Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys
Abstract
1. Introduction
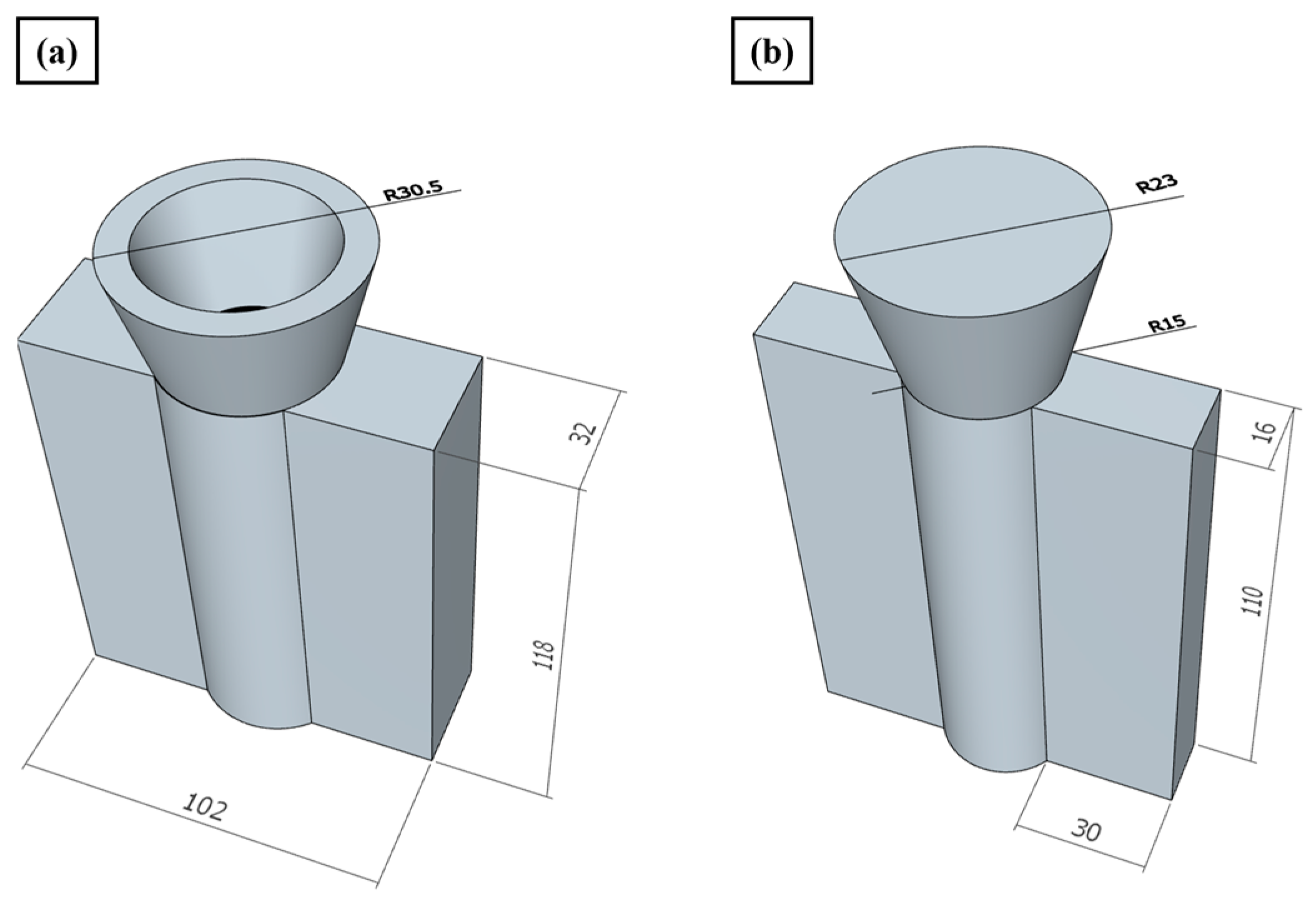
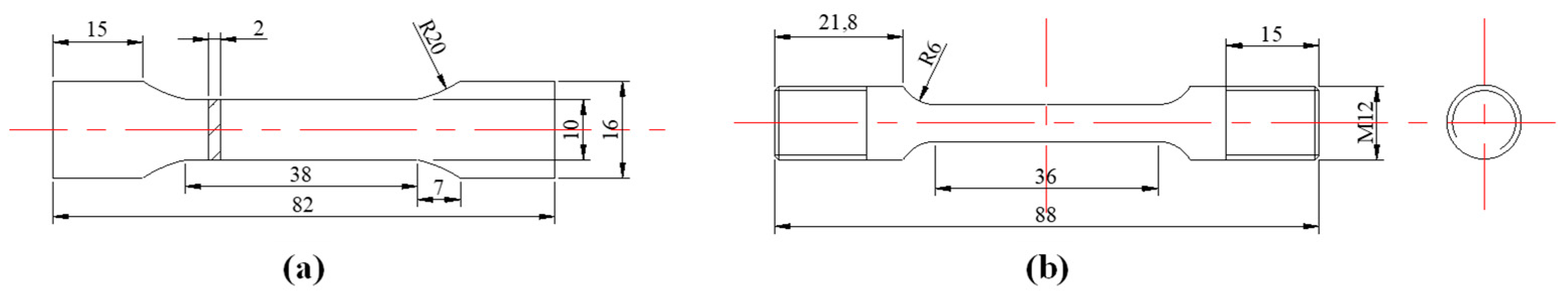
2. Materials and Methods
3. Results and Discussion
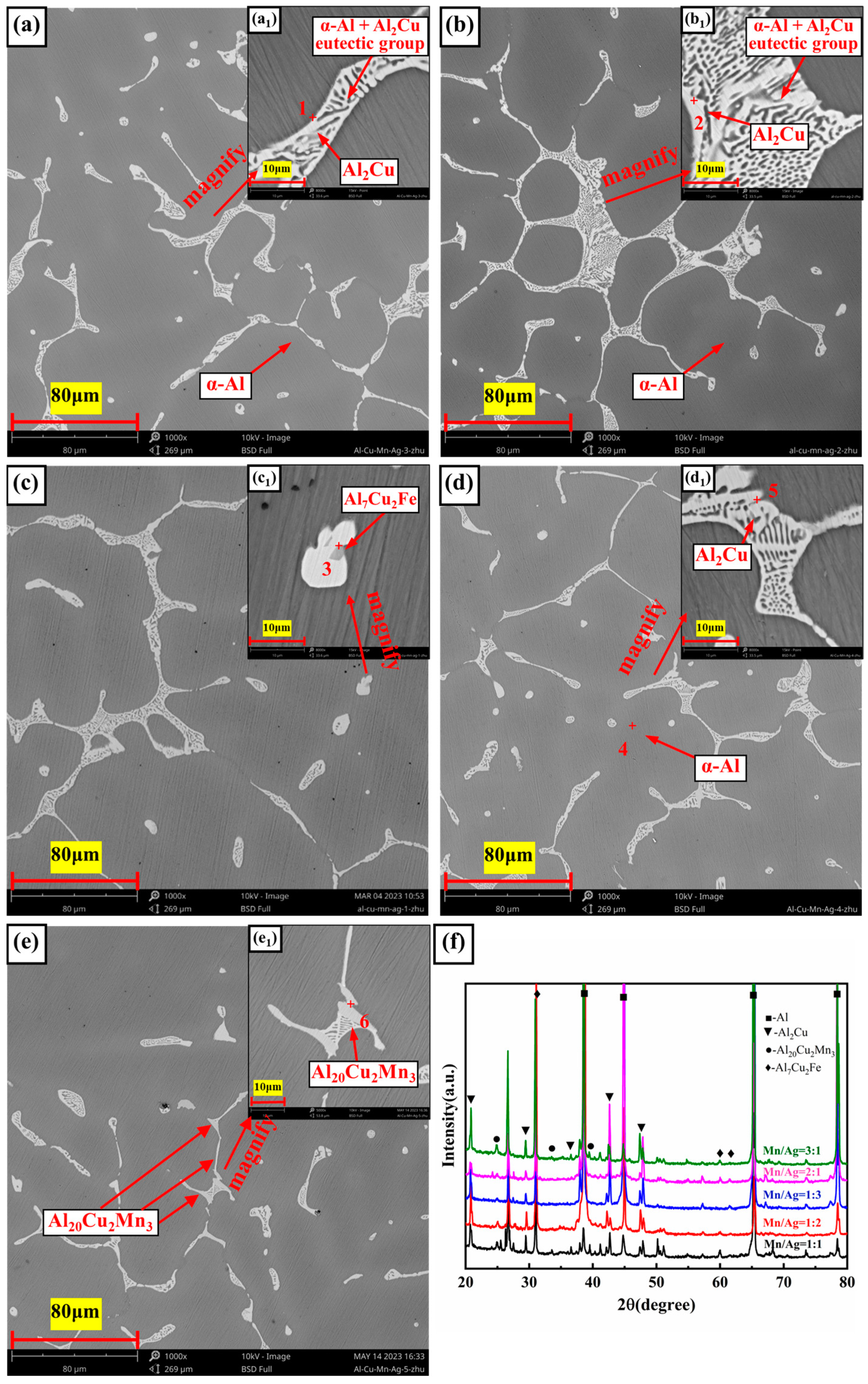
3.1. Microstructure Analysis
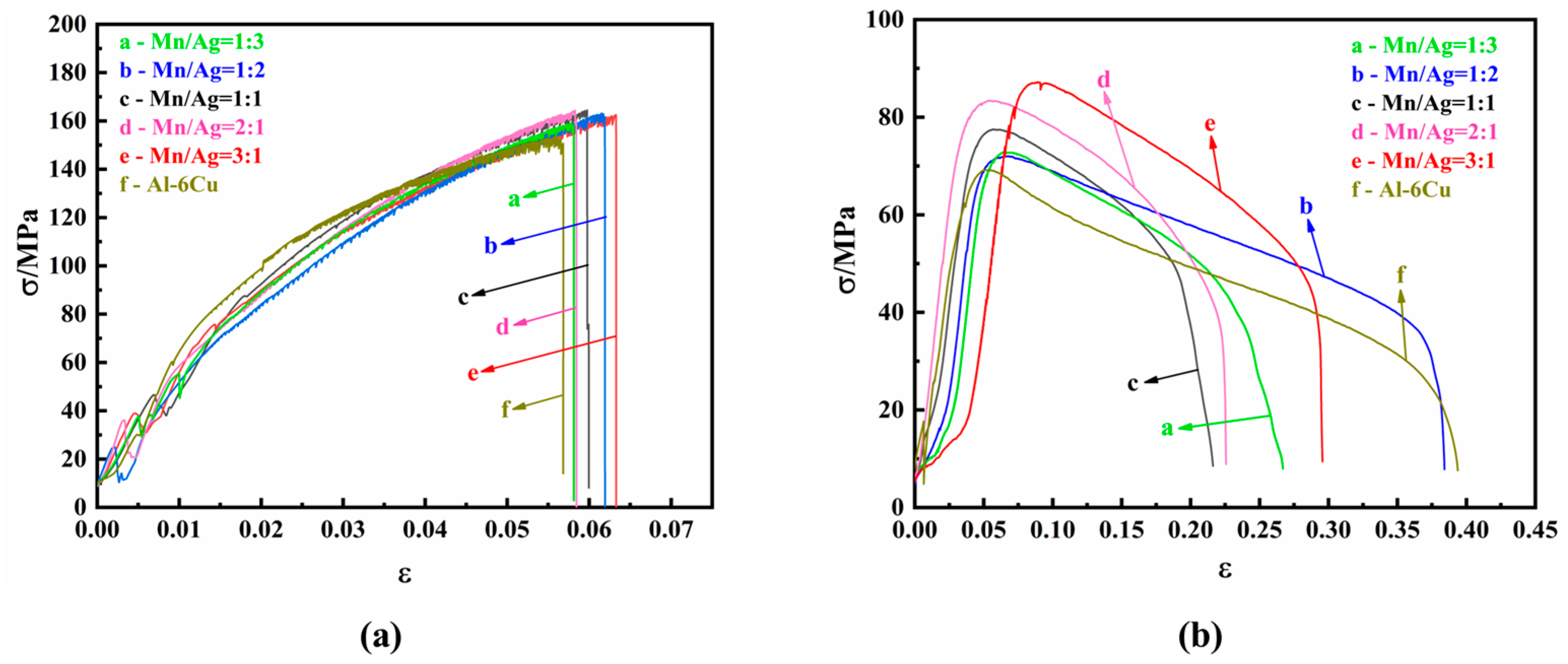
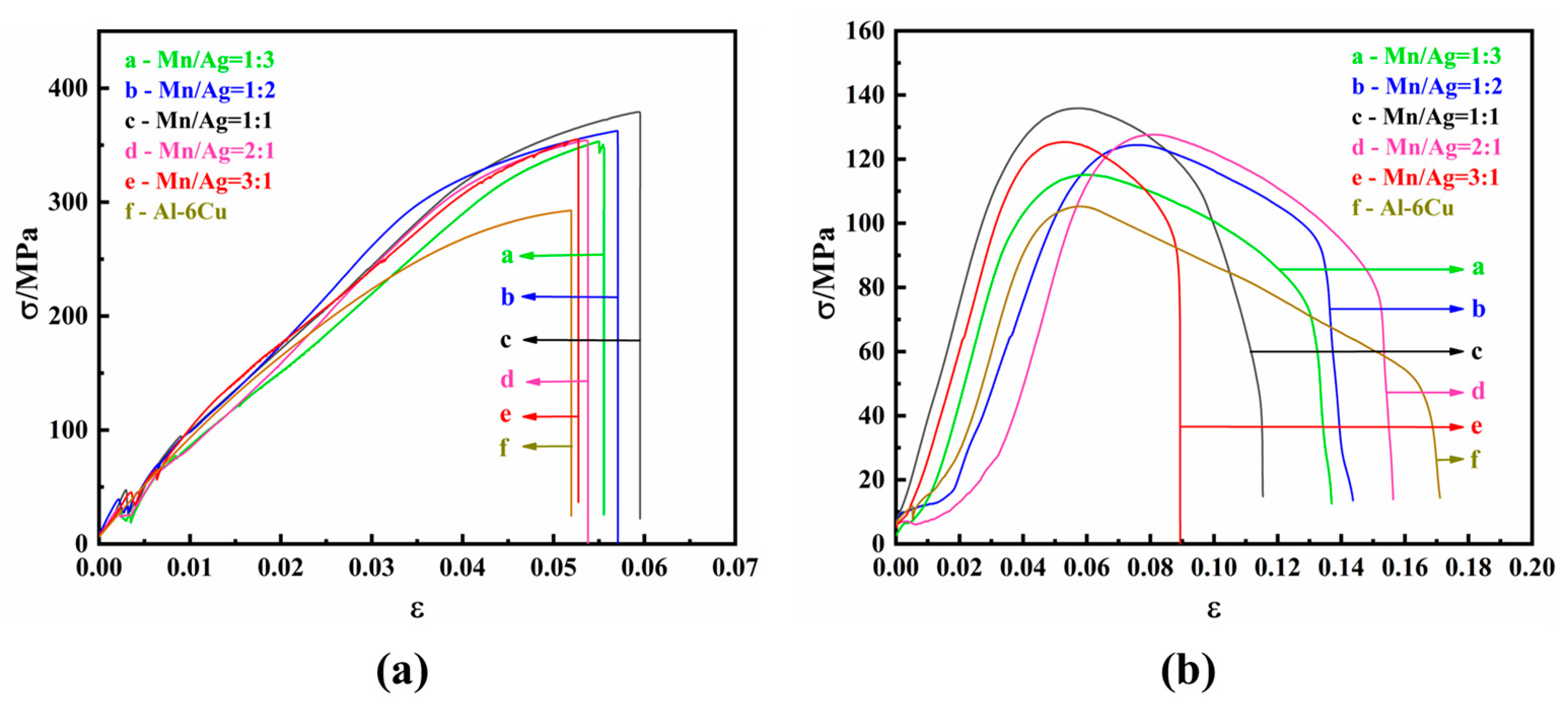
3.2. Tensile Properties of as-Cast Al-Cu-xMn-yAg Alloys
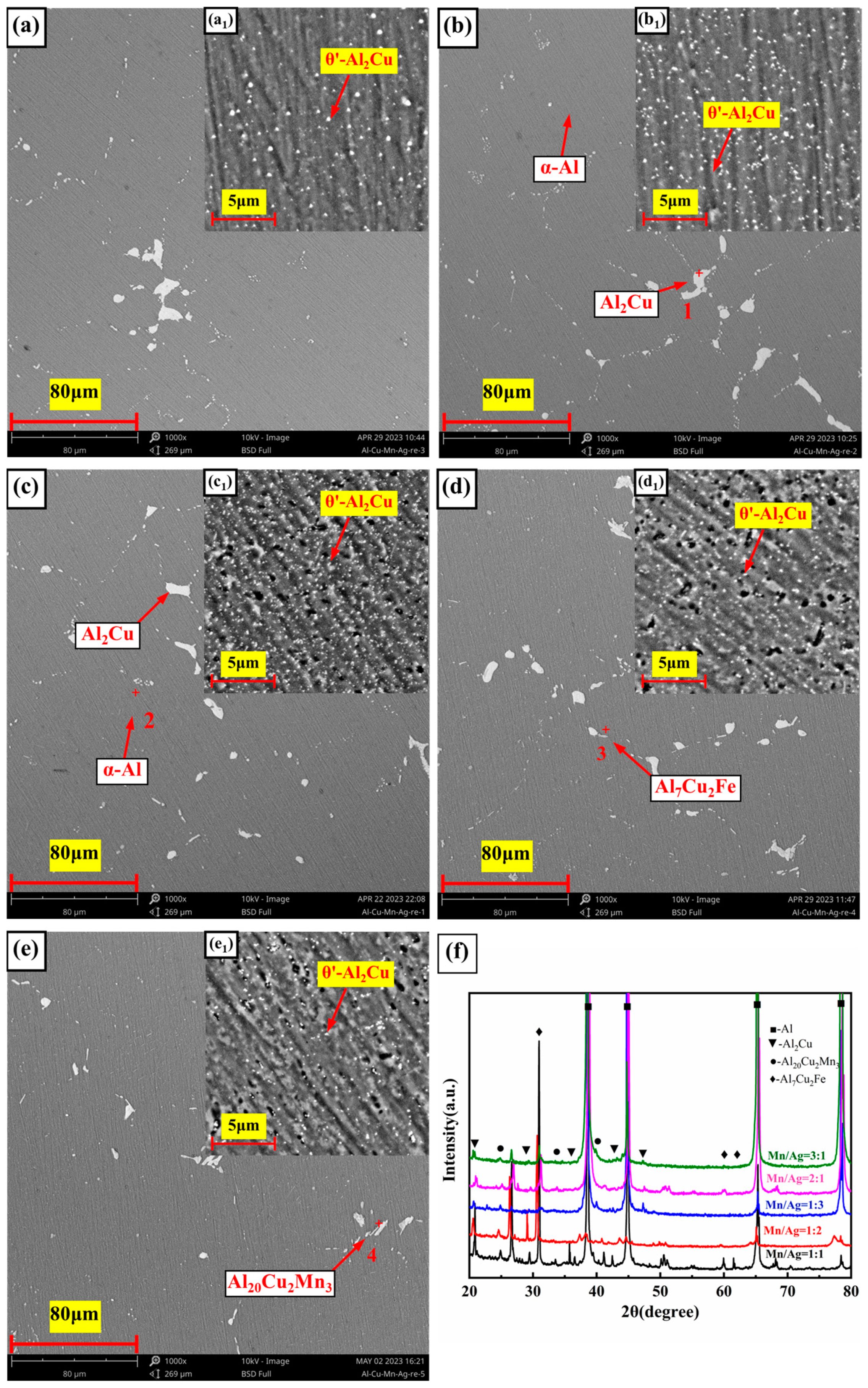
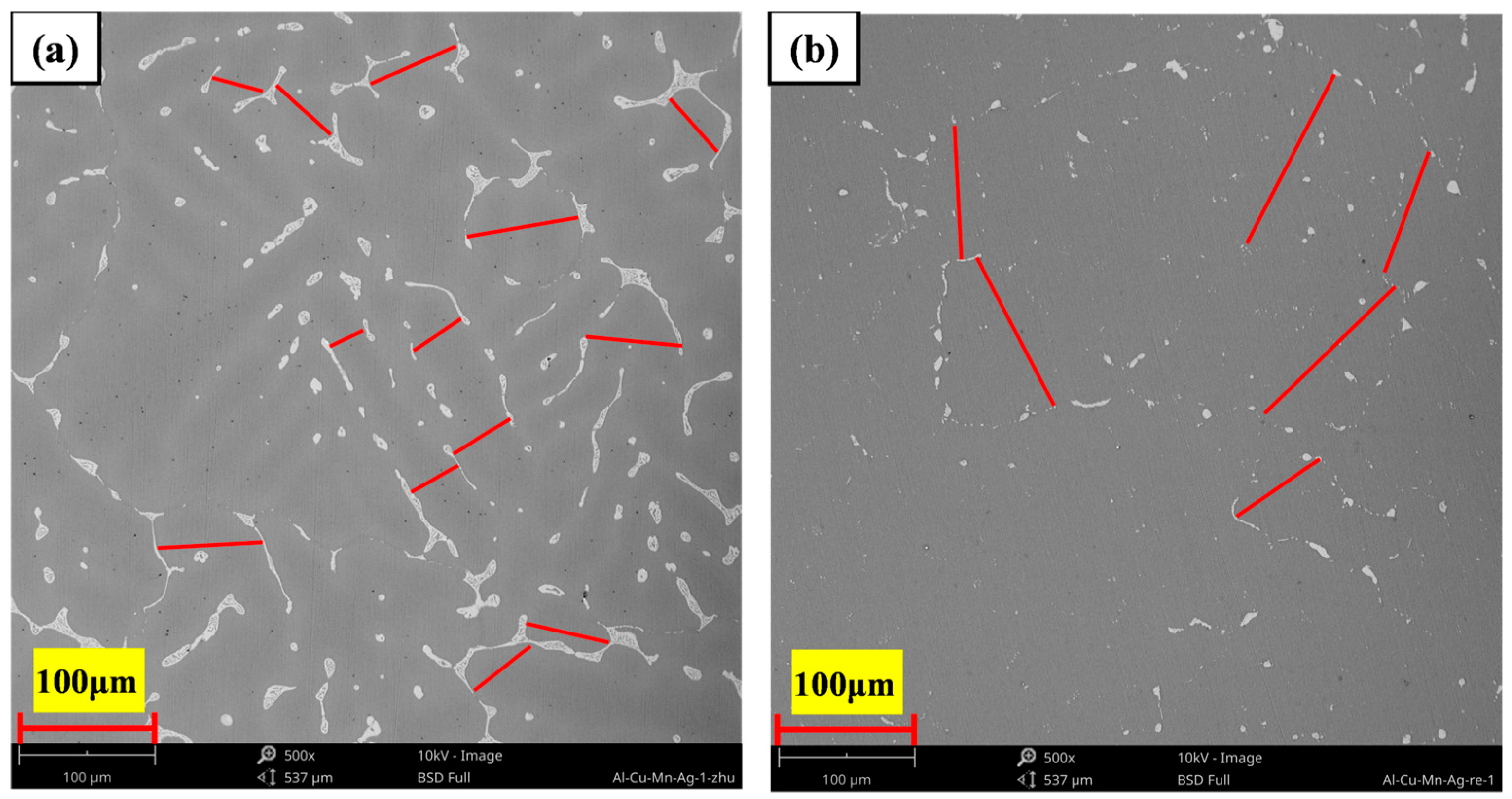
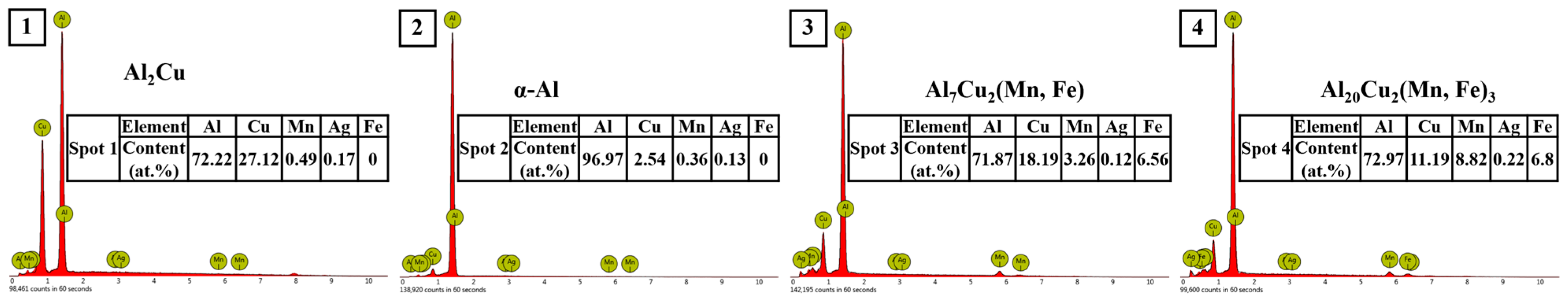
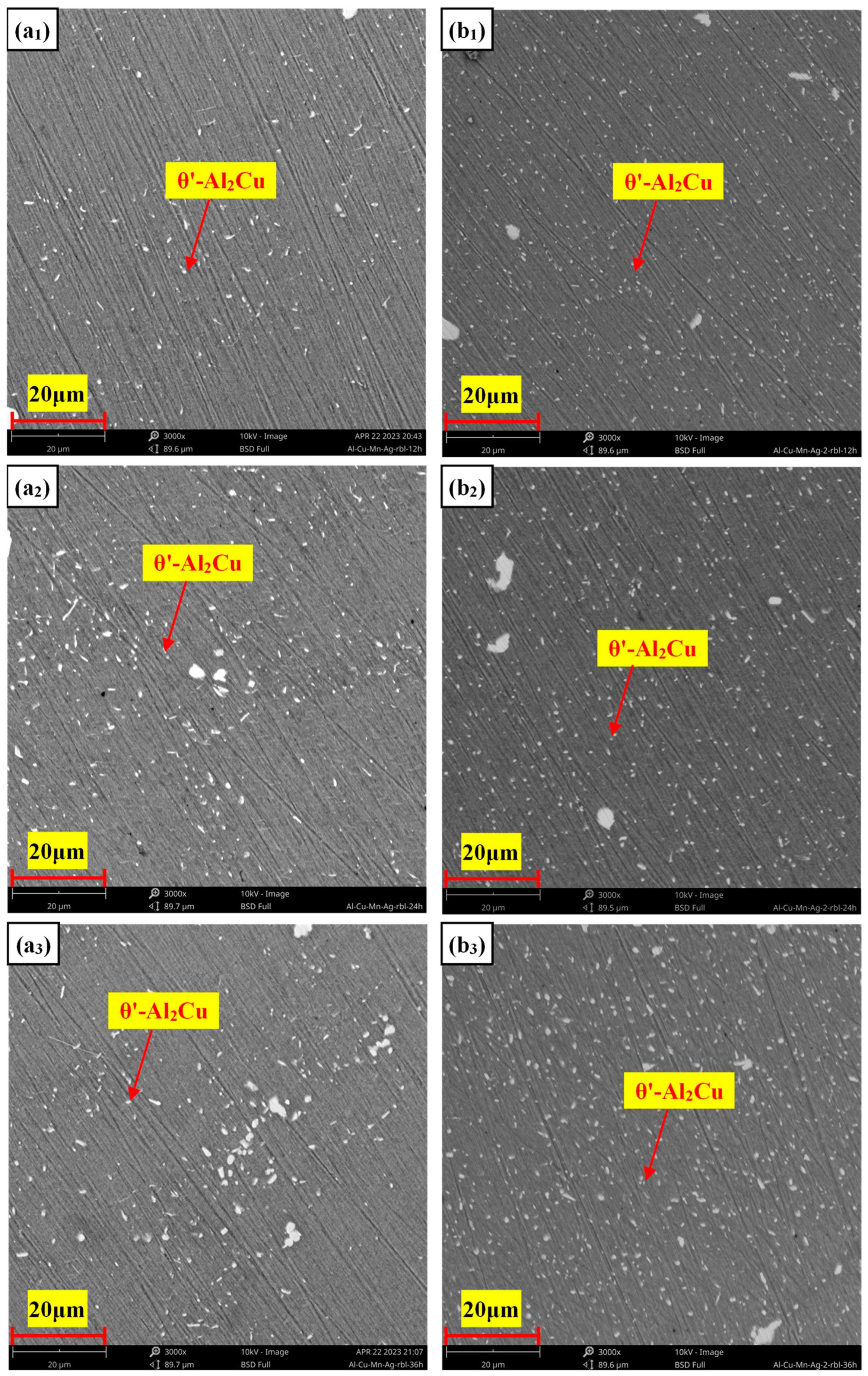
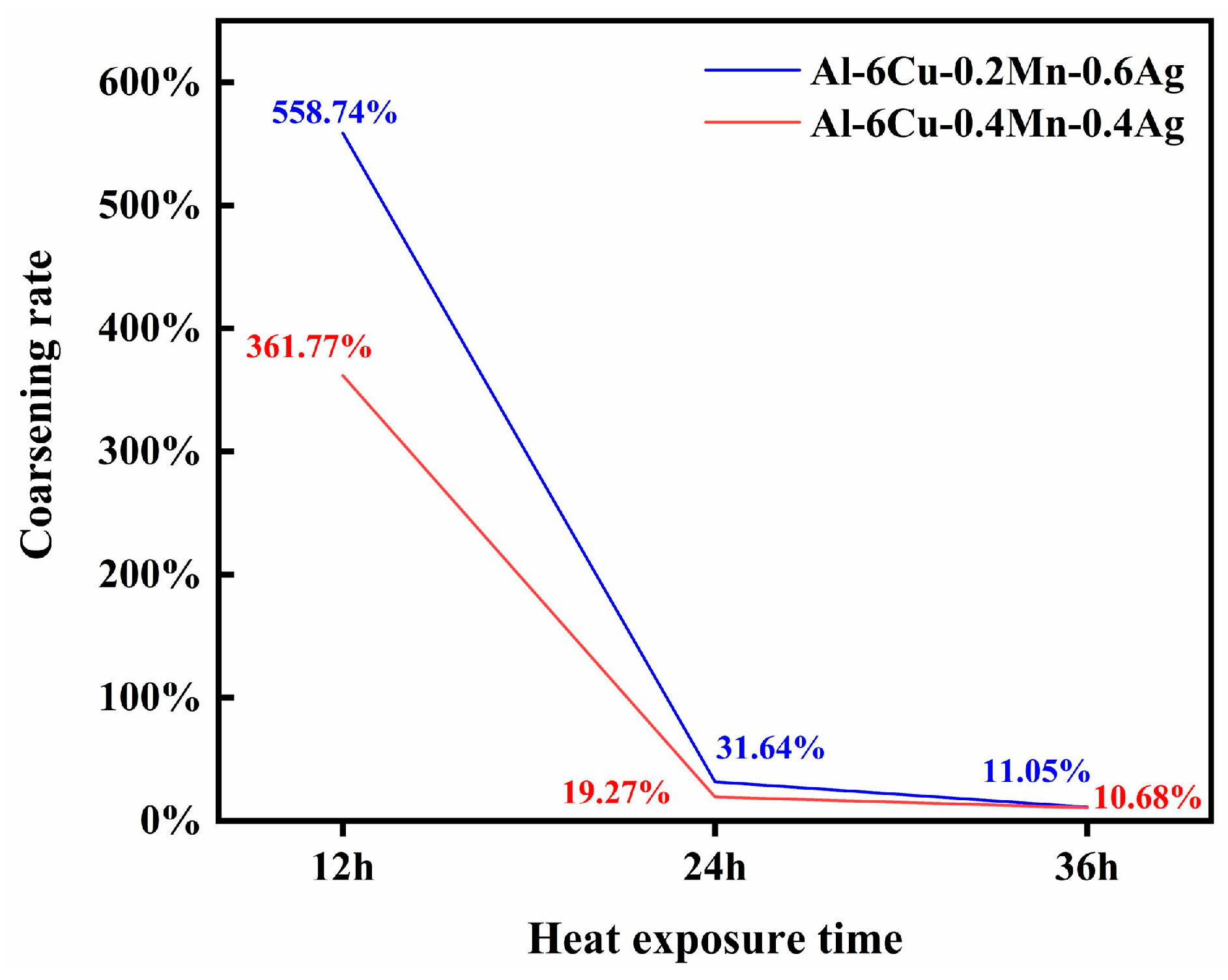
3.3. Microstructure of the Heat-Treated Alloy
3.4. Tensile Properties of the Heat-Treated Alloys
| Materials Composition (wt.%) | Temperature (°C) | σUTS (MPa) | Year | Ref. |
|---|---|---|---|---|
| Al-6Cu-0.4Mn-0.4Ag | 350 | 135.89 | 2024 | Present work |
| Al-5Cu-1.5Ni-0V | 350 | 92.06 | 2023 | [55] |
| Al-5Cu-1.5Ni-0.3V | 350 | 111.81 | 2023 | [55] |
| Al-6.5Cu-2Ni-0.5Zr-0.3Ti-0.25V | 350 | 127.5 | 2023 | [60] |
| Al-6Cu-2Ni-0.5V | 350 | 123.4 | 2023 | [57] |
| 9 wt.%Al3Zr/Al-6Cu-2Ni-0.5V | 350 | 136.9 | 2023 | [57] |
| Al-11.93Si-4.03Cu-0.97Mg-2.67Ni-0.52Fe | 350 | 103 | 2022 | [61] |
| rGO/Al | 350 | 128 | 2020 | [62] |
| (2% Al3Zr + 15.2% Al3Ni)/Al-1Mg-0.8Mn-0.8V | 350 | 82 | 2020 | [63] |
| Al-12.16Si-3.95Cu-1.01Mg-2.54Ni-0.47Fe | 350 | 100 | 2019 | [64] |
| Al-11.98Si-4.02Cu-1.02Mg-2.68Ni-0.62Fe | 350 | 106 | 2019 | [64] |
| Al-12Si-4Cu-2Ni-1Mg-AlNp | 350 | 106 | 2019 | [6] |
| Al-12.95Si-3.57Cu-0.72Mg-0.91Ni-0.53Fe-0.4Er | 350 | 117 | 2019 | [65] |
4. Conclusions
- (1)
- The as-cast and heat-treated Al-Cu-Mn-Ag alloys mainly comprised α-Al and Al2Cu phases. In the as-cast alloy, the Mn/Ag ratio had no noticeable effect on the microstructure of the alloy, and the reticular (θ′-Al2Cu+α-Al) eutectic structure was distributed along the grain boundaries of the primary α-Al grains in the as-cast alloy. After T6 heat treatment, the α-Al dendrite phase was coarsened. The fine nanocrystalline θ′-Al2Cu particles were precipitated from the matrix. With the Mn/Ag ratio increase, precipitation and particle size increased first and then decreased. When the Mn/Ag ratio was 1:1, the precipitated quantity of the θ′-Al2Cu phase reached the highest and the smallest size.
- (2)
- At room temperature, the ultimate tensile strength of the as-cast alloy did not significantly change, and the average tensile strength was about 163.5 MPa. At high temperatures, the ultimate tensile strength of the as-cast alloy increased with the increase of the Mn/Ag ratio, and the highest tensile strength was 87.17 MPa. After heat treatment, the alloy’s ultimate tensile strength and yield strength at room temperature and high temperatures were significantly increased. As the Mn/Ag ratio increased, it increased and then decreased. When the Mn/Ag ratio was 1:1, the ultimate tensile strength at room temperature and high temperatures reached the highest, which were 379.14 MPa and 135.89 MPa, respectively.
- (3)
- In the heat treatment process of the alloy, due to the low thermal diffusion coefficient of Mn, the diffusion of Al and Cu could be blocked to form a blockage of atoms and then promote the nucleation and refinement of the θ′-Al2Cu phase, while the Ag element could also cooperate with the Mn element to further the fine θ′-Al2Cu phase and promote its thermal stability. Through the optimization of the Mn/Ag ratio, we found that when the Mn/Ag ratio was 1:1, the θ′-Al2Cu phase had the best thermal stability, which indicated that the interface energy of the θ′-Al2Cu phase of this ratio alloy was the lowest, which was the most favorable for anti-coursing, so its high-temperature mechanical properties were the highest.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roósz, A.; Exner, H.E. Ternary restricted-equilibrium phase diagrams—II. Practical application: Aluminium-rich corner of the Al-Cu-Mg system. Acta Metall. Mater. 1990, 38, 2009–2016. [Google Scholar] [CrossRef]
- Matvienko, Y.I.; Polishchuk, S.S.; Rud, A.D.; Popov, O.Y.; Demchenkov, S.A.; Fesenko, O.M. Effect of graphite additives on microstructure and mechanical properties of Al–Cu composites prepared by mechanical alloying and sintering. Mater. Chem. Phys. 2020, 254, 123437. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Shen, H.; Liao, H.; Li, H. Alloying effect of Mg on microstructure and mechanical properties at 300 °C of Al-5Cu-1Mn-0.5Ni heat-resistant alloy. Mater. Res. Express 2021, 8, 086515. [Google Scholar] [CrossRef]
- De Bonfils-Lahovary, M.L.; Laffont, L.; Blanc, C. Characterization of intergranular corrosion defects in a 2024 T351 aluminium alloy. Corros. Sci. 2017, 119, 60–67. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Hu, K.; Xu, Q.; Ma, X.; Sun, Q.; Gao, T.; Liu, X. A novel heat-resistant Al-Si-Cu-Ni-Mg base material synergistically strengthened by Ni-rich intermetallics and nano-AlNp microskeletons. J. Mater. Sci. Technol. 2019, 35, 306–312. [Google Scholar] [CrossRef]
- Rofman, O.V.; Mikhaylovskaya, A.V.; Kotov, A.D.; Mochugovskiy, A.G.; Mohamed, A.K.; Cheverikin, V.V.; Short, M.P. AA2024/SiC metal matrix composites simultaneously improve ductility and cracking resistance during elevated temperature deformation. Mater. Sci. Eng. A 2020, 790, 139697. [Google Scholar] [CrossRef]
- Santos-Güemes, R.; Bellón, B.; Esteban-Manzanares, G.; Segurado, J.; Capolungo, L.; LLorca, J. Multiscale modelling of precipitation hardening in Al–Cu alloys: Dislocation dynamics simulations and experimental validation. Acta Mater. 2020, 188, 475–485. [Google Scholar] [CrossRef]
- Da Costa Teixeira, J.; Cram, D.G.; Bourgeois, L.; Bastow, T.J.; Hill, A.J.; Hutchinson, C.R. On the strengthening response of aluminum alloys containing shear-resistant plate-shaped precipitates. Acta Mater. 2008, 56, 6109–6122. [Google Scholar] [CrossRef]
- Kaira, C.S.; Kantzos, C.; Williams, J.J.; De Andrade, V.; De Carlo, F.; Chawla, N. Microstructural evolution and deformation behavior of Al-Cu alloys: A Transmission X-ray Microscopy (TXM) and micropillar compression study. Acta Mater. 2018, 144, 419–431. [Google Scholar] [CrossRef]
- Nie, J.F.; Muddle, B.C. Strengthening of an Al-Cu-Sn alloy by deformation-resistant precipitate plates. Acta Mater. 2008, 56, 3490–3501. [Google Scholar] [CrossRef]
- Mamzurina, O.I.; Amer, S.; Loginova, I.; Glavatskikh, M.; Mochugovskiy, A.; Barkov, R.; Pozdniakov, A. Effect of Zr on Microstructure and Mechanical Properties of the Al-Cu-Yb and Al-Cu-Gd Alloys. Metals 2022, 12, 479. [Google Scholar] [CrossRef]
- Bansal, U.; Singh, M.P.; Mondol, S.; Sinha, S.K.; Makineni, S.K.; Paul, A.; Chattopadhyay, K. The interplay of precipitation of ordered compounds and interfacial segregation in Al-Cu-Hf-Si alloys for high-temperature strength. Acta Mater. 2022, 240, 118355. [Google Scholar] [CrossRef]
- Rakhmonov, J.U.; Bahl, S.; Shyam, A.; Dunand, D.C. Cavitation-resistant intergranular precipitates enhance creep performance of θ′-strengthened Al-Cu based alloys. Acta Mater. 2022, 228, 117788. [Google Scholar] [CrossRef]
- Dar, S.M.; Liao, H. Creep behavior of heat resistant Al-Cu-Mn alloys strengthened by fine (θ′) and coarse (Al20Cu2Mn3) second phase particles. Mater. Sci. Eng. A 2019, 763, 138062. [Google Scholar] [CrossRef]
- Amer, S.M.; Glavatskikh, M.V.; Barkov, R.Y.; Loginova, I.S.; Pozdniakov, A.V. Effect of Mn substitution on Cr in the Al-Cu-Er-Mg-Zr-Fe-Si-Ti cast alloy. J. Alloys Compd. 2024, 983, 173958. [Google Scholar] [CrossRef]
- Hitter, M.M.L.; Varma, S.K. Microstructural characterization, oxidation behavior, and properties of a new Al-Cu-Ni-Mn-Si non-BCC high entropy alloy at 600, 700, and 800 °C. J. Mater. Res. Technol. 2023, 23, 680–686. [Google Scholar] [CrossRef]
- Yakovtseva, O.A.; Bazlov, A.I.; Prosviryakov, A.S.; Emelina, N.B.; Tabachkova, N.Y.; Mikhaylovskaya, A.V. The influence of the Al2O3 particles on the microstructure of the mechanically alloyed Al-Mn-Cu alloy. J. Alloys Compd. 2023, 930, 167452. [Google Scholar] [CrossRef]
- Ruan, S.; Schuh, C.A. Electrodeposited Al-Mn alloys with microcrystalline, nanocrystalline, amorphous and nano-quasicrystalline structures. Acta Mater. 2009, 57, 3810–3822. [Google Scholar] [CrossRef]
- Buttard, M.; Freixes, M.L.; Josserond, C.; Donnadieu, P.; Chéhab, B.; Blandin, J.; Gault, B.; De Geuser, F.; Martin, G. Ageing response and strengthening mechanisms in a new Al-Mn-Ni-Cu-Zr alloy designed for laser powder bed fusion. Acta Mater. 2023, 259, 119271. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al-Si-Cu casting alloys. Mater. Sci. Eng. A 2008, 488, 496–504. [Google Scholar] [CrossRef]
- Shower, P.; Morris, J.R.; Shin, D.; Radhakrishnan, B.; Allard, L.F.; Shyam, A. Temperature-dependent stability of θ′-Al2Cu precipitates investigated with phase field simulations and experiments. Materialia 2019, 5, 100185. [Google Scholar] [CrossRef]
- Shower, P.; Roy, S.; Hawkins, C.S.; Shyam, A. The effects of microstructural stability on the compressive response of two cast aluminum alloys up to 300 °C. Mater. Sci. Eng. A 2017, 700, 519–529. [Google Scholar] [CrossRef]
- Shower, P.; Morris, J.; Shin, D.; Radhakrishnan, B.; Poplawsky, J.; Shyam, A. Mechanisms for stabilizing θ′(Al2Cu) precipitates at elevated temperatures investigated with phase field modeling. Materialia 2019, 6, 100335. [Google Scholar] [CrossRef]
- Poplawsky, J.D.; Milligan, B.K.; Allard, L.F.; Shin, D.; Shower, P.; Chisholm, M.F.; Shyam, A. The synergistic role of Mn and Zr/Ti in producing θ′/L12 co-precipitates in Al-Cu alloys. Acta Mater. 2020, 194, 577–586. [Google Scholar] [CrossRef]
- Jiang, L.; Rouxel, B.; Langan, T.; Dorin, T. Coupled segregation mechanisms of Sc, Zr and Mn at θ′ interfaces enhances the strength and thermal stability of Al-Cu alloys. Acta Mater. 2021, 206, 116634. [Google Scholar] [CrossRef]
- Li, G.; Liao, H.; Zheng, J.; Yang, M.; Qian, L.; Shi, M.; Lu, L. Synergistic effect of joint addition of Sb+Mn on high temperature strengthening in Al-4Cu heat-resistant alloy. Mater. Sci. Eng. A 2022, 851, 143623. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Eggeler, G.; Skrotzki, B. Nucleation of ω phase in an Al-Cu-Mg-Mn-Ag alloy aged at temperatures below 200 °C. Scr. Mater. 2001, 44, 545–551. [Google Scholar] [CrossRef]
- Avateffazeli, M.; Shakil, S.I.; Pirgazi, H.; Shalchi-Amirkhiz, B.; Mohammadi, M.; Haghshenas, M. Fatigue response of a cast Al-Cu-Mg-Ag-TiB2 (A205) alloy: Stress relieved and aged. Mater. Today Commun. 2023, 36, 106581. [Google Scholar] [CrossRef]
- Garg, A.; Howe, J.M. Convergent-beam electron diffraction analysis of the Ω phase in an Al-4.0 Cu-0.5 Mg-0.5 Ag alloy. Acta Metall. Mater. 1991, 39, 1939–1946. [Google Scholar] [CrossRef]
- Muddle, B.C.; Polmear, I.J. The precipitate Ω phase in Al-Cu-Mg-Ag alloys. Acta Metall. 1989, 37, 777–789. [Google Scholar] [CrossRef]
- Nikulin, I.; Malopheyev, S.; Kipelova, A.; Kaibyshev, R. Effect of SPD and friction stir welding on microstructure and mechanical properties of Al-Cu-Mg-Ag sheets. Mater. Lett. 2012, 66, 311–313. [Google Scholar] [CrossRef]
- Nath, S.; Konkati, C.; Gupta, R.K.; Chauhan, A. Quasi-static and dynamic response of AA-2219-T87 aluminium alloy. Mater. Today Commun. 2024, 38, 108443. [Google Scholar] [CrossRef]
- Narasayya, C.V.A.; Rambabu, P.; Mohan, M.K.; Mitra, R.; Prasad, N.E. Tensile Deformation and Fracture Behaviour of an Aerospace Aluminium Alloy AA2219 in Different Ageing Conditions. Procedia Mater. Sci. 2014, 6, 322–330. [Google Scholar] [CrossRef]
- Bartošák, M.; Šulák, I.; Horváth, J.; Jambor, M.; Pilsová, L. Isothermal low-cycle fatigue and fatigue–creep behaviour of 2618 aluminium alloy. Int. J. Fatigue 2024, 179, 108027. [Google Scholar] [CrossRef]
- Schuster, M.; De Luca, A.; Widmer, R.; Maeder, X.; Leinenbach, C. Processability, microstructure and precipitation of a Zr-modified 2618 aluminium alloy fabricated by laser powder bed fusion. J. Alloys Compd. 2022, 913, 165346. [Google Scholar] [CrossRef]
- Malek, B.; Mabru, C.; Chaussumier, M. Fatigue behavior of 2618-T851 aluminum alloy under uniaxial and multiaxial loadings. Int. J. Fatigue 2020, 131, 105322. [Google Scholar] [CrossRef]
- Kumar, N.M.; Kumaran, S.S.; Kumaraswamidhas, L.A. High temperature investigation on EDM process of Al 2618 alloy reinforced with Si3N4, ALN and ZrB2 in-situ composites. J. Alloys Compd. 2016, 663, 755–768. [Google Scholar] [CrossRef]
- Chen, J.; Liao, H.; Wu, Y.; Li, H. Contributions to high temperature strengthening from three types of heat-resistant phases formed during solidification, solution treatment and ageing treatment of Al-Cu-Mn-Ni alloys respectively. Mater. Sci. Eng. A 2020, 772, 138819. [Google Scholar] [CrossRef]
- Rosalie, J.M.; Bourgeois, L. Silver segregation to θ′ (Al2Cu)-Al interfaces in Al-Cu-Ag alloys. Acta Mater. 2012, 60, 6033–6041. [Google Scholar] [CrossRef]
- Garg, A.; Chang, Y.C.; Howe, J.M. Precipitation of the Ω phase in an Al-4.0Cu-0.5Mg alloy. Scr. Metall. Mater. 1990, 24, 677–680. [Google Scholar] [CrossRef]
- Mandal, P.K.; Robi, P.S. Influence of micro-alloying with silver on microstructure and mechanical properties of Al-Cu alloy. Mater. Sci. Eng. A 2018, 722, 99–111. [Google Scholar] [CrossRef]
- Gazizov, M.; Kaibyshev, R. Effect of pre-straining on the aging behavior and mechanical properties of an Al-Cu-Mg-Ag alloy. Mater. Sci. Eng. A 2015, 625, 119–130. [Google Scholar] [CrossRef]
- Gable, B.M.; Shiflet, G.J.; Starke, E.A. Alloy development for the enhanced stability of Ω precipitates in Al-Cu-Mg-Ag alloys. Metall. Mater. Trans. A 2006, 37, 1091–1105. [Google Scholar] [CrossRef]
- Balducci, E.; Ceschini, L.; Morri, A.; Morri, A.; Di Sabatino, M.; Arnberg, L.; Li, Y. High Temperature Behavior of the EN AW-2618A Piston Alloy Containing 0.12wt% Zr: Influence of Heat Treatment. Mater. Today Proc. 2015, 2, 5037–5044. [Google Scholar] [CrossRef]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Ageing characteristics and high-temperature tensile properties of Al-Si-Cu-Mg alloys with micro-additions of Cr, Ti, V and Zr. Mater. Sci. Eng. A 2016, 652, 353–364. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; Li, G.; Chen, J.; Jiang, J.; Shao, W.; Zhen, L. New insights on the initial local corrosion of composite Al7Cu2Fe/Al20Cu2Mn3 intermetallic particles in 2024-T351 aluminum alloy. Corros. Sci. 2022, 208, 110657. [Google Scholar] [CrossRef]
- Dar, S.M.; Liao, H.; Xu, A. Effect of Cu and Mn content on solidification microstructure, T-phase formation and mechanical property of AlCuMn alloys. J. Alloys Compd. 2019, 774, 758–767. [Google Scholar] [CrossRef]
- Kosari, A.; Tichelaar, F.; Visser, P.; Zandbergen, H.; Terryn, H.; Mol, J.M.C. Dealloying-driven local corrosion by intermetallic constituent particles and dispersoids in aerospace aluminium alloys. Corros. Sci. 2020, 177, 108947. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.N.; Mao, C.; Peng, P. Structural, elastic and electronic properties of θ (Al2Cu) and S (Al2CuMg) strengthening precipitates in Al-Cu-Mg series alloys: First-principles calculations. Solid State Commun. 2012, 152, 2100–2104. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Effects of local geometry distortion at the Al/Al2Cu interfaces on solute segregation. Phys. Chem. Chem. Phys. 2020, 22, 4106–4114. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.N.; Shrivastava, P.; Panda, D.; Gunale, B.; Susmitha, K.; Pola, P. Synthesis of Al2Cu intermetallic compound by mechanical alloying. Mater. Today Commun. 2022, 31, 103267. [Google Scholar] [CrossRef]
- Nanev, C.N. Thermodynamic and molecular-kinetic considerations of the initial growth of newly born crystals; crystal size distribution; Dissolution of small crystals during Ostwald ripening due to temperature changes. Prog. Cryst. Growth Charact. Mater. 2023, 69, 100604. [Google Scholar] [CrossRef]
- Cui, J.-G.; Chen, Y.; Fu, X.; Yang, H.; Wang, H.; Li, A.; Pan, L. Effect of V on high-temperature mechanical properties of Al-5Cu-1.5Ni alloy. J. Iron Steel Res. Int. 2023. [Google Scholar] [CrossRef]
- Elgallad, E.M.; Zhang, Z.; Chen, X.G. Effect of two-step aging on the mechanical properties of AA2219 DC cast alloy. Mater. Sci. Eng. A 2015, 625, 213–220. [Google Scholar] [CrossRef]
- Cui, J.; Zeng, G.; Gupta, N.; Luo, Y.; Fu, X.; Yang, H.; Li, A.; Pan, L. Microstructure and Tensile Property of Al3Zr/Al-Cu-Ni-V Composite Prepared by In Situ Reaction. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Yang, H.; Gao, T.; Zhang, H.; Nie, J.; Liu, X. Enhanced age-hardening behavior in Al-Cu alloys induced by in-situ synthesized TiC nanoparticles. J. Mater. Sci. Technol. 2019, 35, 374–382. [Google Scholar] [CrossRef]
- Sanaty-Zadeh, A. Comparison between current models for the strength of particulate-reinforced metal matrix nanocomposites with emphasis on consideration of Hall–Petch effect. Mater. Sci. Eng. A 2012, 531, 112–118. [Google Scholar] [CrossRef]
- Dai, H.; Wang, L.; Dong, B.; Miao, J.; Lin, S.; Chen, H. Microstructure and high-temperature mechanical properties of new-type heat-resisting aluminum alloy Al6.5Cu2Ni0.5Zr0.3Ti0.25V under the T7 condition. Mater. Lett. 2023, 332, 133503. [Google Scholar] [CrossRef]
- Meng, F.; Wu, Y.; Li, W.; Hu, K.; Zhao, K.; Yang, H.; Gao, T.; Sun, Y.; Liu, X. Influence of the evolution of heat-resistant phases on elevated-temperature strengthening mechanism and deformation behavior in Al–Si multicomponent alloys. Curr. Appl. Phys. 2022, 39, 239–247. [Google Scholar] [CrossRef]
- Zan, Y.N.; Zhang, Q.; Zhou, Y.T.; Liu, Z.Y.; Wang, Q.Z.; Wang, D.; Xiao, B.L.; Ren, W.C.; Ma, Z.Y. Introducing graphene (reduced graphene oxide) into Al matrix composites for enhanced high-temperature strength. Compos. Part B Eng. 2020, 195, 108095. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Yang, Y.; Gupta, N.; Yang, C.; Zhao, Y.; Hu, Z. High-Temperature Mechanical Properties of Aluminum Alloy Matrix Composites Reinforced with Zr and Ni Trialumnides Synthesized by In Situ Reaction. Metall. Mater. Trans. A 2020, 51, 214–225. [Google Scholar] [CrossRef]
- Meng, F.; Wu, Y.; Hu, K.; Li, Y.; Sun, Q.; Liu, X. Evolution and Strengthening Effects of the Heat-Resistant Phases in Al-Si Piston Alloys with Different Fe/Ni Ratios. Materials 2019, 12, 2506. [Google Scholar] [CrossRef] [PubMed]
- Chankitmunkong, S.; Eskin, D.G.; Patakham, U.; Limmaneevichitr, C. Microstructure and elevated temperature mechanical properties of a direct-chill cast AA4032 alloy with copper and erbium additions. J. Alloys Compd. 2019, 782, 865–874. [Google Scholar] [CrossRef]
- Bahl, S.; Xiong, L.; Allard, L.F.; Michi, R.A.; Poplawsky, J.D.; Chuang, A.C.; Singh, D.; Watkins, T.R.; Shin, D.; Haynes, J.A.; et al. Aging behavior and strengthening mechanisms of coarsening resistant metastable θ′ precipitates in an Al-Cu alloy. Mater. Des. 2021, 198, 109378. [Google Scholar] [CrossRef]
- Gao, Y.H.; Cao, L.F.; Kuang, J.; Zhang, J.Y.; Liu, G.; Sun, J. Assembling dual precipitates to improve high-temperature resistance of multi-microalloyed Al-Cu alloys. J. Alloys Compd. 2020, 822, 153629. [Google Scholar] [CrossRef]
- Rezaee, M.; Aval, H.J. Effect of lithium on mechanical and corrosion properties of friction stir back extruded Al-Cu-Mg alloy. Kov. Mater.-Met. Mater. 2023, 61, 307–320. [Google Scholar] [CrossRef]
- Shin, D.; Shyam, A.; Lee, S.; Yamamoto, Y.; Haynes, J.A. Solute segregation at the Al/θ′-Al2Cu interface in Al-Cu alloys. Acta Mater. 2017, 141, 327–340. [Google Scholar] [CrossRef]



| Alloys | Mass Fraction/% | Mn/Ag | |||
|---|---|---|---|---|---|
| No. | Cu | Mn | Ag | Al | Weight Ratio |
| #1 | 6 | 0.2 | 0.6 | Bal. | 1:3 |
| #2 | 6 | 0.27 | 0.53 | Bal. | 1:2 |
| #3 | 6 | 0.4 | 0.4 | Bal. | 1:1 |
| #4 | 6 | 0.53 | 0.27 | Bal. | 2:1 |
| #5 | 6 | 0.6 | 0.2 | Bal. | 3:1 |
| Al-6Cu | 6 | - | - | Bal. | - |
| Alloys | Mn/Ag | UTS(σb)/MPa | YS(σs)/MPa | FS/% | El./% |
|---|---|---|---|---|---|
| #1 | 1:3 | 163.19 | 156.99 | 6.20 | 3.2 |
| #2 | 1:2 | 162.53 | 156.65 | 6.33 | 3.31 |
| #3 | 1:1 | 164.00 | 159.65 | 5.99 | 3.18 |
| #4 | 2:1 | 164.34 | 159.63 | 5.85 | 2.94 |
| #5 | 3:1 | 163.68 | 151.40 | 5.81 | 3.13 |
| Al-6Cu | - | 153.55 | 143.38 | 6.82 | 3.36 |
| Alloys | Mn/Ag | UTS(σb)/MPa | YS(σs)/MPa | FS/% | El./% |
|---|---|---|---|---|---|
| #1 | 1:3 | 72.90 | 72.06 | 26.69 | 22.87 |
| #2 | 1:2 | 72.02 | 65.04 | 38.40 | 35.43 |
| #3 | 1:1 | 77.52 | 73.71 | 22.16 | 18.85 |
| #4 | 2:1 | 83.40 | 77.21 | 23.57 | 19.60 |
| #5 | 3:1 | 87.17 | 86.46 | 29.56 | 25.00 |
| Al-6Cu | - | 69.22 | 60.36 | 39.37 | 35.88 |
| Alloys | Mn/Ag | UTS(σb)/MPa | YS(σs)/MPa | FS/% | El./% |
|---|---|---|---|---|---|
| #1 | 1:3 | 353.28 | 328.69 | 5.55 | 2.63 |
| #2 | 1:2 | 362.56 | 336.72 | 5.71 | 2.72 |
| #3 | 1:1 | 379.14 | 345.73 | 5.95 | 2.87 |
| #4 | 2:1 | 354.29 | 329.73 | 5.38 | 2.58 |
| #5 | 3:1 | 355.29 | 328.83 | 5.27 | 2.43 |
| Al-6Cu | - | 292.11 | 261.16 | 5.19 | 2.07 |
| Alloys | Mn/Ag | UTS(σb)/MPa | YS(σs)/MPa | FS/% | El./% |
|---|---|---|---|---|---|
| #1 | 1:3 | 115.15 | 104.21 | 13.69 | 10.90 |
| #2 | 1:2 | 124.42 | 118.31 | 14.37 | 11.02 |
| #3 | 1:1 | 135.89 | 122.26 | 14.83 | 11.92 |
| #4 | 2:1 | 127.69 | 115.75 | 15.63 | 12.55 |
| #5 | 3:1 | 125.37 | 114.23 | 8.94 | 7.05 |
| Al-6Cu | - | 105.39 | 93.21 | 17.09 | 14.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Yang, H.; Wang, H.; Huang, C.; Chen, Y.; Huang, Q.; Li, A.; Pan, L. Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys. Materials 2024, 17, 1371. https://doi.org/10.3390/ma17061371
Fu X, Yang H, Wang H, Huang C, Chen Y, Huang Q, Li A, Pan L. Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys. Materials. 2024; 17(6):1371. https://doi.org/10.3390/ma17061371
Chicago/Turabian StyleFu, Xiangzhou, Hailong Yang, Hanzhang Wang, Chifu Huang, Yongbin Chen, Qiangang Huang, Anmin Li, and Liwen Pan. 2024. "Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys" Materials 17, no. 6: 1371. https://doi.org/10.3390/ma17061371
APA StyleFu, X., Yang, H., Wang, H., Huang, C., Chen, Y., Huang, Q., Li, A., & Pan, L. (2024). Effect of Mn/Ag Ratio on Microstructure and Mechanical Properties of Heat-Resistant Al-Cu Alloys. Materials, 17(6), 1371. https://doi.org/10.3390/ma17061371






