C60- and CdS-Co-Modified Nano-Titanium Dioxide for Highly Efficient Photocatalysis and Hydrogen Production
Abstract
1. Introduction
2. Materials and Methods
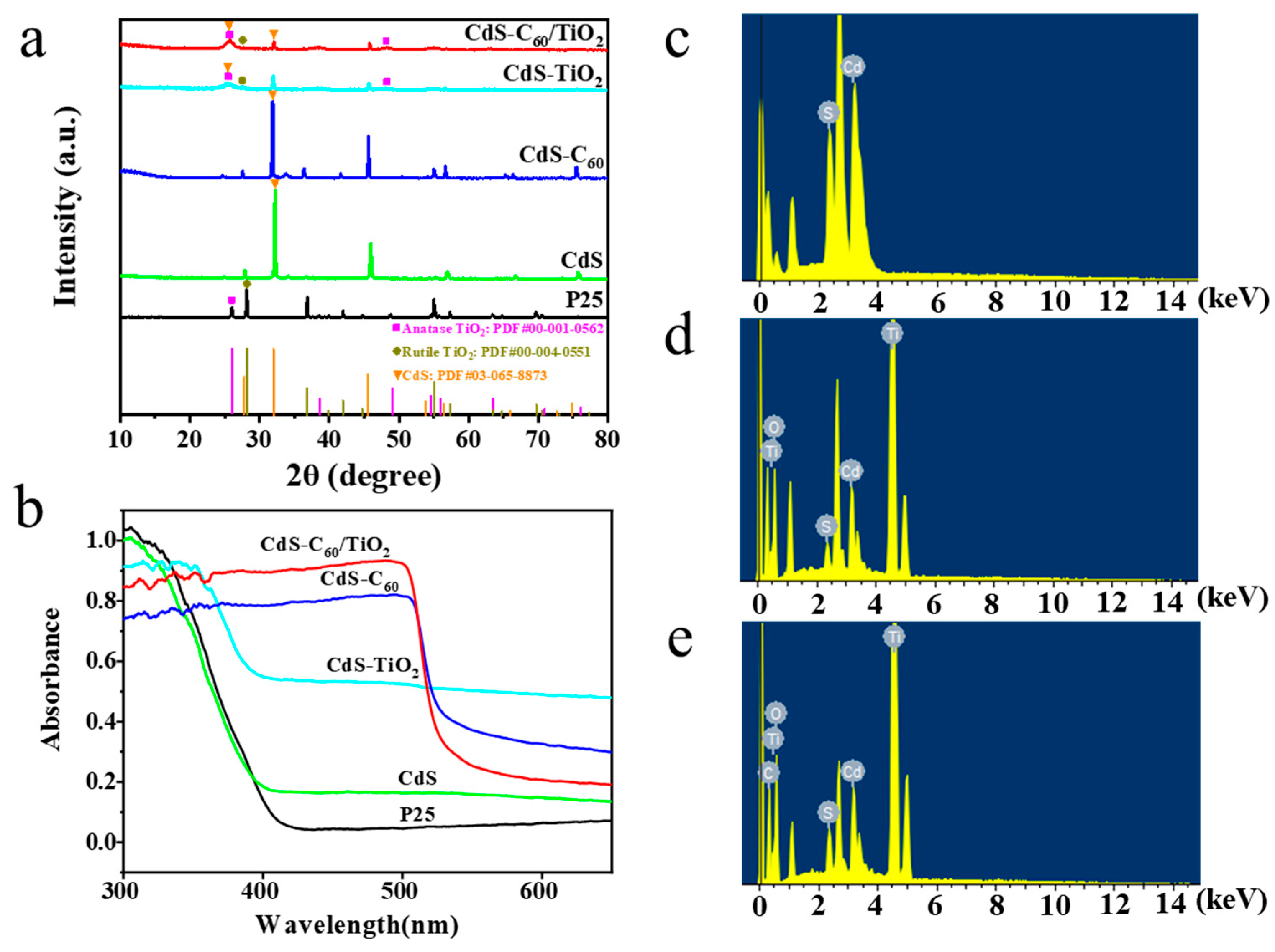
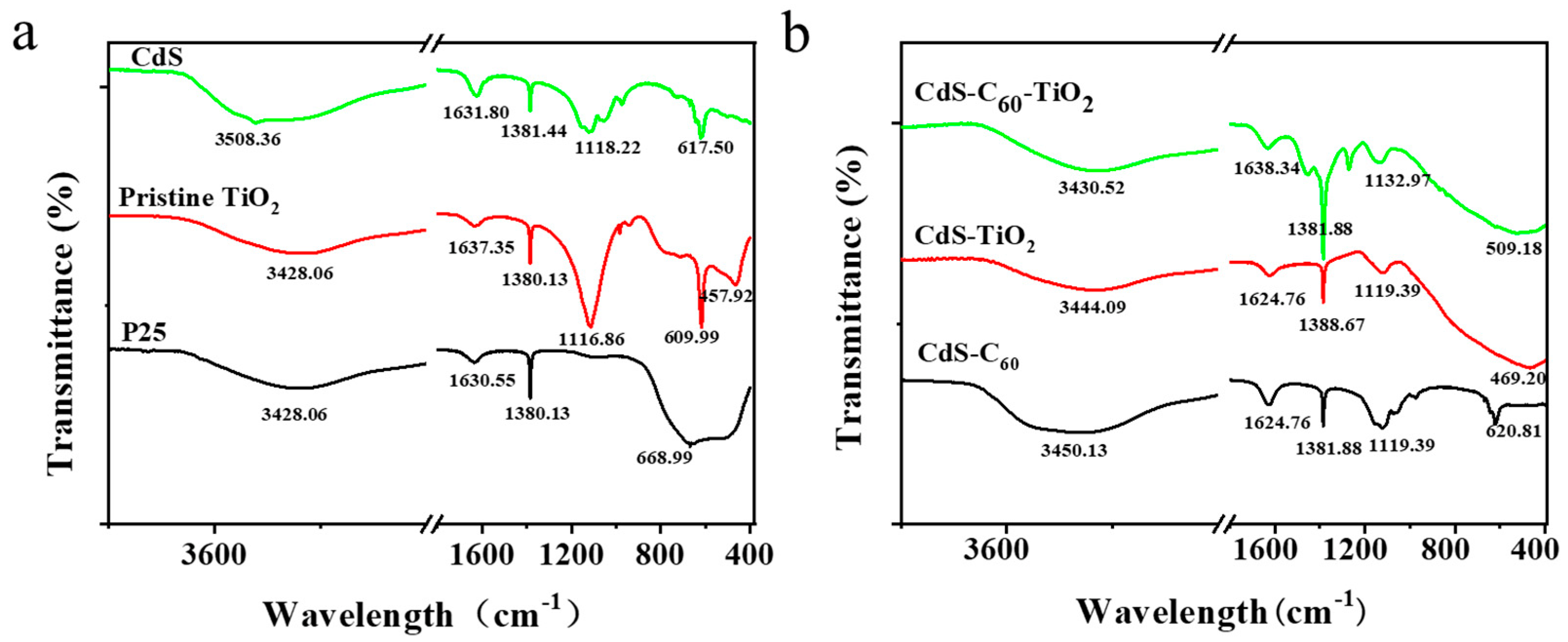
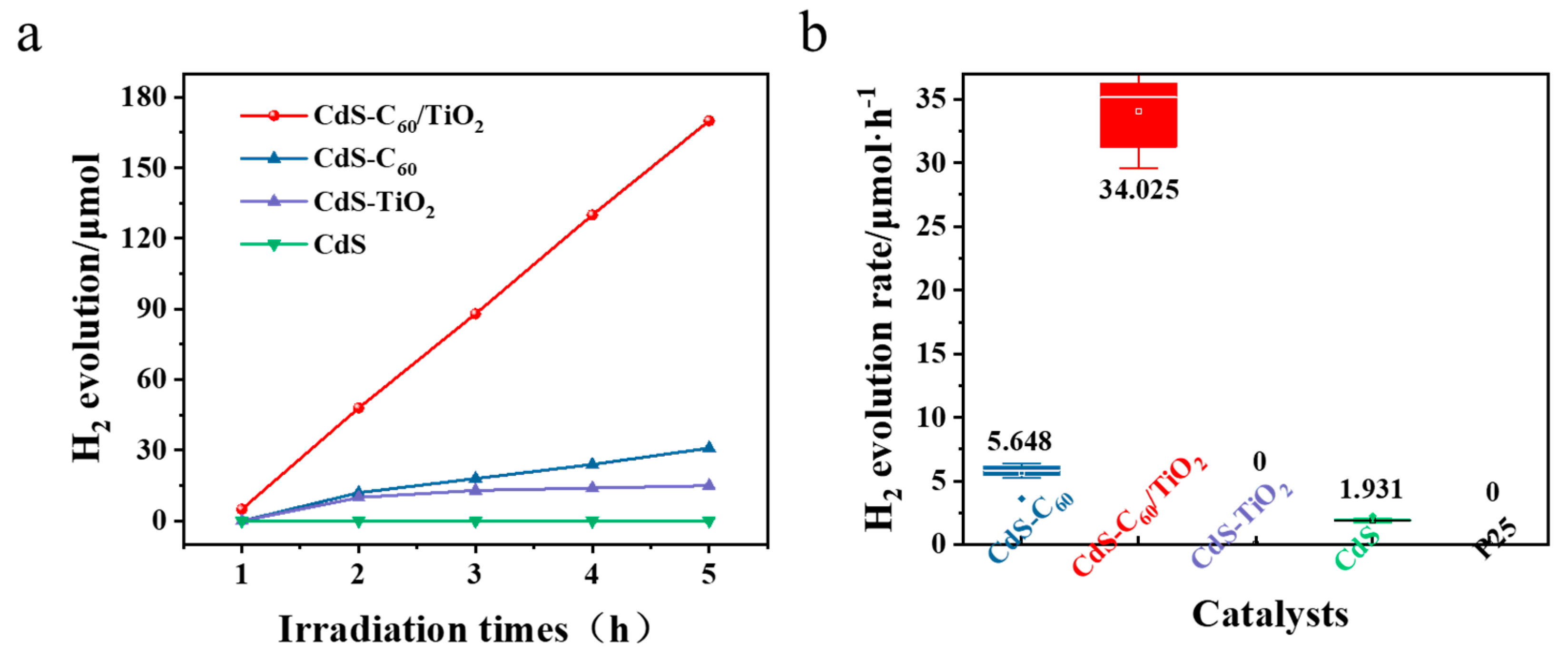
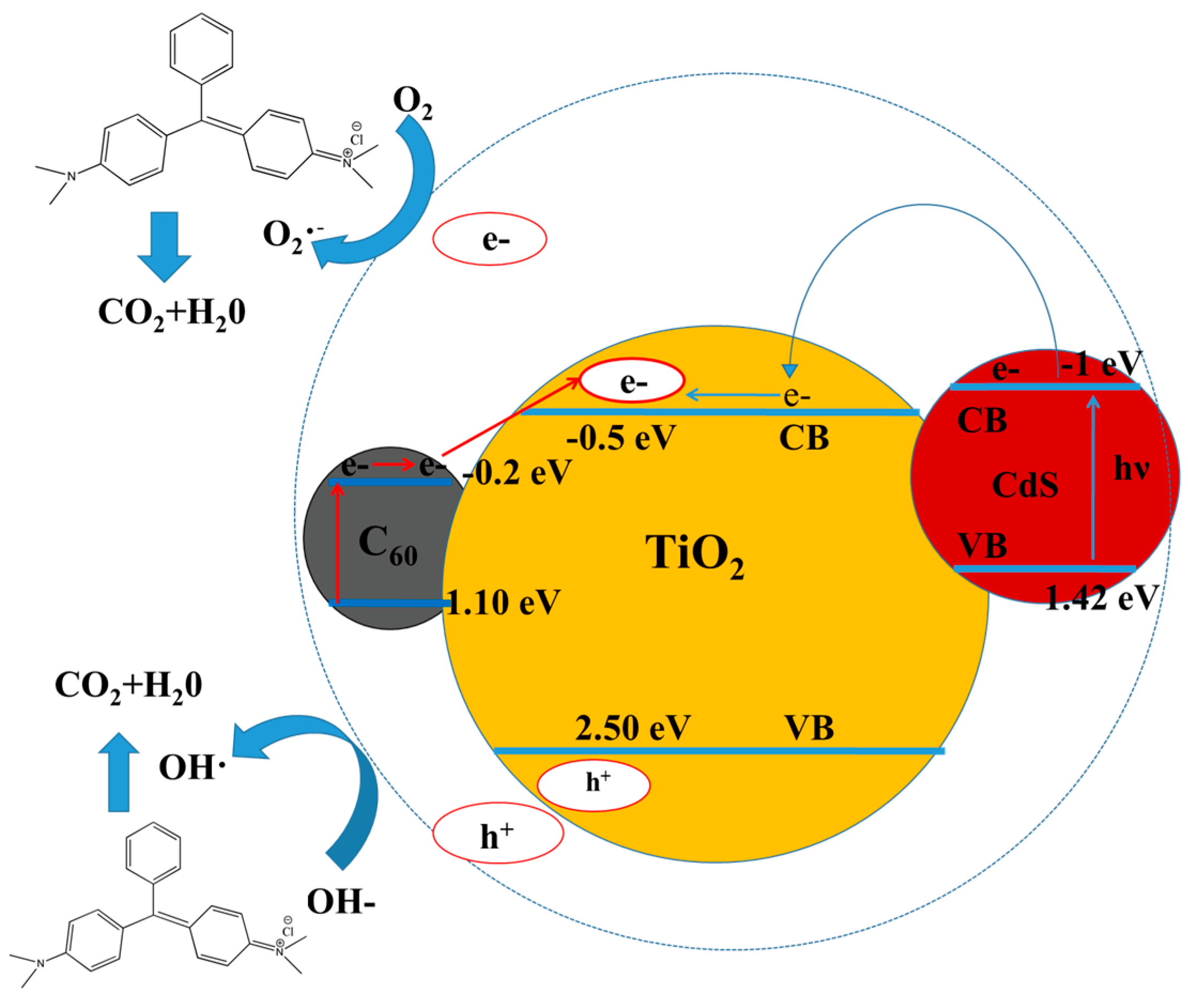
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Q.; Yuan, H.; Du, J.; Ming, M.; Yang, S.; Chen, Y.; Lei, J.; Han, Z. Photocatalytic CO2 reduction with aminoanthraquinone organic dyes. Nat. Commun. 2023, 14, 1087. [Google Scholar] [CrossRef]
- Huang, W.; Su, C.; Zhu, C.; Bo, T.; Zuo, S.; Zhou, W.; Ren, Y.; Zhang, Y.; Zhang, J.; Rueping, M.; et al. Isolated electron trap-induced charge accumulation for efficient photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2023, 62, e202304634. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Ambroová, N.; Koí, K. Photocatalytic reduction of carbon dioxide on TiO2 heterojunction photocatalysts—A review. Materials 2022, 15, 967. [Google Scholar] [CrossRef]
- Singh, V.; Rao, A.; Tiwari, A.; Yashwanth, P.; Lal, M.; Dubey, U.; Aich, S.; Roy, B. Study on the effects of Cl and F doping in TiO2 powder synthesized by a sol-gel route for biomedical applications. J. Phys. Chem. Solids 2019, 134, 262–271. [Google Scholar] [CrossRef]
- Aich, S.; Mishra, M.K.; Sekhar, C.; Satapathy, D.; Roy, B. Synthesis of Al-doped nano Ti-O scaffolds using a hydrothermal route on titanium foil for biomedical applications. Mater. Lett. 2016, 178, 135–139. [Google Scholar] [CrossRef]
- Caglar, A.; Aktas, N.; Kivrak, H. The role and effect of CdS-based TiO2 photocatalysts enhanced with a wetness impregnation method for efficient photocatalytic glucose electrooxidation. Surf. Interfaces 2022, 33, 102250. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Wang, X.; Ma, R.; Ding, H.; Zhang, H. Preparation and characterization of Zr-containing silica residue purification loaded nano-TiO2 composite photocatalysts. Chem. Phys. 2023, 570, 111889. [Google Scholar] [CrossRef]
- Chen, B.C.; Li, P.P.; Wang, B.; Wang, Y.D. Flame-annealed porous TiO2/CeO2 nanosheets for enhenced CO gas sensors. Appl. Surf. Sci. 2022, 593, 153418. [Google Scholar] [CrossRef]
- Chen, Y.J.; Luo, X.; Luo, Y.; Xu, P.W.; He, J.; Jiang, L.; Li, J.J.; Yan, Z.Y.; Wang, J.Q. Efficient charge carrier separation in l-alanine acids derived N-TiO2 nanospheres: The role of oxygen vacancies in tetrahedral Ti4+ sites. Nanomaterials 2019, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, Y.; Zhao, G.; Chen, J.H.; He, S.F.; Zhu, Y.C.; Chai, B.; Ren, Z.D. Preparation of electrolyzed oxidizing water by TiO2 doped IrO2-Ta2O5 electrode with high selectivity and stability for chlorine evolution. J. Electroanal. Chem. 2019, 832, 459–466. [Google Scholar] [CrossRef]
- Li, S.Q.; Li, J.Z.; Zhang, H.X.; Luo, B.; Cai, H.; Nie, S.X.; Sha, J.L. Ultraviolet-light-driven electricity generation by a TiO2/graphene composite in water. ACS Mater. Lett. 2023, 5, 2862–2869. [Google Scholar] [CrossRef]
- Li, G.Q.; Xu, T.; He, R.F.; Li, C.P.; Bai, J. Hollow cadmium sulfide tubes with novel morphologies for enhanced stability of the photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143642. [Google Scholar] [CrossRef]
- Goud, B.S.; Suresh, Y.; Annapurna, S.; Singh, A.K.; Bhikshamaiah, G. Green synthesis and characterization of cadmium sulphide nanoparticles. Mater. Today Proc. 2016, 3, 4003–4008. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Joshi, U.A.; Jang, J.W.; Lee, J.S. Fabrication of CdS nanovires decorated with TiO2 nanopartieles for photocatalytic hydrogen production under visible light irradiation. Int. J. Hydrogen Energ. 2008, 33, 5975–5980. [Google Scholar] [CrossRef]
- Ma, J.Y.; An, Z.H.; Zhang, W.W.; Shen, J.; Qi, Y.L.; Chen, D.H. TiO2/CdS composite photocathode improves the performance and degradation of wastewater in microbial fuel cells. Anal. Sci. Adv. 2022, 3, 188–197. [Google Scholar] [CrossRef]
- Huo, G.N.; Zhang, S.S.; Li, Y.L.; Li, J.X.; Zhao, Y.; Huang, W.P.; Zhang, S.M.; Zhu, B.L. CdS-modified TiO2 nanotubes with heterojunction structure: A photoelectrochemical sensor for glutathione. Nanomaterials 2023, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Li, S.H.; Hui, Y.; Wu, B.S.; Chen, Z.C.; Yun, D.Q.; Deng, L.L.; Zhang, M.L.; Mao, B.W.; Xie, S.Y.; et al. Star-like hexakis[di(ethoxycarbonyl)methano]-C60 with higher electron mobility: An unexpected electron extractor interfaced in photovoltaic perovskites. Nano Energy 2020, 774, 104859. [Google Scholar] [CrossRef]
- Zieleniewska, A.; Lodermeyer, F.; Rotha, A.; Guldi, D.M. Fullerenes-how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhang, Z.; Zhao, C.; Wang, J.; Li, W. Fullerene as an additive for increasing the efficiency of organic solar cells to more than 17%. J. Colloid Interf. Sci. 2021, 601, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wei, Z.Y.; Guan, Z.H.; Shan, N.Y.; Zhao, Y.; Liu, F.; Fu, L.L.; Huang, Z.P.; Humphrey, M.G.; Zhang, C. Covalent chemical functionalization of Ti3C2Tx MXene nanosheets with fullerenes C60 and C70 for enhanced nonlinear optical limiting. J. Mater. Chem. C 2023, 11, 7331–7344. [Google Scholar] [CrossRef]
- Kang, S.; Bozhilov, K.; Myung, N.; Mulchandani, A.; Chen, W. Microbial Synthesis of CdS Nanocrystals in Genetically Engineered E. coli. Angew. Chem. Int. Ed. 2008, 47, 5186–5189. [Google Scholar] [CrossRef]
- Xu, M.; Kang, Y.; Jiang, L.; Jiang, L.; Tremblay, P.; Zhang, T. The one-step hydrothermal synthesis of CdS nanorods modified with carbonized leaves from Japanese raisin trees for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2022, 47, 15516–15527. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Y.J.; Guo, X.; Jin, Z.L. Design and synthesis of ZnCo2O4/CdS for substantially improved photocatalytic hydrogen production. Front. Chem. Sci. Eng. 2023, 17, 606–616. [Google Scholar] [CrossRef]
- Li, J.L.; Xu, X.T.; Liu, X.J.; Qin, W.; Wang, M.; Pan, L.K. Metal-organic frameworks derived cake-like anatase/rutile mixed phase TiO2 for highly efficient photocatalysis. J. Alloys Compd. 2017, 690, 640–646. [Google Scholar] [CrossRef]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–130. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.W. Modulation of thermal conductivity of single-walled carbon nanotubes by fullerene encapsulation: The effect of vacancy defects. Phys. Chem. Chem. Phys. 2023, 11, 7734–7740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.R.; Jia, L.B.; Chen, M.Q.; Li, X.C.; Su, Z.H.; Shang, Y.B.; Jiang, X.F.; Gao, X.Y.; Chen, T.; Wang, M.T.; et al. An improbable amino-functionalized fullerene spacer enables 2D/3D hybrid perovskite with enhanced electron transport in solar cells. Adv. Funct. Mater. 2022, 32, 2201374. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 1992, 32, 249–253. [Google Scholar] [CrossRef]
- Zhou, X.A.; Ye, S.T.; Zhao, S.F.; Song, H.H.; Gong, H.T.; Fan, S.R.; Liu, M.J.; Wang, M.L.; Zhou, W.H.; Liu, J.J.; et al. Unraveling structure sensitivity in the photocatalytic dehydrogenative C-C coupling of acetone to 2,5-Hexanedione over Pt/TiO2 catalysts. ACS Catal. 2023, 13, 11825–11833. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, P.Z.; Zhang, L.P.; Jiang, B.J.; Gao, Y.T.; Hou, F.; Wu, B.G.; Li, Q.; Jiang, Y.; Tian, C.G.; et al. Assembly of TiO2 ultrathin nanosheets with surface lattice distortion for solar-light-driven photocatalytic hydrogen evolution. Appl. Catal. B 2018, 239, 317–323. [Google Scholar] [CrossRef]
- Sambur, J.; Brgoch, J. Unveiling the hidden influence of defects via experiment and data science. Chem. Mater. 2023, 35, 7351–7354. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Arandiyan, H.; Yang, H.; Bai, L.; Mujtaba, J.; Wang, Q.; Liu, S.; Sun, H. Uniform Fe3O4 microflowers hierarchical structures assembled with porous nanoplates as superior anode materials for lithium-ion batteries. Appl. Surf. Sci. 2016, 389, 240–246. [Google Scholar] [CrossRef]
- Rahmati, M.S.; Fazaeli, R.; Saravani, M.G.; Ghiasi, R. Cu–curcumin/MCM-41 as an efficient catalyst for in situ conversion of carbazole to fuel oxygenates: A DOE approach. J. Nanostruct. Chem. 2022, 12, 307–327. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.L.; Wang, X.T.; Wang, L.J.; Weng, W.S.; Yu, X.T.; Xing, G.B.; Xie, J.; Lu, C.; Luo, Y.; et al. Influence of nano-attapulgite on compressive strength and microstructure of recycled aggregate concrete. Cem. Concr. Comp. 2022, 134, 104788. [Google Scholar] [CrossRef]
- Kanjan, N.; Laokul, P.; Maiaugree, W. Photocatalytic activity of nanocrystalline Fe3+-doped anatase TiO2 hollow spheres in a methylene blue solution under visible-light irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 4659–4680. [Google Scholar] [CrossRef]
- Mohajernia, S.; Andryskova, P.; Zoppellaro, G.; Kment, S.; Zboril, R.; Schmidt, J.; Schmuki, P. Influence of Ti3+ defect-type on heterogeneous photocatalytic H2 evolution activity of TiO2. J. Mater. Chem. A. 2020, 8, 1432–1442. [Google Scholar] [CrossRef]
- Belghiti, M.; Tanji, K.; El Mersly, L.; Lamsayety, I.; Ouzaouit, K.; Faqir, H.; Benzakour, I.; Rafqah, S.; Outzourhit, A. Fast and non-selective photodegradation of basic yellow malachite green, tetracycline, and sulfamethazine using a nanosized ZnO synthesized from zinc ore. React. Kinet. Mech. Cat. 2022, 135, 2265–2278. [Google Scholar] [CrossRef]
- Causa, M.; Risse, J.D.J.; Scarongella, M.; Brauer, J.C.; Domingo, E.B.; Moser, J.E.; Stingelin, N.; Banerji, N. The fate of electron-hole pairs in polymer: Fullerene blends for organic photovoltaics. Nat. Commun. 2016, 7, 12556. [Google Scholar] [CrossRef]
- Katal, R.; Farahan, M.H.D.A.; Hu, J.Y. Degradation of acetaminophen in a photocatalytic (batch and continuous system) and photoelectrocatalytic process by application of faceted-TiO2. Sep. Purif. Technol. 2020, 230, 115859. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction photocatalysts. Adv. Mater. 2017, 29, 160694. [Google Scholar] [CrossRef] [PubMed]
- Nagappagari, L.R.; Le, T.D.; Ahemad, M.J.; Oh, G.J.; Shin, G.S.; Lee, K.Y.; Yu, Y.T. Enhancement of bifunctional photocatalytic activity of boron-doped g-C3N4/SnO2 heterojunction driven by plasmonic Ag quantum dots. Mater. Today Nano 2023, 22, 100325. [Google Scholar] [CrossRef]
- Islam, M.R.; Chakraborty, A.K.; Gafur, M.A.; Rahman, M.A.; Rahman, M.H. Easy preparation of recyclable thermally stable visible-light-active graphitic-C3N4/TiO2 nanocomposite photocatalyst for efficient decomposition of hazardous organic industrial pollutants in aqueous medium. Res. Chem. Intermed. 2019, 45, 11753–11773. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Pham, T.D.; Nguyen, M.V.; Thuan, D.V.; Trung, D.Q.; Thao, P.; Trang, H.T.; Nguyen, V.N.; Tran, D.T.; Minh, D.N.; et al. Advanced NiMoO4/g-C3N4 Z-scheme heterojunction photocatalyst for efficient conversion of CO2 to valuable products. J. Alloys Compd. 2020, 842, 155860. [Google Scholar] [CrossRef]
- Boettche, S.W.; Oene, S.Z.; Lonerga, M.C.; Surendranat, Y.; Ardo, S.; Broze, C.; Kempler, P.A. Potentially confusing: Potentials in electrochemistry. ACS Energy Lett. 2021, 6, 261–266. [Google Scholar] [CrossRef]
- Pasarán, A.C.; Luke, T.L.; Zarazúa, I.; Rosa, E.D.; Ramírez, R.F.; Sanal, K.C.; Ordaz, A.A. Co-sensitized TiO2 electrodes with different quantum dots for enhanced hydrogen evolution in photoelectrochemical cells. J. Appl. Electrochem. 2019, 49, 475–484. [Google Scholar] [CrossRef]
- Mao, Z.; Lin, H.; Xu, M.; Miao, J.; He, S.J.; Li, Q. Fabrication of Co-doped CdSe quantum dot-sensitized TiO2 nanotubes by ultrasound-assisted method and their photoelectrochemical properties. J. Appl. Electrochem. 2018, 48, 147–155. [Google Scholar] [CrossRef]
- Oh, W.C.; Zhang, F.J.; Chen, M.L. Preparation of MWCNT/TiO2 composites by using MWCNTs and titanium (IV) alkoxide precursors in benzene and their photocatalytic effect and bactericidal activity. Bull. Korean Chem. Soc. 2009, 30, 2637–2642. [Google Scholar]
- Li, Y.S.; Jiang, F.L.; Xiao, Q.; Li, R.; Li, K.; Zhang, M.F.; Zhang, A.Q.; Sun, S.F.; Liu, Y. Enhanced photocatalytic activities of TiO2 nanocomposites doped with water-soluble mercapto-capped CdTe quantum dots. Appl. Catal. B 2010, 101, 118–129. [Google Scholar] [CrossRef]
- AI-Ekabi, H.; Serpone, N. Kinetic studies in heterogeneous photocatalysis. 1. Photocatalytic degradation of chlorinated phenols in aerated aqueous solutions over TiO2 supported on a glass matrix. J. Phys. Chem. 1988, 92, 5726–5731. [Google Scholar] [CrossRef]
- AI-Ekabi, H.; Serpone, N.; Wang, X.H.; Li, J.G.; Kamiyama, H.; Moriyoshi, Y.; Ishigaki, T. Wavelength-sensitive photocatalytic degradation of methyl orange in aqueous suspension over Iron(III) doped TiO2 nanopowders under UV and visible light irradiation. J. Phys. Chem. B 2006, 110, 6804–6809. [Google Scholar]
- Korshin, G.; Chow, C.W.K.; Fabris, R.; Drikas, M. Absorbance spectroscopy-based examination of effects of coagulation on the reactivity of fractions of natural organic matter with varying apparent molecular weights. Water Res. 2009, 43, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Halomoan, I.; Yulizar, Y.; Surya, R.M.; Apriandanu, D.O. Facile preparation of CuO-Gd2Ti2O7 using acmella uliginosa leaf extract for photocatalytic degradation of malachite green. Mater. Res. Bull. 2022, 150, 111726. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Surya, R.M.; Zhang, Q.Q.; Liu, E.Z.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Wu, D.; Li, C.; Zhang, D.; Wang, L.; Zhang, X.; Shi, Z.; Lin, Q. Enhanced photocatalytic activity of Gd3+ doped TiO2 and Gd2O3 modified TiO2 prepared via ball milling method. J. Rare Earths 2019, 37, 845–852. [Google Scholar] [CrossRef]
- Zhang, M.F.; Liang, X.F.; Liu, Y. Co-CNT/TiO2 composites efectively improved the photocatalytic degradation of malachite green. Ionics 2022, 30, 521–527. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liang, X.; Gao, Y.; Liu, Y. C60- and CdS-Co-Modified Nano-Titanium Dioxide for Highly Efficient Photocatalysis and Hydrogen Production. Materials 2024, 17, 1206. https://doi.org/10.3390/ma17051206
Zhang M, Liang X, Gao Y, Liu Y. C60- and CdS-Co-Modified Nano-Titanium Dioxide for Highly Efficient Photocatalysis and Hydrogen Production. Materials. 2024; 17(5):1206. https://doi.org/10.3390/ma17051206
Chicago/Turabian StyleZhang, Meifang, Xiangfei Liang, Yang Gao, and Yi Liu. 2024. "C60- and CdS-Co-Modified Nano-Titanium Dioxide for Highly Efficient Photocatalysis and Hydrogen Production" Materials 17, no. 5: 1206. https://doi.org/10.3390/ma17051206
APA StyleZhang, M., Liang, X., Gao, Y., & Liu, Y. (2024). C60- and CdS-Co-Modified Nano-Titanium Dioxide for Highly Efficient Photocatalysis and Hydrogen Production. Materials, 17(5), 1206. https://doi.org/10.3390/ma17051206






