An Overview of the Non-Energetic Valorization Possibilities of Plastic Waste via Thermochemical Processes
Abstract
1. Introduction
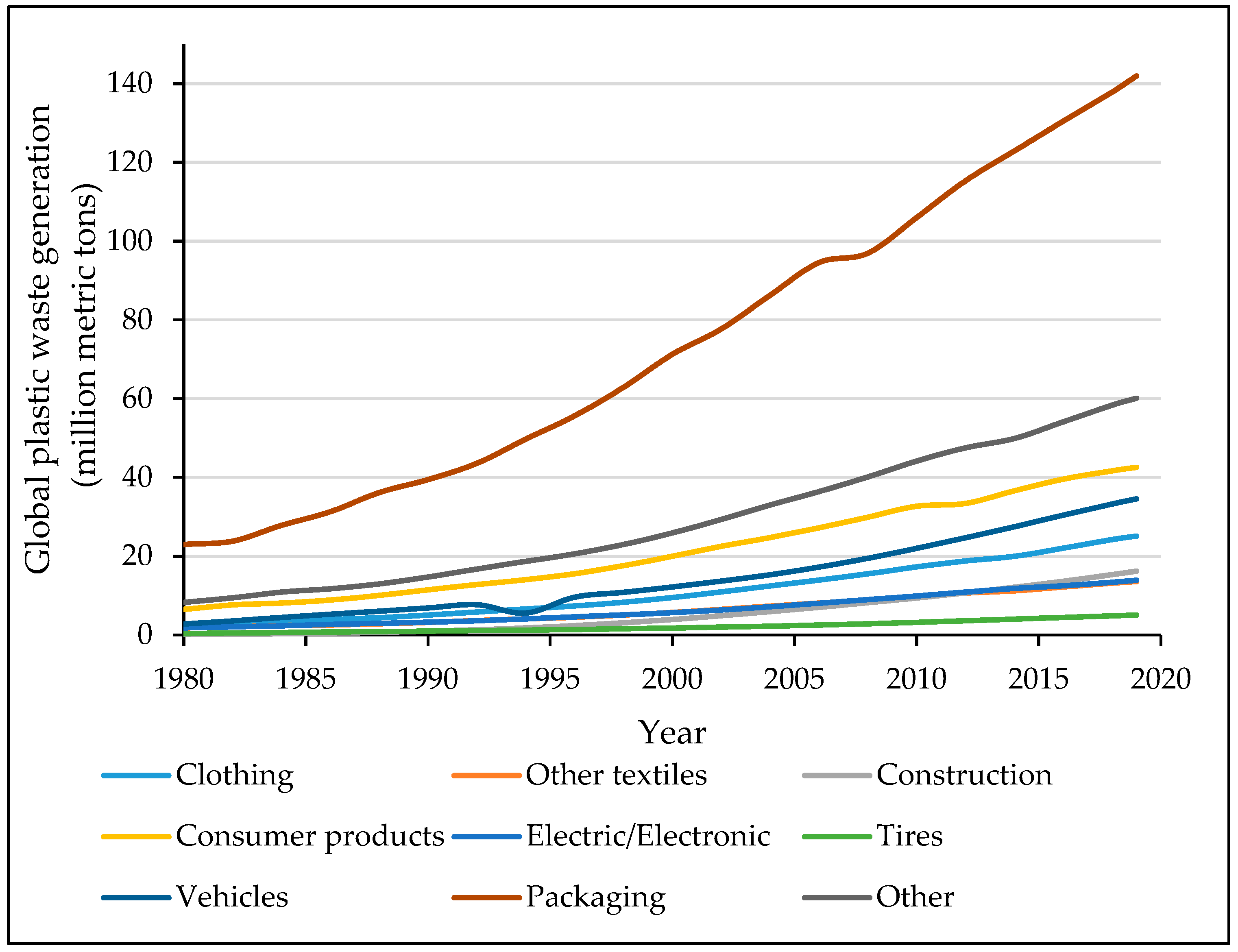
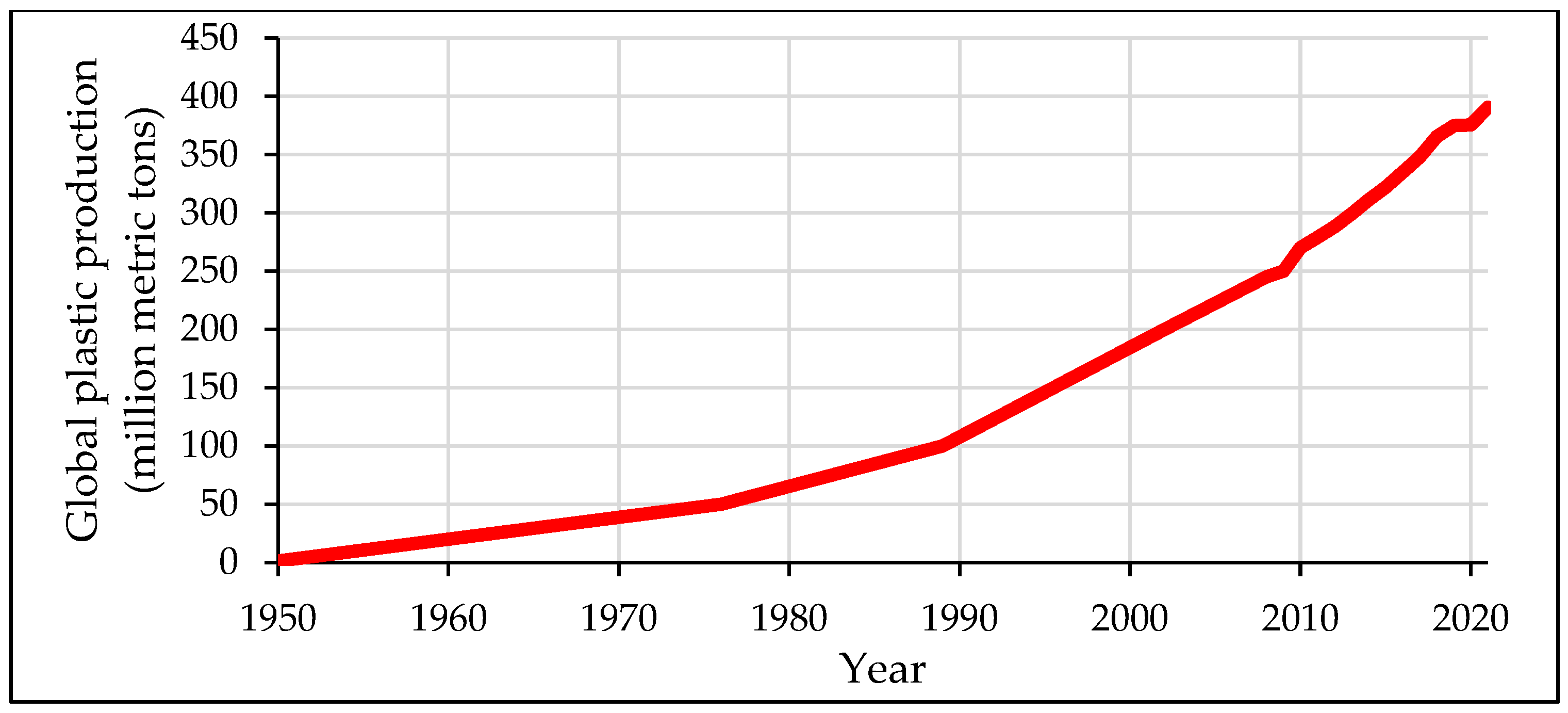
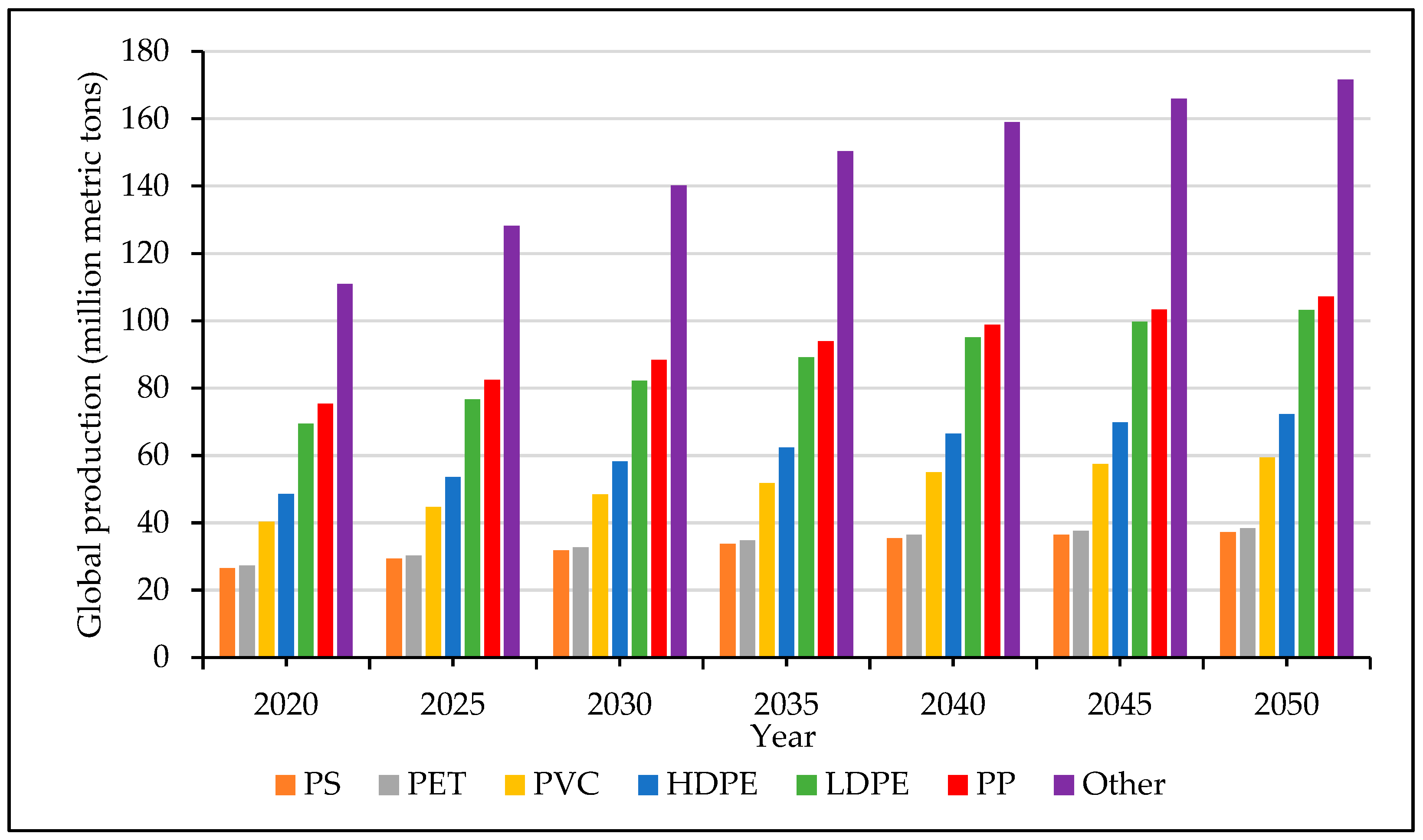
2. Challenges Facing Plastic Recycling
2.1. Quality of Plastic Waste
2.2. Purity
3. Alternative Plastic Waste Treatment Processes
3.1. Solvent-Based Processes
3.2. Depolymerization Processes
3.2.1. Solvolysis
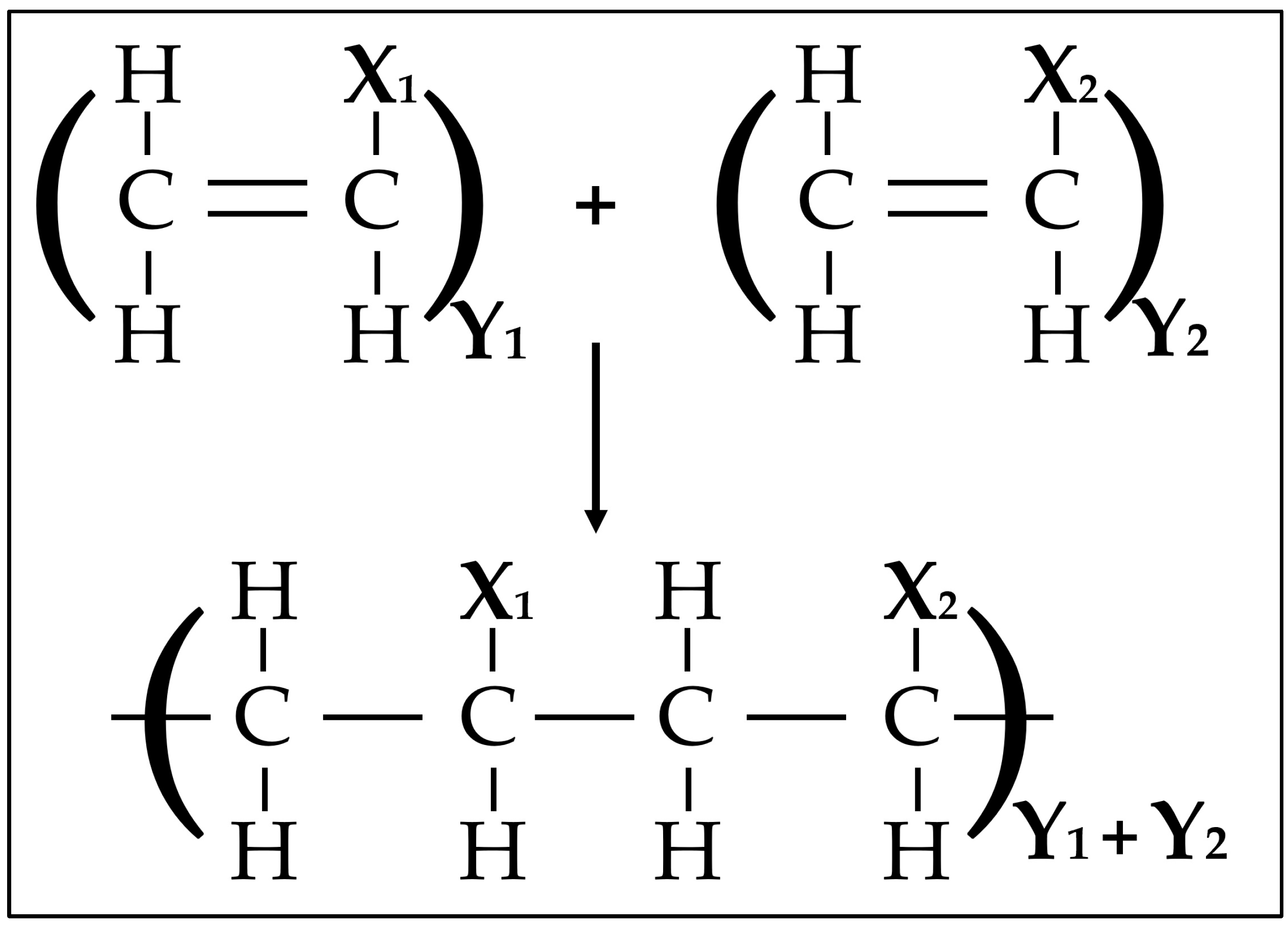
3.2.2. Pyrolysis and Gasification
Liquid-Oriented Processes
Carbonaceous Materials and Gaseous Product-Oriented Processes
- Gasification
- Combined Carbonaceous Materials and Hydrogen-Production-Oriented Processes
4. Possible Uses of Plastic Waste Pyrolysis Products in Industry
4.1. Energetic Applications
4.2. Monomer Production
4.3. Benzene, Toluene, and Xylene (BTX)
4.4. Hydrocarbon-Based Lubricants
4.5. Phase Change Materials
4.6. Refrigerants
5. Challenges and Future Prospects
5.1. Thermochemical Processing of Thermoplastics
5.2. Thermochemical Processing of Fiber-Filled/Reinforced Plastics
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Servera-Francés, D.; Fuentes-Blasco, M.; Piqueras-Tomás, L. The Importance of Sustainable Practices in Value Creation and Consumers’ Commitment with Companies’ Commercial Format. Sustainability 2020, 12, 9852. [Google Scholar] [CrossRef]
- Statista. Annual Production of Plastics Worldwide from 1950 to 2021. Statista. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 22 December 2023).
- Kumar, S.; Singh, E.; Mishra, R.; Kumar, A.; Caucci, S. Utilization of Plastic Wastes for Sustainable Environmental Management: A Review. ChemSusChem 2021, 14, 3985–4006. [Google Scholar] [CrossRef]
- Statista. Global Thermoplastic Production by Type 2050. Statista. Available online: https://www.statista.com/statistics/1192886/thermoplastics-production-volume-by-type-globally/ (accessed on 22 December 2023).
- Krawczak, P. Towards a circular, low-carbon emission plastics industry. Express Polym. Lett. 2022, 16, 901. [Google Scholar] [CrossRef]
- Statista. Global Plastic Waste Generation by Application. Statista. Available online: https://www.statista.com/statistics/1339124/global-plastic-waste-generation-by-application/ (accessed on 22 December 2023).
- PlasticsEurope. ReShaping Plastics: Pathways to a Circular, Climate Neutral Plastics System in Europe. Available online: https://plasticseurope.org/wp-content/uploads/2022/04/SYSTEMIQ-ReShapingPlastics-April2022.pdf (accessed on 15 November 2023).
- PlasticsEurope. The Plastics Transition. Available online: https://plasticseurope.org/wp-content/uploads/2023/11/1814354_Roadmap-copychange_112023.pdf (accessed on 15 November 2023).
- Choi, J.; Yang, I.; Kim, S.; Cho, S.Y.; Lee, S. Upcycling Plastic Waste into High Value-Added Carbonaceous Materials. Macromol. Rapid Commun. 2022, 43, 2100467. [Google Scholar] [CrossRef]
- Klotz, M.; Haupt, M.; Hellweg, S. Potentials and limits of mechanical plastic recycling. J. Ind. Ecol. 2023, 27, 1043–1059. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; Organisation for Economic Co-operation and Development: Paris, France, 2022; Available online: https://www.oecd-ilibrary.org/environment/global-plastics-outlook_de747aef-en (accessed on 22 December 2023).
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. The Environmental Impacts of Plastics and Micro-Plastics Use, Waste and Pollution: EU and National Measures|Think Tank|European Parliament. Available online: https://www.europarl.europa.eu/thinktank/en/document/IPOL_STU(2020)658279 (accessed on 22 December 2023).
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Kirk-Othmer Encyclopedia of Chemical Technology; WILEY Online Library: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Bråte, I.; Allan, I.; Thomas, K.; Halsband, C. Report Made for the Norwegian Environment Agency: Microplastics in Marine Environments; Occurrence, Distribution and Effects. Akvaplan Niva. Available online: https://www.researchgate.net/publication/273089847_Report_made_for_the_Norwegian_Environment_Agency_Microplastics_in_marine_environments_Occurrence_distribution_and_effects (accessed on 20 October 2023).
- Da Costa, J.; da Costa Duarte, A. Microplastics—Occurrence, Fate and Behaviour in the Environment. Compr. Anal. Chem. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Zhou, Y.; Zhang, L.; Ge, Z.; Wang, X.; Xu, W. β-Cyclodextrin-Stabilized Au Nanoparticles for the Detection of Butyl Benzyl Phthalate. ACS Appl. Nano Mater. 2019, 2, 2743–2751. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Yalikun, N.; Wang, H.; Wang, C.; Jiang, H. A comprehensive review of separation technologies for waste plastics in urban mine. Resour. Conserv. Recycl. 2023, 197, 107087. [Google Scholar] [CrossRef]
- Serranti, S.; Bonifazi, G. Techniques for separation of plastic wastes. In Use of Recycled Plastics in Eco-efficient Concrete; Elsevier: Amsterdam, The Netherlands, 2019; pp. 9–37. ISBN 978-0-08-102676-2. [Google Scholar]
- Volk, R.; Stallkamp, C.; Steins, J.J.; Yogish, S.P.; Müller, R.C.; Stapf, D.; Schultmann, F. Techno-economic assessment and comparison of different plastic recycling pathways: A German case study. J. Ind. Ecol. 2021, 25, 1318–1337. [Google Scholar] [CrossRef]
- Uzosike, C.C.; Yee, L.H.; Padilla, R.V. Small-Scale Mechanical Recycling of Solid Thermoplastic Wastes: A Review of PET, PEs, and PP. Energies 2023, 16, 1406. [Google Scholar] [CrossRef]
- Lange, J.-P. Managing Plastic Waste—Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- SABIC-NORYLTM Resin. Available online: https://www.sabic.com/en/products/specialties/noryl-resins/noryl-resin (accessed on 22 December 2023).
- SABIC-VALOXTM FR Resins. Available online: https://www.sabic.com/en/products/polymers/polybutylene-terephthalate-pbt/valox-fr-resins (accessed on 22 December 2023).
- Aid, S.; Eddhahak, A.; Khelladi, S.; Ortega, Z.; Chaabani, S.; Tcharkhtchi, A. On the miscibility of PVDF/PMMA polymer blends: Thermodynamics, experimental and numerical investigations. Polym. Test. 2019, 73, 222–231. [Google Scholar] [CrossRef]
- Dong, H.; Zhong, J.; Isayev, A.I. Manufacturing Polypropylene (PP)/Waste EPDM Thermoplastic Elastomers Using Ultrasonically Aided Twin-Screw Extrusion. Polymers 2021, 13, 259. [Google Scholar] [CrossRef]
- Bano, S.; Iqbal, T.; Ramzan, N.; Farooq, U. Study of Surface Mechanical Characteristics of ABS/PC Blends Using Nanoindentation. Processes 2021, 9, 637. [Google Scholar] [CrossRef]
- Bärwinkel, S.; Seidel, A.; Hobeika, S.; Hufen, R.; Mörl, M.; Altstädt, V. Morphology Formation in PC/ABS Blends during Thermal Processing and the Effect of the Viscosity Ratio of Blend Partners. Materials 2016, 9, 659. [Google Scholar] [CrossRef]
- Jie, X.; Li, W.; Slocombe, D.; Gao, Y.; Banerjee, I.; Gonzalez-Cortes, S.; Yao, B.; AlMegren, H.; Alshihri, S.; Dilworth, J.; et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons. Nat. Catal. 2020, 3, 902–912. [Google Scholar] [CrossRef]
- Wyss, K.M.; Silva, K.J.; Bets, K.V.; Algozeeb, W.A.; Kittrell, C.; Teng, C.H.; Choi, C.H.; Chen, W.; Beckham, J.L.; Yakobson, B.I.; et al. Synthesis of Clean Hydrogen Gas from Waste Plastic at Zero Net Cost. Adv. Mater. 2023, 35, 2306763. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S.; Van Geem, K.M.; Roosen, M.; Delbeke, E.I.; De Meester, S. Challenges and opportunities of solvent-based additive extraction methods for plastic recycling. Waste Manag. 2020, 104, 148–182. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Hejmej, A.; Kotula-Balak, M.; Bilińska, B. Antiandrogenic and Estrogenic Compounds: Effect on Development and Function of Male Reproductive System. In Steroids—Clinical Aspect; Intech Open: London, UK, 2011. [Google Scholar] [CrossRef]
- McKeen, L.W. Thermoplastic Elastomers. In The Effect of Creep and Other Time Related Factors on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2015; pp. 355–372. ISBN 978-0-323-35313-7. [Google Scholar] [CrossRef]
- Narain, R. Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier Science: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-816806-6. [Google Scholar]
- Muguruma, H. Biosensors: Enzyme Immobilization Chemistry. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 64–71. ISBN 978-0-12-809894-3. [Google Scholar] [CrossRef]
- Kim, K.J.; Dhevi, M.; Lee, J.S.; Cho, Y.D.; Choe, E.K. Mechanism of glycolysis of nylon 6,6 and its model compound by ethylene glycol. Polym. Degrad. Stab. 2006, 91, 1545–1555. [Google Scholar] [CrossRef]
- Shojaei, B.; Abtahi, M.; Najafi, M. Chemical recycling of PET: A stepping-stone toward sustainability. Polym. Adv. Technol. 2020, 31, 2912–2938. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765–3789. [Google Scholar] [CrossRef]
- Mancini, S.D.; Zanin, M. Post Consumer Pet Depolymerization by Acid Hydrolysis. Polym.-Plast. Technol. Eng. 2007, 46, 135–144. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Brivio, L.; Meini, S.; Sponchioni, M.; Moscatelli, D. Chemical recycling of polyethylene terephthalate (PET) to monomers: Mathematical modeling of the transesterification reaction of bis(2-hydroxyethyl) terephthalate to dimethyl terephthalate. Chem. Eng. Sci. 2024, 284, 119466. [Google Scholar] [CrossRef]
- Lin, P.-L.; Fang, H.-W.; Tseng, T.; Lee, W.-H. Effects of hydroxyapatite dosage on mechanical and biological behaviors of polylactic acid composite materials. Mater. Lett. 2007, 61, 3009–3013. [Google Scholar] [CrossRef]
- Hongthong, S.; Leese, H.S.; Allen, M.J.; Chuck, C.J. Assessing the Conversion of Various Nylon Polymers in the Hydrothermal Liquefaction of Macroalgae. Environments 2021, 8, 34. [Google Scholar] [CrossRef]
- Xayachak, T.; Haque, N.; Parthasarathy, R.; King, S.; Emami, N.; Lau, D.; Pramanik, B.K. Pyrolysis for plastic waste management: An engineering perspective. J. Environ. Chem. Eng. 2022, 10, 108865. [Google Scholar] [CrossRef]
- Daligaux, V.; Richard, R.; Manero, M.-H. Deactivation and Regeneration of Zeolite Catalysts Used in Pyrolysis of Plastic Wastes—A Process and Analytical Review. Catalysts 2021, 11, 770. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Experimental study of catalytic pyrolysis of polyethylene and polypropylene over USY zeolite and separation to gasoline and diesel-like fuels. J. Anal. Appl. Pyrolysis 2017, 127, 31–37. [Google Scholar] [CrossRef]
- Kassargy, C.; Awad, S.; Burnens, G.; Kahine, K.; Tazerout, M. Gasoline and diesel-like fuel production by continuous catalytic pyrolysis of waste polyethylene and polypropylene mixtures over USY zeolite. Fuel 2018, 224, 764–773. [Google Scholar] [CrossRef]
- Tang, S.; He, Y.; Deng, X.; Cui, X. Thermal Catalytic-Cracking Low-Density Polyethylene Waste by Metakaolin-Based Geopolymer NaA Microsphere. Molecules 2022, 27, 2557. [Google Scholar] [CrossRef] [PubMed]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Lu, C.; Xiao, H.; Chen, X. Simple pyrolysis of polystyrene into valuable chemicals. e-Polymers 2021, 21, 428–432. [Google Scholar] [CrossRef]
- Kaminsky, W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021, 8, 100023. [Google Scholar] [CrossRef]
- Greenwood, A. INSIGHT: US Agilyx Takes Big Step in Producing Naphtha, Propylene from Waste Plastics. ICIS Explore. Available online: https://www.icis.com/explore/resources/news/2018/09/27/10262364/insight-us-agilyx-takes-big-step-in-producing-naphtha-propylene-from-waste-plastics (accessed on 25 October 2023).
- Dhahak, A.; Hild, G.; Rouaud, M.; Mauviel, G.; Burkle-Vitzthum, V. Slow pyrolysis of polyethylene terephthalate: Online monitoring of gas production and quantitative analysis of waxy products. J. Anal. Appl. Pyrolysis 2019, 142, 104664. [Google Scholar] [CrossRef]
- Cheng, L.; Gu, J.; Wang, Y.; Zhang, J.; Yuan, H.; Chen, Y. Polyethylene high-pressure pyrolysis: Better product distribution and process mechanism analysis. Chem. Eng. J. 2020, 385, 123866. [Google Scholar] [CrossRef]
- Kumagai, S.; Yoshioka, T. Feedstock Recycling via Waste Plastic Pyrolysis. J. Jpn. Petrol. Inst. 2016, 59, 243–253. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; Zhang, K.; Phan, A.N. Monomer recovery through advanced pyrolysis of waste high density polyethylene (HDPE). Green Chem. 2018, 20, 1813–1823. [Google Scholar] [CrossRef]
- Cai, N.; Li, X.; Xia, S.; Sun, L.; Hu, J.; Bartocci, P.; Fantozzi, F.; Williams, P.T.; Yang, H.; Chen, H. Pyrolysis-catalysis of different waste plastics over Fe/Al2O3 catalyst: High-value hydrogen, liquid fuels, carbon nanotubes and possible reaction mechanisms. Energy Convers. Manag. 2021, 229, 113794. [Google Scholar] [CrossRef]
- Aboul-Enein, A.A.; Awadallah, A.E. Production of nanostructure carbon materials via non-oxidative thermal degradation of real polypropylene waste plastic using La2O3 supported Ni and Ni–Cu catalysts. Polym. Degrad. Stab. 2019, 167, 157–169. [Google Scholar] [CrossRef]
- Artetxe, M.; Lopez, G.; Amutio, M.; Barbarias, I.; Arregi, A.; Aguado, R.; Bilbao, J.; Olazar, M. Styrene recovery from polystyrene by flash pyrolysis in a conical spouted bed reactor. Waste Manag. 2015, 45, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, H.; Andrew Lin, K.-Y.; Kwon, E.E.; Lee, J. COVID-19 mask waste to energy via thermochemical pathway: Effect of Co-Feeding food waste. Energy 2021, 230, 120876. [Google Scholar] [CrossRef] [PubMed]
- Chanda, M. Chemical aspects of polymer recycling. Adv. Ind. Eng. Polym. Res. 2021, 4, 133–150. [Google Scholar] [CrossRef]
- Klean Industries. Large Scale Plastics Pyrolysis System to Diesel Fuel|Cogeneration & Recycling Technology|Klean Industries. Available online: https://kleanindustries.com/waste-challenges-innovations/plastic-pyrolysis-recycling/spr-japan/ (accessed on 25 December 2023).
- IChemE. Plastics: Towards a Circular Economy. Available online: https://www.thechemicalengineer.com/features/plastics-towards-a-circular-economy/ (accessed on 25 December 2023).
- Vadxx Energy. Vadxx Energy Establishes Waste Plastic to EcoFuelTM Facility in Akron|Ohio’s Polymer Industry Resource. PolymerOhio. Available online: https://polymerohio.org/vadxx-energy-establishes-waste-plastic-ecofuel-facility-akron/ (accessed on 25 December 2023).
- Solving the Problem of Plastic Waste; Recycling Technologies: Swindon, UK; Available online: https://recyclingtechnologies.co.uk/technology/ (accessed on 25 December 2023).
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- 2015 Plastics-To-Fuel Project Developer’s Guide; Ocean Recovery Alliance. Available online: https://www.americanchemistry.com/content/download/8085/file/2015-Plastics-to-Fuel-Project-Developers-Guide.pdf (accessed on 27 February 2024).
- Shea, E. Alterra Looks to Blow the Lid Off Plastics Recycling. Alterra Energy. Available online: https://alterraenergy.com/news/alterra-looks-to-blow-the-lid-off-plastics-recycling-2/ (accessed on 25 December 2023).
- Project Susteen TCR500. Susteen Technologies. Available online: https://demoplants21.best-research.eu/projects/info/3823/8JBaZy (accessed on 27 February 2024).
- Dogu, O.; Pelucchi, M.; Van De Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Zare, A.A.D.; Yari, M. Comprehensive examination and analysis of thermodynamics in a multi-generation hydrogen, heat, and power system based on plastic waste gasification integrated biogas -fueled chemical looping combustion. Process Saf. Environ. Prot. 2024, 183, 976–991. [Google Scholar] [CrossRef]
- Saebea, D.; Ruengrit, P.; Arpornwichanop, A.; Patcharavorachot, Y. Gasification of plastic waste for synthesis gas production. Energy Rep. 2020, 6, 202–207. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Xiong, Y. High quality H2-rich syngas production from pyrolysis-gasification of biomass and plastic wastes by Ni–Fe@Nanofibers/Porous carbon catalyst. Int. J. Hydrogen Energy 2019, 44, 26193–26203. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.-J.; Chen, H. Plastic wastes derived carbon materials for green energy and sustainable environmental applications. Environ. Funct. Mater. 2022, 1, 34–48. [Google Scholar] [CrossRef]
- Cai, N.; Yang, H.; Zhang, X.; Xia, S.; Yao, D.; Bartocci, P.; Fantozzi, F.; Chen, Y.; Chen, H.; Williams, P.T. Bimetallic carbon nanotube encapsulated Fe-Ni catalysts from fast pyrolysis of waste plastics and their oxygen reduction properties. Waste Manag. 2020, 109, 119–126. [Google Scholar] [CrossRef]
- Gim, H.; Park, J.H.; Choi, W.Y.; Yang, J.; Kim, D.; Lee, K.-H.; Lee, J.W. Plastic waste residue-derived boron and nitrogen co-doped porous hybrid carbon for a modified separator of a lithium sulfur battery. Electrochim. Acta 2021, 380, 138243. [Google Scholar] [CrossRef]
- Lee, G.; Eui Lee, M.; Kim, S.-S.; Joh, H.-I.; Lee, S. Efficient upcycling of polypropylene-based waste disposable masks into hard carbons for anodes in sodium ion batteries. J. Ind. Eng. Chem. 2022, 105, 268–277. [Google Scholar] [CrossRef]
- Abbas, A.; Yi, Y.M.; Saleem, F.; Jin, Z.; Veksha, A.; Yan, Q.; Lisak, G.; Lim, T.M. Multiwall carbon nanotubes derived from plastic packaging waste as a high-performance electrode material for supercapacitors. Int. J. Energy Res. 2021, 45, 19611–19622. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.G.; Yun, H.; Deng, S.; Kim, Y.J.; Lee, J.E.; Kwak, S.K.; Lee, K.B. Solving two environmental issues simultaneously: Waste polyethylene terephthalate plastic bottle-derived microporous carbons for capturing CO2. Chem. Eng. J. 2020, 397, 125350. [Google Scholar] [CrossRef]
- Zhang, H.; Pap, S.; Taggart, M.A.; Boyd, K.G.; James, N.A.; Gibb, S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020, 258, 113698. [Google Scholar] [CrossRef]
- Hiremath, V.; Lim, A.C.; Nagaraju, G.; Seo, J.G. Promoting Discarded Packing Waste into Value-Added 2D Porous Carbon Flakes for Multifunctional Applications. ACS Sustain. Chem. Eng. 2019, 7, 11944–11954. [Google Scholar] [CrossRef]
- Karim, A.V.; Selvaraj, A. Graphene composites in photocatalytic oxidation of aqueous organic contaminants—A state of art. Process Saf. Environ. Prot. 2021, 146, 136–160. [Google Scholar] [CrossRef]
- Zhang, S.; Hao, A.; Nguyen, N.; Oluwalowo, A.; Liu, Z.; Dessureault, Y.; Park, J.G.; Liang, R. Carbon nanotube/carbon composite fiber with improved strength and electrical conductivity via interface engineering. Carbon 2019, 144, 628–638. [Google Scholar] [CrossRef]
- Dai, L.; Karakas, O.; Cheng, Y.; Cobb, K.; Chen, P.; Ruan, R. A review on carbon materials production from plastic wastes. Chem. Eng. J. 2023, 453, 139725. [Google Scholar] [CrossRef]
- Saygin, D.; Blanco, H.; Boshell, F.; Cordonnier, J.; Rouwenhorst, K.; Lathwal, P.; Gielen, D. Ammonia Production from Clean Hydrogen and the Implications for Global Natural Gas Demand. Sustainability 2023, 15, 1623. [Google Scholar] [CrossRef]
- Saygin, D.; Gielen, D. Zero-Emission Pathway for the Global Chemical and Petrochemical Sector. Energies 2021, 14, 3772. [Google Scholar] [CrossRef]
- Polverino, P.; D’Aniello, F.; Arsie, I.; Pianese, C. Study of the energetic needs for the on-board production of Oxy-Hydrogen as fuel additive in internal combustion engines. Energy Convers. Manag. 2019, 179, 114–131. [Google Scholar] [CrossRef]
- Edwards, R.; Mahieu, V.; Griesemann, J.-C.; Larivé, J.-F.; Rickeard, D.J. Well-to-Wheels Analysis of Future Automotive Fuels and Powertrains in the European Context, Version 4a. SAE Trans. 2014, 1072–1084. [Google Scholar] [CrossRef]
- Olah, G.A.; Molnár, Á. Hydrocarbon Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-41782-8. [Google Scholar] [CrossRef]
- The Future of Petrochemicals—Analysis. IEA. Available online: https://www.iea.org/reports/the-future-of-petrochemicals (accessed on 4 January 2024).
- de Geofroy, A. BioBTX and Agilyx Announce Collaboration for the Production of Circular Aromatic Chemicals|Agilyx. Available online: https://www.agilyx.com/biobtx-and-agilyx-announce-collaboration-for-the-production-of-circular-aromatic-chemicals/ (accessed on 4 January 2024).
- Gancedo, J.; Li, H.; Walz, J.S.; Faba, L.; Ordoñez, S.; Huber, G.W. Investigation into the shape selectivity of zeolites for conversion of polyolefin plastic pyrolysis oil model compound. Appl. Catal. A Gen. 2024, 669, 119484. [Google Scholar] [CrossRef]
- Zheng, D.; Cheng, J.; Wang, X.; Yu, G.; Xu, R.; Dai, C.; Liu, N.; Wang, N.; Chen, B. Influences and mechanisms of pyrolytic conditions on recycling BTX products from passenger car waste tires. Waste Manag. 2023, 169, 196–207. [Google Scholar] [CrossRef]
- Mortier, R.M.; Fox, M.F.; Orszulik, S.T. (Eds.) Chemistry and Technology of Lubricants; Springer: Dordrecht, The Netherlands, 2010; ISBN 978-1-4020-8661-8. [Google Scholar] [CrossRef]
- Holweger, W. Fundamentals of Lubricants and Lubrication. In Tribology—Fundamentals and Advancements; Gegner, J., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-1135-1. [Google Scholar] [CrossRef]
- Strategic Market Research. Phase Change Materials Market Size, Share, Growth Analysis, 2030. Available online: https://www.strategicmarketresearch.com/market-report/phase-change-materials-market (accessed on 22 December 2023).
- Raoux, S.; Wuttig, M. (Eds.) Phase Change Materials; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-84873-0. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Organic Phase Change Materials for Thermal Energy Storage: Influence of Molecular Structure on Properties. Molecules 2021, 26, 6635. [Google Scholar] [CrossRef]
- Soliman, F.S. Paraffin—An Overview; InTech Open: London, UK, 2020; ISBN 978-1-83880-595-1. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Wang, X.; Wu, D. Innovative design of superhydrophobic thermal energy-storage materials by microencapsulation of n-docosane with nanostructured ZnO/SiO2 shell. Appl. Energy 2019, 237, 549–565. [Google Scholar] [CrossRef]
- Camin, D.L.; Forziati, A.F.; Rossini, F.D. Physical Properties of n-Hexadecane, n-Decylcyclopentane, n-Decylcyclohexane, 1-Hexadecene and n-Decylbenzene. J. Phys. Chem. 1954, 58, 440–442. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid–liquid microPCM for thermal energy storage. Appl. Energy 2010, 87, 1529–1534. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Wang, X.; Zhang, Y.; Zhang, Q. Preparation of n-tetradecane-containing microcapsules with different shell materials by phase separation method. Sol. Energy Mater. Sol. Cells 2009, 93, 1817–1822. [Google Scholar] [CrossRef]
- Vélez, C.; Khayet, M.; Ortiz de Zárate, J.M. Temperature-dependent thermal properties of solid/liquid phase change even-numbered n-alkanes: N-Hexadecane, n-octadecane and n-eicosane. Appl. Energy 2015, 143, 383–394. [Google Scholar] [CrossRef]
- Vélez, C.; de Zárate, J.M.O.; Khayet, M. Thermal properties of n-pentadecane, n-heptadecane and n-nonadecane in the solid/liquid phase change region. Int. J. Therm. Sci. 2015, 94, 139–146. [Google Scholar] [CrossRef]
- European Commission. Information for Importers of Quipment Containing Fluorinated Greenhouse Gases on Their Obligations under the EU F-Gas Regulation. 2016. Available online: https://www.certifico.com/component/attachments/download/4314 (accessed on 20 December 2023).
- EU F-Gas Regulation: The Beginning of the End for Climate-Damaging Fluorinated Gases—ECOS. Available online: https://ecostandard.org/news_events/eu-f-gas-regulation-the-beginning-of-the-end-for-climate-damaging-fluorinated-gases/ (accessed on 26 December 2023).
- Purohit, P.; Höglund-Isaksson, L. Global emissions of fluorinated greenhouse gases 2005–2050 with abatement potentials and costs. Atmos. Chem. Phys. 2017, 17, 2795–2816. [Google Scholar] [CrossRef]
- Bravo, I.; Díaz-de-Mera, Y.; Aranda, A.; Moreno, E.; Nutt, D.R.; Marston, G. Radiative efficiencies for fluorinated esters: Indirect global warming potentials of hydrofluoroethers. Phys. Chem. Chem. Phys. 2011, 13, 17185–17193. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Van Dril, T. Decarbonisation Options for Large Volume Organic Chemicals production, SABIC Geleen. In Energy and Climate Change; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2021; PBL publication number 3718. [Google Scholar]
- Mazloum, S.; Awad, S.; Allam, N.; Aboumsallem, Y.; Loubar, K.; Tazerout, M. Modelling plastic heating and melting in a semi-batch pyrolysis reactor. Appl. Energy 2021, 283, 116375. [Google Scholar] [CrossRef]
- Kusenberg, M.; Eschenbacher, A.; Djokic, M.R.; Zayoud, A.; Ragaert, K.; De Meester, S.; Van Geem, K.M. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: To decontaminate or not to decontaminate? Waste Manag. 2022, 138, 83–115. [Google Scholar] [CrossRef] [PubMed]
- JEC Group: Trends in the Global Composites Industry 2020–2025. Available online: https://www.textiletechnology.net/technology/news/JEC-Group-Trends-in-the-global-composites-industry-2020-2025-23733 (accessed on 26 December 2023).
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Branfoot, C.; Folkvord, H.; Keith, M.; Leeke, G.A. Recovery of chemical recyclates from fibre-reinforced composites: A review of progress. Polym. Degrad. Stab. 2023, 215, 110447. [Google Scholar] [CrossRef]
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology readiness level assessment of composites recycling technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent Progress in Carbon Fiber Reinforced Polymers Recycling: A Review of Recycling Methods and Reuse of Carbon Fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef]
| Additive Type | Examples |
|---|---|
| Plasticizers | Benzyl butyl phthalate |
| Di-isoheptyl phthalate | |
| Di-isobutyl phthalate | |
| Dibutyl phthalate | |
| Bis (2-ethylhexyl) phthalate | |
| Bis(2-methoxyethyl) phthalate | |
| Tris(2-chloroethyl) phosphate | |
| Stabilizers | Arsenic compounds |
| Triclosan | |
| Organic tin compounds | |
| Bisphenol A (BPA) | |
| Octylphenol | |
| Cadmium compounds | |
| Colorant | Titanium dioxide |
| Cobalt (II) diacetate | |
| Chromium compounds | |
| Curing agents | Formaldehyde |
| 4,4′-Diaminodiphenylmethane | |
| 2,2′-dichloro-4,4′-methylenedianiline |
| Blend | Properties | Ref. | |
|---|---|---|---|
| Homopolymer–homopolymer | Polyphenylene oxide (PPO)–polystyrene (PS) | Known as NOYRLTM, may be designed to replace metallic parts in mechanical assemblies | [23] |
| Polyethylene terephthalate (PET)–polybutylene terephthalate (PBT) | Heat and chemical resistance, along with excellent processability | [24] | |
| poly(methyl methacrylate) (PMMA)–polyvinylidene fluoride (PVDF) | Combines the rigidity of PMMA and the flexibility of PVDF while lowering the melting point | [25] | |
| Homopolymer–copolymer | Polypropylene (PP)–EPDM rubber | Increased tensile strength | [26] |
| Polycarbonate (PC)–acrylonitrile butadiene styrene (ABS) | Improved toughness, processability, and thermal stability | [27,28] |
| Polymer/Feedstock | Type | Operating Conditions | Products | Ref. |
|---|---|---|---|---|
| PET | Acid hydrolysis | Depolymerization of PET occurs at 100 °C in 96 h and may add catalysts, such as MSO4 | Terephthalic acid (TPA) and ethylene glycol (EG) | [41] |
| PET | Alkaline hydrolysis | Carried out in an alkaline solution of NaOH or KOH with a concentration range of 4–20 wt%, with the reaction taking 3–5 h at 210–250 °C | Ethylene glycol (EG) and terephthalic salts | [42] |
| PET | Transesterification (methanol) | Depolymerized at 65 °C in a stirred reactor, catalyzed using Na2CO3 and a MeOH/EG molar ratio > 15 for 90 min | DMT + EG | [43] |
| Polylactic acid (PLA) | Hydrolysis | Carried out at a temperature of 60–80 °C and takes 15–50 days | Lactic acid | [44] |
| Nylon polymers (nylon 6, nylon 6/6, nylon 12, and nylon 6/12) | Acid hydrolysis | Carried out at 350 °C for 10 min | ϵ-caprolactam | [45] |
| Polymer/Feedstock | Type | Operating Conditions | Products | Ref. |
|---|---|---|---|---|
| LDPE | High-pressure pyrolysis | High pressure up to 51 bar, initial temperature of 330–380 °C, exceed the set temperature by 100 °C at a rate of 150 °C/min, stirring at 200 rpm | Aromatic compounds, isoparaffins, and cycloalkanes | [56] |
| PET | Catalytic pyrolysis | Heating at 700 °C in the presence of a Ca(OH)2 catalyst | Benzene (used for lubricants, dyes, and detergents) | [57] |
| HDPE | Catalytic cold plasma pyrolysis | 18 h at room temperature, then calcinated to 500 °C for 6 h in the presence of HZSM-5 zeolite or sulfated zirconia catalyst | Ethylene | [58] |
| HDPE, LDPE, PP, PS | Catalytic pyrolysis | Stirring at 50 °C until the mixture becomes slurry, then heating at 105 °C for 12 h and calcination at 800 °C for 2 h at a rate of 20 °C/min in the presence of an Fe/Al2O3 catalyst | Amorphous carbon, carbon nanotubes, and hydrogen | [59] |
| PP | Catalytic pyrolysis | Stirring at 100 °C, then drying in an oven at 120 °C and calcination at atmospheric pressure at 500 °C using Ni-Cu/La2O3 catalyst | Multiwalled carbon nanotubes and carbon nanofibers | [60] |
| PS | Flash pyrolysis | Operating at 500 °C | Styrene with byproducts (toluene, ethyl benzene, and α-methyl styrene) | [61] |
| Single-use face masks (PP)/food waste | Single-step pyrolysis | Operating at 900 °C | Hydrocarbon mixtures and hydrogen | [62] |
| Technology Provider | Capacity in Tons per Day | Feedstock | Products | Technology Utilized | Location |
|---|---|---|---|---|---|
| Alterra energy | 60 | HDPE, LDPE, PP, PS, and “other” types of plastics | Syncrude and diesel | Rotary kiln | Akron, OH, USA |
| Nexus | 50 | HDPE, LDPE, PP, and PS with contamination ≤ 1% PVC and ≤2% PET | Light crude, diesel, gasoline, kerosene blendstocks, and wax | Melting vessel | Atlanta, GA, USA |
| Agilyx | 10–50 | Film HDPE, LDPE, PP, and PS | Light synthetic crude oil | Dual screw reactor | Tigard, OR, USA |
| Recycling Technologies | 20 | Soft and flexible packaging (films), multilayered and laminated plastics (crisp packets), and complex and contaminated plastics (food trays) | Low-sulfur hydrocarbon Plaxx—wax | Fluidized bed | Swindon, UK |
| Plastic Energy | 20–30 | Rigid and film HDPE, LDPE, PP, and PS | Raw diesel, light oil, and synthetic gas components | Stirred-tank reactor | Sevilla, Spain |
| Susteen Technologies | 12 | Mainly residual biomass and sewage sludge | Green crude, diesel, gasoline, and jet fuel | Screw with recirculation | Sulzbach-Rosenberg, Germany |
| PHJK | 12–14 | Unsorted plastic waste | Light crude oil and diesel | Rotary kiln | Laihia, Finland |
| PCM | Melting Point (°C) | Latent Heat (kJ/kg) | Thermal Conductivity (W/m.K) |
|---|---|---|---|
| n-Tetradecane | 6 | 228–230 | 0.14 |
| n-Pentadecane | 10 | 205 | 0.2 |
| n-Hexadecane | 18 | 237 | 0.2 |
| n-Heptadecane | 22 | 213 | 0.145 |
| n-Octadecane | 28 | 245 | 0.148 |
| n-Docosane | 44.5 | 249 | 0.2 |
| Gas | GWP (AR49, 100 Year) |
|---|---|
| CO2 | 1 |
| Ammonia (NH3) | 0 |
| Nitrous oxide | 298 |
| Hydrocarbons | |
| Methane | 25 |
| Propane (R-290) | 3 |
| Isobutane (R-600a) | 3 |
| Propylene (R-1270) | <1 |
| HFC | |
| R134a | 1430 |
| R407C | 1774 |
| R410A | 2088 |
| R404A | 3922 |
| HFC-125 | 3500 |
| PFC-14 | 7390 |
| SF6 | 22,800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, K.; Awad, S.; Krawczak, P.; Al Takash, A.; Faraj, J.; Khaled, M. An Overview of the Non-Energetic Valorization Possibilities of Plastic Waste via Thermochemical Processes. Materials 2024, 17, 1460. https://doi.org/10.3390/ma17071460
Moussa K, Awad S, Krawczak P, Al Takash A, Faraj J, Khaled M. An Overview of the Non-Energetic Valorization Possibilities of Plastic Waste via Thermochemical Processes. Materials. 2024; 17(7):1460. https://doi.org/10.3390/ma17071460
Chicago/Turabian StyleMoussa, Kazem, Sary Awad, Patricia Krawczak, Ahmad Al Takash, Jalal Faraj, and Mahmoud Khaled. 2024. "An Overview of the Non-Energetic Valorization Possibilities of Plastic Waste via Thermochemical Processes" Materials 17, no. 7: 1460. https://doi.org/10.3390/ma17071460
APA StyleMoussa, K., Awad, S., Krawczak, P., Al Takash, A., Faraj, J., & Khaled, M. (2024). An Overview of the Non-Energetic Valorization Possibilities of Plastic Waste via Thermochemical Processes. Materials, 17(7), 1460. https://doi.org/10.3390/ma17071460








