Effect of Tungsten Inert Gas Remelting on Microstructure and Corrosion Resistance of Q450NQR1 High-Strength Weathering Steel-Welded Joints
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
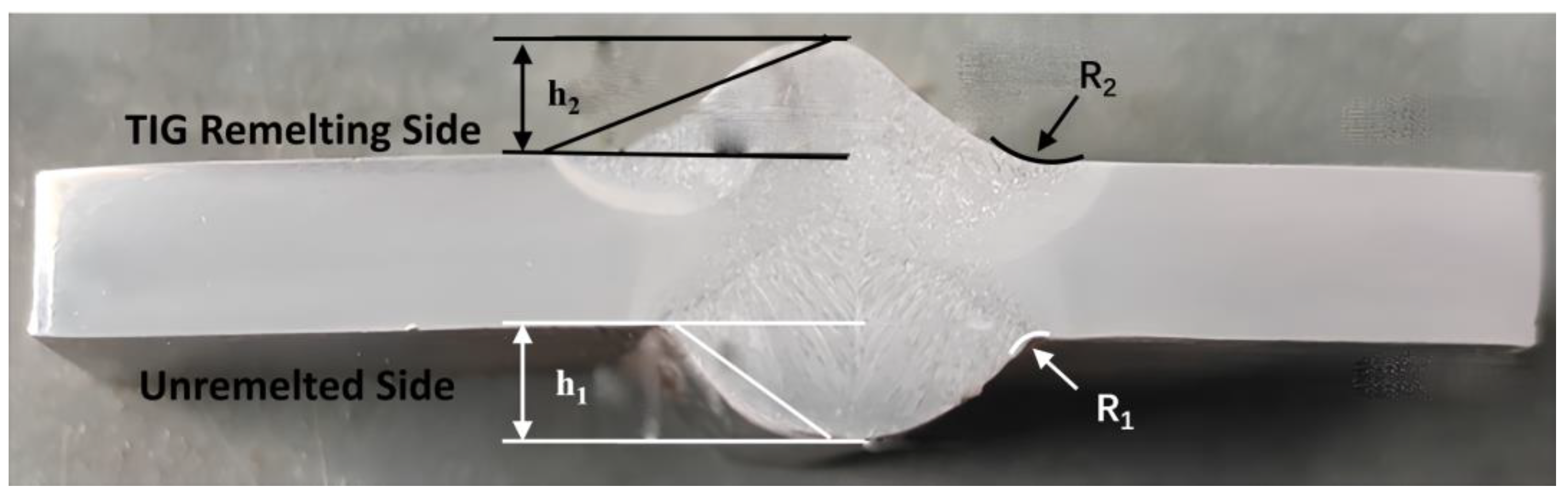
3.1. Weld Toe Stress Concentration
3.2. Effect of TIG Remelting on Microstructure of Welded Joint
3.3. Effect of TIG Remelting on Corrosion Resistance of Welded Joint
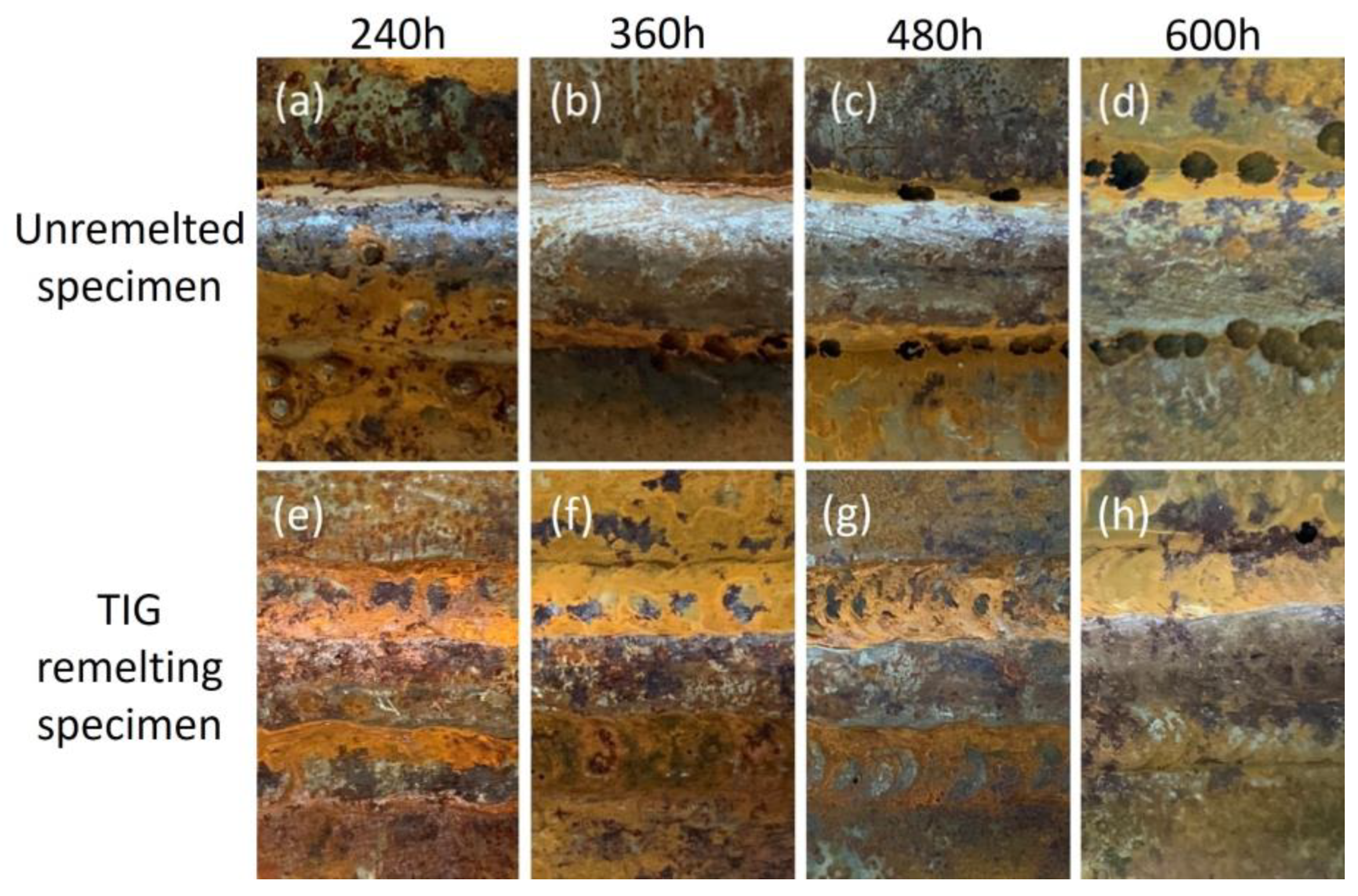
3.3.1. Macroscopic Morphology Analysis of Corrosion
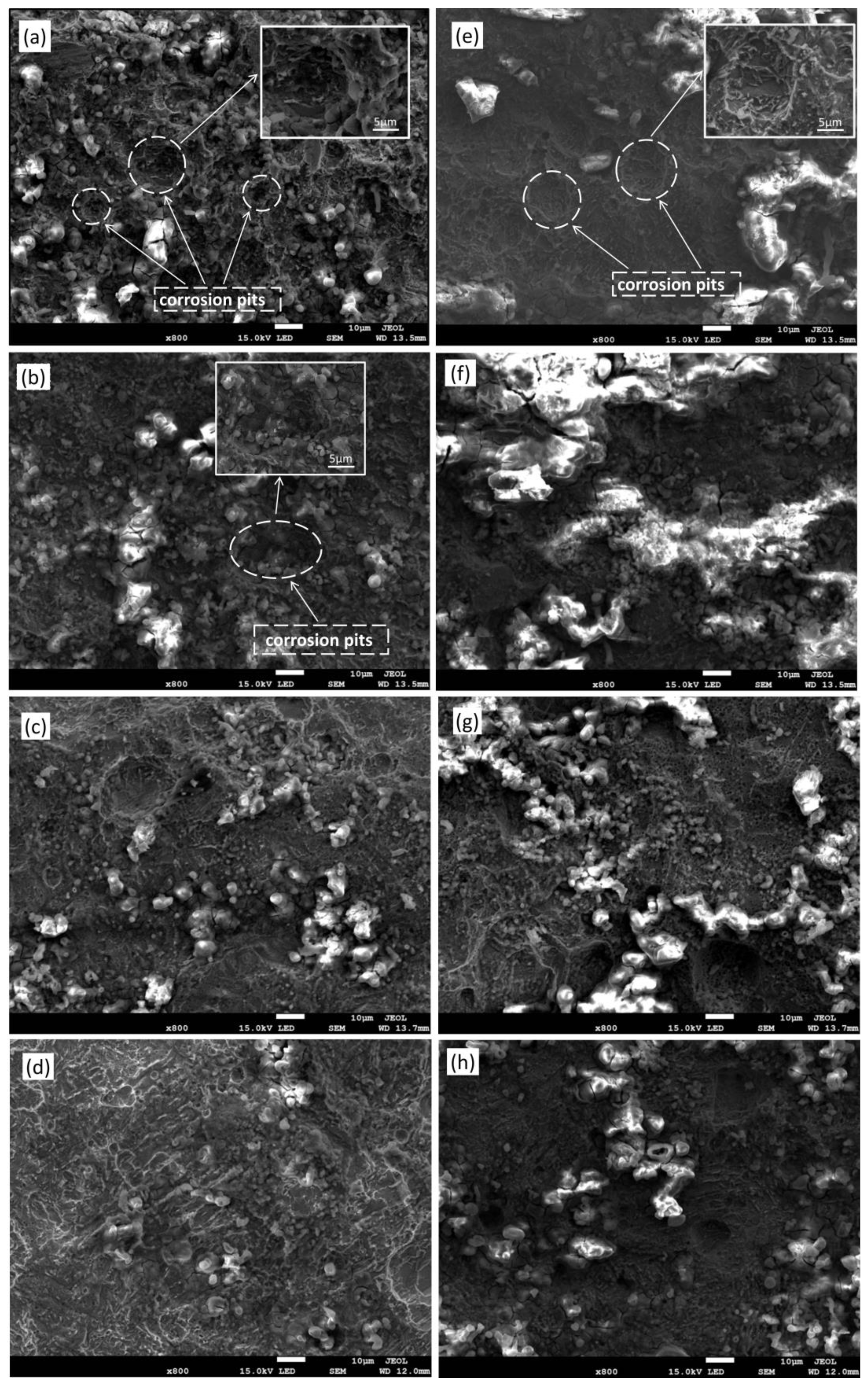
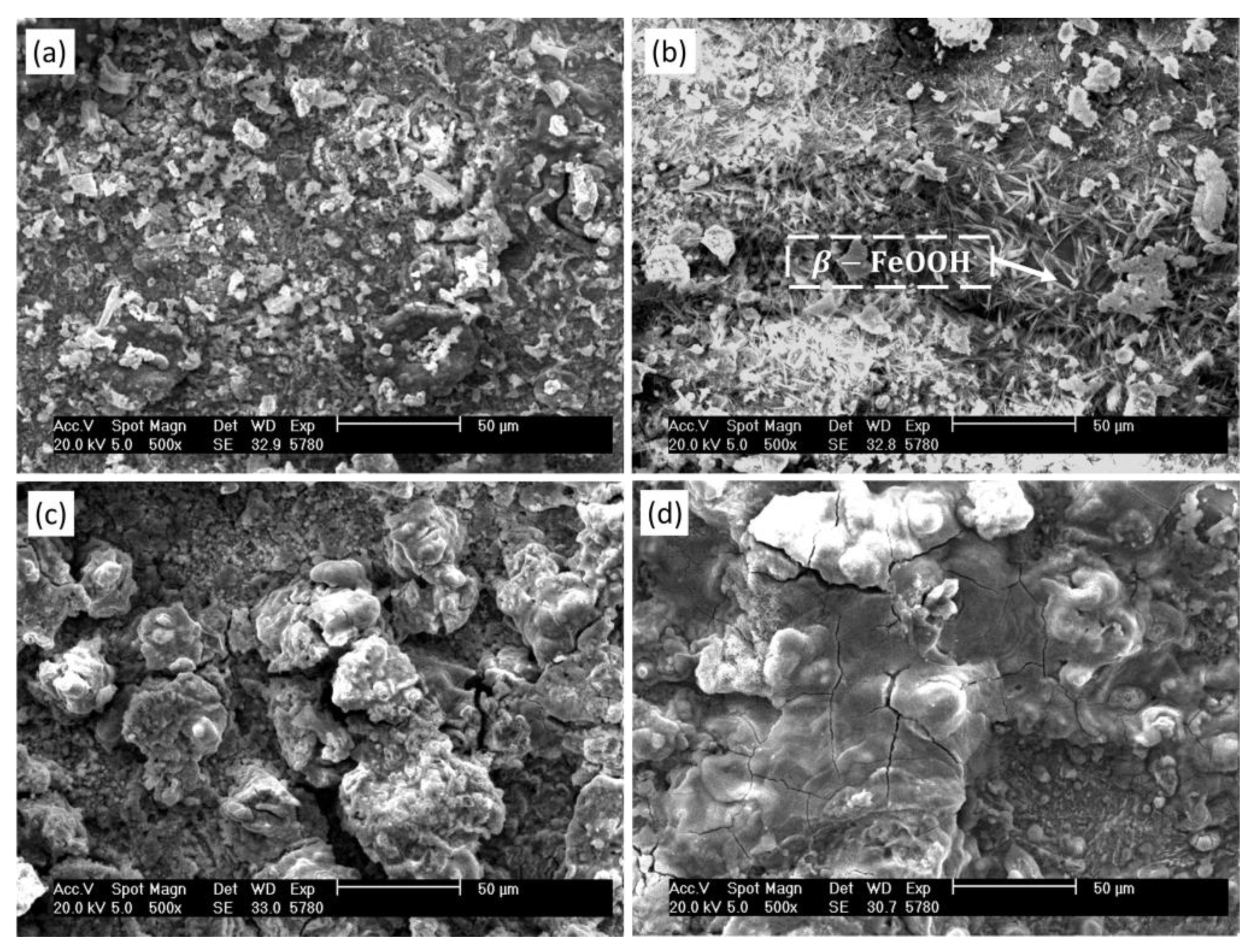
3.3.2. Micro-Morphology Analysis of Corrosion
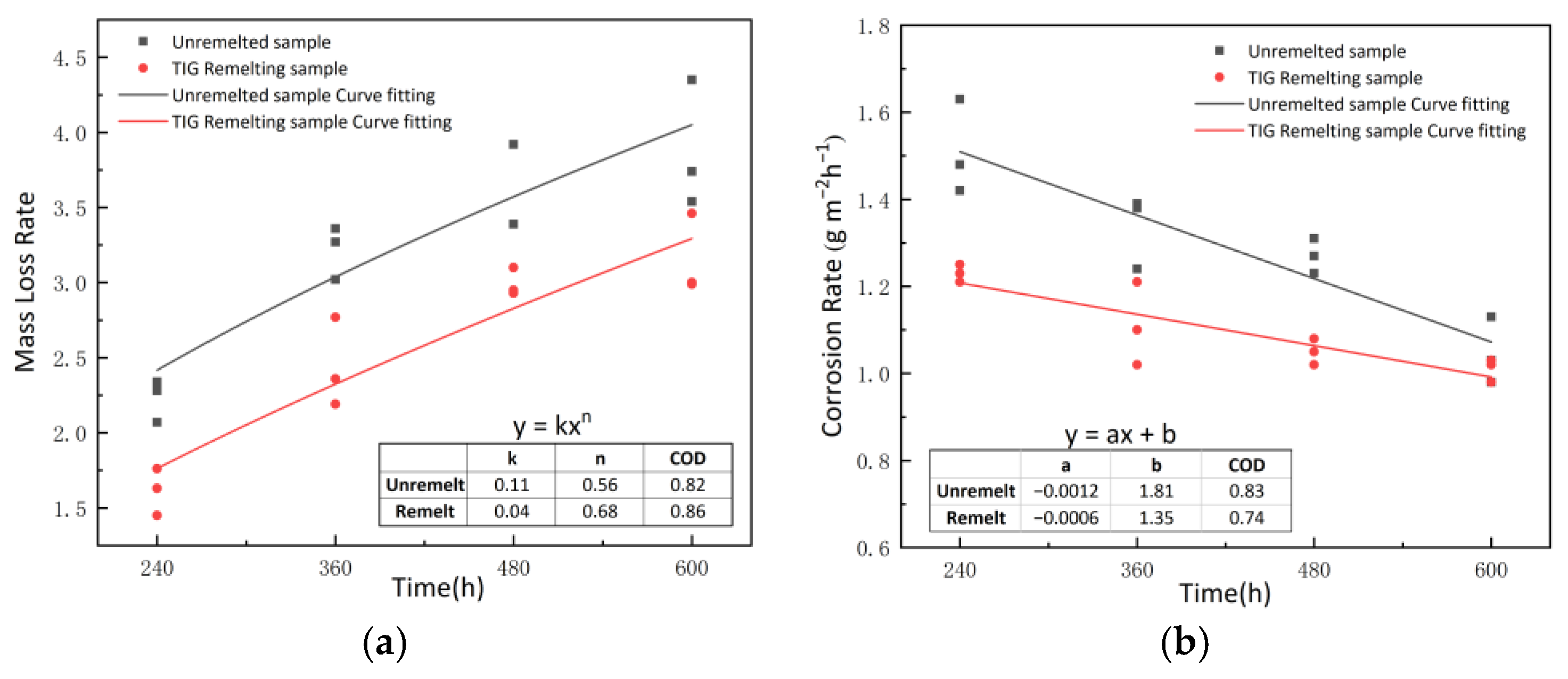
3.4. Corrosion Weight Loss Rate and Corrosion Rate
3.5. Analysis of Corrosion Products
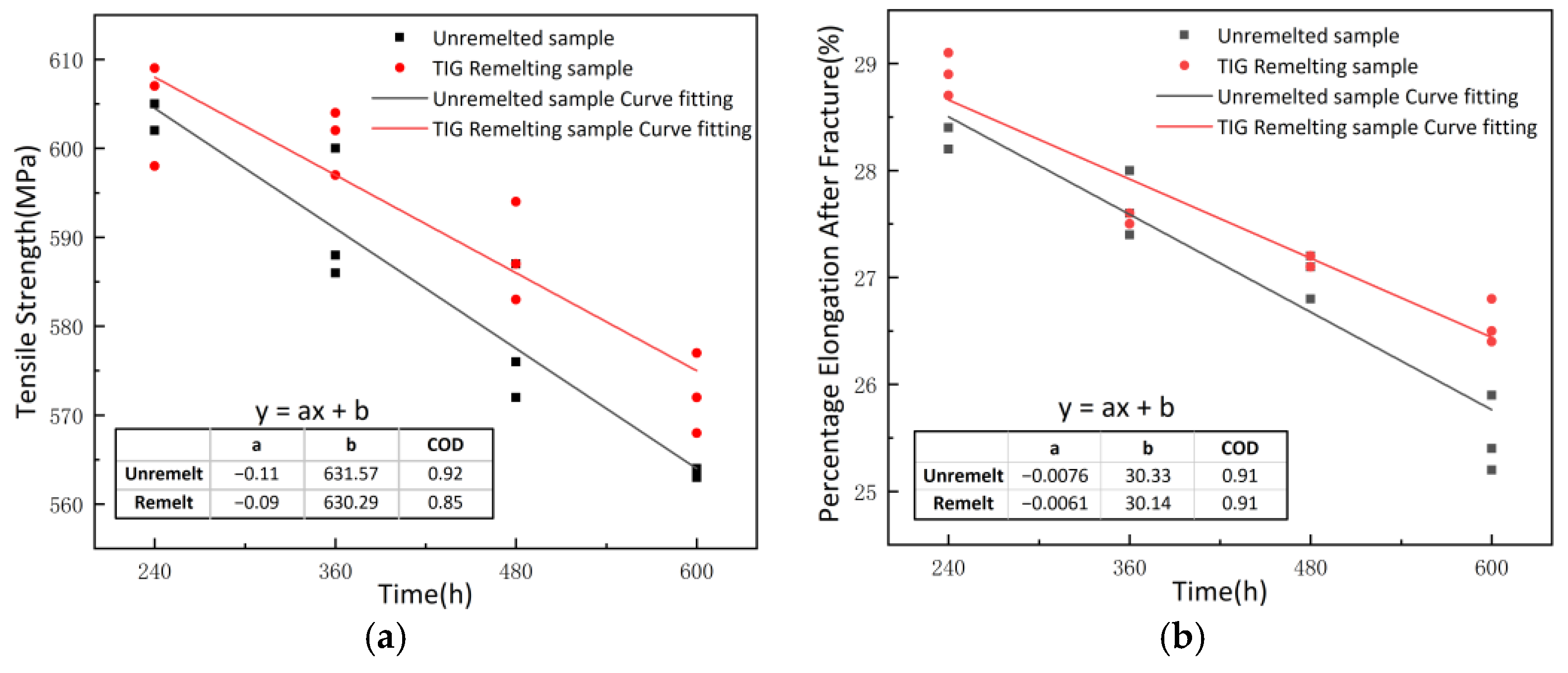
3.6. Analysis of Tensile Performance and Mechanical Degradation of Corroded Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maduraimuthu, V.; Vasantharaja, P.; Vasudevan, M.; Panigrahi, B.S. Microstructure and mechanical properties of 9Cr-0.5Mo-1.8W–VNb (P92) steel weld joints processed by fusion welding. Mater. Sci. Eng. A 2021, 813, 141186. [Google Scholar] [CrossRef]
- Dominika, P.; Paweł, M.; Dariusz, B. Fatigue Analysis of Welded Joints Using a Thin-Walled Al/Fe Explosive Welded Transition Joints. Materials 2023, 16, 6259. [Google Scholar] [CrossRef]
- Tereshchenko, N.A.; Yakovleva, I.L.; Fedorov, M.A.; Gizatullin, A.B.; Esiev, T.S. Structure and Impact Strength of Weld Joints Manufactured from a Pipe Steel with the Use of Hybrid Laser-Arc Welding. Phys. Met. Metallogr. 2022, 123, 535–541. [Google Scholar] [CrossRef]
- Vidyarthy, R.S.; Dwivedi, D.K. Analysis of the Corrosion Behavior of an A-TIG Welded SS 409 Weld Fusion Zone. J. Mater. Eng. Perform. 2017, 26, 5375–5384. [Google Scholar] [CrossRef]
- Lieth, H.M.; Jabbar, M.A.; Jassim, R.J.; Al-Sabur, R. Optimize the corrosion behavior of AISI 204Cu stainless steel in different environments under previous cold working and welding. Metall. Res. Technol. 2023, 120, 415. [Google Scholar] [CrossRef]
- Palsson, N.S.; Wongpinkaew, K.; Khamsuk, P.; Sorachot, S.; Pongsaksawad, W. Outdoor atmospheric corrosion of carbon steel and weathering steel exposed to the tropical-coastal climate of Thailand. Mater. Corros. 2019, 71, 1019–1034. [Google Scholar] [CrossRef]
- Gardiner, C.P.; Melchers, R.E. Corrosion of mild steel by coal and iron ore. Corros. Sci. 2002, 44, 2665–2673. [Google Scholar] [CrossRef]
- Menéndez, E.; Argiz, C.; Sanjuán, M.Á. Chloride Induced Reinforcement Corrosion in Mortars Containing Coal Bottom Ash and Coal Fly Ash. Materials 2019, 12, 1933. [Google Scholar] [CrossRef]
- Gavrilov, P.; Ivanov, V. Analysis of rail profile 610 E1 joints welded by means of mobile rail welding machine. In Proceedings of the 17th International Scientific Conference: Engineering for Rural Development, Jelgava, Latvia, 23–25 May 2018; pp. 1969–1977. [Google Scholar] [CrossRef]
- Deepak, J.R.; Bupesh Raja, V.K. Microstructure and metallurgical property investigation of welded IRSM 41-97 rail steel joints. Mater. Today Proc. 2021, 47, 4827–4832. [Google Scholar] [CrossRef]
- Zhu, P.; Jin, C.; Shi, C.Y. Residual Stress State of TIG Remelted Zone in the Weld Toe after Chilling Treatment. AMR 2011, 189, 461–466. [Google Scholar] [CrossRef]
- Abboud, J.H. Microstructure and erosion characteristic of nodular cast iron surface modified by tungsten inert gas. Mater. Des. 2012, 35, 677–684. [Google Scholar] [CrossRef]
- Zhu, L.; Cui, Y.; Cao, J.; Tian, R.; Cai, Y.; Xu, C.; Han, J.; Tian, Y. Effect of TIG remelting on microstructure, corrosion and wear resistance of coating on surface of 4Cr5MoSiV1 (AISI H13). Surf. Coat. Technol. 2021, 405, 126547. [Google Scholar] [CrossRef]
- Al-Karawi, H.A. Fatigue life estimation of welded structures enhanced by combined thermo-mechanical treatment methods. J. Constr. Steel Res. 2021, 187, 106961. [Google Scholar] [CrossRef]
- Mitelea, I.; Bordeaşu, I.; Riemschneider, E.; Uţu, I.D.; Crăciunescu, C.M. Cavitation erosion improvement following TIG surface-remelting of gray cast iron. Wear 2022, 496, 204282. [Google Scholar] [CrossRef]
- ASTM G31-21; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Guo, S.; Xu, L.; Zhang, L.; Chang, W.; Lu, M. Corrosion of alloy steels containing 2% chromium in CO2 environments. Corros. Sci. 2012, 63, 246–258. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.Y.; Ding, C.G.; Zhu, P. T joints fatigue strength improving by hybrid heat treatment process after tungsten inert-gas arc remelt ing. Weld. Join. 2008, 2, 23–26. [Google Scholar]
- Yang, Z.; Wang, F.; Wang, S.; Song, B. Intragranular Ferrite Formation Mechanism and Mechanical Properties of Non-quenched-and-tempered Medium Carbon Steels. Steel Res. Int. 2008, 79, 390–395. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, X.; Zhang, Z.; Huang, Y.; Ma, L.; Li, X.; Zhang, D. Roles of grain refinement in the rust formation and corrosion resistance of weathering steels. Corros. Sci. 2023, 224, 111561. [Google Scholar] [CrossRef]
- Wu, H.; Lei, H.; Chen, Y.F.; Qiao, J. Comparison on corrosion behaviour and mechanical properties of structural steel exposed between urban industrial atmosphere and laboratory simulated environment. Constr. Build. Mater. 2019, 211, 228–243. [Google Scholar] [CrossRef]
- Guo, J.; Yang, S.; Shang, C.; Wang, Y.; He, X. Influence of carbon content and microstructure on corrosion behaviour of low alloy steels in a Cl− containing environment. Corros. Sci. 2009, 51, 242–251. [Google Scholar] [CrossRef]
- Ferro, P.; Berto, F.; James, N.M. Asymptotic residual stress distribution induced by multipass welding processes. Int. J. Fatigue 2017, 101, 421–429. [Google Scholar] [CrossRef]
- Ngoula, D.T.; Beier, H.; Vormwald, M. Fatigue crack growth in cruciform welded joints: Influence of residual stresses and of the weld toe geometry. Int. J. Fatigue 2017, 101, 253–262. [Google Scholar] [CrossRef]
- He, S.; He, S.Q.; Yu, P. Corrosion behaviour of carbon steel in Beibu Gulf tidal zone. Corros. Eng. Sci. Technol. 2023, 58, 116–123. [Google Scholar] [CrossRef]
- Rémazeilles, C.; Refait, P.H. On the formation of β-FeOOH in chloride-containing environments. Corros. Sci. 2007, 49, 844–857. [Google Scholar] [CrossRef]


| Materials | Chemical Composition (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | P | S | Cu | Ni | Cr | Fe | |
| Q450NQR1 | ≤0.12 | 0.70–1.50 | ≤0.40 | ≤0.002 | ≤0.008 | 0.25–0.45 | 0.05–0.40 | 0.40–0.90 | Bal. |
| HTW-55 | ≤0.12 | ≤1.60 | ≤0.60 | ≤0.025 | ≤0.025 | - | 0.20–0.60 | 0.30–0.90 | Bal. |
| Steel Plate Number | Welding Process | Voltage (V) | Current (A) | Welding Speed (cm min−1) | Shielding Gas Flow Rate (L min−1) |
|---|---|---|---|---|---|
| Q450NQR1 | MAG | 26 | 260 | 55 | 18 |
| TIG | 16 | 170 | 25 | 15 |
| Welding Process | Weld Reinforcement h (mm) | Transition Angle (rad) | Curvature Radius R (mm) |
|---|---|---|---|
| Unremelted(MAG) | 3.8 | 0.63 | 2 |
| TIG remelting | 2.6 | 0.38 | 8 |
| Corrosion Time (h) | Unremelted Sample | Mass Loss Rate | Corrosion Rate (g m−2 h−1) | Remelted Sample | Mass Loss Rate | Corrosion Rate (g m−2 h−1) |
|---|---|---|---|---|---|---|
| 240 | A1 | 2.34 | 1.63 | B1 | 1.63 | 1.21 |
| A2 | 2.07 | 1.42 | B2 | 1.76 | 1.25 | |
| A3 | 2.28 | 1.48 | B3 | 1.45 | 1.23 | |
| 360 | A4 | 3.36 | 1.24 | B4 | 2.19 | 1.02 |
| A5 | 3.27 | 1.38 | B5 | 2.77 | 1.21 | |
| A6 | 3.02 | 1.39 | B6 | 2.36 | 1.1 | |
| 480 | A7 | 3.92 | 1.31 | B7 | 2.95 | 1.02 |
| A8 | 3.39 | 1.23 | B8 | 2.93 | 1.08 | |
| A9 | 3.92 | 1.27 | B9 | 3.1 | 1.05 | |
| 600 | A10 | 3.74 | 1.03 | B10 | 2.99 | 1.02 |
| A11 | 4.35 | 1.13 | B11 | 3.46 | 0.98 | |
| A12 | 3.54 | 0.98 | B12 | 3.00 | 1.03 |
| Corrosion Time (h) | Unremelted Sample | Tensile Strength (MPa) | Percentage Elongation after Fracture (%) | Remelted Sample | Tensile Strength (MPa) | Percentage Elongation after Fracture (%) |
|---|---|---|---|---|---|---|
| 240 | A1 | 605 | 28.4 | B1 | 607 | 29.1 |
| A2 | 605 | 28.2 | B2 | 609 | 28.7 | |
| A3 | 602 | 28.4 | B3 | 598 | 28.9 | |
| 360 | A4 | 600 | 28 | B4 | 604 | 27.6 |
| A5 | 586 | 27.6 | B5 | 597 | 27.6 | |
| A6 | 588 | 27.4 | B6 | 602 | 27.5 | |
| 480 | A7 | 587 | 26.8 | B7 | 594 | 27.2 |
| A8 | 572 | 27.1 | B8 | 587 | 27.1 | |
| A9 | 576 | 27.2 | B9 | 583 | 27.2 | |
| 600 | A10 | 564 | 25.9 | B10 | 577 | 26.4 |
| A11 | 563 | 25.2 | B11 | 572 | 26.8 | |
| A12 | 563 | 25.4 | B12 | 568 | 26.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Guo, R.; Li, Z.; Hu, Q.; Liu, M.; Zhu, L.; Kong, X. Effect of Tungsten Inert Gas Remelting on Microstructure and Corrosion Resistance of Q450NQR1 High-Strength Weathering Steel-Welded Joints. Materials 2024, 17, 1198. https://doi.org/10.3390/ma17051198
Li X, Liu Y, Guo R, Li Z, Hu Q, Liu M, Zhu L, Kong X. Effect of Tungsten Inert Gas Remelting on Microstructure and Corrosion Resistance of Q450NQR1 High-Strength Weathering Steel-Welded Joints. Materials. 2024; 17(5):1198. https://doi.org/10.3390/ma17051198
Chicago/Turabian StyleLi, Xuemei, Yang Liu, Rui Guo, Zicheng Li, Qingming Hu, Meng Liu, Lei Zhu, and Xiangxia Kong. 2024. "Effect of Tungsten Inert Gas Remelting on Microstructure and Corrosion Resistance of Q450NQR1 High-Strength Weathering Steel-Welded Joints" Materials 17, no. 5: 1198. https://doi.org/10.3390/ma17051198
APA StyleLi, X., Liu, Y., Guo, R., Li, Z., Hu, Q., Liu, M., Zhu, L., & Kong, X. (2024). Effect of Tungsten Inert Gas Remelting on Microstructure and Corrosion Resistance of Q450NQR1 High-Strength Weathering Steel-Welded Joints. Materials, 17(5), 1198. https://doi.org/10.3390/ma17051198




