Abstract
Bi-based YbMg2Bi1.98 Zintl compounds represent promising thermoelectric materials. Precise composition and appropriate doping are of great importance for this complex semiconductor. Here, the influence of Zn substitution for Mg on the microstructure and thermoelectric properties of p-type YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23) was investigated. Polycrystalline samples were prepared using induction melting and densified with spark plasma sintering. X-ray diffraction confirmed that the major phase of the samples possesses the trigonal CaAl2Si2-type crystal structure, and SEM/EDS indicated the presence of minor secondary phases. The electrical conductivity increases and the lattice thermal conductivity decreases with more Zn doping in YbMg1.85−xZnxBi1.98, whereas the Seebeck coefficient has a large reduction. The band gap decreases with increasing Zn concentration and leads to bipolar conduction, resulting in an increase in the thermal conductivity at higher temperatures. Figure of merit ZT values of 0.51 and 0.49 were found for the samples with x = 0 and 0.05 at 773 K, respectively. The maximum amount of Zn doping is suggested to be less than x = 0.1.
1. Introduction
Exploring sustainable energy and improving low energy conversion efficiency are top priorities to solve the increasing energy shortage. As core materials for power generation devices in thermoelectric (TE) technology, TE materials allow the interconversion of waste thermal energy and electrical energy without additional energy consumption and have been intensively investigated because of their numerous applications in industrial waste heat recovery, power generation, solid state cooling, and space probes [1,2]. The performance of a TE material is generally evaluated by the term “figure of merit ZT”, which is given as ZT = S2σT/κ, where S, σ, κ, and T represent the Seebeck coefficient, the electrical conductivity, the thermal conductivity, and the absolute temperature, respectively. κ generally comes from two sources, i.e., the electronic contribution κe, which is proportional to σ based on the Wiedemann–Franz relationship, and the lattice contribution κl [3]. High-performance TE materials are expected to possess low κ, and one of the effective solutions for high ZT is to search for alloy materials with intrinsic low κ values. On the other hand, the balance between σ and S is vital since these parameters are coupled and normally work in opposite directions. The electronic transport properties can be better described by the term “power factor PF”, defined as S2σ, and high PF is required [4]. Zintl compounds have complex crystal structures that often lead to intrinsic low lattice thermal conductivity, and their covalent bonding within the polyanionic framework allows maintenance of reasonable carrier mobility. These characteristics of Zintl compounds make them fit well into the concept of phonon–glass electron–crystal [5,6]. High ZT can be achieved by balancing the conflicting electron and phonon transports [7,8,9,10].
Among the Zintl phases, the typical 1-2-2 type Sb-based AB2Sb2 compounds (where A stands for Yb, Eu, Ca, Sr, and Ba; B stands for Cd, Zn, and Mg) have demonstrated promising TE performance in the 500–800 K temperature range [8,11]. Gascoin et al. reported a maximum ZT of 0.56 at 773 K for CaxYb1−xZn2Sb2 with x = 1 [8]. Later, Wang et al. obtained a peak ZT of 1.2 at 700 K for YbCd2−xZnxSb2 with x = 0.4 [11]. With a layered CaAl2Si2 structure, the 1-2-2 compounds consist of a layered covalently bonded polyanionic framework that enables high mobility for charge transport (“electron–crystal”) and weakly bonded cations that facilitate phonon scattering (“phonon–glass”) [5,6,12,13,14]. The ZT values of the pristine 1-2-2 phases are generally lower than 0.5, and enhanced TE performance with maximal ZT values above unity can be finely tuned and achieved by doping or alloying for the formation of solid solutions. Strategies for enhancing ZT include orbital engineering [15,16], band engineering [17,18,19], as well as point defect or microstructure engineering [20,21]. The analogous Bi-based 1-2-2 type Zintl phases have received less attention compared to Sb-based compounds. May et al. studied the TE properties of CaMg2Bi2, EuMg2Bi2, and YbMg2Bi2 between 2 and 650 K with the polycrystalline samples prepared by melting and hot pressing and found that YbMg2Bi2 exhibited the highest ZT value approaching 0.4 near 625 K [22]. Recently, Shuai et al. demonstrated that for samples synthesized using high-energy ball milling and hot pressing, a significantly enhanced ZT of approximately 1.3 can be achieved at 873 K for (Eu0.5Yb0.5)1−xCaxMg2Bi2 samples with x = 0.4 [18]. The authors suggest that the nanostructures as well as the alloying effect contribute to the high ZT. Shuai et al. found through composition optimization that YbMg2Bi1.98 with a minor Bi deficiency exhibits improved TE performance [23]. Subsequent studies have focused either on substitution at the cationic A2+ site of AMg2Bi2 Zintl compounds [16,17] or substitution at either site in the anionic framework [24,25]. It has been shown that the equivalent substitution of Zn for Cd or Mg has successfully led to a greater ZT in YbCd2−xZnxSb2 [11], CaMg2−xZnxBi1.98 [25], and YbMg2−xZnxSb2 [26] owing to the enhanced phonon scattering induced by point defects and/or orbital alignment. Co-doping of Zn at the Mg site and Li, Na, K [25], or Yb [27] at the Ca site in CaMg2Bi1.98 samples synthesized using ball milling and spark plasma sintering (SPS) were explored by Guo et al. It is reported that a competitive peak ZT of approximately 1.0 was achieved for Ca0.995Li0.005Mg1.9Zn0.1Bi1.98 at 873 K [25] and Ca0.65Yb0.35Mg1.9Zn0.1Bi1.98 at 773 K [27]. Guo et al. further studied the defect formation energy, band structure, phonon dispersion, and TE properties of CaMg2Bi2 with Zn doping at either the Ca or the Mg site experimentally and theoretically using density functional theory [16]. They revealed that Zn doping at the Ca site is more effective in increasing the carrier concentration and thus σ due to more remarkable lowering of the formation energy of Ca vacancy than that of Mg vacancy [16]. Based on the literature, we synthesized samples of YbMg2−xZnxBi1.98 with an actual composition of approximately YbMg1.85−xZnxBi1.98 using high-frequency induction melting followed by ball milling and SPS. The doping of Zn at the Mg site increases σ and lowers κl, and these effects are explained by the electronegativity and mass differences between Zn and Mg. However, the bipolar effect occurs at lower temperatures with increasing Zn concentrations, leading to a lower ZT. The overall effects of Zn partially replacing Mg alone on the PF and ZT are less satisfactory. The maximum amount of Zn doping is suggested to be less than x = 0.1.
2. Materials and Methods
Polycrystalline samples with a nominal composition of YbMg2−xZnxBi1.98 (x = 0, 0.2, 0.3, 0.4, 0.6) were prepared from high-purity granules of Yb (99.95%) from Xingtai Zhongyan, Beijing, China, and Mg (99.95%), Bi (99.99%), and Zn (99.995%) from Zhongnuo New Materials, Beijing, China. The starting elements were kept and weighed in an argon-filled glovebox where the levels of O2 and moisture were kept below 0.01 ppm. Then, the elements were placed in a corundum crucible covered with a lid. The reactants in the corundum crucibles were melted in an argon-filled quartz tube using high-frequency induction melting (Shanghai Renjun High Frequency Equipment, Shanghai, China). The melting process was controlled by adjusting the heating electric current and the heating time. The heating process started with a low current for a few minutes and then shifted to higher current holding for a few more minutes for thorough melting. In this work, the appropriate electric current and holding time were identified and adopted after several attempts to obtain single-phase samples. To provide homogeneous powder for sintering, the as-melted samples were powdered in an agate mortar and moved to a stainless-steel jar (with milling balls) filled with argon inside the glovebox. Ball millings of the samples were then carried out for 3 h in a planetary ball mill (QM-3SP4, Nanjing, China). In an argon-filled glovebox, the resulting powders were taken out of the stainless-steel jar and placed into a graphite die with carbon paper underneath to allow for easy demolding. The densifications of the samples with a diameter of 15 mm were then conducted at 873 K for 5 min using SPS (LABOX-225, SINTER LAND INC., Niigata-ken, Japan) in vacuum at an axial pressure of 50 MPa.
Powder X-ray diffraction (XRD) patterns were collected from 10° to 110° 2θ in increments of 0.02° in a step-scan mode with Cu Kα radiation (Rigaku D/Max 2500 V, Tokyo, Japan) for structure characterization. The XRD data were refined for quantitative phase analysis with the Rietveld method using the Fullprof program [28]. Field-emission scanning electron microscope (FE-SEM) (Hitachi SU8020, Tokyo, Japan) and energy-dispersive X-ray spectroscopy (EDS) (Oxford X-MAX80, Oxford, UK) were carried out to examine the microstructures and compositions of the SPS sintered samples. The microstructures were observed on polished and freshly fractured surfaces, separately. The chemical compositions of the samples and the phases were the average of at least three EDS point measurements. The samples were found to be Mg and Zn deficient. The actual sample compositions were measured to be YbMg1.85Bi2.05 (denoted as ~YbMg1.85−xZnxBi1.98 with x = 0 thereafter), YbMg1.75Zn0.05Bi1.94 (x = 0.05), YbMg1.77Zn0.08Bi1.93 (x = 0.08), YbMg1.69Zn0.13Bi1.94 (x = 0.13), and YbMg1.63Zn0.23Bi1.87 (x = 0.23) due to the evaporation of Mg and Zn during the melting process. Rectangular specimens for electronic transport measurements and disc specimens for thermal measurements were cut from the SPS sintered disks. Simultaneously measurements of σ and S were performed using an Advance Riko ZEM-3M10 (Yokohama, Japan) from room temperature up to 773 K under high-purity helium. The thermal diffusivity D measurements were conducted in a NETZSCH LFA 467 HyperFlash (Selb, Germany) under a nitrogen atmosphere employing a laser flash method. Experimental measurements of the specific heat capacity CP of partial samples were carried out using PtRh crucibles with Al2O3 lining under an argon atmosphere up to 803 K in a NETZSCH DSC 404C (Selb, Germany) according to the sapphire method. The measured CP values were compared with the calculated temperature-dependent CP values determined using the corrected Dulong–Petit law from Agne et al. [29] for each measured composition. The bulk densities d of the SPS sintered samples were measured using a METTLER TOLEDO AG285 (Shanghai, China) based on Archimedes’ principle. The thermal conductivity κ was then determined using the equation κ = DCpd.
3. Results and Discussion
3.1. Phase Analysis
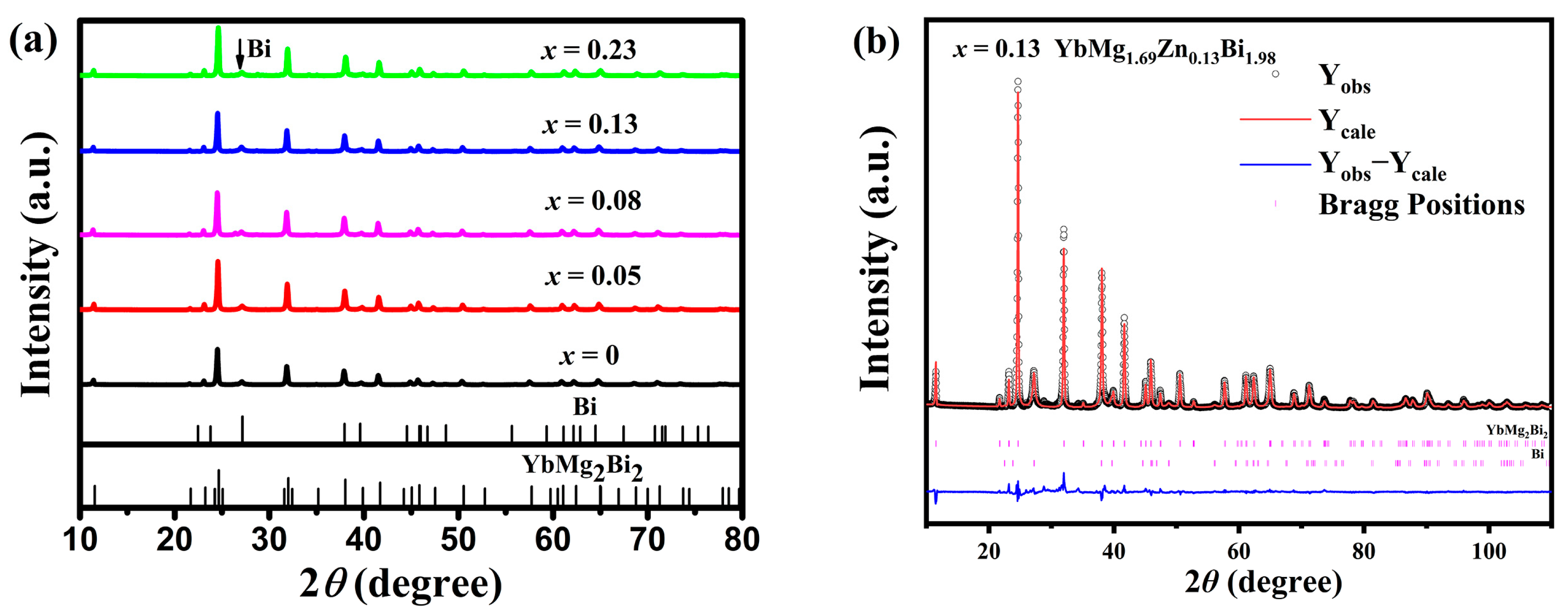
The room temperature XRD patterns of YbMg1.85−xZnxBi1.98 samples with x = 0, 0.05, 0.08, 0.13, and 0.23 are presented in Figure 1a. The majority of the diffraction peaks for all the samples indicate the YbMg2Bi2 phase, which is trigonal CaAl2Si2-type structure with space group (No. 164) [30,31]. A diffraction peak at ca. 27.1° 2θ is observed in all samples, as indicated by the arrow in Figure 1a, and is identified as the strongest peak of the Bi phase. Additional weak peaks at ca. 28.7 and 34.2° 2θ with a peak height less than two for samples with x ≥ 0.08 are detected, indicating the existence of other trace phases. Structure refinements for the 1-2-2 and Bi phases were carried out on the XRD patterns of the samples. For the 1-2-2 phase with a CaAl2Si2-type structure, the Mg/Zn atoms co-occupy the 2d site (1/3, 2/3, z), and their site occupancies (Occ. Mg/Zn) were fixed according to the EDS measured compositions. The occupancies of Mg and Zn on the 2d site are less than 1 for the Mg/Zn-deficient samples. Figure 1b shows the Rietveld refinement of the XRD pattern for the sample with x = 0.13. The structural parameters for the 1-2-2 phase, the amounts (m in wt. %) and the X-ray theoretical densities (ρ) for both the 1-2-2 and Bi phases derived from Rietveld refinement, the bulk densities (d) measured using Archimedes’ method, and the relative theoretical density (% T. D.) are listed in Table 1. It is seen that with more Zn doping, the unit cell parameters a and c and the volume V decrease slightly. This is reasonable in consideration of a covalent radii of 1.379 Å for Zn and 1.598 Å for Mg according to Ref. [32]. The amounts of the secondary phases obtained from the refinements are found to be around 10–17 wt. % of the samples, as further revealed by SEM and EDS.
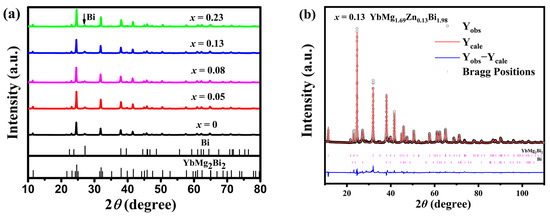
Figure 1.
(a) Powder XRD patterns of the YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, and 0.23) samples and (b) Rietveld refinement of the XRD pattern for the sample with x = 0.13.

Table 1.
Refined structural parameters for the 1-2-2 phase in YbMg1.85−xZnxBi1.98 samples. The 1-2-2 phase has a space group (No. 164) and the following atomic Wyckoff positions: Yb, 1a (0, 0, 0); Mg/Zn, 2d (1/3, 2/3, z); and Bi, 2d (1/3, 2/3, z).
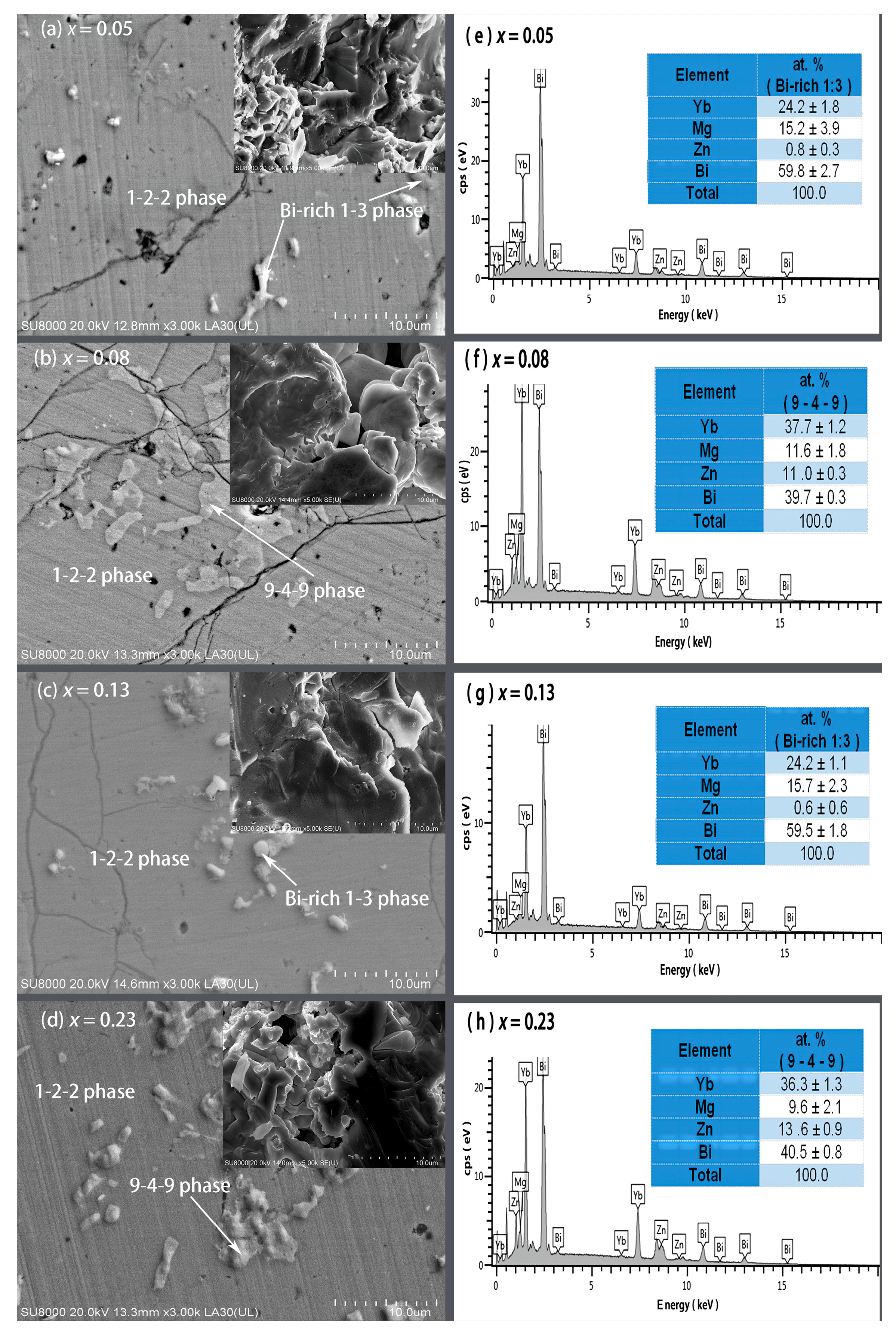
Figure 2 presents the FE-SEM images on polished and fractured surfaces and EDS spectra with average compositions of the secondary phases for the YbMg1.85−xZnxBi1.98 samples with x = 0.05, 0.08, 0.13, and 0.23. Based on the EDS compositional analysis on the polished surfaces, the matrix for all the samples are the 1-2-2 phase, and the secondary phases are revealed to be in the form of the Yb9(Mg, Zn)4.5−δBi9 phase (denoted as 9-4-9 phase) and Bi-rich phase (denoted as Bi-rich 1–3 phase) with a likely formula of Yb(Mg, Zn)0.5−0.7Bi2.3 (Figure 2e–h). The fractured morphology for the sample with x = 0.05 exhibits a lamellar dense structure, whereas pores and voids were observed in samples with x ≥ 0.08, which may be caused by the evaporation of Mg and Zn. The bulk densities (d) were measured to be 7.130, 7.197, 7.271, 7.314, and 7.159 g cm−3 for x = 0, 0.05, 0.08, 0.13, and 0.23, respectively. From the X-ray theoretical densities of the 1-2-2 phase ρ122 and the Bi phase ρBi, the theoretical densities of the samples were estimated using the relation , and the % T. D. was then obtained (Table 1). It is noted that the values of % T. D. exceed 98% except for the sample with x = 0. The sample with x = 0.05 has the highest % T. D., which is in good agreement with the observed fractured morphology.
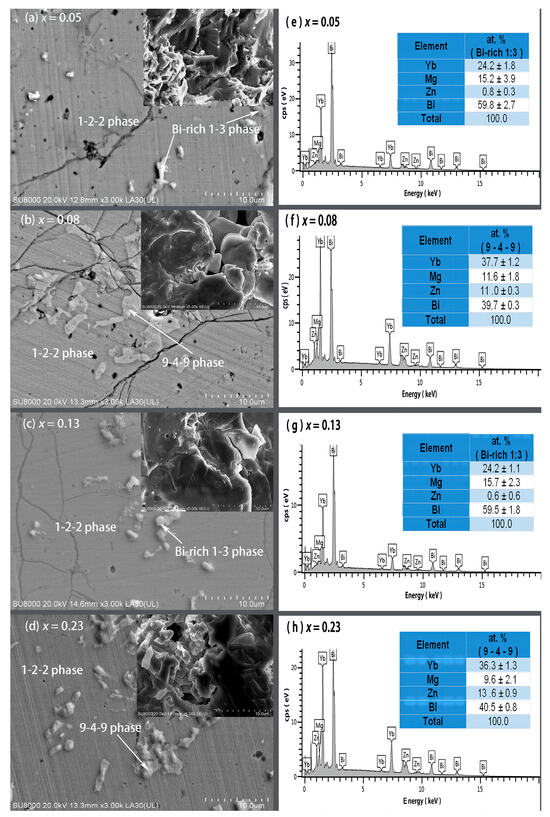
Figure 2.
FE-SEM images on the polished and fractured (inset) surfaces for YbMg1.85−xZnxBi1.98 with (a) x = 0.05, (b) x = 0.08, (c) x = 0.13, and (d) x = 0.23 and the corresponding EDS spectra of the secondary phases in YbMg1.85−xZnxBi1.98 with (e) x = 0.05, (f) x = 0.08, (g) x = 0.13, and (h) x = 0.23.
3.2. Electronic and Thermal Transport Properties
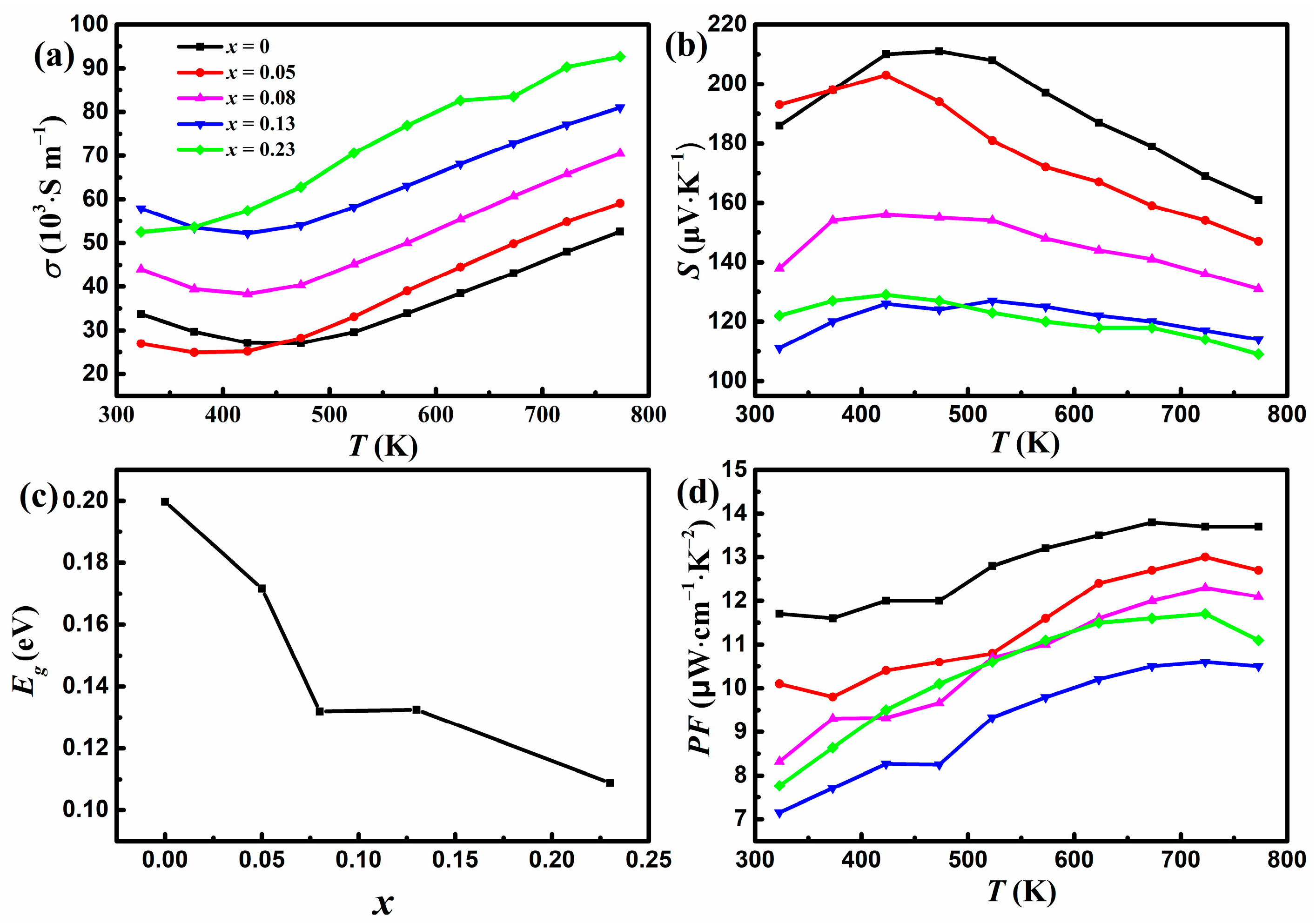
Figure 3a shows the variation of σ with temperature for YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23). The σ values for samples with x ≤ 0.13 exhibit an initial decrease until 423 K and then an upward trend as the temperature increases, whereas σ values for samples with x = 0.23 increase monotonously with temperature. Similar behaviors have been reported for Ca1−xYbxMg2Bi2 [23] and CaMg2−xZnxBi1.98 [25]. The initial decrease in σ indicates the typical behavior of degenerate semiconductors, and the increase in σ above 423 K exhibits the characteristics of intrinsic semiconductors. From the intrinsic region above 523 K, the band gap Eg can be estimated using Arrhenius law , where kB is Boltzmann’s constant. Values of 0.16, 0.16, 0.125, 0.09, and 0.07 eV were obtained for x = 0, 0.05, 0.08, 0.13, and 0.23, respectively. The Eg value of 0.16 eV for x = 0 is close to that of 0.18 eV for YbMg2Bi2 reported in Ref. [18]. The σ increases as more Zn replaces Mg, which can be understood by the electronegativity of the elements in the polyanionic framework. The electronegativities of Mg, Zn, and Bi are 1.31, 1.65, and 2.02, respectively. The introduction of Zn with greater electronegativity strengthens the covalent bond nature through the formation of Zn-Bi bonding in the polyanionic framework and thus enables good mobility of charge carriers, resulting in better electron transport [6].
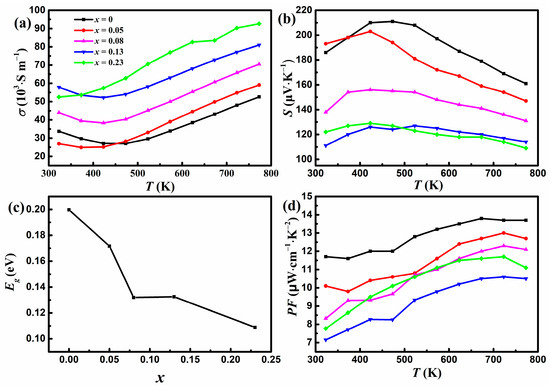
Figure 3.
Variations in (a) the electrical conductivity and (b) the Seebeck coefficient with temperature. (c) Variation in Eg with Zn concentration x. (d) Variation in the power factor with temperature for YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23).
Figure 3b shows that all the samples have positive values of S, indicating a p-type conductive behavior. The variations in σ and S for x = 0 are consistent with the results of YbMg2Bi2 in Ref. [23]. In accordance with σ, temperature-dependent S initially increases, exhibiting a degenerate semiconducting behavior, and then decreases with increasing temperature, showing a bipolar effect due to the intrinsic carrier excitation. The value of the maximum Seebeck coefficient Smax for x = 0 is 211 μV K−1 and drops to 129 μV K−1 for x = 0.23. The S vs. T curve with a peak Smax can be used to estimate the band gap Eg with the Goldsmid–Sharp formula Eg = 2e|S|maxTmax, where Tmax is the temperature corresponding to Smax and e is the electron charge [33]. A relationship between Eg and the concentration of doped Zn is shown in Figure 3c. It is found that although the Eg values calculated from the S vs. T curves are greater than those obtained from the σ vs. T curves, they show similar trends. Eg decreases with increasing Zn concentrations. The narrowing of Eg is in favor of the increase in charge carrier concentration and is therefore beneficial for σ. Meanwhile, narrow band gap semiconductors are prone to intrinsic carrier excitation. The narrowing of Eg, which coincides with the variations in σ and S with Zn concentrations, reveals that the occurrence temperature of the bipolar effect decreases with increasing Zn concentrations. The overall S under bipolar conduction can be expressed using the following formula [34]:
where subscripts e and h express electron and hole carriers, respectively. The decline in S with Zn concentration can be attributed to the competition between the positive contribution Sh from holes and the negative contribution Se from the electrons. As shown in Figure 3d, all the Zn-doped samples exhibit lower PF values than the undoped samples. The doping of Zn for Mg results in a decrease in PF up to x = 0.13 because the remarkable reduction of S predominates in the calculation of PF. The obtained maximum PF of 13.8 μW cm−1 K−2 for x = 0 at 673 K is close to the value reported for YbMg2Bi2 at 700 K [23].
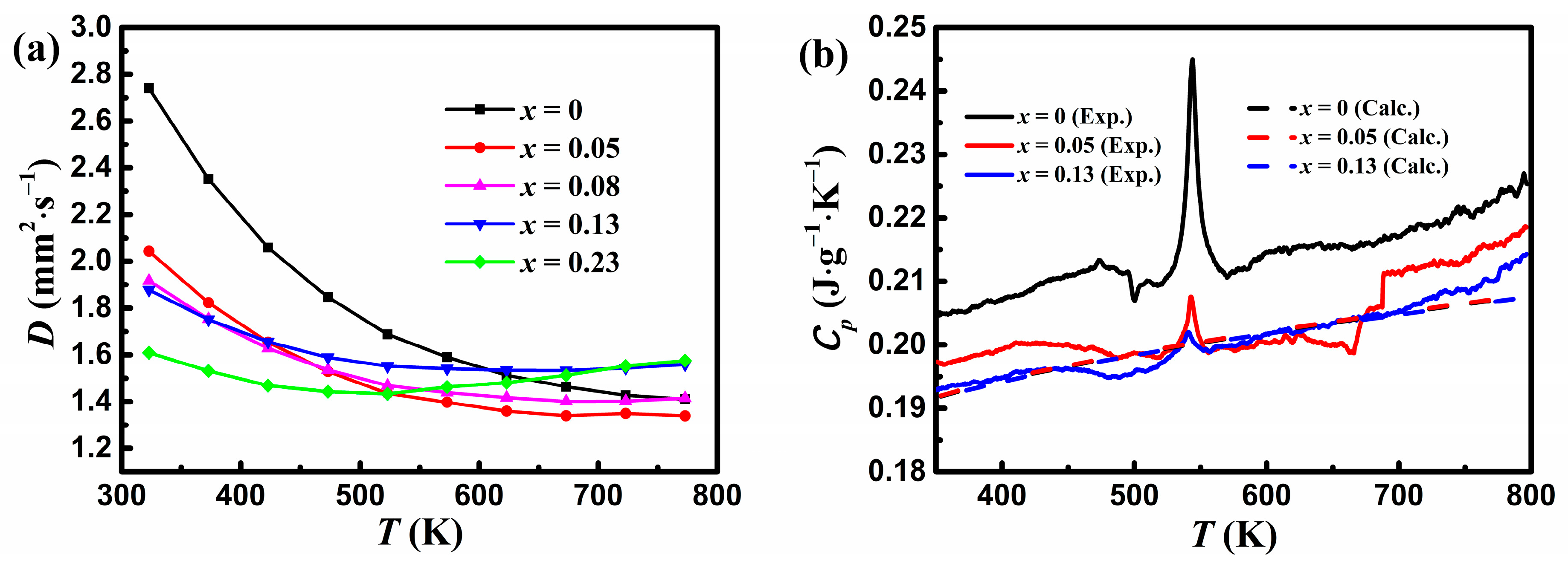
Figure 4a shows the variation in the thermal diffusivity D with temperature for YbMg2−xZnxBi1.98. It is seen that D for samples with x ≤ 0.08 decreases monotonously with increasing temperature, whereas D for samples with x ≥ 0.13 increases at higher temperatures, implying a bipolar diffusivity due to the decrease in the band gap. D for x = 0.23 shows the lowest value at 323 K but the highest value at 773 K. This phenomenon has been observed in Ref. [23]. It has been reported that a widening band gap and/or increasing carrier concentration by doping Eu or Yb at the Sm2+ site in SmMg2Bi2 [17], trace monovalent ion Ag+ at the Mg2+/Zn2+ sites in YbMg2−xZnxSb2 [26], or Sn at the Bi site in Yb0.8−xCaxMg0.2Mg2Bi1.96 [35] can lead to an increase in the temperature for the intrinsic carrier excitation, thus effectively resulting in suppressed bipolar diffusion.
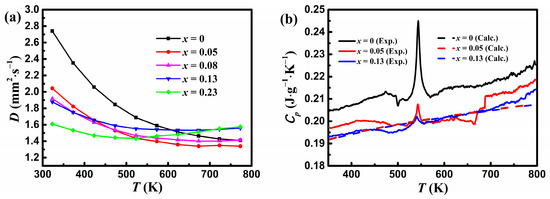
Figure 4.
Variations in (a) the thermal diffusivity for YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23) and (b) the measured and calculated specific heat capacity with temperature. The dashed lines represent the calculated Cp obtained from the corrected Dulong–Petit law in Agne et al. [29].
Figure 4b shows variations in the measured specific heat capacity with temperature for samples with x = 0, 0.05, and 0.13. A peak at ca. 544 K that corresponds to the melting point of Bi is assigned to the Bi phase, which is similar to that reported for YbMg2Bi2-based alloys in Ref. [23]. The experimental Cp decreases with Zn concentration as expected from the estimated values based on the Dulong–Petit limit. A Debye temperature of 309 K and a Cp value of 121 J mol−1 K−1 (i.e., 0.189 J g−1 K−1) at 300 K for YbMg2Bi2 were obtained from the measurements of Cp over the temperature range of 1.8–300 K [36]. As a comparison, the calculated CP values obtained using the corrected Dulong–Petit law from Agne et al. [29] for measured EDS compositions are also presented in Figure 4b. It is evident that the calculated CP is in good agreement with the experimental Cp. In view of the negligible effect of the minor variation of Cp values on the total κ, the calculated CP values are used in this paper to determine the total κ using the relationship κ = DCpd.
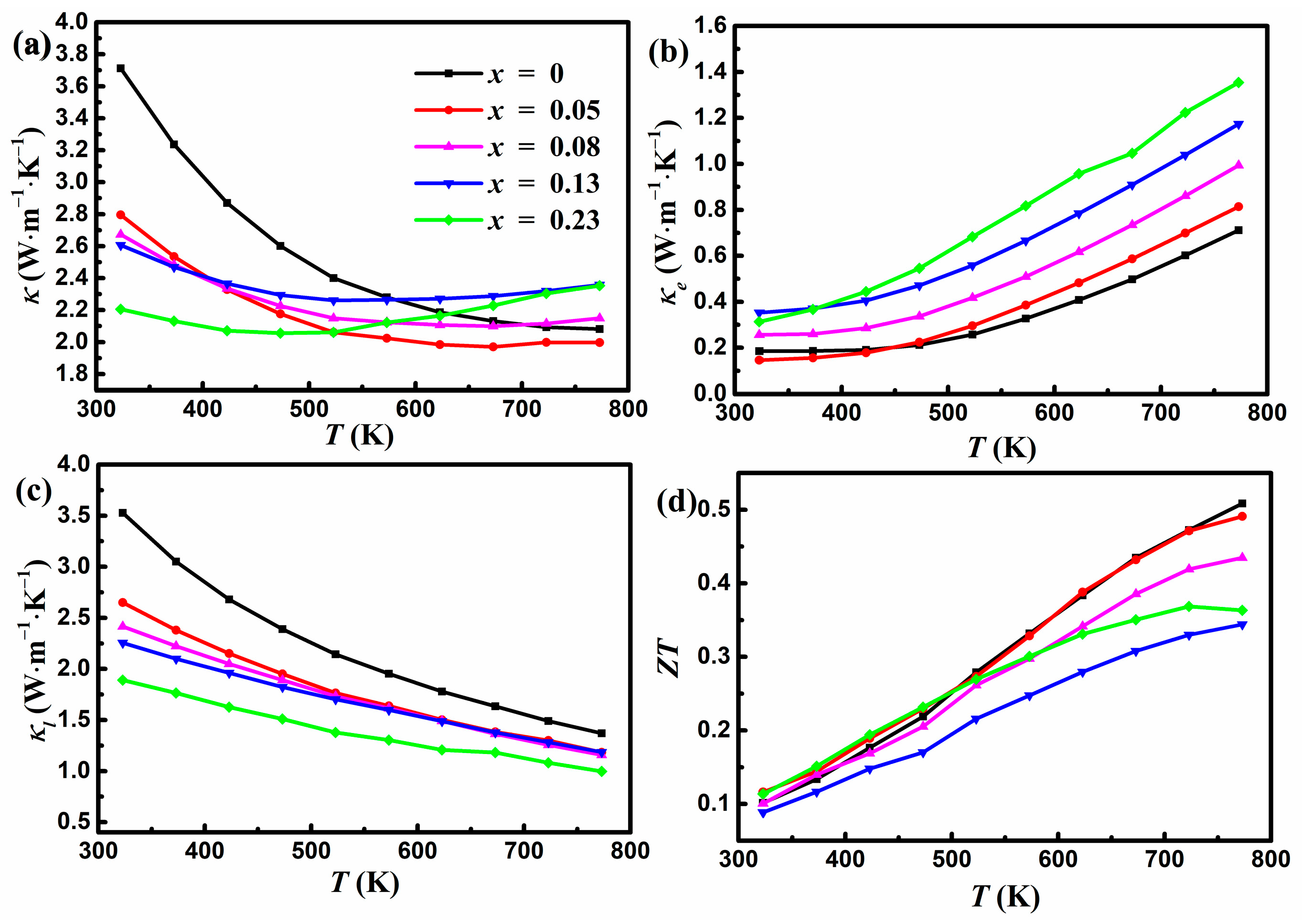
As shown in Figure 5a, the total κ exhibits a similar temperature-dependent tendency as D. κ at 323 K is reduced from 3.7 W m−1 K−1 for x = 0 to 2.2 W m−1 K−1 for x = 0.23, a reduction of about 41%. However, κ at 773 K increases slightly from 2.1 W m−1 K−1 for x = 0 to 2.4 W m−1 K−1 for x = 0.23, with a minimum κ of 2.0 W m−1 K−1 for x = 0.05. It is noted that the total κ of the undoped YbMg1.85Bi2.05 sample is slightly greater compared to those given in Ref. [23], where samples were prepared using high-energy ball milling and then hot pressing or SPS. This can be ascribed to the existence of coarse secondary phases in this work and no thorough ball milling to form the nanostructures. It is known that TE properties vary sensitively with different preparation methods [5]. Mechanical alloying effectively enhances boundary scattering and increases the densities of point defects and nanostructures, resulting in a reduced κ. Figure 5b presents the κe calculated using κe = LσT. The Lorentz number L can be estimated using the following equation [37]:
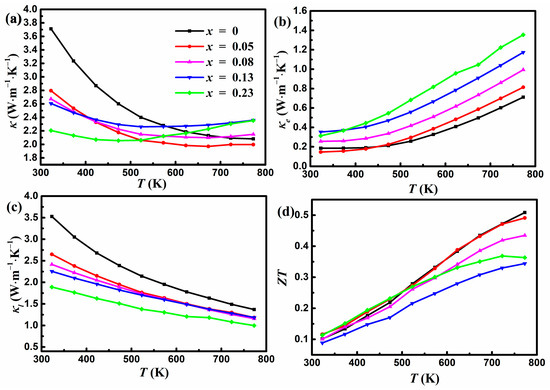
Figure 5.
Variations in (a) the total thermal conductivity, (b) the electronic thermal conductivity, (c) the lattice thermal conductivity, and (d) ZT with temperature for YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23).
In Equation (2), the unit of L is 10−8 W Ω K−2, and that of S is μV K−1. It is seen that κe increases with the increase in Zn concentration, which is consistent with the change in σ. The corresponding κL presented in Figure 5c was obtained by subtracting κe from the total κ. The reduction in κL with increasing Zn concentration can be ascribed to the enhanced phonon scattering owing to the mass field fluctuation and the disorder induced by the introduction of Zn at Mg sites. The undoped κL follows the T−1 law very well, indicating the dominant anharmonic Umklapp processes [38], while the doped samples deviate from the T−1 law with a significant drop near room temperature. This is ascribed to the contribution from point defect scattering induced by the addition of Zn content and can be understood using the Callaway model [18,39]. At higher temperatures, the contribution from the point defect scattering becomes smaller compared to that from the Umklapp scattering. In addition, the existence of pores also contributes to the reduction in κL. κL contributes to most of the total κ in the lower temperature regime, while κe increases with temperature and gradually becomes comparable to κL in the higher temperature regime. Figure 5d shows the variation of ZT as a function of temperature. The undoped sample shows a peak ZT of 0.51 at 773 K, which is consistent with the results of Ref. [22]. The Zn-doped sample with x = 0.05 exhibits nearly identical ZT to the undoped sample, yielding a maximum ZT of 0.49. The doping of Zn for Mg within the polyanionic double-layers could not optimize the TE performance due to the reduced PF and the increased κ at higher temperatures in the Zn-doped samples. The values of σ increase and values of κL decrease with increasing x in YbMg1.85−xZnxBi1.98, which is beneficial to ZT. However, Zn doping also significantly reduces S and causes an increase in κe and a bipolar effect, which is detrimental to the PF and κ, consequently leading to a lower ZT.
4. Conclusions
A series of Zn-doped YbMg1.85−xZnxBi1.98 (x = 0, 0.05, 0.08, 0.13, 0.23) Zintl alloys have been prepared using high-frequency induction melting, ball milling, and SPS. XRD and SEM/EDS analysis of all the samples revealed that the matrix of the alloys belongs to the trigonal system with a (164) space group and a CaAl2Si2-type structure. The secondary phases are Yb9(Mg, Zn)4.5−δBi9 phases, and/or Bi and Bi-rich 1–3 phases. With increasing Zn concentration in p-type YbMg1.85−xZnxBi1.98, σ increases due to the electronegativity difference between Zn and Mg, and κl decreases with increasing phonon scattering induced by more point defects together with the pores. However, the band gap decreases as more Zn is added, and bipolar conduction occurs, leading to a substantial decrease in S and therefore PF. The values of κ for samples with x ≥ 0.08 increase with temperature in the higher temperature regime, resulting in lower ZT values. The samples with x = 0 and 0.05 reach maximum ZT values of 0.51 and 0.49 at 773 K, respectively. It is suggested that the maximum amount of Zn doping is x ≤ 0.1, and synergistic optimization by co-doping at either site of the AMg2Bi2 Zintl compounds is essential for improving TE performance.
Author Contributions
S.W.: methodology, investigation, formal analysis, and writing original draft; N.Q.: investigation and formal analysis; G.W.: methodology; Z.X. and L.M.: resources; X.C.: resources and funding acquisition; J.Y.: writing—review and editing, supervision, and resources and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Nos. 51961006 and 52062002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- d’Angelo, M.; Galassi, C.; Lecis, N. Thermoelectric Materials and Applications: A Review. Energies 2023, 16, 6409. [Google Scholar] [CrossRef]
- Mukherjee, M.; Srivastava, A.; Singh, A.K. Recent advances in designing thermoelectric materials. J. Mater. Chem. C 2022, 10, 12524–12555. [Google Scholar] [CrossRef]
- Dadhich, A.; Saminathan, M.; Kumari, K.; Perumal, S.; Rao, M.S.R.; Sethupathi, K. Physics and technology of thermoelectric materials and devices. J. Phys. D Appl. Phys. 2023, 56, 333001. [Google Scholar] [CrossRef]
- Wolf, M.; Hinterding, R.; Feldhoff, A. High Power Factor vs. High zT-A Review of Thermoelectric Materials for High-Temperature Application. Entropy 2019, 21, 1058. [Google Scholar] [CrossRef]
- Shuai, J.; Mao, J.; Song, S.; Zhang, Q.; Chen, G.; Ren, Z. Recent progress and future challenges on thermoelectric Zintl materials. Mater. Today Phys. 2017, 1, 74–95. [Google Scholar] [CrossRef]
- Kauzlarich, S.M.; Brown, S.R.; Snyder, G.J. Zintl phases for thermoelectric devices. Dalton Trans. 2007, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chanakian, S.; Zevalkink, A. Crystal chemistry and thermoelectric transport of layered AM2X2 compounds. Inorg. Chem. Front. 2018, 5, 1744–1759. [Google Scholar] [CrossRef]
- Gascoin, F.; Ottensmann, S.; Stark, D.; Haïle, S.M.; Snyder, G.J. Zintl Phases as Thermoelectric Materials: Tuned Transport Properties of the Compounds CaxYb1−xZn2Sb2. Adv. Funct. Mater. 2005, 15, 1860–1864. [Google Scholar] [CrossRef]
- Zeier, W.G.; Zevalkink, A.; Gibbs, Z.M.; Hautier, G.; Kanatzidis, M.G.; Snyder, G.J. Thinking Like a Chemist: Intuition in Thermoelectric Materials. Angew. Chem. Int. Ed. 2016, 55, 6826–6841. [Google Scholar] [CrossRef]
- Imasato, K.; Wood, M.; Anand, S.; Kuo, J.J.; Snyder, G.J. Understanding the High Thermoelectric Performance of Mg3Sb2-Mg3Bi2 Alloys. Adv. Energy Sustain. Res. 2022, 3, 2100208. [Google Scholar] [CrossRef]
- Wang, X.-J.; Tang, M.-B.; Chen, H.-H.; Yang, X.-X.; Zhao, J.-T.; Burkhardt, U.; Grin, Y. Synthesis and high thermoelectric efficiency of Zintl phase YbCd2−xZnxSb2. Appl. Phys. Lett. 2009, 94, 092106. [Google Scholar] [CrossRef]
- Tiadi, M.; Trivedi, V.; Kumar, S.; Jain, P.K.; Yadav, S.K.; Gopalan, R.; Satapathy, D.K.; Battabyal, M. Enhanced Thermoelectric Efficiency in P-Type Mg3Sb2: Role of Monovalent Atoms Codoping at Mg sites. ACS Appl. Mater. Interfaces 2023, 15, 20175–20190. [Google Scholar] [CrossRef] [PubMed]
- Takagiwa, Y.; Sato, Y.; Zevalkink, A.; Kanazawa, I.; Kimura, K.; Isoda, Y.; Shinohara, Y. Thermoelectric properties of EuZn2Sb2 Zintl compounds: zT enhancement through Yb substitution for Eu. J. Alloys Compd. 2017, 703, 73–79. [Google Scholar] [CrossRef]
- Beretta, D.; Neophytou, N.; Hodges, J.M.; Kanatzidis, M.G.; Narducci, D.; Martin-Gonzalez, M.; Beekman, M.; Balke, B.; Cerretti, G.; Tremel, W.; et al. Thermoelectrics: From history, a window to the future. Mater. Sci. Eng. R 2019, 138, 100501. [Google Scholar] [CrossRef]
- Wood, M.; Aydemir, U.; Ohno, S.; Snyder, G.J. Observation of valence band crossing: The thermoelectric properties of CaZn2Sb2–CaMg2Sb2 solid solution. J. Mater. Chem. A 2018, 6, 9437–9444. [Google Scholar] [CrossRef]
- Guo, M.; Liu, M.; Zhu, J.; Zhu, Y.; Guo, F.; Cai, W.; Zhang, Y.; Zhang, Q.; Sui, J. Mechanism of Thermoelectric Performance Enhancement in CaMg2Bi2-Based Materials with Different Cation Site Doping. Small 2024, 20, 2306251. [Google Scholar] [CrossRef]
- Saparamadu, U.; Tan, X.; Sun, J.; Ren, Z.; Song, S.; Singh, D.J.; Shuai, J.; Jiang, J.; Ren, Z. Achieving high-performance p-type SmMg2Bi2 thermoelectric materials through band engineering and alloying effects. J. Mater. Chem. A 2020, 8, 15760–15766. [Google Scholar] [CrossRef]
- Shuai, J.; Geng, H.; Lan, Y.; Zhu, Z.; Wang, C.; Liu, Z.; Bao, J.; Chu, C.W.; Sui, J.; Ren, Z. Higher thermoelectric performance of Zintl phases (Eu0.5Yb0.5)1−xCaxMg2Bi2 by band engineering and strain fluctuation. Proc. Natl. Acad. Sci. USA 2016, 113, E4125–E4132. [Google Scholar] [CrossRef]
- Imasato, K.; Kang, S.D.; Ohno, S.; Snyder, G.J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2 for enhanced thermoelectric performance. Mater. Horiz. 2018, 5, 59–64. [Google Scholar] [CrossRef]
- Zhou, T.; Song, J.; Lei, X.; Zhang, Q.; Bi, J.; Gao, D.; Jiang, J.; Wang, C. Achieving n-type conduction in YbMg2Sb2-based compounds through defect engineering and doping. Acta Mater. 2022, 223, 117467. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Zhang, Y.; Guo, L.; Yang, J.; Luo, S.; Lu, X.; Chen, K.; Chai, H.; Wang, G.; et al. Enhanced thermoelectric properties of YbZn2Sb2−xBix through a synergistic effect via Bi-doping. Chem. Eng. J. 2019, 374, 589–595. [Google Scholar] [CrossRef]
- May, A.F.; McGuire, M.A.; Singh, D.J.; Ma, J.; Delaire, O.; Huq, A.; Cai, W.; Wang, H. Thermoelectric transport properties of CaMg2Bi2, EuMg2Bi2, and YbMg2Bi2. Phys. Rev. B 2012, 85, 035202. [Google Scholar] [CrossRef]
- Shuai, J.; Liu, Z.; Kim, H.S.; Wang, Y.; Mao, J.; He, R.; Sui, J.; Ren, Z. Thermoelectric properties of Bi-based Zintl compounds Ca1−xYbxMg2Bi2. J. Mater. Chem. A 2016, 4, 4312–4320. [Google Scholar] [CrossRef]
- Liang, Z.; Shang, H.; Xu, C.; Shi, X.; Zhang, F.; Ren, W.; Song, S.; Ding, F.; Ren, Z. New insights into the effect of chemical bonding strength on thermoelectric performance and stability in YbMg2Bi2 toward practical thermoelectric applications. Mater. Today Phys. 2022, 28, 100858. [Google Scholar] [CrossRef]
- Guo, M.; Guo, F.; Zhu, J.; Yin, L.; Qin, H.; Zhang, Q.; Cai, W.; Sui, J. Enhanced Thermoelectric Properties of p-Type CaMg2Bi2 via a Synergistic Effect Originated from Zn and Alkali-Metal Co-doping. ACS Appl. Mater. Interfaces 2020, 12, 6015–6021. [Google Scholar] [CrossRef]
- Yang, X.; Gu, Y.; Li, Y.; Guo, K.; Zhang, J.; Zhao, J.-T. The equivalent and aliovalent dopants boosting the thermoelectric properties of YbMg2Sb2. Sci. China Mater. 2020, 63, 437–443. [Google Scholar] [CrossRef]
- Guo, M.; Zhu, J.; Guo, F.; Zhang, Q.; Cai, W.; Sui, J. Enhanced thermoelectric performance of P-type CaMg2Bi1.98 and optimized CaAl2Si2-type Zintl phase module with equal cross-section area. Mater. Today Phys. 2020, 15, 100270. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Agne, M.T.; Imasato, K.; Anand, S.; Lee, K.; Bux, S.K.; Zevalkink, A.; Rettie, A.J.E.; Chung, D.Y.; Kanatzidis, M.G.; Snyder, G.J. Heat capacity of Mg3Sb2, Mg3Bi2, and their alloys at high temperature. Mater. Today Phys. 2018, 6, 83–88. [Google Scholar] [CrossRef]
- May, A.F.; McGuire, M.A.; Singh, D.J.; Custelcean, R.; Jellison, G.E., Jr. Structure and properties of single crystalline CaMg2Bi2, EuMg2Bi2, and YbMg2Bi2. Inorg. Chem. 2011, 50, 11127–11133. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.; Gallagher, A.; Baumbach, R.; Siegrist, T. Synthesis and characterization of the divalent samarium Zintl-phases SmMg2Bi2 and SmMg2Sb2. J. Solid State Chem. 2015, 231, 217–222. [Google Scholar] [CrossRef]
- Pauling, L. Atomic Radii and Interatomic Distances in Metals. J. Am. Chem. Soc. 1947, 69, 542–553. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Bardeen, J.; Shockley, W. Deformation Potentials and Mobilities in Non-Polar Crystals. Phys. Rev. 1950, 80, 72–80. [Google Scholar] [CrossRef]
- Zhou, T.; Jiang, J.; Wang, C. Enhancement of thermoelectric performance of YbMg2Bi2-based materials by alloying and doping with suppressed bipolar effect. IOP Conf. Ser. Earth Environ. Sci. 2020, 467, 012023. [Google Scholar] [CrossRef]
- Pakhira, S.; Tanatar, M.A.; Johnston, D.C. Magnetic, thermal, and electronic-transport properties of EuMg2Bi2 single crystals. Phys. Rev. B 2020, 101, 214407. [Google Scholar] [CrossRef]
- Kim, H.-S.; Gibbs, Z.M.; Tang, Y.; Wang, H.; Snyder, G.J. Characterization of Lorenz number with Seebeck coefficient measurement. APL Mater. 2015, 3, 041506. [Google Scholar] [CrossRef]
- Morelli, D.T.; Jovovic, V.; Heremans, J.P. Intrinsically minimal thermal conductivity in cubic I-V-VI2 semiconductors. Phys. Rev. Lett. 2008, 101, 035901. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J. Model for Lattice Thermal Conductivity at Low Temperatures. Phys. Rev. 1959, 113, 1046–1051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).