CrossRef]
- Yuan, B.G.; Yu, H.P.; Li, C.F.; Sun, D.L. Effect of Hydrogen on Fracture Behavior of Ti-6Al-4V Alloy by in-Situ Tensile Test. Int. J. Hydrogen Energy 2010, 35, 1829–1838. [Google Scholar] [CrossRef]
- Chang, Y.; Breen, A.J.; Tarzimoghadam, Z.; Kürnsteiner, P.; Gardner, H.; Ackerman, A.; Radecka, A.; Bagot, P.A.J.; Lu, W.; Li, T.; et al. Characterizing Solute Hydrogen and Hydrides in Pure and Alloyed Titanium at the Atomic Scale. Acta Mater. 2018, 150, 273–280. [Google Scholar] [CrossRef]
- Nelson, H.G. Hydrogen embrittlement. Treatise Mater. Sci. Technol. 1983, 25, 275–359. [Google Scholar] [CrossRef]
- Conforto, E.; Caillard, D. A Fast Method for Determining Favourable Orientation Relationships and Interface Planes: Application to Titanium-Titanium Hydrides Transformations. Acta Mater. 2007, 55, 785–798. [Google Scholar] [CrossRef]
- Brosh, E.; Navi, N.U.; Rosen, B.A.; Eliaz, N. Microvoids in Electrochemically Hydrogenated Titanium-Based Alloys. Int. J. Hydrogen Energy 2021, 46, 27234–27242. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.J. Effect of Microstructure Control of High-Strength Steel on Hydrogen Diffusivity, Trap Activation Energy, and Cracking Resistance in Sour Environments. Corros. Sci. Technol. 2023, 22, 131–136. [Google Scholar] [CrossRef]
- Metalnikov, P.; Eliezer, D.; Ben-Hamu, G. Hydrogen Trapping in Additive Manufactured Ti–6Al–4V Alloy. Mater. Sci. Eng. A 2021, 811, 141050. [Google Scholar] [CrossRef]
- Wu, W.; He, G.; Huang, J.; Zhang, A.; Liu, X.; Ouyang, Z.; Sun, Z.; Guan, L.; Chu, S.; Li, P.; et al. Influence of Electrochemically Charged Hydrogen on Mechanical Properties of Ti–6Al–4V Alloy Additively Manufactured by Laser Powder-Bed Fusion (L-PBF) Process. Mater. Sci. Eng. A 2023, 866, 144339. [Google Scholar] [CrossRef]
- Deconinck, L.; Villa Vidaller, M.T.; Bernardo Quejido, E.; Jägle, E.A.; Depover, T.; Verbeken, K. In-Situ Hydrogen Embrittlement Evaluation of as-Built and Heat Treated Laser Powder Bed Fused Ti-6Al-4V versus Conventionally Cold Rolled Ti-6Al-4V. Addit. Manuf. 2023, 76, 103768. [Google Scholar] [CrossRef]
- Kim, J.; Plancher, E.; Tasan, C.C. Hydrogenation-Induced Lattice Expansion and Its Effects on Hydrogen Diffusion and Damage in Ti–6Al–4V. Acta Mater. 2020, 188, 686–696. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Xia, J.; Chen, Y. Effect of Hydrogen Precharging on Mechanical and Electrochemical Properties of Pure Titanium. Adv. Eng. Mater. 2020, 22, 1901182. [Google Scholar] [CrossRef]
- Pushilina, N.; Panin, A.; Syrtanov, M.; Kashkarov, E.; Kudiiarov, V.; Perevalova, O.; Laptev, R.; Lider, A.; Koptyug, A. Hydrogen-Induced Phase Transformation and Microstructure Evolution for Ti-6Al-4V Parts Produced by Electron Beam Melting. Metals 2018, 8, 301. [Google Scholar] [CrossRef]
- Zhang, H.; Leygraf, C.; Wen, L.; Huang, F.; Chang, H.; Jin, Y. The Formation of Hydride and Its Influence on Ti–6Al–4V Alloy Fracture Behavior. Int. J. Hydrogen Energy 2023, 48, 36169–36184. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, W.; Zhang, G.; Wang, B. Surface and Phase Transformation Characteristics of Titanium Hydride Film under Irradiation of Pulsed Ion Beam. Appl. Surf. Sci. 2013, 285, 557–563. [Google Scholar] [CrossRef]
- Wen, J.; Allain, N.; Fleury, E. Hydrogen Evolution and Its Effects on Cold Rolling Behavior in Commercial Pure Titanium. Mater. Charact. 2016, 121, 139–148. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards. Anal Chem. 1973, 45, 944A. [CrossRef]
- Luo, L.; Su, Y.; Guo, J.; Fu, H. Formation of Titanium Hydride in Ti-6Al-4V Alloy. J. Alloys Compd. 2006, 425, 140–144. [Google Scholar] [CrossRef]
- Moshtaghi, M.; Loder, B.; Safyari, M.; Willidal, T.; Hojo, T.; Mori, G. Hydrogen Trapping and Desorption Affected by Ferrite Grain Boundary Types in Shielded Metal and Flux-Cored Arc Weldments with Ni Addition. Int. J. Hydrogen Energy 2022, 47, 20676–20683. [Google Scholar] [CrossRef]
- Pichler, S.; Bendo, A.; Mori, G.; Safyari, M.; Moshtaghi, M. Inhibition of Grain Growth by Pearlite Improves Hydrogen Embrittlement Susceptibility of the Ultra-Low Carbon Ferritic Steel: The Influence of H-Assisted Crack Initiation and Propagation Mechanisms. J. Mater. Sci. 2023, 58, 13460–13475. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, W.C.; Jain, J.; Huang, E.W.; Lee, S.Y. Hydrogen Embrittlement of a Boiler Water Wall Tube in a District Heating System. Metals 2022, 12, 1276. [Google Scholar] [CrossRef]
- Hwang, H.K.; Shin, D.H.; Kim, S.J. Hydrogen Embrittlement Characteristics by Slow Strain Rate Test of Aluminum Alloy for Hydrogen Valve of Hydrogen Fuel Cell Vehicle. Corros. Sci. Technol. 2022, 21, 503–513. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, S.; Lecomte, J.S.; Schuman, C.; Peltier, L.; Shen, X.; Song, W. Crystallographic Orientation Dependence of Hydride Precipitation in Commercial Pure Titanium. Acta Mater. 2020, 183, 329–339. [Google Scholar] [CrossRef]
- Wang, Q.; Lecomte, J.S.; Schuman, C.; Peltier, L. Nanoindentation Study of Hydride Diffusion Layer in Commercial Pure Titanium. Mater. Sci. Eng. A 2022, 832, 142428. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of Strain Rate on Hydrogen Embrittlement in Low-Carbon Martensitic Steel. Int. J. Hydrogen Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Martin, M.L.; Somerday, B.P.; Ritchie, R.O.; Sofronis, P.; Robertson, I.M. Hydrogen-Induced Intergranular Failure in Nickel Revisited. Acta Mater. 2012, 60, 2739–2745. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, H.; Lin, M.; Zhao, K.; Xiao, S.; Vinogradov, A.; Qiao, L.; Ortiz, M.; He, J.; Zhang, Z. Hydrogen-Enhanced Grain Boundary Vacancy Stockpiling Causes Transgranular to Intergranular Fracture Transition. Acta Mater. 2022, 239, 118279. [Google Scholar] [CrossRef]
- Lee, D.H.; Zhao, Y.; Lee, S.Y.; Ponge, D.; Jägle, E.A. Hydrogen-Assisted Failure in Inconel 718 Fabricated by Laser Powder Bed Fusion: The Role of Solidification Substructure in the Embrittlement. Scr Mater. 2022, 207, 114308. [Google Scholar] [CrossRef]


| Mechanical Properties | Uncharged Sample | SH Sample | HP Sample |
|---|---|---|---|
| Ultimate tensile strength (MPa) | 986 ± 6 | 1000 ± 6 | 963 ± 18 |
| Yield strength (MPa) | 904 ± 16 | 900 ± 13 | 874 ± 10 |
| Uniform elongation (%) | 7.07 ± 0.80 | 6.57 ± 0.05 | 6.27 ± 0.16 |
| Total elongation (%) | 13.24 ± 1.25 | 11.41 ± 1.53 | 8.89 ± 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-D.; Singh, C.; Lee, D.-H.; Kim, Y.S.; Lee, T.; Lee, S.Y. Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase. Materials 2024, 17, 1178. https://doi.org/10.3390/ma17051178
Nguyen T-D, Singh C, Lee D-H, Kim YS, Lee T, Lee SY. Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase. Materials. 2024; 17(5):1178. https://doi.org/10.3390/ma17051178
Chicago/Turabian StyleNguyen, Tien-Dung, Chetan Singh, Dong-Hyun Lee, You Sub Kim, Taeho Lee, and Soo Yeol Lee. 2024. "Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase" Materials 17, no. 5: 1178. https://doi.org/10.3390/ma17051178
APA StyleNguyen, T.-D., Singh, C., Lee, D.-H., Kim, Y. S., Lee, T., & Lee, S. Y. (2024). Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase. Materials, 17(5), 1178. https://doi.org/10.3390/ma17051178






