Electrical and Electro-Thermal Characteristics of (Carbon Black-Graphite)/LLDPE Composites with PTC Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites and Samples for Measurements
2.3. Sample Conditioning
3. Instruments and Methods
3.1. Scanning Electron Microscopy
3.2. Differential Scanning Calorimetry
3.3. FTIR Spectroscopy
3.4. DC Measurements
3.5. AC Measurements
3.6. Measurement of the Electro-Thermal Effect
4. Results and Discussion
4.1. Structural Characterization
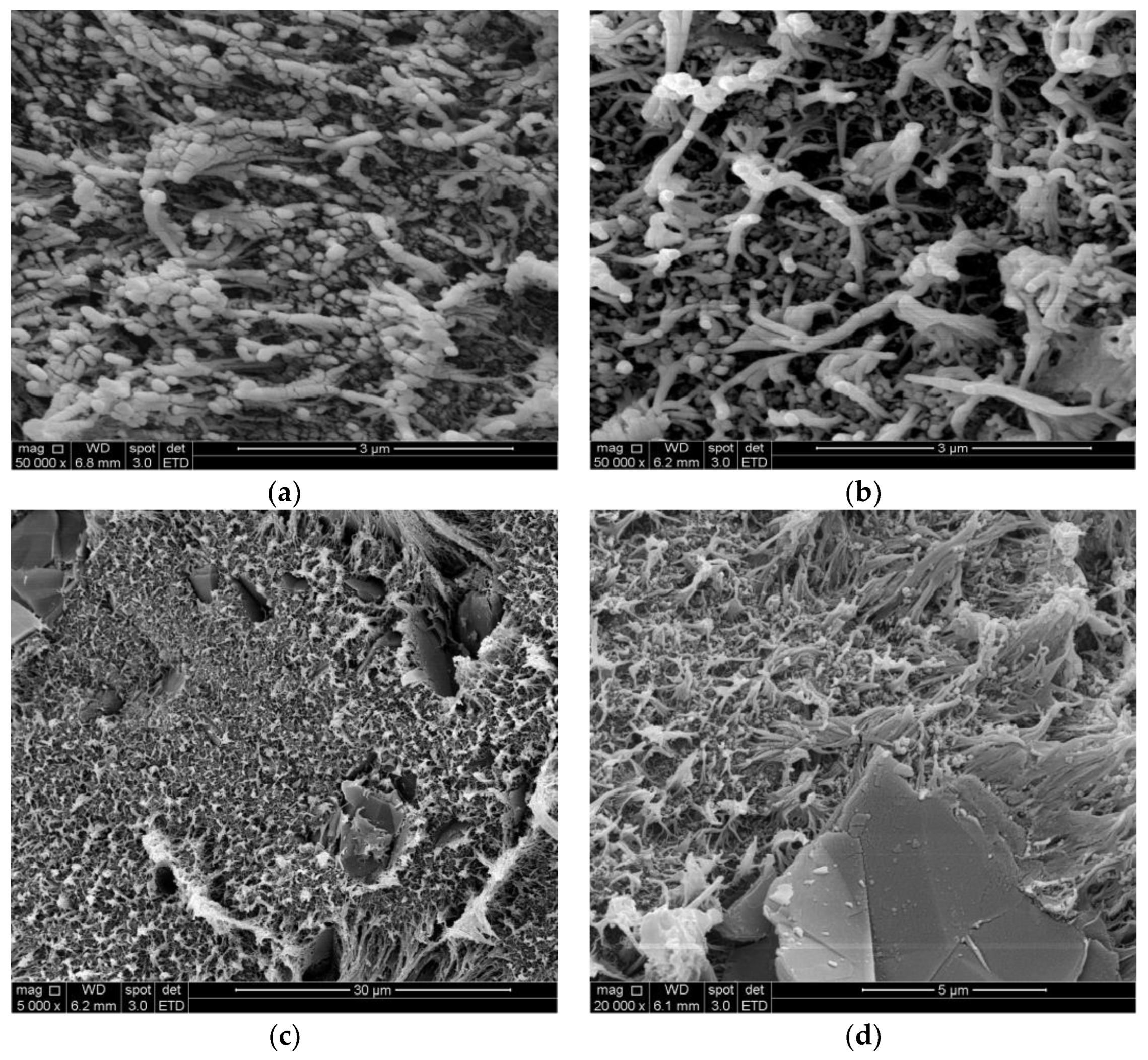
4.1.1. SEM (Scanning Electron Microscopy)
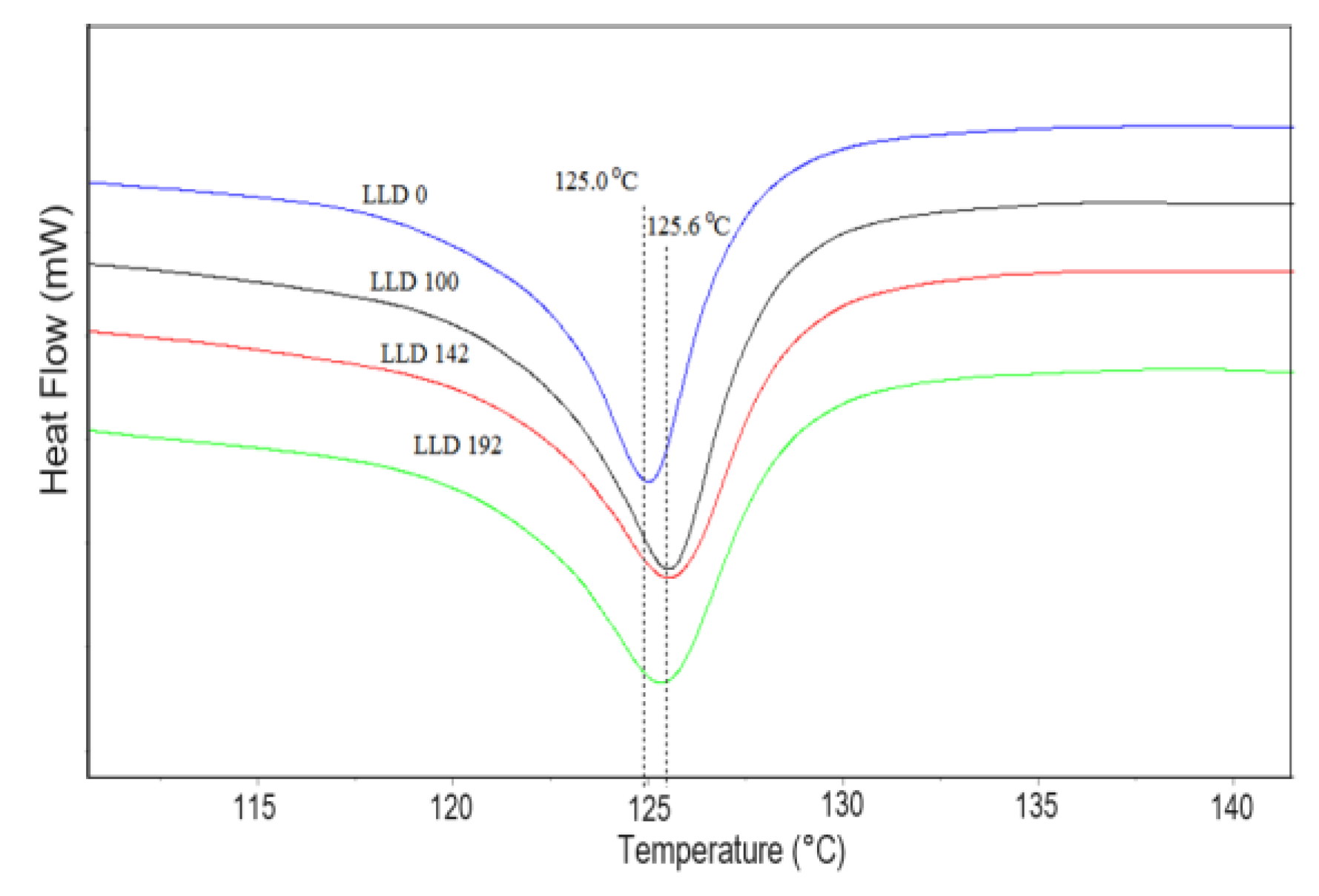
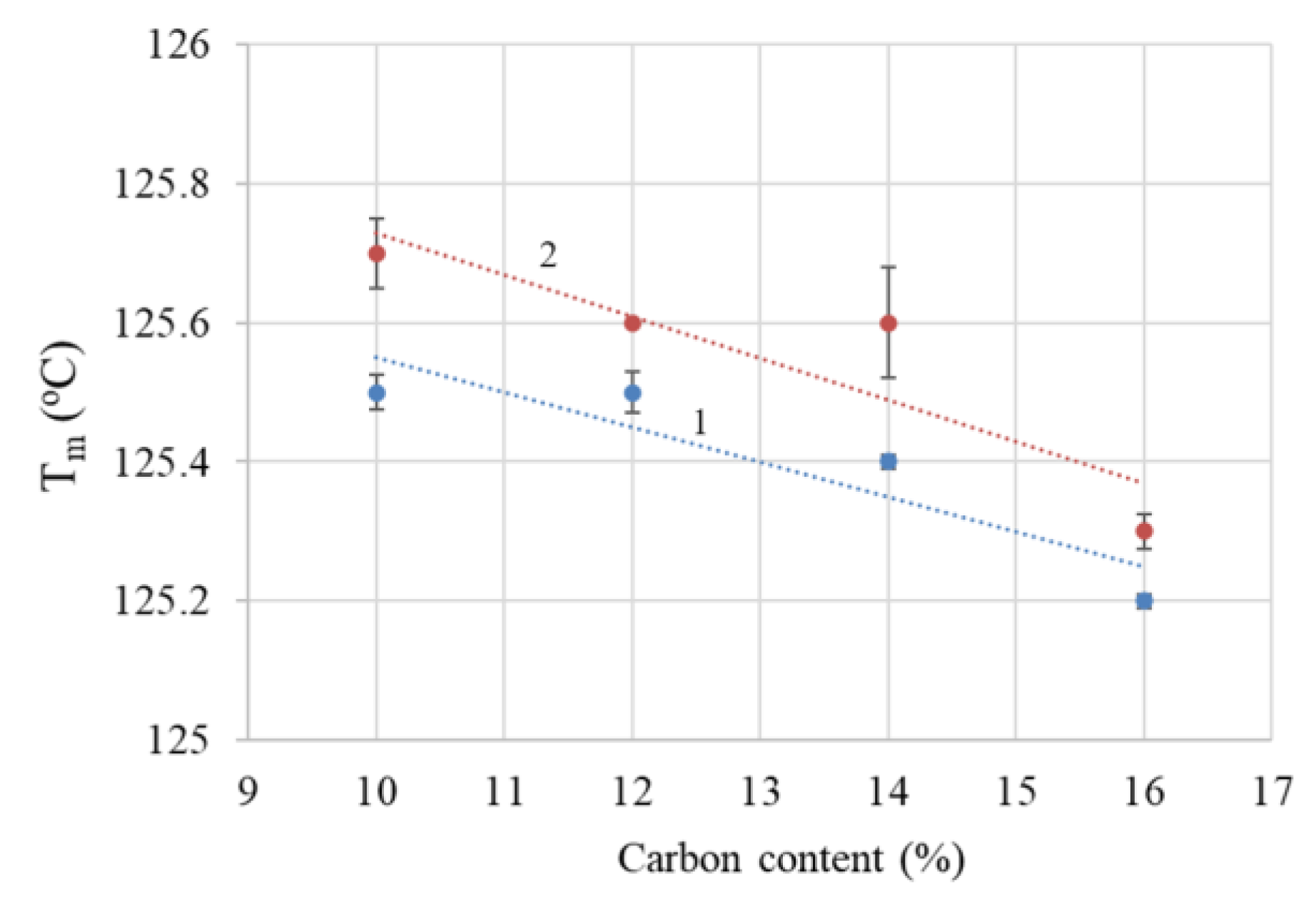
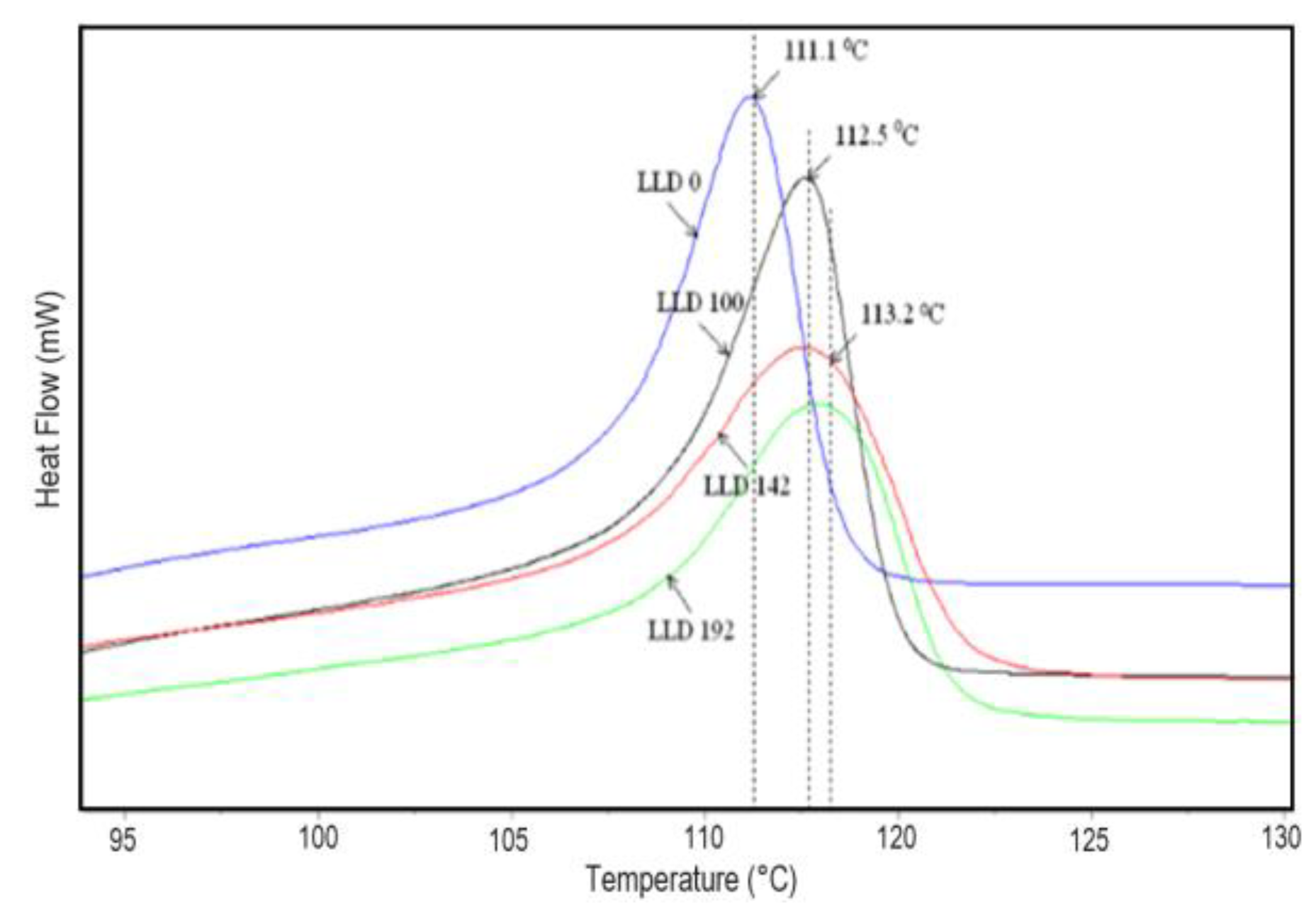
4.1.2. Differential Scanning Calorimetry
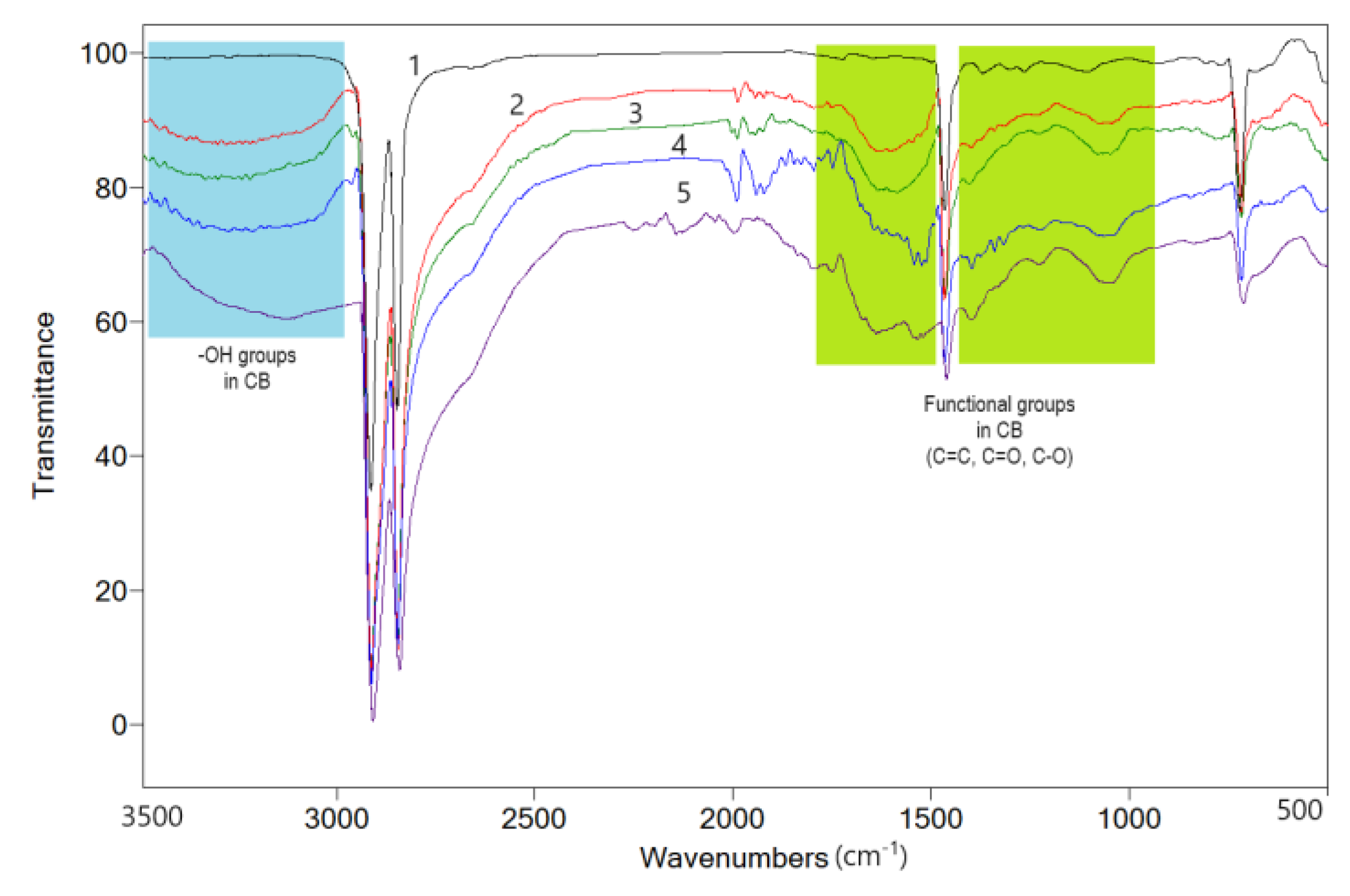
4.1.3. FTIR
4.2. Electrical Properties
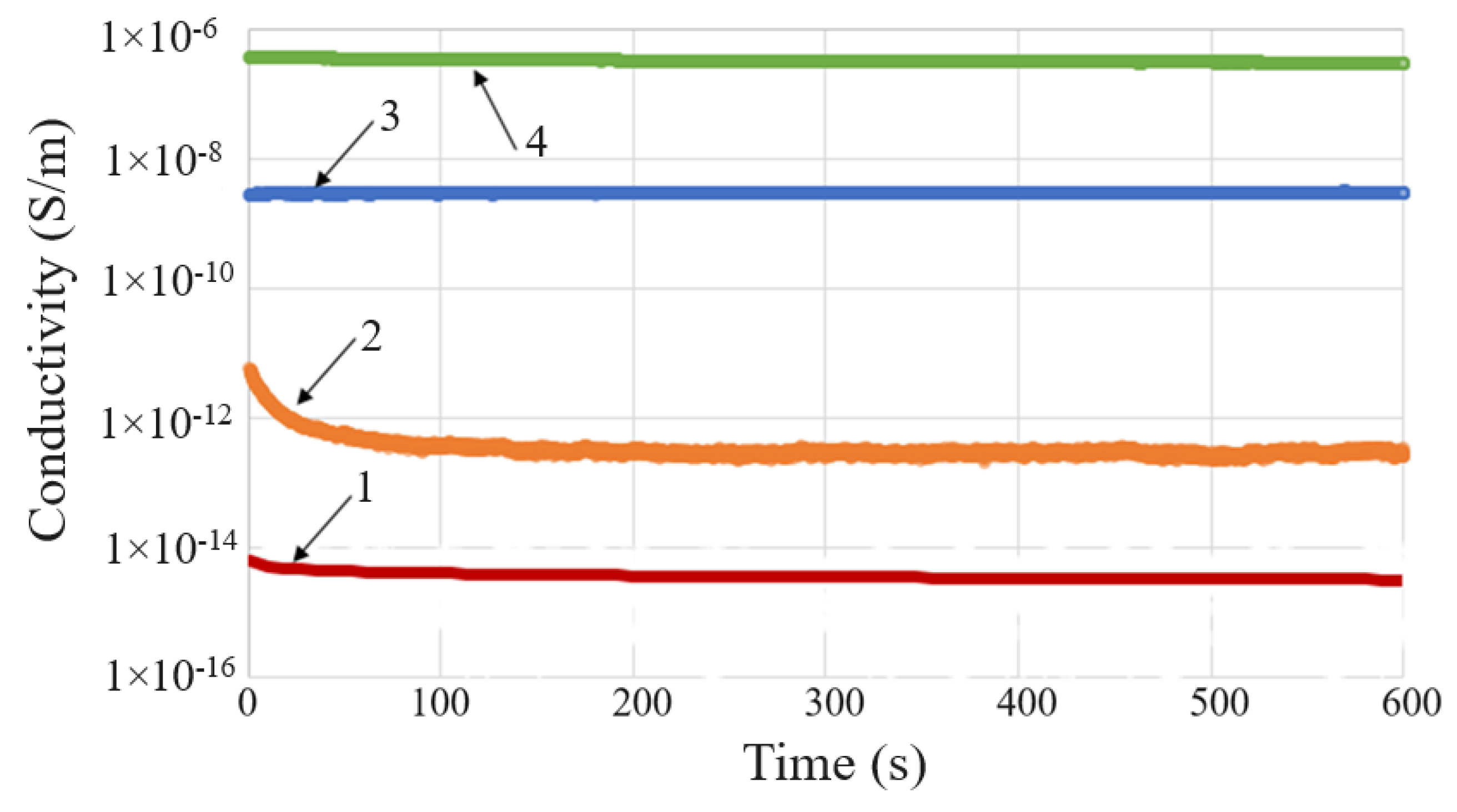
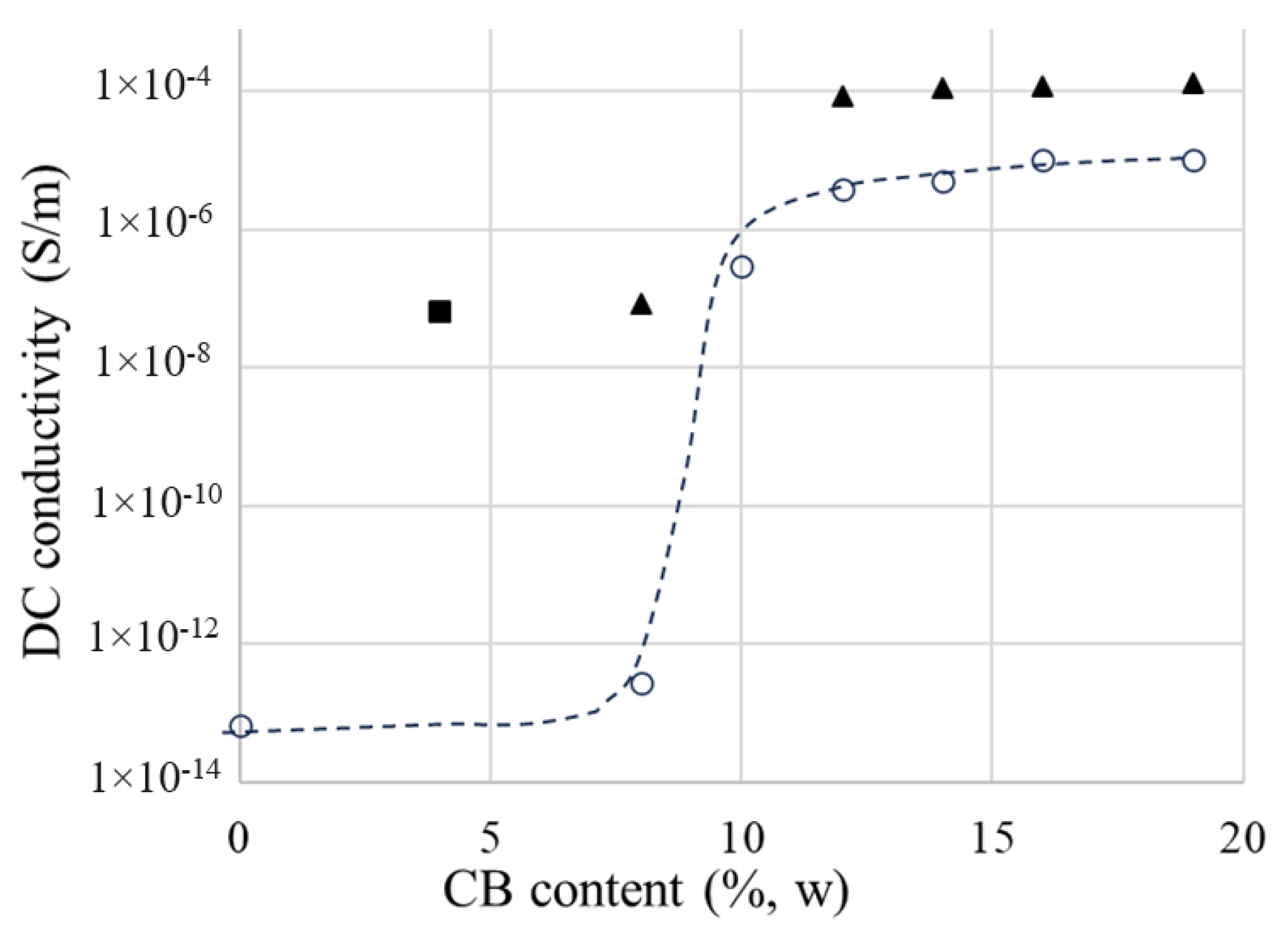
4.2.1. DC Conductivity
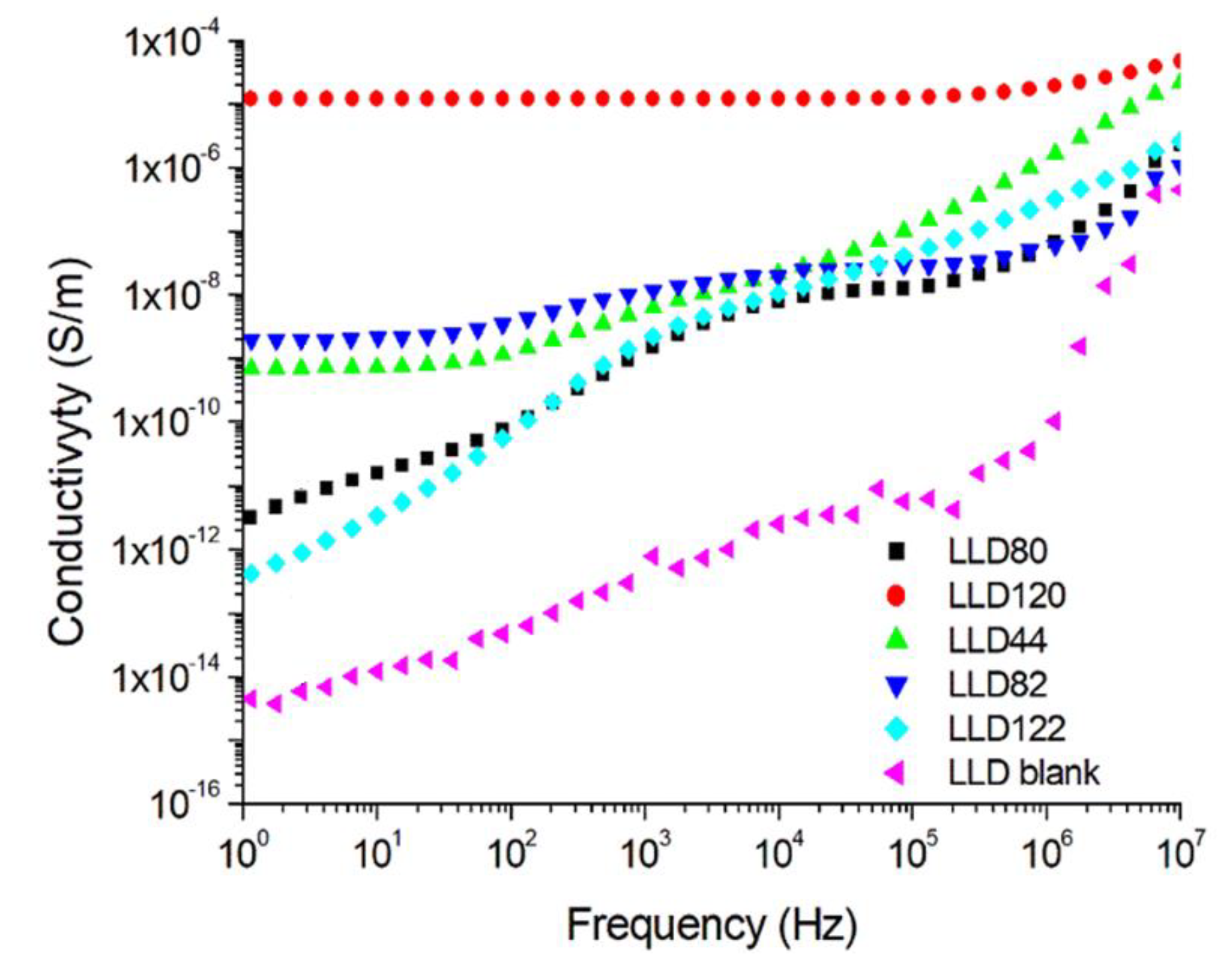
4.2.2. AC Conductivity
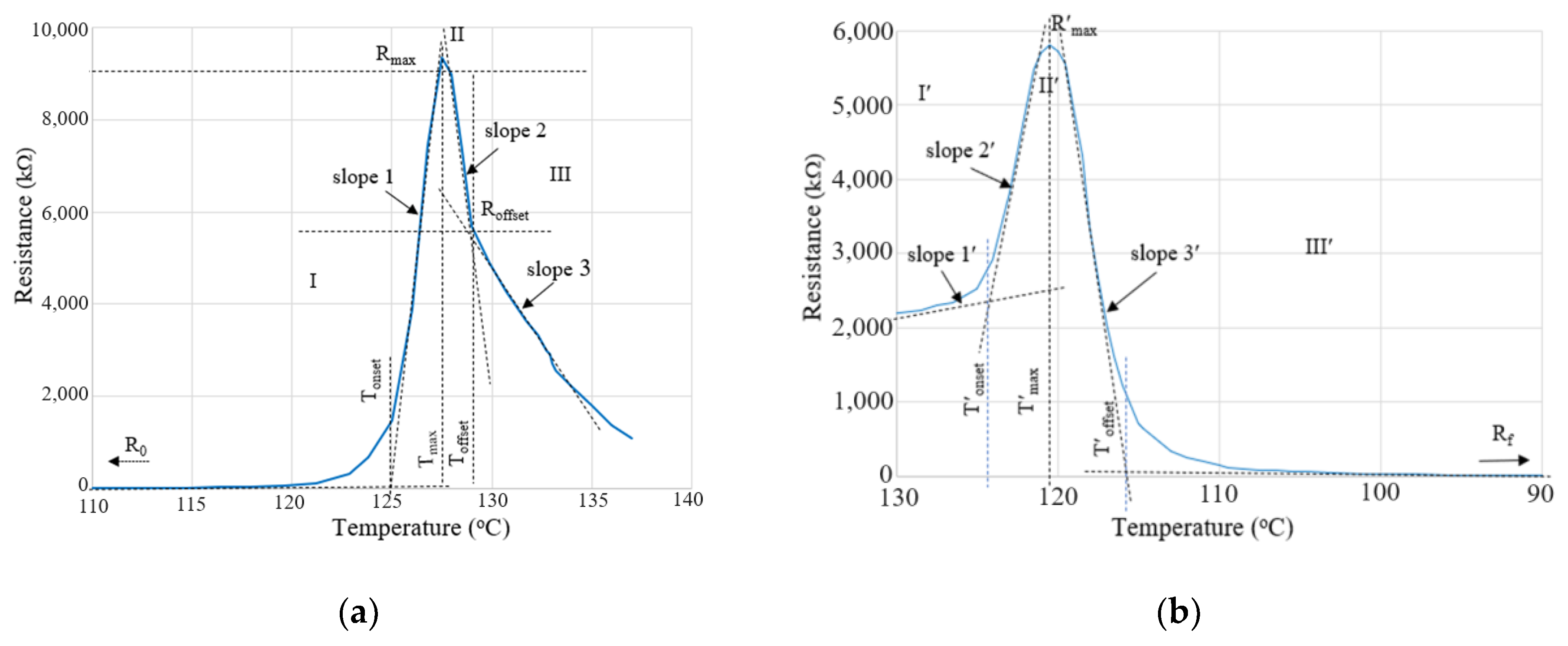
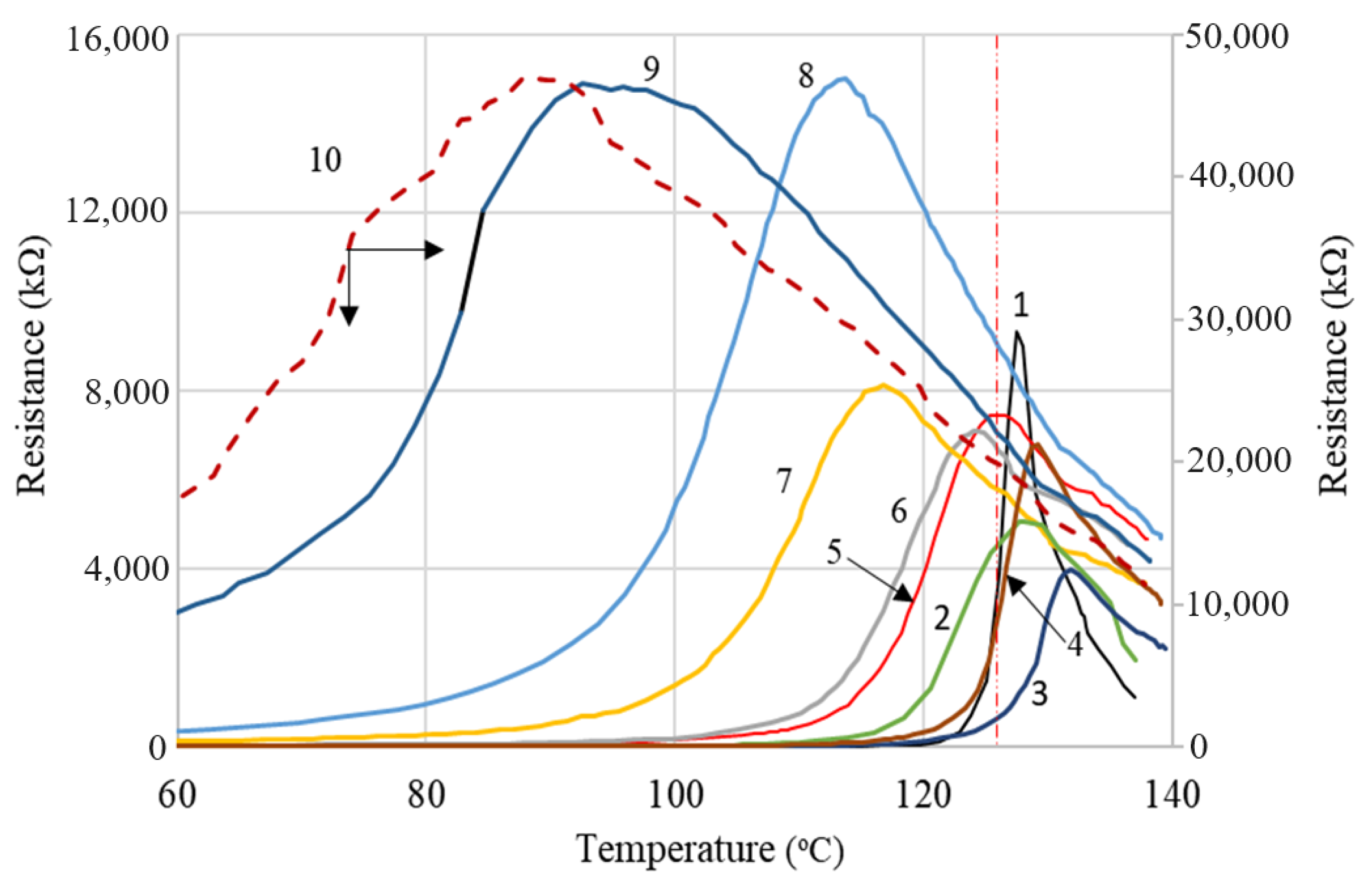
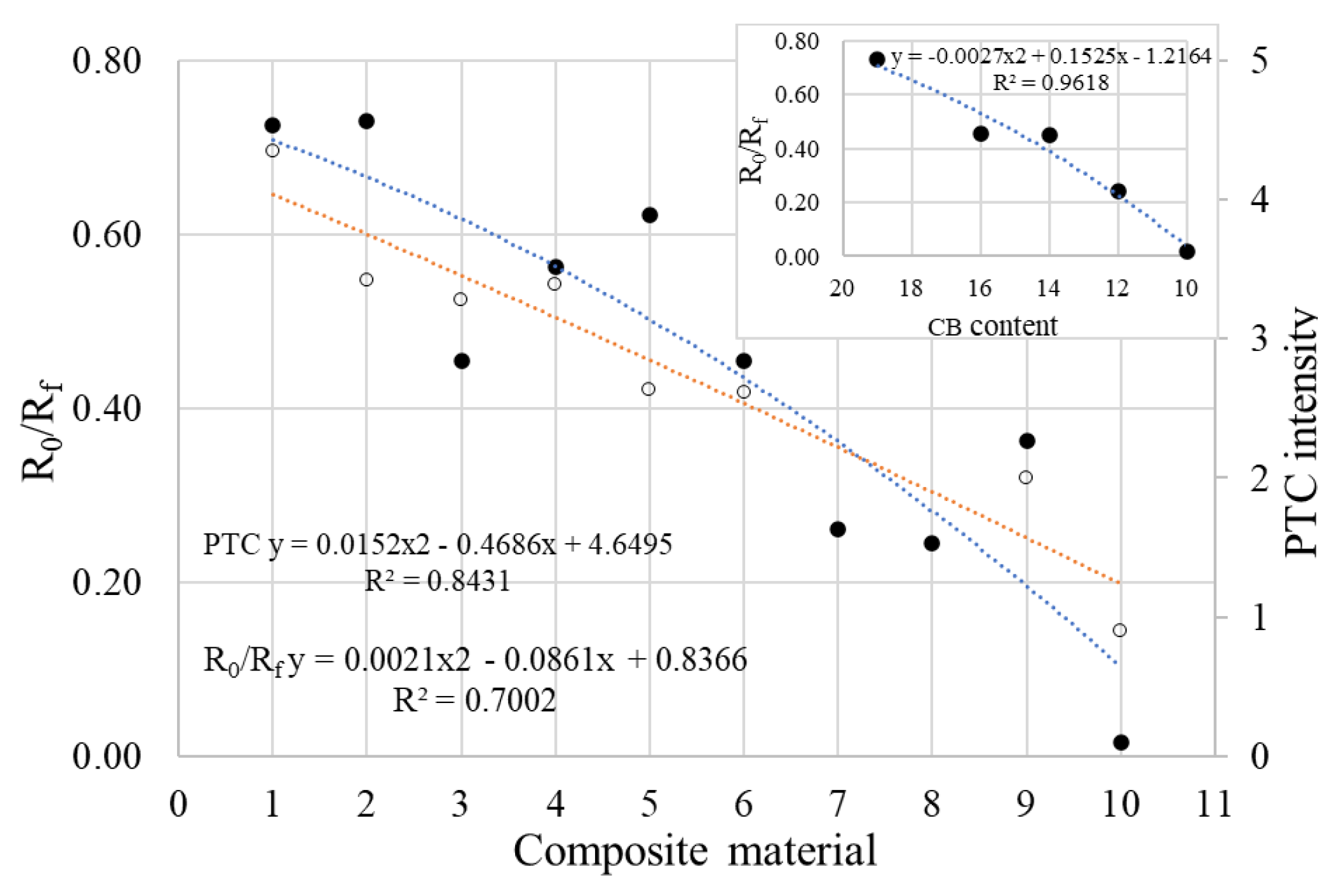
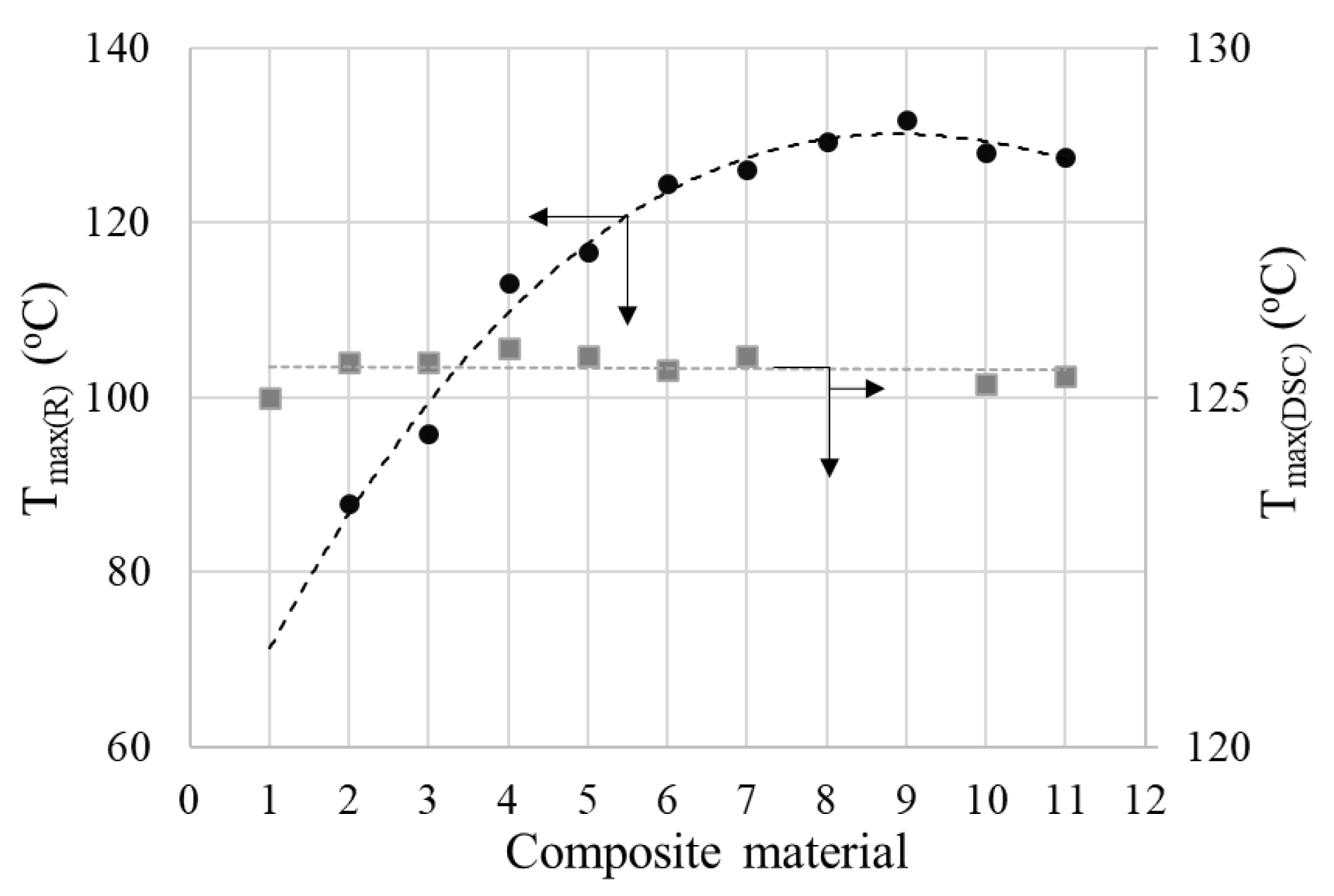
4.3. Resistivity (Resistance) vs. Temperature (PTC Behavior)
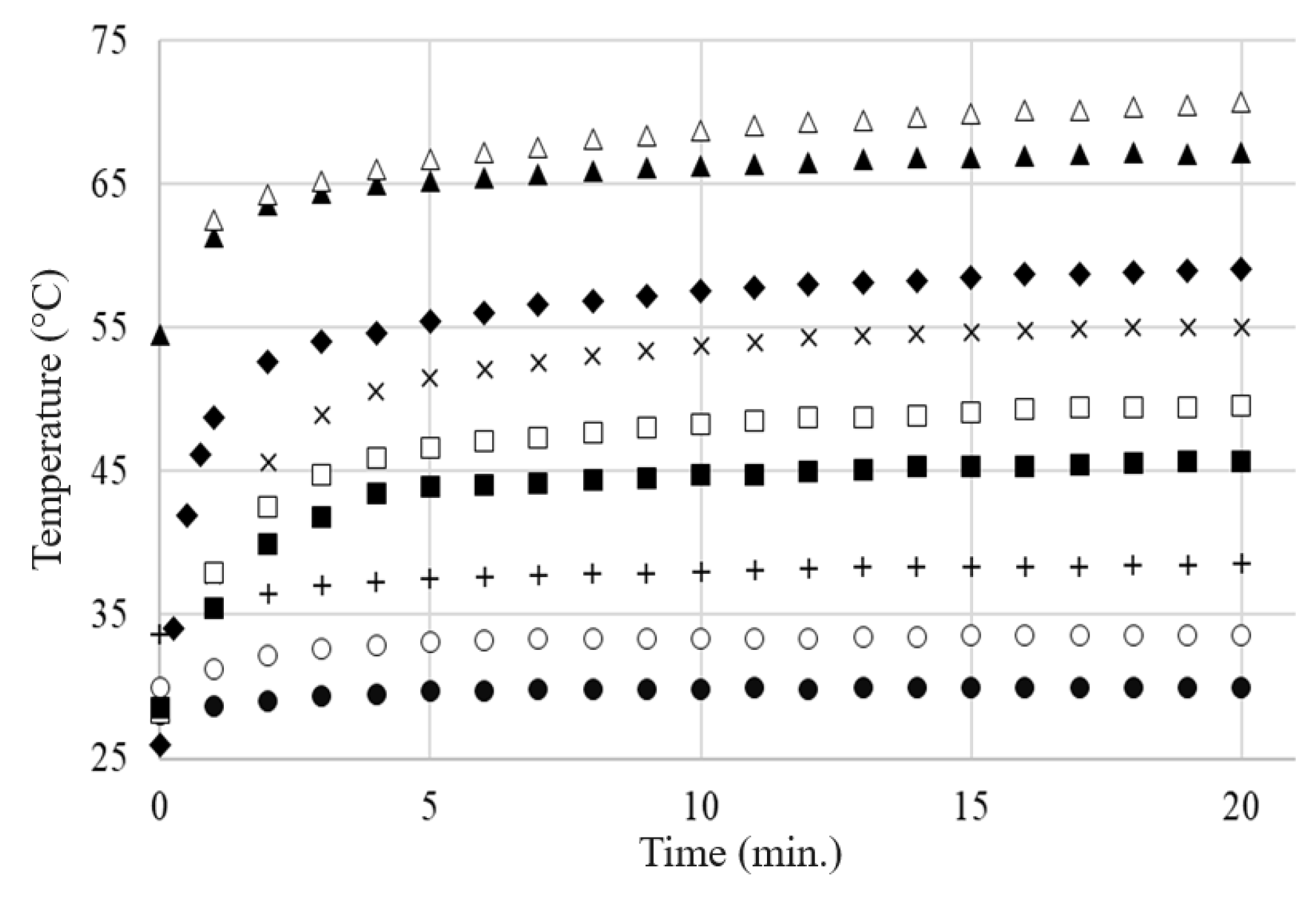
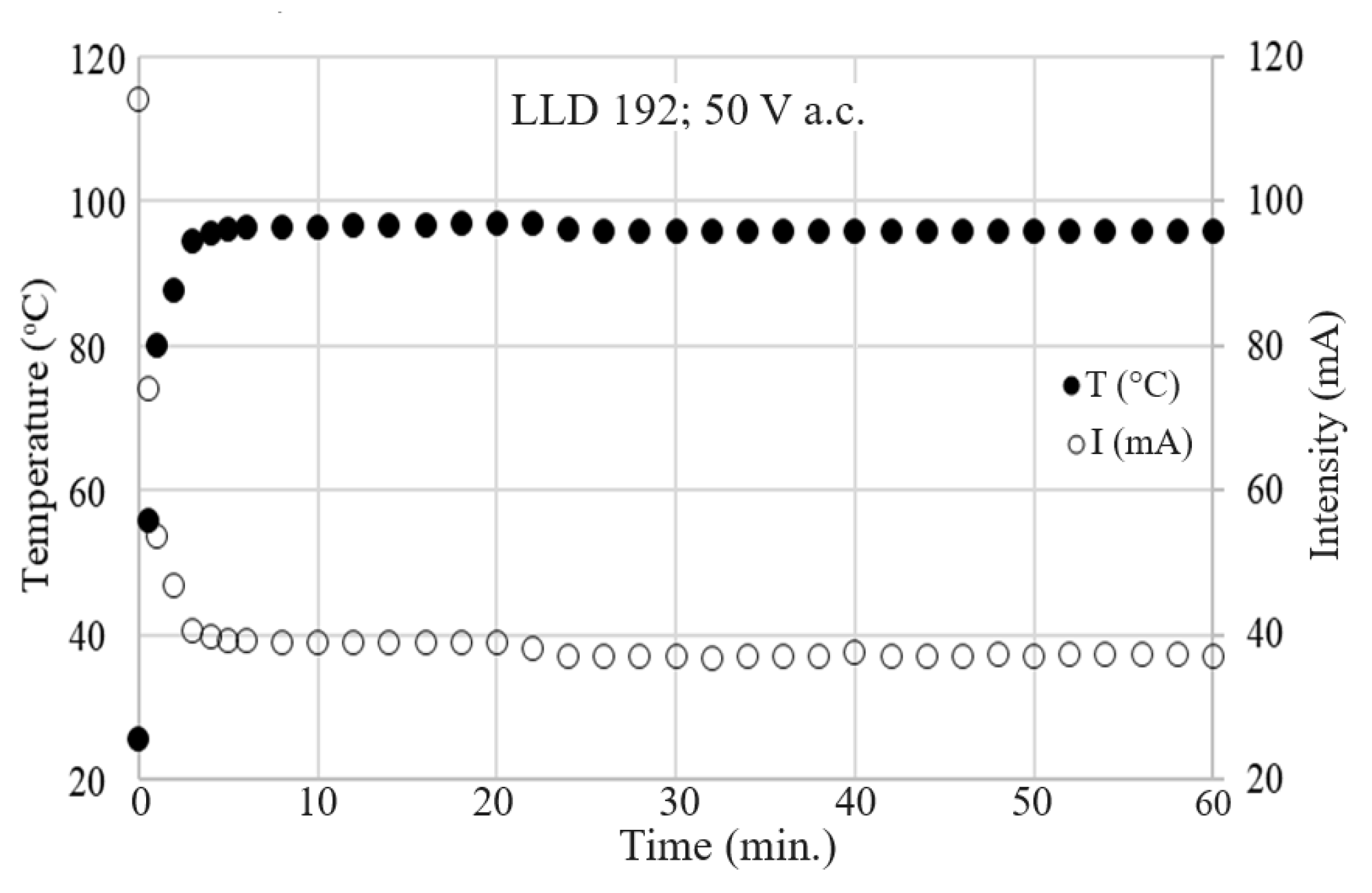
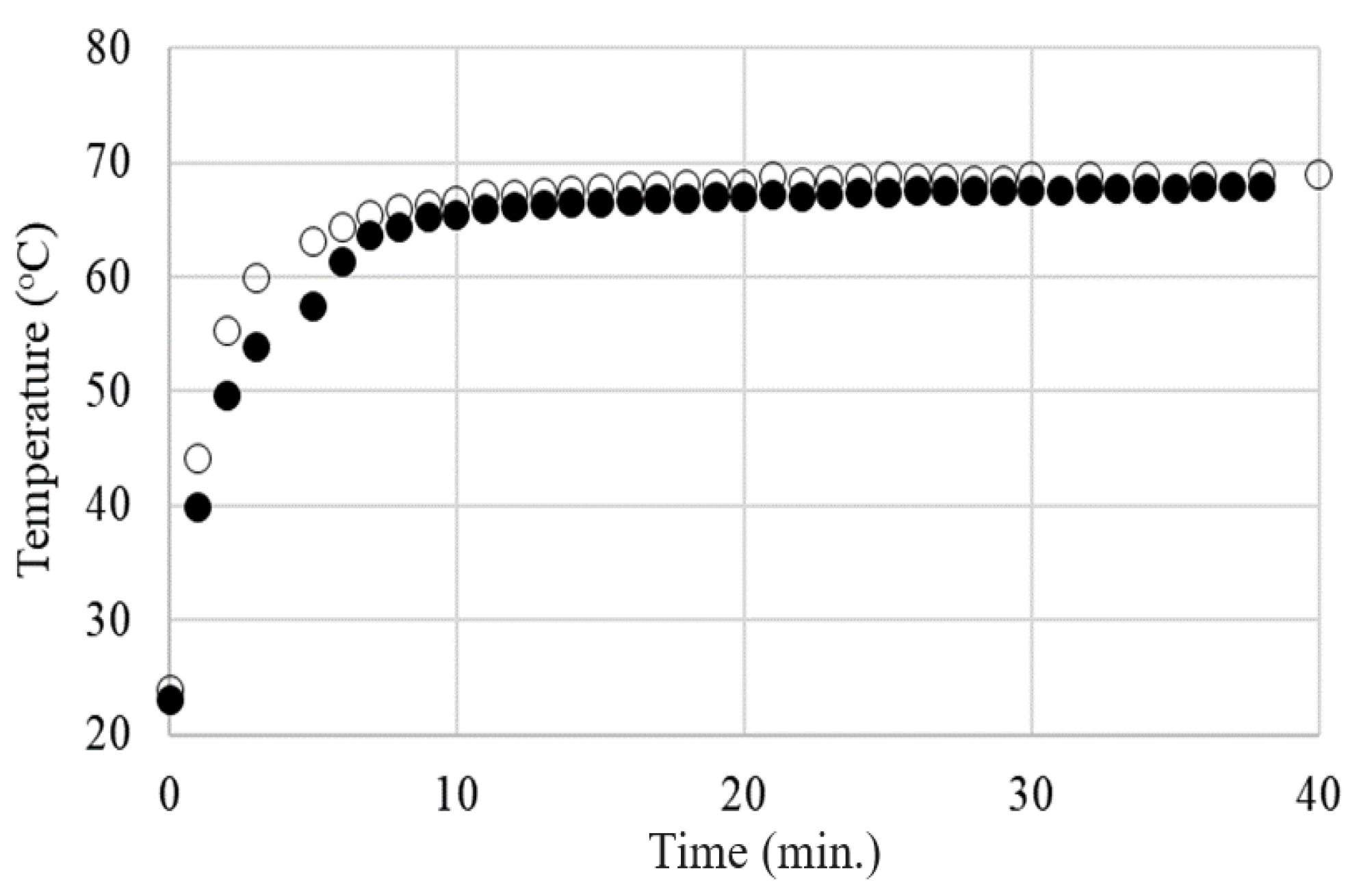
4.4. Electrothermal Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Sun, B.; Yu, C.; Wu, J.; Zhang, J.; Jiang, P. Highly conductive polymer nanocomposites for emerging high voltage power cable shields: Experiment, simulation and applications. High Volt. 2020, 5, 387–396. [Google Scholar] [CrossRef]
- Park, S.-H.; Hwang, J.; Park, G.-S.; Ha, J.-H.; Zhang, M.; Kim, D.; Yun, D.-J.; Lee, S.; Lee, S.H. Modeling the electrical resistivity of polymer composites with segregated structures. Nat. Commun. 2019, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-F.; Kuan, H.-C.; Ma, C.-C.M.; Chen, C.-H. Mechanical and electrical properties of multi-wall carbon nanotube/poly(lactic acid) composites. J. Phys. Chem. Solids 2008, 69, 1395–1398. [Google Scholar] [CrossRef]
- Miyasaka, K.; Watanabe, K.; Jojima, E.; Aida, H.; Sumita, M.; Ishikawa, K. Electrical conductivity of carbon-polymer composites as a function of carbon content. J. Mater. Sci. 1982, 17, 1610–1616. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Han, X.; Jing, Y.; Du, M.; Lu, C.; Zhang, K. Low-dimensional Thermoelectric Materials. In Flexible Thermoelectric Polymers and Systems; Wiley: Hoboken, NJ, USA, 2022; pp. 209–238. [Google Scholar]
- Xi, Y.; Yamanaka, A.; Bin, Y.; Matsuo, M. Electrical properties of segregated ultrahigh molecular weight polyethylene/multiwalled carbon nanotube composites. J. Appl. Polym. Sci. 2007, 105, 2868–2876. [Google Scholar] [CrossRef]
- Gümüş, E.; Yağımlı, M.; Arca, E. Investigation of the Dielectric Properties of Graphite and Carbon Black-Filled Composites as Electromagnetic Interference Shielding Coatings. Appl. Sci. 2023, 13, 8893. [Google Scholar] [CrossRef]
- Wu, M.; Wu, F.; Ren, Q.; Jia, X.; Luo, H.; Shen, B.; Wang, L.; Zheng, W. Tunable electromagnetic interference shielding performance of polypropylene/carbon black composites via introducing microcellular structure. Compos. Commun. 2022, 36, 101363. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kim, M.S.; Ahn, D.; Yeo, S.Y.; Lee, S. Electrical percolation threshold of carbon black in a polymer matrix and its application to antistatic fibre. Sci. Rep. 2019, 9, 6338. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Dai, C.; Gao, J.; Gou, B.; Bi, S.; Yu, P.; Chen, X. Reinforcement effects of graphite fluoride on breakdown voltage rating and Pyro-Resistive properties of carbon Black/Poly (vinylidene fluoride) composites. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107947. [Google Scholar] [CrossRef]
- Ding, X.; Wang, J.; Zhang, S.; Wang, J.; Li, S. Carbon black-filled polypropylene as a positive temperature coefficient material: Effect of filler treatment and heat treatment. Polym. Bull. 2016, 73, 369–383. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, P.; Li, J.; Xu, D.; Bin, Y. Study on filler content dependence of the onset of positive temperature coefficient (PTC) effect of electrical resistivity for UHMWPE/LDPE/CF composites based on their DC and AC electrical behaviors. Polymer 2014, 55, 2103–2112. [Google Scholar] [CrossRef]
- Mamunya, Y.; Maruzhenko, O.; Kolisnyk, R.; Iurzhenko, M.; Pylypenko, A.; Masiuchok, O.; Godzierz, M.; Krivtsun, I.; Trzebicka, B.; Pruvost, S. Pyroresistive Properties of Composites Based on HDPE and Carbon Fillers. Polymers 2023, 15, 2105. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.; Hanemann, T.; Rapp, B.E.; Finnah, G. Enhanced PTC Effect in Polyamide/Carbon Black Composites. Materials 2022, 15, 5400. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Zhao, L.; Zou, J.; Zhang, P. High positive temperature coefficient effect of resistivity in conductive polystyrene/polyurethane composites with ultralow percolation threshold of MWCNTs via interpenetrating structure. React. Funct. Polym. 2020, 151, 104562. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.-H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, B.-b. Positive temperature coefficient effect and mechanism of compatible LLDPE/HDPE composites doping conductive graphite powders. J. Appl. Polym. Sci. 2018, 135, 46453. [Google Scholar] [CrossRef]
- Makuuchi, K.; Cheng, S. Radiation Processing of Polymer Materials and Its Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xu, H.-P.; Dang, Z.-M.; Shi, D.-H.; Bai, J.-B. Remarkable selective localization of modified nanoscaled carbon black and positive temperature coefficient effect in binary-polymer matrix composites. J. Mater. Chem. 2008, 18, 2685–2690. [Google Scholar] [CrossRef]
- Bao, Y.; Xu, L.; Pang, H.; Yan, D.-X.; Chen, C.; Zhang, W.-Q.; Tang, J.-H.; Li, Z.-M. Preparation and properties of carbon black/polymer composites with segregated and double-percolated network structures. J. Mater. Sci. 2013, 48, 4892–4898. [Google Scholar] [CrossRef]
- Setnescu, R.; Lungulescu, E.M. Novel PTC Composites for Temperature Sensors (and Related Applications). In Wireless Sensor Networks; Jaydip, S., Mingqiang, Y., Fenglei, N., Hao, W., Eds.; IntechOpen: Rijeka, Croatia, 2023; Chapter 6. [Google Scholar]
- Wei, Y.; Li, Z.; Liu, X.; Dai, K.; Zheng, G.; Liu, C.; Chen, J.; Shen, C. Temperature-resistivity characteristics of a segregated conductive CB/PP/UHMWPE composite. Colloid Polym. Sci. 2014, 292, 2891–2898. [Google Scholar] [CrossRef]
- Li, X.; Guo, Q.; Yang, F.; Sun, X.; Li, W.; Yao, Z. Electrical Properties of LLDPE/LLDPE-g-PS Blends with Carboxylic Acid Functional Groups for Cable Insulation Applications. ACS Appl. Polym. Mater. 2020, 2, 3450–3457. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, S.; Zheng, X.; Liu, Z.; Yang, W.; Yang, M. Distinct positive temperature coefficient effect of polymer–carbon fiber composites evaluated in terms of polymer absorption on fiber surface. Phys. Chem. Chem. Phys. 2016, 18, 8081–8087. [Google Scholar] [CrossRef] [PubMed]
- Ohe, K.; Naito, Y. A New Resistor Having An Anomalously Large Positive Temperature Coefficient. Jpn. J. Appl. Phys. 1971, 10, 99. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, C.-A.; Wang, P.; Sumita, M. Temperature dependence of electrical resistivity for carbon black filled ultra-high molecular weight polyethylene composites prepared by hot compaction. Carbon 2005, 43, 2544–2553. [Google Scholar] [CrossRef]
- Nakamura, S.; Tomimura, T.; Sawa, G. Dielectric properties of carbon black-polyethylene composites below the percolation threshold. In Proceedings of the 1999 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (Cat. No.99CH36319), Austin, TX, USA, 17–20 October 1999; Volume 291, pp. 293–296. [Google Scholar]
- Hu, X.; Shi, Y.; Wang, Y.; Liu, L.-Z.; Ren, Y.; Wang, Y. Crystallization, structure, morphology, and properties of linear low-density polyethylene blends made with different comonomers. Polym. Eng. Sci. 2021, 61, 2406–2415. [Google Scholar] [CrossRef]
- Setnescu, R.; Lungulescu, M.; Bara, A.; Caramitu, A.; Mitrea, S.; Marinescu, V.; Culicov, O. Thermo-Oxidative Behavior of Carbon Black Composites for Self-Regulating Heaters. Adv. Eng. Forum 2019, 34, 66–80. [Google Scholar] [CrossRef]
- Setnescu, R.; Lungulescu, E.-M.; Marinescu, V.E. Polymer Composites with Self-Regulating Temperature Behavior: Properties and Characterization. Materials 2023, 16, 157. [Google Scholar] [CrossRef]
- Durmuş, A.; Woo, M.; Kaşgöz, A.; Macosko, C.W.; Tsapatsis, M. Intercalated linear low density polyethylene (LLDPE)/clay nanocomposites prepared with oxidized polyethylene as a new type compatibilizer: Structural, mechanical and barrier properties. Eur. Polym. J. 2007, 43, 3737–3749. [Google Scholar] [CrossRef]
- Ilie, S.; Setnescu, R.; Lungulescu, E.M.; Marinescu, V.; Ilie, D.; Setnescu, T.; Mares, G. Investigations of a mechanically failed cable insulation used in indoor conditions. Polym. Test. 2011, 30, 173–182. [Google Scholar] [CrossRef]
- Stancu, C.; Notingher, P.V.; Panaitescu, D.M.; Marinescu, V. Electrical Properties of Polyethylene Composites with Low Content of Neodymium. Polym.-Plast. Technol. Eng. 2015, 54, 1135–1143. [Google Scholar] [CrossRef]
- Kita, Y.; Kasai, Y.; Hashimoto, S.; Iiyama, K.; Takamiya, S. Application of Brightness of Scanning Electron Microscope Images to Measuring Thickness of Nanometer-Thin SiO2 Layers on Si Substrates. Jpn. J. Appl. Phys. 2001, 40, 5861. [Google Scholar] [CrossRef]
- Müller, M.T.; Dreyße, J.; Häußler, L.; Krause, B.; Pötschke, P. Influence of talc with different particle sizes in melt-mixed LLDPE/MWCNT composites. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1680–1691. [Google Scholar] [CrossRef]
- Kundu, P.P.; Biswas, J.; Kim, H.; Choe, S. Influence of film preparation procedures on the crystallinity, morphology and mechanical properties of LLDPE films. Eur. Polym. J. 2003, 39, 1585–1593. [Google Scholar] [CrossRef]
- Liu, M.; Horrocks, A.R. Effect of Carbon Black on UV stability of LLDPE films under artificial weathering conditions. Polym. Degrad. Stab. 2002, 75, 485–499. [Google Scholar] [CrossRef]
- Lobo, H.; Bonilla, J.V. Handbook of Plastics Analysis; CRC Press: Boca Raton, FL, USA, 2003; Volume 68. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez C, V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Bai, B.C.; Kim, Y.R. Effective Surface Structure Changes and Characteristics of Activated Carbon with the Simple Introduction of Oxygen Functional Groups by Using Radiation Energy. Surfaces 2024, 7, 12–25. [Google Scholar] [CrossRef]
- Zappielo, C.D.; Nanicuacua, D.M.; dos Santos, W.N.; da Silva, D.L.; Dall’Antônia, L.H.; Oliveira, F.M.d.; Clausen, D.N.; Tarley, C.R. Solid phase extraction to on-line preconcentrate trace cadmium using chemically modified nano-carbon black with 3-mercaptopropyltrimethoxysilane. J. Braz. Chem. Soc. 2016, 27, 1715–1726. [Google Scholar] [CrossRef]
- Veca, L.M.; Nastase, F.; Banciu, C.; Popescu, M.; Romanitan, C.; Lungulescu, M.; Popa, R. Synthesis of macroporous ZnO-graphene hybrid monoliths with potential for functional electrodes. Diam. Relat. Mater. 2018, 87, 70–77. [Google Scholar] [CrossRef]
- Hristea, G.; Iordoc, M.; Lungulescu, E.-M.; Bejenari, I.; Volf, I. A sustainable bio-based char as emerging electrode material for energy storage applications. Sci. Rep. 2024, 14, 1095. [Google Scholar] [CrossRef]
- Setnescu, R.; Jipa, S.; Setnescu, T.; Kappel, W.; Kobayashi, S.; Osawa, Z. IR and X-ray characterization of the ferromagnetic phase of pyrolysed polyacrylonitrile. Carbon 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Domingo-García, M.; Garzón, F.L.; Pérez-Mendoza, M. On the characterization of chemical surface groups of carbon materials. J. Colloid Interface Sci. 2002, 248, 116–122. [Google Scholar] [CrossRef]
- Thomassin, J.-M.; Jérôme, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Ren, M.; Liu, X.; Jia, X.; Luo, C.; Zhang, L.; Alamo, R.G. Memory of crystallization in the melt of commercial linear low density polyethylenes processed in an open twin-screw extruder. Thermochim. Acta 2023, 720, 179423. [Google Scholar] [CrossRef]




| Abbreviation | Full Name/Description |
|---|---|
| AC | Alternating Current |
| CB | Carbon Black |
| (CB, Gr)/LLD | Composites with LLDPE matrix and CB and Gr |
| CF | Carbon Fibers |
| CPC | Conductive Polymer Composite |
| DC | Direct Current |
| DSC | Differential Scanning Calorimetry |
| ΔH | Transition enthalpy (from DSC) |
| ε′, ε″ | complex relative permitivities (AC) |
| FTIR | Fourier Transform InfraRed spectroscopy |
| ϕ | Fraction (either mass or volume) of conductive filler within the composite |
| ϕc | Critical concentration of the filler |
| Gr | Graphite |
| HDPE | High Density Polyethylene |
| LDPE | Low Density Polyethylene |
| LLDPE | Linear Low-Density Polyethylene |
| NTC | Negative Temperature Coefficient |
| PTC | Positive Temperature Coefficient |
| R | Electrical resistance |
| ρ | Electrical resistivity |
| ρDC | Direct current resistivity |
| ρV | Volume resistivity |
| RMS voltage | Root Mean Square voltage (effective voltage = 0.707 of peak voltage, in AC measurements) |
| σ′, σ″ | complex conductivities (AC) |
| σdc | Direct current conductivity |
| SEM | Scanning Electron Microscopy |
| Tc, Tc(DSC) (see the footnote for Tm) | peak temperature of crystallization endotherm (in DSC) |
| Teq | Equilibrium temperature (Teq denotes the practically constant value of the surface temperature reached after few minutes of sample exposure to electric field, in T vs. t, U measurements) |
| Tm; Tm(DSC) (The notation Tm(DSC) is used for better distinguish between the maximum of temperatures in either DSC and R vs. T measurements) | Peak temperature of crystallinity melting in DSC |
| Toffset | Offset temperature in DSC or R vs. T-heating measurements |
| T′offset | Offset temperature in R vs. T-cooling measurements |
| Tonset | Onset temperature in DSC or R vs. T-heating measurements |
| UHMWPE | Ultra-High Molecular Weight Polyethylene |
| Sample Code | Polymeric Matrix | Filler | Total C (%, w) | |
|---|---|---|---|---|
| LLDPE (%, w) | Carbon Black (%, w) | Graphite (%, w) | ||
| LLD 0 | 100 (neat) | 0 | 0 | 0 |
| LLD 44 | 92 | 4 | 4 | 8 |
| LLD 80 | 92 | 8 | 0 | 8 |
| LLD 82 | 90 | 8 | 2 | 10 |
| LLD 100 | 90 | 10 | 0 | 10 |
| LLD 120 | 88 | 12 | 0 | 12 |
| LLD 122 | 86 | 12 | 2 | 14 |
| LLD 140 | 86 | 14 | 0 | 14 |
| LLD 142 | 84 | 14 | 2 | 16 |
| LLD 160 | 84 | 16 | 0 | 16 |
| LLD 162 | 82 | 16 | 2 | 18 |
| LLD 190 | 81 | 19 | 0 | 19 |
| LLD 192 | 79 | 19 | 2 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungulescu, E.-M.; Stancu, C.; Setnescu, R.; Notingher, P.V.; Badea, T.-A. Electrical and Electro-Thermal Characteristics of (Carbon Black-Graphite)/LLDPE Composites with PTC Effect. Materials 2024, 17, 1224. https://doi.org/10.3390/ma17051224
Lungulescu E-M, Stancu C, Setnescu R, Notingher PV, Badea T-A. Electrical and Electro-Thermal Characteristics of (Carbon Black-Graphite)/LLDPE Composites with PTC Effect. Materials. 2024; 17(5):1224. https://doi.org/10.3390/ma17051224
Chicago/Turabian StyleLungulescu, Eduard-Marius, Cristina Stancu, Radu Setnescu, Petru V. Notingher, and Teodor-Adrian Badea. 2024. "Electrical and Electro-Thermal Characteristics of (Carbon Black-Graphite)/LLDPE Composites with PTC Effect" Materials 17, no. 5: 1224. https://doi.org/10.3390/ma17051224
APA StyleLungulescu, E.-M., Stancu, C., Setnescu, R., Notingher, P. V., & Badea, T.-A. (2024). Electrical and Electro-Thermal Characteristics of (Carbon Black-Graphite)/LLDPE Composites with PTC Effect. Materials, 17(5), 1224. https://doi.org/10.3390/ma17051224









