Preparation of Antimony-Doped Tin Oxide Fly Ash Antistatic Composite and Its Properties in Filling EVA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Powder
2.3. Preparation of EVA Composite Material
2.4. Testing Methods
3. Results and Discussion
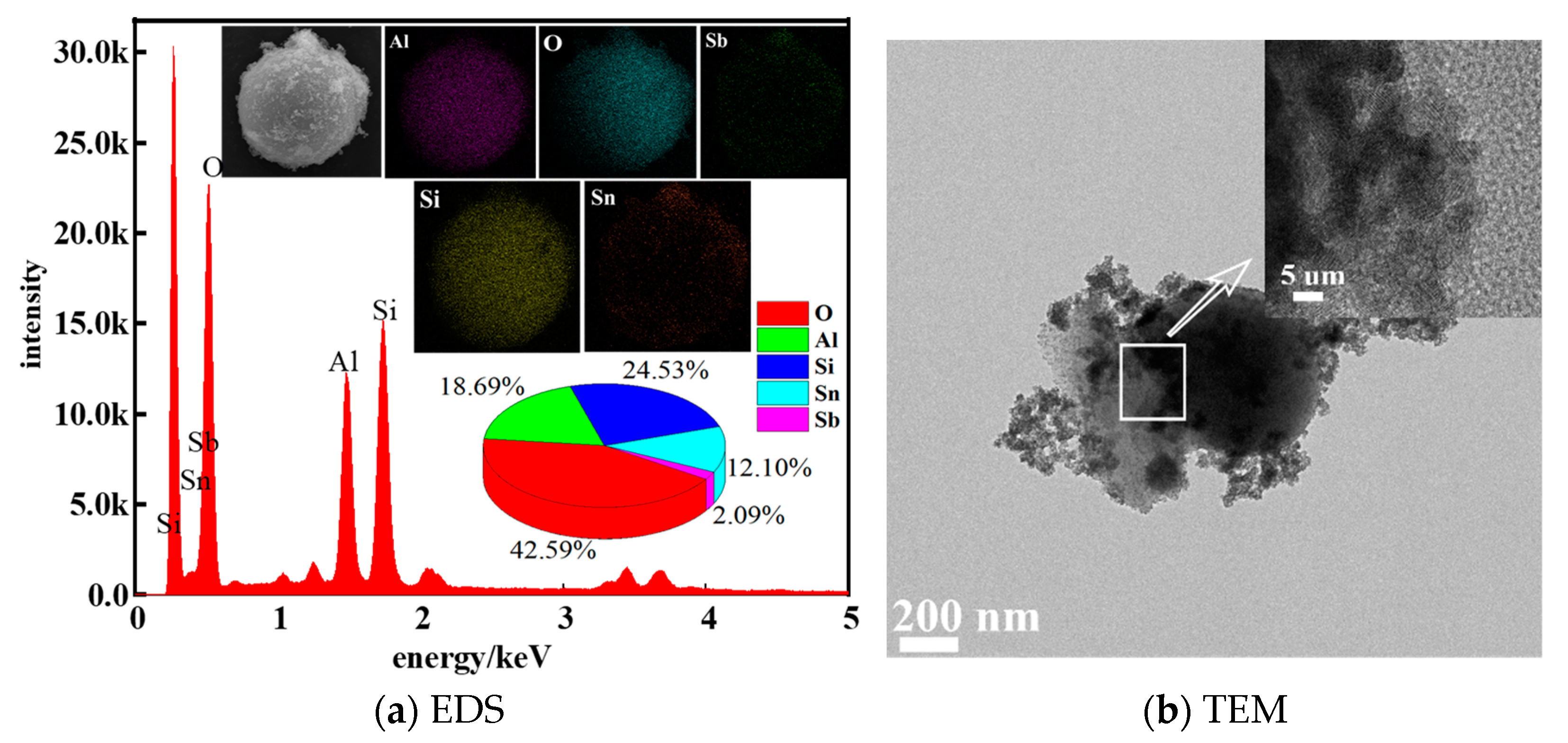
3.1. SEM and EDS Analysis of Composite Powder
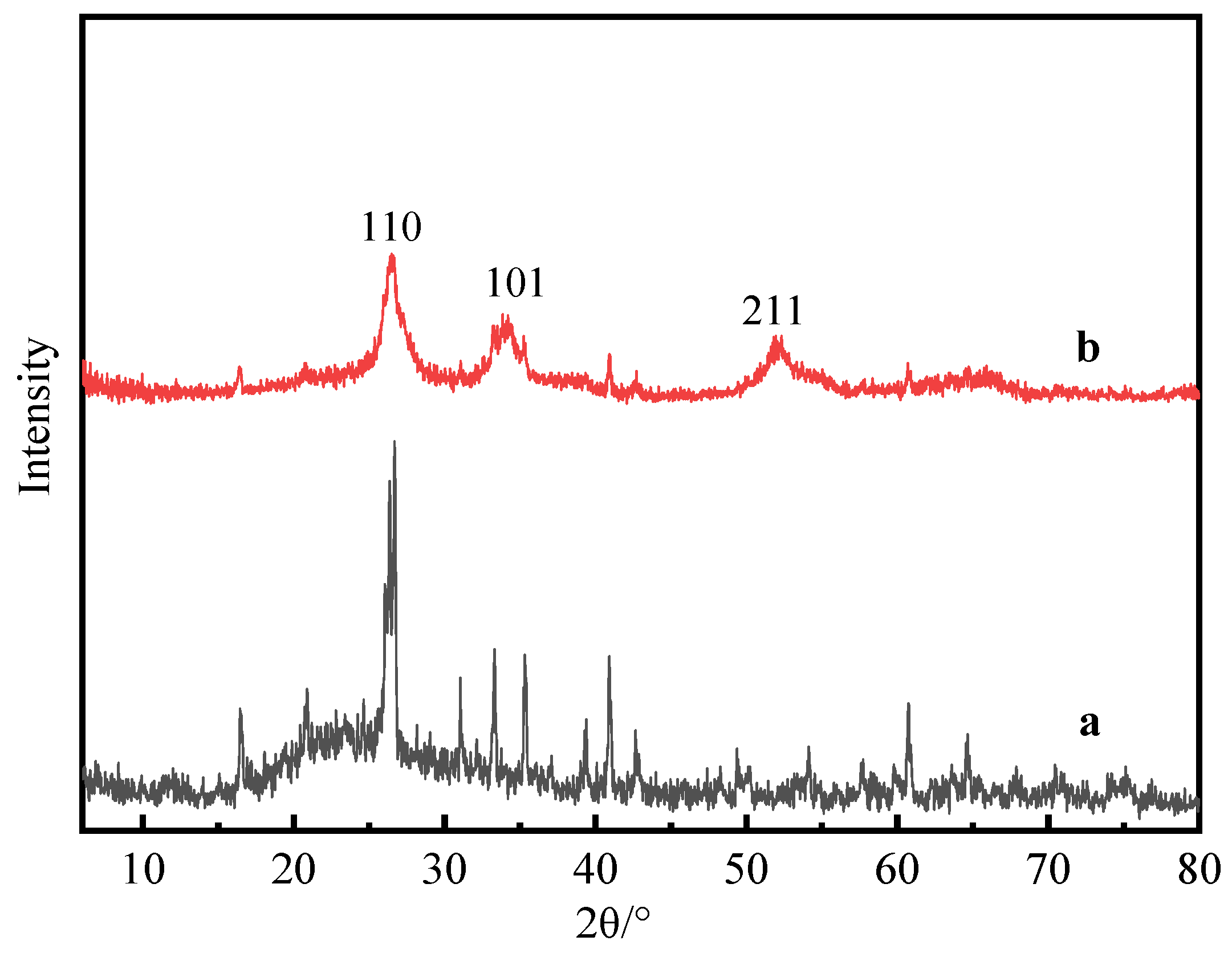
3.2. XRD Analysis of Composite Powder
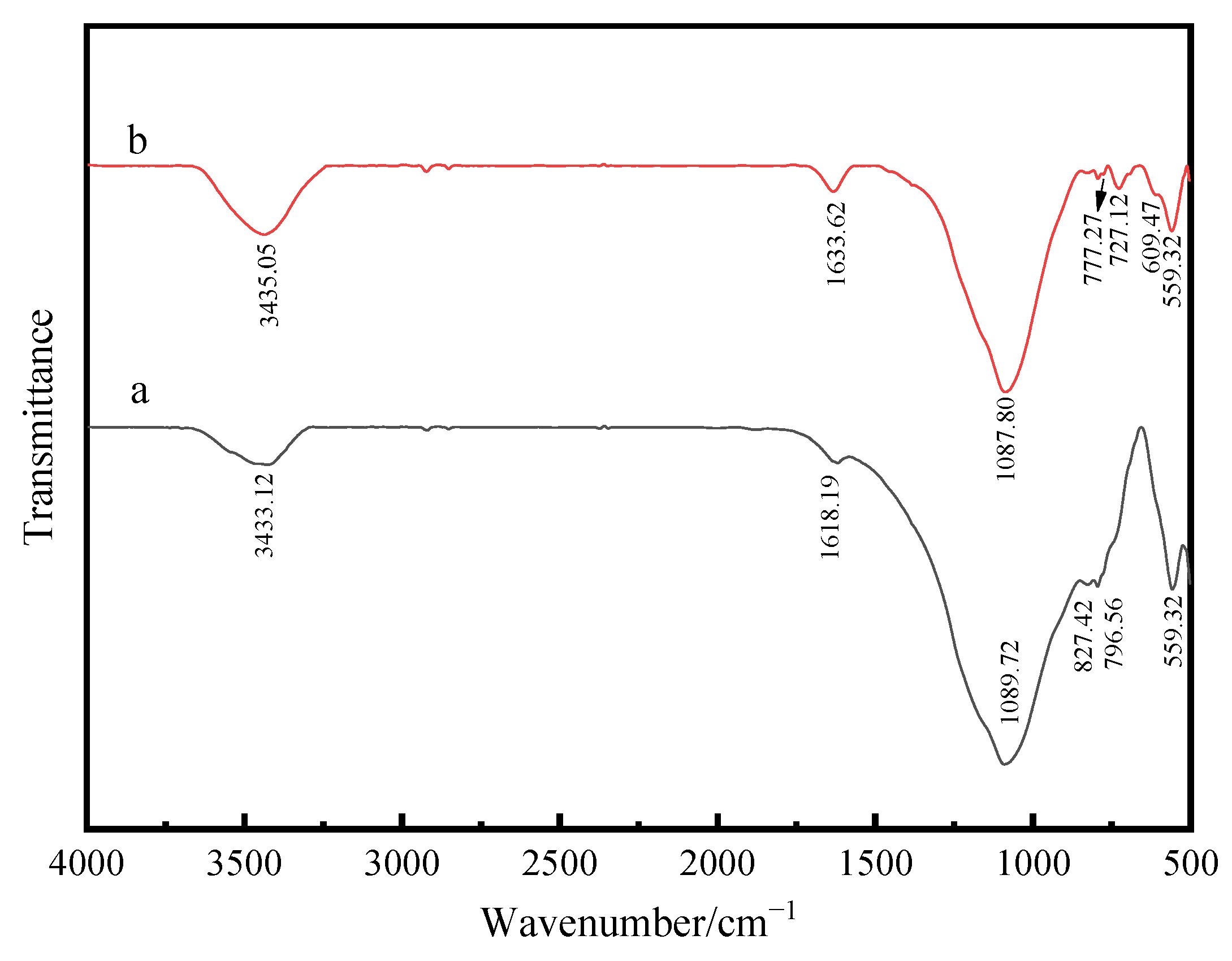
3.3. FTIR Analysis of Composite Powder
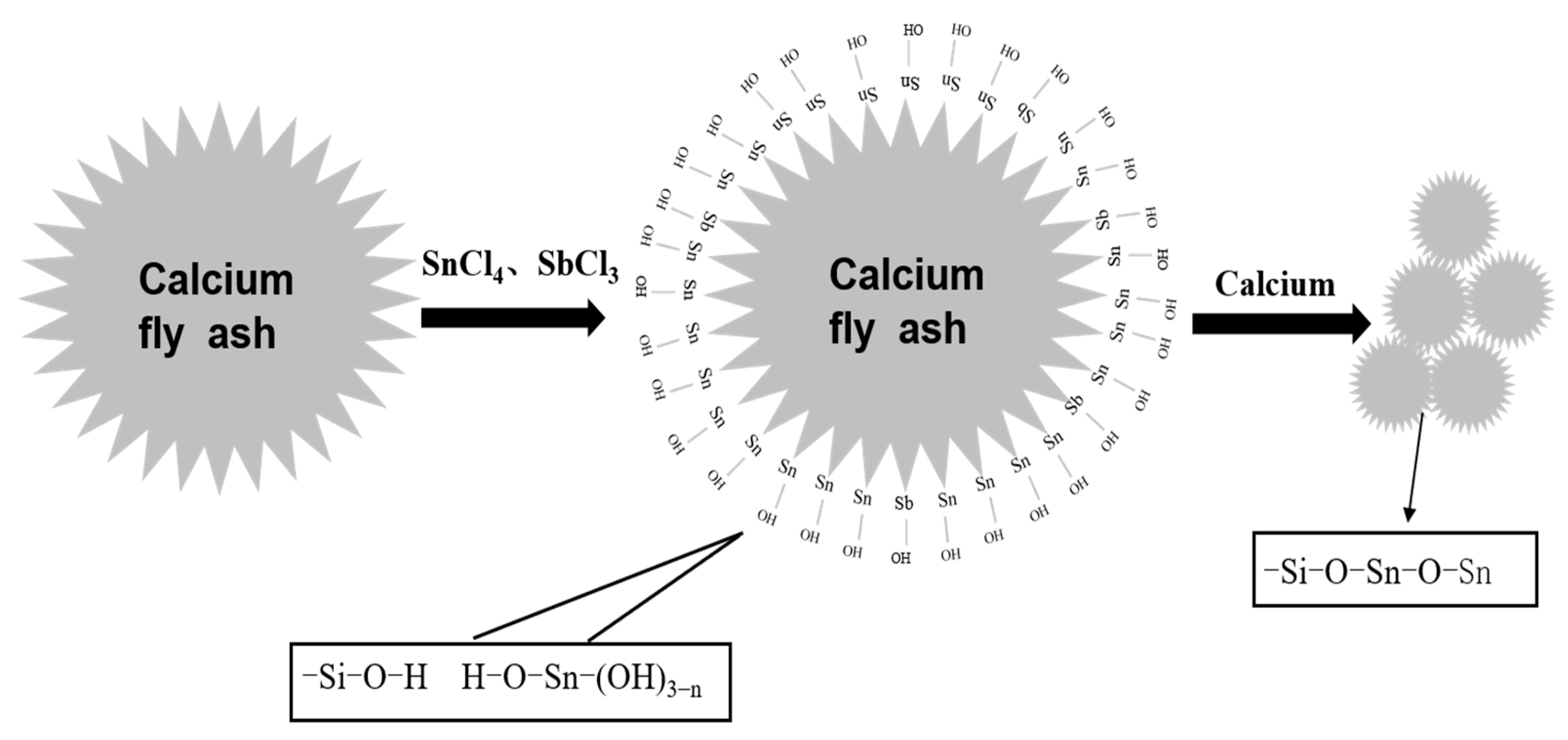
3.4. Preparation Mechanism of Composite Powder
3.5. Performance Analysis of EVA Filled with Composite Powder
3.6. Analysis of Cross-Section Morphology of EVA Filled with Composite Powder
4. Conclusions
- (1)
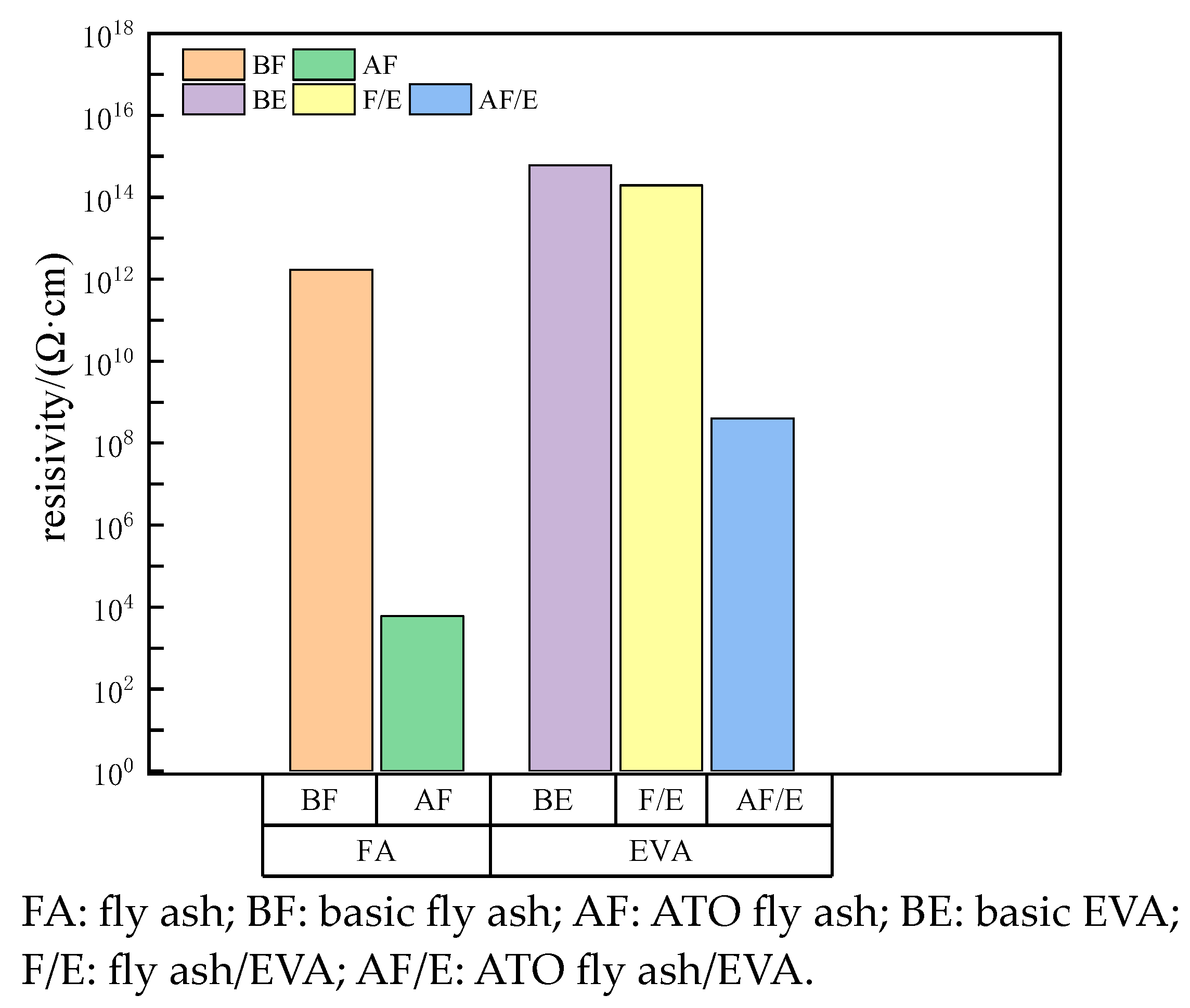
- ATO was successfully coated on the surface of calcined fly ash, and the volume electrical resistivity of calcined fly ash was decreased from 1.72 × 1012 Ω·cm to 6 × 103 Ω·cm, which belonged to the antistatic powder;
- (2)
- According to the analysis of XRD, SEM, EDS and FTIR, Si-OH in fly ash and Sn (Sb)-OH of ATO are condensed to form a Si-O-Sn (Sb) bond, which is calcined at high temperatures to generate Sb-SnO2 with a rutile structure and conductive function. Nano-antimony-doped tin oxide particles are uniformly distributed on the surface of fly ash;
- (3)
- The EVA-filling experiment showed that the tensile strength and elongation at the break of ATO fly ash/EVA were better than those of calcined fly ash/EVA. ATO fly-ash composite powder-filling EVA had little effect on the melting index. Compared with calcined fly ash, ATO fly ash has fewer spherical particles in the EVA section after filling, so it has better compatibility with EVA;
- (4)
- The surface electrical resistivity of calcined fly ash filled with EVA is high, and its antistatic performance cannot meet the requirements. The surface electrical resistivity of EVA filled with ATO fly-ash composite powder is significantly reduced, which makes EVA have good antistatic properties. ATO fly ash can improve the antistatic performance of EVA without deteriorating the performance of the matrix;
- (5)
- Compared with calcined fly ash, the limiting oxygen index of ATO fly ash composite powder filled with EVA increased more, which enhanced the flame retardant performance of EVA, indicating that there was a synergistic flame-retardant effect between fly ash and antimony-doped tin oxide.
- (6)
- ATO fly-ash composite powder has a good application prospect in the field of polymer-material modification. The filled EVA can be widely used in the construction industry, wire and cable and other fields, which greatly reduces the harm caused by static electricity. ATO is expensive when used alone as an antistatic material, so coating it on economic materials such as fly ash can meet the antistatic requirements while reducing costs. The laboratory preparation method of this material is complicated, and its production process needs to be optimized during industrial production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vertepa, A.V.; Starostina, I.A.; Kuzina, N.A.; Perukhin, Y.V.; Stoyanov, O.V. Modification of High-Pressure Polyethylene and Ethylene–Vinyl Acetate Copolymer with Sepiolite Chain-Layered Silicate. Poly. Sci. Ser. D 2023, 16, 381–385. [Google Scholar] [CrossRef]
- Yan, J.; He, Y.; Liu, L.; Li, X.; Shen, W.; Xu, M. High-efficient flame retardant ethylene-vinyl acetate composites by incorporating monomolecular IFR and its pyrolysis behavior. J. Anal. Appl. Pyrolysis 2023, 175, 106210. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Lin, S.; Guo, J. Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents. e-Polymers 2022, 22, 370–378. [Google Scholar] [CrossRef]
- Su, Y.; Yin, H.; Wang, X.; Yong, M.; Sravanthi, V.; Guo, Z.; Song, G. Preparation and properties of ethylene-acrylate salt ionomer/polypropylene antistatic alloy. Adv. Compos. Hybrid. Mater. 2021, 4, 104–113. [Google Scholar] [CrossRef]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A review on recent advancements in electrochemical detection of dopamine using carbonaceous nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- Isobe, Y.; Fuse, M.; Kobayashi, K. Additive element effects on electronic conductivity of zirconium oxide film. J. Nucl. Sci. Technol. 1994, 31, 546–551. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Feng, B.; Duan, K.; Weng, J. Synthesis and characterization of antimony-doped tin oxide (ATO) nanoparticles with high conductivity using a facile ammonia-diffusion co-precipitation method. J. Alloy. Compd. 2015, 634, 37–42. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Liu, B.; Yin, Z. Preparation and Properties of Antimony Doped Tin Oxide Nanopowders and Their Conductivity. Mater. Res. Bull. 2016, 83, 354–359. [Google Scholar] [CrossRef]
- Mishra, M.; Sahu, S.K.; Mangaraj, P.; Beig, G. Assessment of hazardous radionuclide emission due to fly ash from fossil fuel combustion in industrial activities in India and its impact on public. J. Environ. Manag. 2023, 328, 116908. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Sobczyk, A.T.; Czech, T.; Marchewicz, A.; Krupa, A. Recovery of cenospheres from solid waste produced by coal-fired power plants. Clean. Waste. Syst. 2023, 6, 100109. [Google Scholar] [CrossRef]
- Li, M.; Chen, P.; Li, J. Effect of content and grading ratios of fly ash cenospheres on the flexural properties of the flexural properties of the epoxy resin composites. Acta Mater. Compos. Sin. 2017, 34, 345–351. [Google Scholar] [CrossRef]
- Porąbka, A.; Jurkowski, K.; Laska, J. Fly ash used as a reinforcing and flame-retardant filler in low-density polyethylene. Polimery 2015, 60, 251–257. [Google Scholar] [CrossRef]
- Yang, X.; Guan, L.; Yang, C. Preparation of aluminium doped zinc oxide nanopowder and its antistatic modification of PET. Eng. Plast. Appl. 2024, 52, 138–145. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, C.; Wang, Z.; Yao, G.; Zou, Y.; Yang, R. Preparation and mechanism of nano antimony-doped in oxide @ fly ash antistatic composite powder. J. China Coal Soc. 2022, 47, 3483–3492. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, S.; Lu, J.; Du, M.; Jiao, Z.; Zou, J. Preparation of Hollow Double-Shell SiO2@ATO Microspheres and Applications in Thermal Insulation Coatings. Ceram. Int. 2023, 49, 28062–28070. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Yang, R.; Wang, H. Preparation and Electrical Properties of Wollastonite Coated with Antimony-Doped Tin Oxide Nanoparticles. Powder Technol. 2019, 342, 397–403. [Google Scholar] [CrossRef]
- Tiwari, S.; Gehlot, C.L.; Srivastava, D. Synergistic influence of CaCO3 nanoparticle on the mechanical and thermal of fly ash reinforced epoxy polymer composites. Mater. Today-Proc. 2021, 43, 3375–3385. [Google Scholar] [CrossRef]
- Miyajima, Y.; Nakamura, Y.; Konishi, Y.; Ishikawa, K.; Wang, W.; Takata, N. Effect of Low-Temperature Annealing on Electrical Resistivity and Mechanical Properties of Laser-Powder Bed Fused AlSi10Mg Alloy. Mater. Sci. Eng. A 2023, 871, 144876. [Google Scholar] [CrossRef]
- Chayoukhi, S.; Dhifelaoui, H.; Boucherou, N.; Boukhachem, A.; Amlouk, M.; Zghal, A. Structural, optical and mechanical investigations on pure and Co-doped SnO2 thin films samples. Inorg. Chem. Commun. 2023, 149, 110391. [Google Scholar] [CrossRef]
- Bhakta, N.; Chakrabarti, P.K. XRD analysis, Raman, AC conductivity and dielectric properties of Co and Mn co-doped SnO2 nanoparticles. Appl. Phys. A 2019, 125, 73. [Google Scholar] [CrossRef]
- Lacroix, M.R.; Gao, X.; Liu, Y.; Strauss, S.H. Unusually sharp FTIR ν (OH) bands and very weak OH⋯ F hydrogen bonds in M2 (H2O) 1, 2B12F12 hydrates (MNaCs). J. Fluor. Chem. 2019, 217, 105–108. [Google Scholar] [CrossRef]
- Solanki, A.; Singh, L.P.; Karade, S.R.; Sharma, U. Mineralogy of tricalcium aluminate hydration with silica nanoparticles. Constr. Build. Mater. 2022, 340, 127707. [Google Scholar] [CrossRef]
- Palchowdhury, S.; Mukherjee, K.; Maroncelli, M. Rapid water dynamics structures the OH-stretching spectra of solitary water in ionic liquids and dipolar solvents. J. Chem. Phys. 2022, 157, 084502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, J.; Jiang, Y.; Ju, A.; Zhu, D.; Zhang, J.; Wei, C. Synthesis and characterization of composite conductive powders prepared by Sb-SnO2-coated coal gasification fine slag porous microbeads. Powder Technol. 2021, 385, 409–417. [Google Scholar] [CrossRef]
- Yang, N.; Kuang, S.; Yue, Y. Infrared spectra analysis of several common anhudrous carbonate minerals. J. Mineral. Petrol. 2015, 35, 37–42. [Google Scholar] [CrossRef]
- Rajan, Z.S.H.S.; Binninger, T.; Kooyman, P.J.; Susac, D.; Mohamed, R. Organometallic chemical deposition of crystalline iridium oxide nanoparticles on antimony-doped tin oxide support with high-performance for the oxygen evolution reaction. Catal. Sci. Technol. 2020, 10, 3938–3948. [Google Scholar] [CrossRef]
- Mokhtari, S.; Faghihian, H.; Pourshirband, N.; Sharafi-Badr, P. Synthesis of Fe3O4-SiO2@ZnO Nanocomposite: A RSM Study towards Sulfasalazine Photodegradation. Inorg. Chem. Commun. 2024, 161, 112002. [Google Scholar] [CrossRef]
- Hoang, N.T. Synthesis of a Novel Ti/TiO2 Blue/SnO2-Sb@Ni-La Electrode and the Way to Improve the Degradation Efficiency of Some Organic Pollutants in Electrochemical Process Using Ti/TiO2 Blue/SnO2-Sb@Ni-La as Anode. J. Environ. Chem. Eng. 2024, 12, 112109. [Google Scholar] [CrossRef]
- Haustein, E.; Kuryłowicz-Cudowska, A. Effect of Particle Size of Fly Ash Microspheres (FAMs) on the Selected Properties of Concrete. Minerals 2022, 12, 847. [Google Scholar] [CrossRef]
- Palomba, D.; Vazquez, G.E.; Díaz, M.F. Prediction of elongation at break for linear polymers. Chemometr. Intell. Lab. 2014, 139, 121–131. [Google Scholar] [CrossRef]
- Alves, B.F.; Silva, B.K.; Silva, C.A.; Celestino, G.G.; Nunes, R.C.; Lucas, E.F. Preparation and evaluation of polymeric nanocomposites based on EVA/montmorillonite, EVA/palygorskite and EVA/halloysite as pour point depressants and flow improvers of waxy systems. Fuel 2023, 333, 126540. [Google Scholar] [CrossRef]
- Singh Manola, M.; Singh, B.; Singla, M.K.; Kumar, R. Investigation of melt flow index of dual metal reinforced ABS polymer for FDM filament fabrication. Mater. Today-Proc. 2023. [Google Scholar] [CrossRef]
- Obed D’Souza, R.; Shettigar, Y.P.; Prajwal Byndoor, D.; Sudhakar, S.S.; Ahmed, N.; Shetty, R. Experimental Analysis on the Mechanical Properties of Glass-Epoxy composite with Fly ash as a filler material. IOP Conf. Ser. Mater. Sci. Eng. 2018, 376, 012065. [Google Scholar] [CrossRef]
- Filippi, S.; Cappello, M.; Polacco, G. Limiting oxygen index reduction in bitumen modified with nanoclays. Fire Saf. J. 2020, 111, 102929. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, M.; Arora, S. Thermal degradation and flammability studies of wood coated with fly ash intumescent composites. J. Indian Acad. Wood Sci. 2013, 10, 125–133. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, Q. Synthesis of Melamine Cyanuric Based Flame Retardant via Hydrogen Bond Self-Assembly and in-Situ Dispersion Strategies for Improving Comprehensive Performance of Epoxy Resin. Compos. Part Appl. Sci. Manuf. 2024, 176, 107826. [Google Scholar] [CrossRef]
- Zhuo, J.; Wang, X.; Gao, J.; Wei, Y.; Sha, J. Influence of hollow glass microsphere on flame retardancy of ethylene-vinyl acetate/9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide composites. Ferroelectrics 2023, 610, 171–185. [Google Scholar] [CrossRef]
- Yang, W.; Jiang, T.; Li, W.; Zhang, G. Fabrication and thermal insulating properties of ATO/PVB nanocomposites for energy saving glass. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2013, 28, 384–393. [Google Scholar] [CrossRef]
- Sun, Y.; Song, J.; Liu, Y. Fly ash/antimony trioxide as flame retardancy and smoke Suppressants for flexible poly(vinylchloride). Plastics Sci. Technol. 2022, 50, 40–44. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, J.; Xu, W. Study on synergistic flame retardance of Sb2O3 in EVA/MH composites. China Plast. Ind. 2019, 49, 115–118. [Google Scholar] [CrossRef]
- Cao, R.; Yang, J.; Li, G.; Niu, M. Effect of sulfuric acid on cementitious composites containing ethylene-vinyl acetate and styrene-butadiene rubber. J. Build. Eng. 2023, 80, 108024. [Google Scholar] [CrossRef]
- Imren, D.; Boztuĝ, A.; Basan, S. Investigation of miscibility of poly(vinyl chloride) with poly(ethylene-co-vinyl acetate) by viscosimetric method. Mater. Res. Innov. 2006, 10, 187–192. [Google Scholar] [CrossRef]



| Chemical Elements | Si | Al | Fe | Ca | K | Ti | Mg | Na | Sx | Else |
|---|---|---|---|---|---|---|---|---|---|---|
| Mass Fraction/% | 50.00 | 23.67 | 9.34 | 6.25 | 4.00 | 2.38 | 1.19 | 0.723 | 0.685 | 0.017 |
| Sample | EVA | Calcined Fly Ash/EVA | ATO@fly Ash/EVA |
|---|---|---|---|
| Tensile strength (MPa) | 6.10 | 6.38 | 6.50 |
| Elongation at break (%) | 434 | 323.6 | 409.23 |
| Melt index (g/10 min) | 2.90 | 5 | 2.50 |
| Surface resistivity (Ω) | 6.0 × 1014 | 1.95 × 1014 | 4.0 × 108 |
| Limit oxygen index (%) | 19.8 | 22 | 23.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Wang, C.; Zhao, C.; Yao, G.; Wang, Z.; Yang, R. Preparation of Antimony-Doped Tin Oxide Fly Ash Antistatic Composite and Its Properties in Filling EVA. Materials 2024, 17, 1183. https://doi.org/10.3390/ma17051183
Qiu Y, Wang C, Zhao C, Yao G, Wang Z, Yang R. Preparation of Antimony-Doped Tin Oxide Fly Ash Antistatic Composite and Its Properties in Filling EVA. Materials. 2024; 17(5):1183. https://doi.org/10.3390/ma17051183
Chicago/Turabian StyleQiu, Ying, Caili Wang, Chunxue Zhao, Guoxin Yao, Zhixue Wang, and Runquan Yang. 2024. "Preparation of Antimony-Doped Tin Oxide Fly Ash Antistatic Composite and Its Properties in Filling EVA" Materials 17, no. 5: 1183. https://doi.org/10.3390/ma17051183




