Porous High-Entropy Oxide Anode Materials for Li-Ion Batteries: Preparation, Characterization, and Applications
Abstract
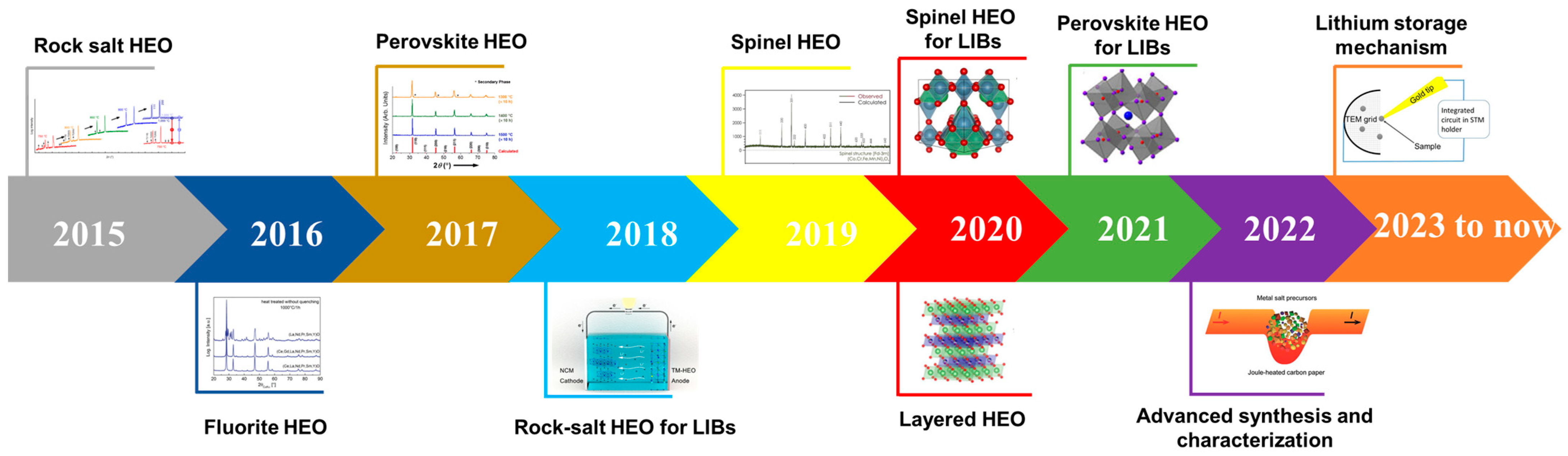
:1. Introduction
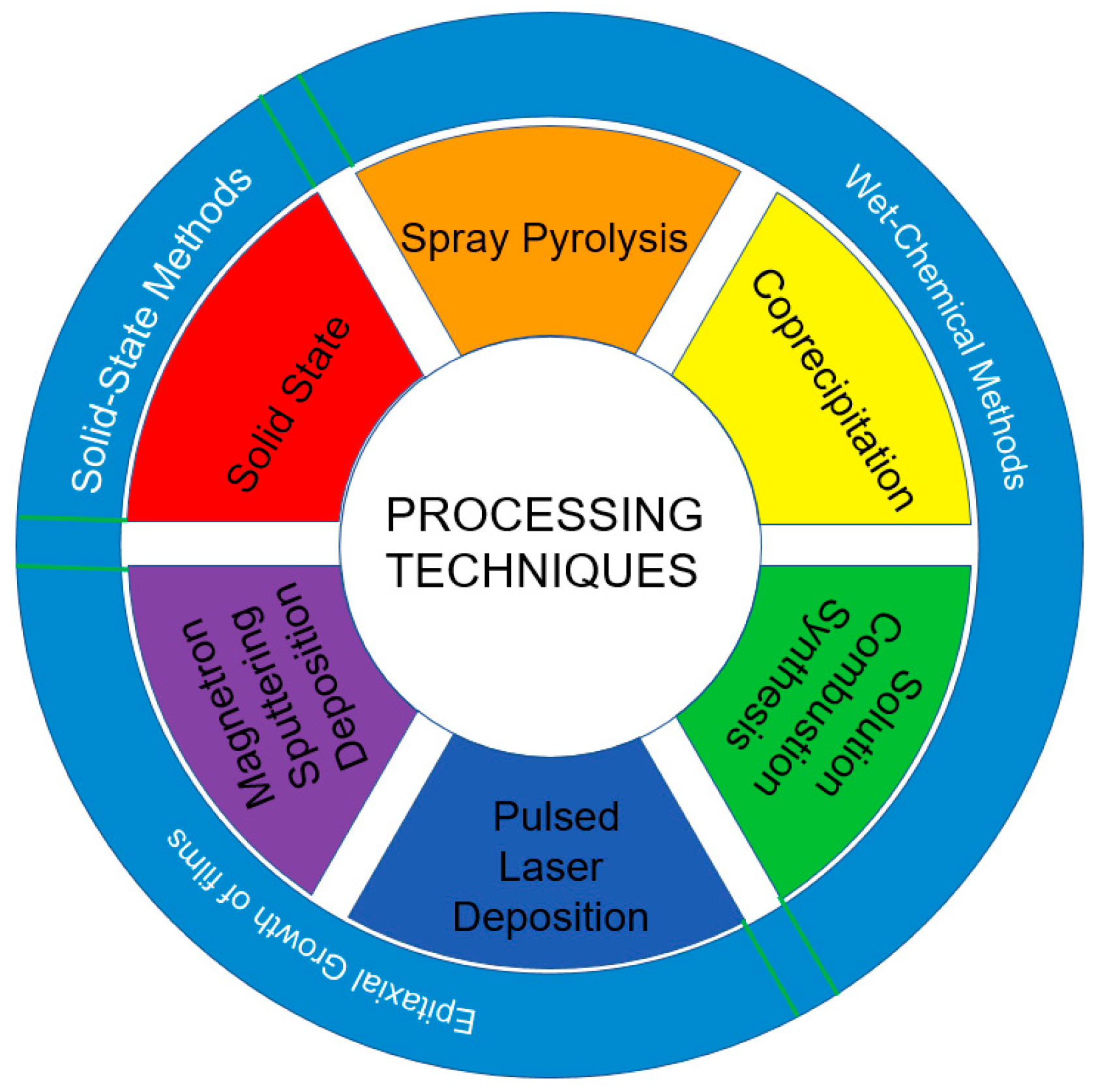
2. Preparation Methods of Porous HEOs
2.1. Solid-State Reaction Method
2.2. Wet Chemical Methods
2.3. Epitaxial Growth of Films
3. Characterization of Porous HEOs
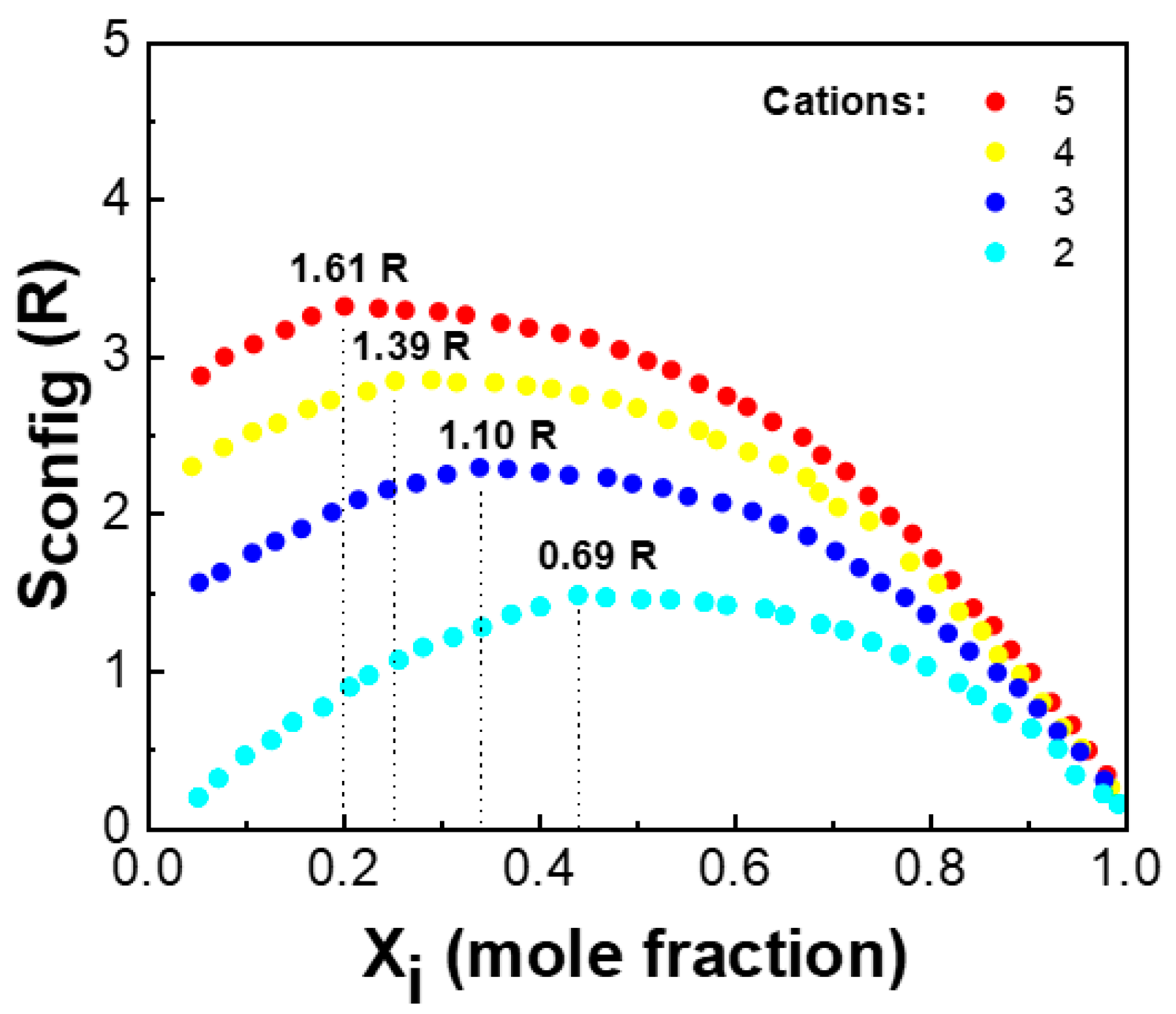
3.1. Crystal Structures of Porous HEOs
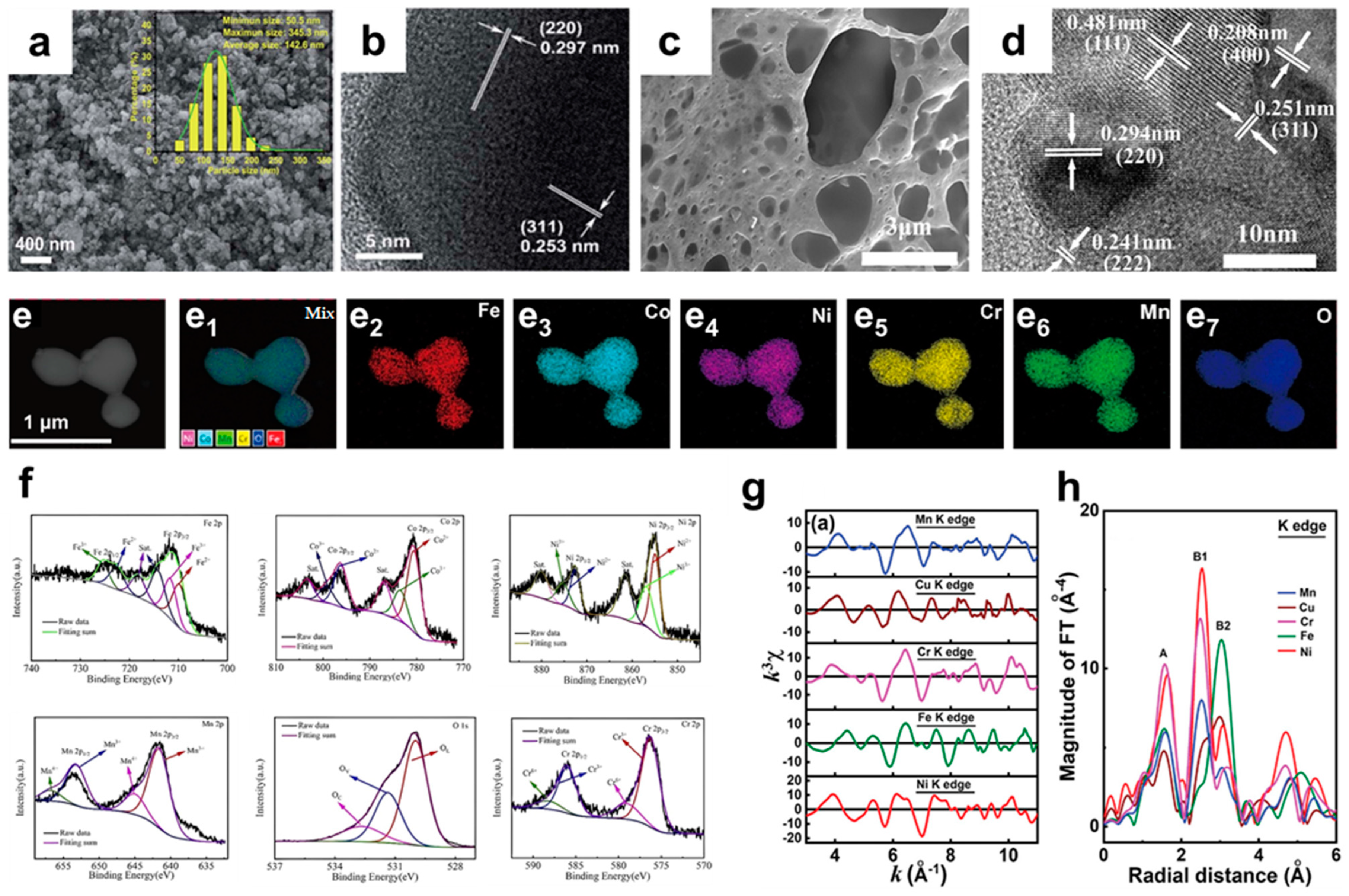
3.2. Structural Characterization of Porous HEOs
3.3. Phase Structure Characterization of Porous HEOs
3.4. Mophological Characterization of Porous HEOs
4. Application of Porous HEOs in Lithium-Ion Battery Anodes
4.1. Electrochemical Properties
4.2. Lithium Storage Mechanism
5. Summary and Outlook
- (1)
- Design and development of new ingredients: Compositional design using high-throughput computing and machine learning and other means to assist high-entropy oxides exploitation, including compositional modulation in a wider range. At the same time, it is necessary to reduce the use of expensive elements as well as harmful elements.
- (2)
- Development of new preparation methods: The low-energy, fast, and efficient preparation of HEOs requires the precise control of the synthesis conditions based on the two key factors of the desired size and morphology, as well as a high yield.
- (3)
- Design of a new structure: Conventional nanoparticles tend to exhibit low initial coulombic efficiencies during electrochemical cycling, limiting the practical application of HEOs. The development of novel microstructures, such as biphasic high-entropy oxides, doped second phases, localized heterogeneous structures, etc., is expected to generate a new lithiation mechanism during charging and discharging, thus improving the lithium storage performance.
- (4)
- Exploration of lithium storage mechanisms: HEOs with multiple active sites lead to complex lithium storage mechanisms, which are still subject to some controversy in the existing reports. Some advanced characterization techniques should be applied to reveal the truth and focus on the phase transitions of single-element effects and the synergistic effects among multiple elements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.J.; Han, J.; Lee, K.; Lee, Y.J.; Kim, B.G.; Jung, K.N.; Kim, B.J.; Lee, S.W. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 2022, 601, 217–222. [Google Scholar] [CrossRef]
- Aini, Q.; Irmawati, Y.; Karunawan, J.; Pasha, M.H.R.; Alini, A.; Iskandar, F.; Sumboja, A. Para grass-derived porous carbon-rich SiOx/C as a stable anode for lithium-ion batteries. Nature 2023, 37, 11397–11405. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zhao, S.; Lai, W.; Sun, B.; Notten, P.; Wang, G. Oxygen redox chemistry in lithium-rich cathode materials for Li-ion batteries: Understanding from atomic structure to nano-engineering. Nano Mater. Sci. 2022, 4, 322–338. [Google Scholar] [CrossRef]
- Dubarry, M.; Costa, N.; Matthews, D. Data-driven direct diagnosis of Li-ion batteries connected to photovoltaics. Nat. Commun. 2023, 14, 3138. [Google Scholar] [CrossRef]
- Nguyen Thi, X.M.; Le, K.M.; Phung, Q.; Truong, D.Q.; Nguyen, H.V.; Nguyen, Q.N.; Huynh, T.T.K.; Pham, L.T.; Van, M.T.; Le, P.M.L. Improving the electrochemical performance of lithium-ion battery using silica/carbon anode through prelithiation techniques. Battery Energy 2023, 2, 20230003. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yan, Y.J.; Zhang, Y.G.; Chen, Y.X.; Peng, X.Y.; Wang, X.; Zhao, W.M.; Qin, C.L.; Liu, Q.; Liu, X.J.; et al. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium–sulfur batteries. Carbon Energy 2023, 5, e306. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Liu, X.; Chen, Y.; Zhao, Y.; Zhang, Y.; Han, Q.; Qin, C.; Bakenov, Z.; Wang, Y.; et al. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li–S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Lee, B.S.; Oh, S.H.; Choi, Y.J.; Yi, M.J.; Kim, S.H.; Kim, S.Y.; Sung, Y.E.; Shin, S.Y.; Lee, Y.J.; Yu, S.H. SiO-induced thermal instability and interplay between graphite and SiO in graphite/SiO composite anode. Nat. Commun. 2023, 14, 150. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Biesold, G.M.; Sewell, C.D.; Hao, S.M.; Huang, J.Y.; Zhang, W.; Lai, Y.K.; Lin, Z.Q. Recent advance in silicon-based electrodes: From fundamental research toward practical applications. Adv. Mater. 2021, 33, 2004577. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.W.; Kim, S.S.; Senthil, C.; Jung, H.Y. Freestanding conversion-type anode via one-pot formation for flexible Li-ion battery. Chem. Eng. J. 2022, 427, 130937. [Google Scholar] [CrossRef]
- Ma, Y.J.; Ma, Y.; Wang, Q.S.; Schweidler, S.; Botros, M.; Fu, T.T.; Hahn, H.; Brezesinski, T.; Breitung, B. High-entropy energy materials: Challenges and new opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, X.K.; Zhang, H.J.; Jia, Q.L.; Zhang, S.W.; Lei, W. High-entropy oxide: A future anode contender for lithium-ion battery. EcoMat. 2022, 4, e12261. [Google Scholar] [CrossRef]
- Salian, A.; Mandal, S. Entropy stabilized multicomponent oxides with diverse functionality—A review. Crit. Rev. Solid State Mater. Sci. 2021, 47, 142–193. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, N.; Chamas, M.; Hu, C.F.; Grasso, S. Sustainable high-entropy ceramics for reversible energy storage: A short review. Int. J. Appl. Ceram. Technol. 2021, 18, 1560–1569. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Y.J.; Sun, C.; Ni, Q.; Wang, C.Z.; Jin, H.B. High entropy spinel-structure oxide for electrochemical application. Chem. Eng. J. 2022, 431, 133448. [Google Scholar] [CrossRef]
- Murthy, A.A.; Subiantoro, A.; Norris, S.; Fukuta, M. A review on expanders and their performance in vapour compression refrigeration systems. Int. J. Refrig. 2019, 106, 427–446. [Google Scholar] [CrossRef]
- Chen, K.P.; Pei, X.T.; Tang, L.; Cheng, H.R.; Li, Z.M.; Li, C.W.; Zhang, X.W.; An, L.N. A five-component entropy-stabilized fluorite oxide. J. Eur. Ceram. Soc. 2018, 38, 4161–4164. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Wang, D.; Hein, C.; Kautenburger, R.; Clemens, O.; Hahn, H. Rare earth and transition metal based entropy stabilised perovskite type oxides. J. Eur. Ceram. Soc. 2018, 38, 2318–2327. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Bérardan, D.; Franger, S.; Dragoe, D.; Meena, A.K.; Dragoe, N. Colossal dielectric constant in high entropy oxides. Phys. Status Solidi RRL 2016, 10, 328–333. [Google Scholar] [CrossRef]
- Liu, Z.; Yuan, X.; Zhang, S.; Wang, J.; Huang, Q.; Yu, N.; Zhu, Y.; Fu, L.; Wang, F.; Chen, Y.; et al. Three-dimensional ordered porous electrode materials for electrochemical energy storage. NPG Asia Mater. 2019, 11, 12. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.D.; Duan, C.Q.; Mao, J.; Dong, Y.; Dong, K.Z.; Wang, Z.Y.; Luo, S.H.; Liu, Y.G.; Qi, X.W. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Marques, O.J.B.J.; Walter, M.D.; Timofeeva, E.V.; Segre, C.U.; Kim, D.E.; Hong, S.L. Effect of initial structure on performance of high-entropy oxide anodes for li-ion batteries. Batteries 2023, 9, 115. [Google Scholar] [CrossRef]
- Sarkar, A.; Djenadic, R.; Usharani, N.J.; Sanghvi, K.P.; Chakravadhanula, V.S.K.; Gandhi, A.S.; Hahn, H.; Bhattacharya, S.S. Nanocrystalline multicomponent entropy stabilised transition metal oxides. J. Eur. Ceram. Soc. 2017, 37, 747–754. [Google Scholar] [CrossRef]
- Triolo, C.; Maisuradze, M.; Li, M.; Liu, Y.C.; Ponti, A.; Pagot, G.; Di Noto, V.; Aquilanti, G.; Pinna, N.; Giorgetti, M.; et al. Charge storage mechanism in electrospun spinel-structured high-entropy (Mn0.2Fe0.2Co0.2Ni0.2Zn0.23O4 oxide nanofibers as anode material for li-ion batteries. Small 2023, 19, 2304585. [Google Scholar] [CrossRef]
- Minouei, H.; Tsvetkov, N.; Kheradmandfard, M.; Han, J.H.; Kim, D.E.; Hong, S.L. Tuning the electrochemical performance of high-entropy oxide nanopowder for anode Li-ion storage via structural tailoring. J. Power Sources 2022, 549, 232041. [Google Scholar] [CrossRef]
- Shin, D.; Chae, S.; Park, S.; Seo, S.; Choi, W. Rational engineering of high-entropy oxides for Li-ion battery anodes with finely tuned combustion syntheses. NPG Asia Mater. 2023, 15, 54. [Google Scholar] [CrossRef]
- Bayraktar, D.O.; Lokcu, E.; Ozgur, C.; Erdil, T.; Toparli, C. Effect of synthesis environment on the electrochemical properties of (FeMnCrCoZn)3O4 high-entropy oxides for li-ion batteries. Int. J. Energy Res. 2022, 46, 22124–22133. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A novel technique to synthesize advanced materials. Research 2019, 2019, 4219812. [Google Scholar] [CrossRef]
- Tallarita, G.; Licheri, R.; Garroni, S.; Orrù, R.; Cao, G. Novel processing route for the fabrication of bulk high-entropy metal diborides. Scr. Mater. 2019, 158, 100–104. [Google Scholar] [CrossRef]
- Meisenheimer, P.B.; Kratofil, T.J.; Heron, J.T. Giant enhancement of exchange coupling in entropy-stabilized oxide heterostructures. Sci. Rep. 2017, 7, 13344. [Google Scholar] [CrossRef]
- Yang, Z.M.; Zhang, K.; Qiu, N.; Zhang, H.B.; Wang, Y.; Chen, J. Effects of helium implantation on mechanical properties of (Al0.31Cr0.20Fe0.14Ni0.35)O high entropy oxide films. Chin. Phys. B 2019, 28, 046201. [Google Scholar] [CrossRef]
- Bunpang, K.K.; Singkammo, S.; Cann, D.P.; Raengthon, N. Titanate-based high-entropy perovskite oxides relaxor ferroelectrics. Sci. Rep. 2024, 14, 6017. [Google Scholar] [CrossRef]
- Dong, Q.; Hong, M.; Gao, J.L.; Li, T.Y.; Cui, M.J.; Li, S.K.; Qiao, H.Y.; Brozena, A.H.; Yao, Y.G.; Wang, X.Z.; et al. Rapid synthesis of high-entropy oxide microparticles. Small. 2022, 18, 2104761. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, N.; Wu, B.Z.; Yang, Z.M.; Sun, S.; Wang, Y. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries. RSC Adv. 2020, 10, 9736–9744. [Google Scholar] [CrossRef]
- Chellali, M.R.; Sarkar, A.; Nandam, S.H.; Bhattacharya, S.S.; Breitung, B.; Hahn, H.; Velasco, L. On the homogeneity of high entropy oxides: An investigation at the atomic scale. Scr. Mater. 2019, 166, 58–63. [Google Scholar] [CrossRef]
- Kamecki, B.; Karczewski, J.; Cempura, G.; Jasinski, P.; Molin, S. Evaluation of structural and electrical properties of multicomponent spinel oxide thin films deposited via spray pyrolysis technique. Mater. Charact. 2023, 203, 113097. [Google Scholar] [CrossRef]
- Sarkar, A.; Velasco, L.; Wang, D.; Wang, Q.; Talasila, G.; Biasi, L.; Kübel, C.; Brezesinski, T.; Bhattacharya, S.S.; Hahn, H. High entropy oxides for reversible energy storage. Nat. Commun. 2018, 9, 3400. [Google Scholar] [CrossRef]
- Goncalves, J.M.; Ghorbani, A.; Ritter, T.G.; Lima, I.S.; Saray, M.T.; Phakatkar, A.H.; Silva, V.D.; Pereira, R.S.; Yarin, A.L.; Angnes, L. Multimetallic glycerolate as a precursor template of spherical porous high-entropy oxide microparticles. J. Colloid Interface Sci. 2023, 641, 643–652. [Google Scholar] [CrossRef]
- Salian, A.; Pujar, P.; Vardhan, R.V.; Cho, H.W.; Kim, S.; Mandal, S. Evolution of high dielectric permittivity in low-temperature solution combustion-processed phase-pure high entropy oxide (CoMnNiFeCr)O for thin film transistors. J. Eur. Ceram. Soc. 2023, 5, 2608–2623. [Google Scholar] [CrossRef]
- He, L.; Kang, H.J.; Hou, G.Y.; Qiao, X.S.; Jia, J.; Qin, W.; Wu, X.H. Low-temperature synthesis of nano-porous high entropy spinel oxides with high grain boundary density for oxygen evolution reaction. Chem. Eng. J. 2023, 460, 141675. [Google Scholar] [CrossRef]
- Nikiforova, G.E.; Kondrat’eva, O.N.; Tyurin, A.V.; Ryumin, M.A.; Guskov, V.N.; Gavrichev, K.S. Thermophysical properties of M′-LuTaO4: Structural and calorimetric studies. J. Alloys Compd. 2019, 803, 1016–1022. [Google Scholar] [CrossRef]
- Spiridigliozzi, L.; Dell’Agli, G.; Callone, E.; Dirè, S.; Campostrini, R.; Bettotti, P.; Bortolotti, M.; Speranza, G.; Sglavo, V.M.; Biesuz, M. A structural and thermal investigation of Li-doped high entropy (Mg,Co,Ni, Cu, Zn)O obtained by co-precipitation. J. Alloys Compd. 2022, 927, 166933. [Google Scholar] [CrossRef]
- Kotsonis, G.N.; Rost, C.M.; Harris, D.T.; Maria, J.P. Epitaxial entropy-stabilized oxides: Growth of chemically diverse phases via kinetic bombardment. MRS Commun. 2018, 8, 1371–1377. [Google Scholar] [CrossRef]
- Sharma, Y.; Musico, B.L.; Gao, X.; Hua, C.; May, A.F.; Herklotz, A.; Rastogi, A.; Mandrus, D.; Yan, J.; Lee, H.N.; et al. Single-crystal high entropy perovskite oxide epitaxial films. Phys. Rev. Mater. 2018, 2, 060404. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Zhang, X.; Choi, S.; Kim, D.; Lee, L.Y.S.; Lee, K. Stabilization, characterization, and electrochemical applications of high-entropy oxides: Critical assessment of crystal phase–properties relationship. Adv. Funct. Mater. 2022, 32, 2205142. [Google Scholar] [CrossRef]
- Biesuz, M.; Chen, J.; Bortolotti, M.; Speranza, G.; Esposito, V.; Sglavo, V.M. Ni-free high-entropy rock salt oxides with Li superionic conductivity. J. Mater. Chem. A 2022, 10, 23603–23616. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Stygar, M.; Mikuła, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co,Cr,Fe,Mn,Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Ko, S.T.; Lee, T.; Qi, J.; Zhang, D.W.; Peng, W.T.; Wang, X.; Tsai, W.C.; Sun, S.K.; Wang, Z.K.; Bowman, W.J.; et al. Compositionally complex perovskite oxides: Discovering a new class of solid electrolytes with interface-enabled conductivity improvements. Matter 2023, 6, 2395–2418. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.Y.; Hu, C.Z.; Yeh, Y.T.; Chen, R.K.; Luo, J. Single-phase duodenary high-entropy fluorite/pyrochlore oxides with an order-disorder transition. Acta Mater. 2021, 211, 116858. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Walczak, K.; Plewa, A.; Ghica, C.; Zajac, W.; Trenczek, Z.A.; Zajac, M.; Tobo, J.; Molenda, J. NaMn0.2Fe0.2Co0.2Ni0.2Ti0.2O2 high-entropy layered oxide—Experimental and theoretical evidence of high electrochemical performance in sodium batteries. Energy Storage Mater. 2022, 47, 500–514. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Bian, X.; Hatakeyama, T.; Li, H.; Ichitsubo, T. Influences of enhanced entropy in layered rock-salt oxide cathodes for lithium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 4369–4381. [Google Scholar] [CrossRef]
- Xiang, H.Z.; Xie, H.X.; Chen, Y.X.; Zhang, H.; Mao, A.Q.; Zheng, C.H. Porous spinel-type (Al0.2CoCrFeMnNi)0.58O4-δ high-entropy oxide as a novel high-performance anode material for lithium-ion batteries. J. Mater. Sci. 2021, 56, 8127–8142. [Google Scholar] [CrossRef]
- Ponti, A.; Triolo, C.; Petrovicova, B.; Ferretti, A.M.; Pagot, G.; Xu, W.L.; Di, N.V.; Pinna, N.; Santangelo, S. Structure and magnetism of electrospun porous high-entropy (Cr1/5Mn1/5Fe1/5Co1/5Ni1/5)3O4, (Cr1/5Mn1/5Fe1/5Co1/5Zn1/5)3O4 and (Cr1/5Mn1/5Fe1/5Ni1/5Zn1/5)3O4 spinel oxide nanofibers. Phys. Chem. Chem. Phys. 2023, 25, 2212–2226. [Google Scholar] [CrossRef]
- Yang, X.B.; Wang, H.Q.; Song, Y.Y.; Liu, K.T.; Huang, T.T.; Wang, X.Y.; Zhang, C.F.; Li, J. Low-temperature synthesis of a porous high-entropy transition-metal oxide as an anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces. 2022, 14, 26873–26881. [Google Scholar] [CrossRef]
- Wang, Q.S.; Sarkar, A.; Li, Z.Y.; Lu, Y.; Velasco, L.; Bhattacharya, S.S.; Brezesinski, T.; Hahn, H.; Breitung, B. High entropy oxides as anode material for Li-ion battery applications: A practical approach. Electrochem. Commun. 2019, 100, 121–125. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kübel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2016, 5, 102–109. [Google Scholar] [CrossRef]
- Jiang, S.C.; Hu, T.; Gild, J.; Zhou, N.X.; Nie, J.Y.; Qin, M.; Harrington, T. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Wang, J.B.; Cui, Y.Y.; Wang, Q.S.; Wang, K.; Huang, X.H.; Stenzel, D.; Sarkar, A.; Azmi, R.; Bergfeldt, T.; Bhattacharya, S.S.; et al. Lithium containing layered high entropy oxide structures. Sci. Rep. 2020, 10, 18430. [Google Scholar] [CrossRef]
- Wang, K.; Hua, W.B.; Huang, X.H.; Stenzel, D.; Wang, J.B.; Ding, Z.M.; Cui, Y.Y.; Wang, Q.S.; Ehrenberg, H.; Breitung, B.; et al. Synergy of cations in high entropy oxide lithium ion battery anode. Nat. Commun. 2023, 14, 1487. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Zherebtsov, D.A.; Starikov, A.Y.; Sherstyuk, D.P.; Gudkova, S.A.; Taskaev, S.A. The new extremely substituted high entropy (Ba,Sr,Ca,La)Fe6-x(Al,Ti,Cr,Ga,In,Cu,W)xO19 microcrystals with magnetoplumbite structure. Ceram. Int. 2020, 46, 9656–9660. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Lu, Y.; Wang, Q.; Liu, J.; Wang, Y. High-entropy environmental barrier coating for the ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 2574–2579. [Google Scholar] [CrossRef]
- Kirnbauer, A.; Spadt, C.; Koller, C.M.; Kolozsvári, S.; Mayrhofer, P.H. High-entropy oxide thin films based on Al–Cr–Nb–Ta–Ti. Vacuum 2019, 168, 108850. [Google Scholar] [CrossRef]
- Feltrin, A.C.; Akhtar, F. High-temperature oxidation kinetics of a metastable dual-phase diboride and a high-entropy diboride. J. Eur. Ceram. Soc. 2023, 43, 7363–7372. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Z.; Liu, S.; Li, G.; Gao, X. High-entropy perovskite oxide nanofibers as efficient bidirectional electrocatalyst of liquid-solid conversion processes in lithium-sulfur batteries. Nano Energy 2023, 106, 108037. [Google Scholar] [CrossRef]
- Jothi, P.R.; Liyanage, W.; Jiang, B.; Paladugu, S.; Olds, D.; Gilbert, D.A.; Page, K. Persistent structure and frustrated magnetism in high entropy rare-earth zirconates. Small 2022, 18, 2101323. [Google Scholar] [CrossRef]
- Yan, X.; Wang, C.; Ai, T.; Li, Z.; Niu, Y. Synthesis of porous (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high entropy oxide catalysts for peroxymonosulfate activation toward tetracycline degradation. Inorg. Chem. Commun. 2023, 150, 110547. [Google Scholar] [CrossRef]
- Li, H.; Duan, Y.; Zhao, Z.; Cheng, X.; Bian, W.; Xiao, Z. Atomic-scale storage mechanism in ultra-small size (FeCuCrMnNi)3O4/rGo with super-stable sodium storage and accelerated kinetics. Chem. Eng. J. 2023, 469, 143951. [Google Scholar] [CrossRef]
- Duan, C.Q.; Tian, K.H.; Li, X.L.; Wang, D.; Sun, H.Y.; Zheng, R.G.; Wang, Z.Y.; Liu, Y.G. New spinel high-entropy oxides (FeCoNiCrMnXLi)3O4 (X = Cu, Mg, Zn) as the anode material for lithium-ion batteries. Ceram. Int. 2021, 47, 32025–32032. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Tsai, C.C.; Patra, J.; Clemens, O.; Chang, J.K.; Ting, J.M. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in lithium-ion batteries. Chem. Eng. J. 2022, 430, 132658. [Google Scholar] [CrossRef]
- An, Y.; Wan, K.; Song, M.; Zhao, L. Thermal insulating and mechanical properties of high entropy pyrochlore oxide ceramic with hierarchical porous structures. Ceram. Int. 2024, 50, 4699–4707. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Li, H.; Wang, L.; Chen, K.; An, L. Porous (Ce0.2Zr0.2Ti0.2Sn0.2Ga0.2)O2–δ high-entropy ceramics with both high strength and low thermal conductivity. J. Eur. Ceram. Soc. 2021, 41, 309–314. [Google Scholar] [CrossRef]
- Han, B.; Pan, Q.; Chen, Y.; Liu, D.; Zhou, C.; Xia, K.; Gao, Q. High-entropy perovkite oxide washcoated porous alumina ceramic as a superb catalyst for activating peroxymonosulfate to eliminate refractory organic pollutants. Chem. Eng. J. 2023, 455, 140828. [Google Scholar] [CrossRef]
- Marques, O.J.; Chen, C.; Timofeeva, E.V.; Segre, C.U. Local structure and conversion chemistry of high-entropy oxides as Li-ion anodes. J. Power Source 2023, 564, 232852. [Google Scholar] [CrossRef]
- Dong, L.S.; Wang, Z.G.; Mi, C.; Zhao, W.M.; Qin, C.L.; Luo, C.; Wang, Z.F. Defect-rich hierarchical porous spinel MFe2O4 (M = Ni, Co, Fe, Mn) as high-performance anode for lithium ion batteries. Mater. Today Chem. 2024, 35, 101853. [Google Scholar] [CrossRef]
- Dong, L.S.; Wang, Z.G.; Li, Y.Y.; Jin, C.H.; Dong, F.B.; Zhao, W.M.; Qin, C.L.; Wang, Z.F. Spinel-structured, multi-component transition metal oxide (Ni,Co,Mn)Fe2O4−x as long-life lithium-ion battery anode material. Batteries 2023, 9, 54. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-anionic and-cationic compounds: New high entropy materials for advanced Li-ion batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Moździerz, M.; Dąbrowa, J.; Stępień, A.; Zajusz, M.; Stygar, M.; Zając, W.; Danielewski, M.; Świerczek, K. Mixed ionic-electronic transport in the high-entropy (Co,Cu,Mg,Ni,Zn)1−xLixO oxides. Acta Mater. 2021, 208, 116735. [Google Scholar] [CrossRef]
- Xiao, B.; Wu, G.; Wang, T.D.; Wei, Z.G.; Sui, Y.W.; Shen, B.L.; Qi, J.Q.; Wei, F.X.; Zheng, J.C. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries. Nano Energy 2022, 95, 106962. [Google Scholar] [CrossRef]
- Luo, X.F.; Patra, J.; Chuang, W.T.; Nguyen, T.X.; Ting, J.M.; Li, J.; Pao, C.W.; Chang, J.K. Charge-discharge mechanism of high-entropy Co-free spinel oxide toward Li+ storge examined using operando quick-scanning X-ray absorption spectroscopy. Adv. Sci. 2022, 9, 2201219. [Google Scholar] [CrossRef]
- Shin, H.; Lee, Y.K.; Lu, W. Structural degradation of graphite anode induced by dissolved manganese ions in lithium-ion batteries. J. Power Sources 2023, 6, e12662. [Google Scholar] [CrossRef]
- Ryu, K.; Lee, M.J.; Lee, K.B.; Lee, S.W. ZnO-embedded expanded graphite composite anodes with controlled charge storage mechanism enabling operation of lithium-ion batteries at ultra-low temperatures. Energy Environ. Mater. 2022, 3, 2200086. [Google Scholar] [CrossRef]
- Luo, C.; Wang, Z.G.; Chen, Y.X.; Zhao, Y.M.; Han, Q.Q.; Qin, C.L.; Wang, Z.F. Eutectic-derived bimodal porous Ni@NiO nanowire networks for high-performance Li-ion battery anodes. Int. J. Energy Res. 2022, 46, 24654–24666. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Zhang, W.Q.; Zhang, Y.G.; Li, Y.Y.; Qin, C.L.; Zhao, W.M.; Bakenov, Z. Dual-network nanoporous NiFe2O4/NiO composites for high performance Li-ion battery anodes. Chem. Eng. J. 2020, 388, 124207. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Wang, Y.C.; Zhang, Y.G.; Li, Y.Y.; Zhao, W.M.; Qin, C.L.; Mukanova, A.; Bakenov, Z. Bimodal nanoporous NiO@Ni-Si network prepared by dealloying method for stable Li-ion storage. J. Power Sources 2020, 449, 227750. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Huang, Y.; Du, F.; Zeng, Y. Entropy stabilization effect and oxygen vacancies enabling spinel oxide highly reversible lithium-ion storage. ACS Appl. Mater. Interfaces 2021, 13, 58674–58681. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Chang, J.K.; Ting, J.M. High entropy spinel oxide nanoparticles for superior lithiation–delithiation performance. J. Mater. Chem. A 2020, 8, 18963–18973. [Google Scholar] [CrossRef]
- Mozdzierz, M.; Swierczek, K.; Dabrowa, J.; Gajewska, M.; Hanc, A.; Feng, Z.H.; Cieslak, J.; Kadziolka, G.M.; Plotek, J.; Marzec, M.; et al. High-Entropy Sn0.8(Co0.2Mg0.2Mn0.2Ni0.2Zn0.2)2.2O4 conversion- alloying anode material for Li-ion cells: Altered lithium storage mechanism, activation of Mg, and origins of the improved cycling stability. ACS Appl. Mater. Interfaces 2022, 14, 42057–42070. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.M.; Sun, S.; Wang, Y.; Cui, Y.H. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O) with superior lithium storage performance. J. Alloys Compd. 2019, 777, 767–774. [Google Scholar] [CrossRef]
- Cai, Z.P.; Ma, C.; Kong, X.Y.; Wu, X.Y.; Wang, K.X.; Chen, J.S. High-performance PEO-based all-solid-state battery achieved by Li-conducting high entropy oxides. ACS Appl. Mater. Interfaces 2022, 14, 57047–57054. [Google Scholar] [CrossRef]
- Biesuz, M.; Spiridigliozzi, L.; Dell’Agli, G.; Bortolotti, M.; Sglavo, V.M. Synthesis and sintering of (Mg, Co, Ni, Cu, Zn)O entropy-stabilized oxides obtained by wet chemical methods. J. Mater. Sci. 2018, 53, 8074–8085. [Google Scholar] [CrossRef]
- Breitun, B.; Wang, Q.S.; Schiele, A.; Tripkovic, D.; Sarkar, A.; Velasco, L.; Wang, D.; Bhattacharya, S.S.; Hahn, H.; Brezesinski, T. Gassing behavior of high-entropy oxide anode and oxyfluoride cathode probed using differential electrochemical mass spectrometry. Batter. Supercaps 2020, 3, 361–369. [Google Scholar] [CrossRef]
- Wei, J.L.; Rong, K.; Li, X.L.; Wang, Y.C.; Qiao, Z.A.; Fang, Y.X.; Dong, X.J. Deep eutectic solvent assisted facile synthesis of low-dimensional hierarchical porous high-entropy oxides. Nano Res. 2022, 15, 2756–2763. [Google Scholar] [CrossRef]
- Liu, X.F.; Xing, Y.Y.; Xu, K.; Zhang, H.J.; Gong, M.X.; Jia, Q.L.; Zhang, S.W.; Lei, W. Kinetically accelerated lithium storage in high-Entropy (LiMgCoNiCuZn)O enabled by oxygen vacancies. Small 2022, 18, 2200524. [Google Scholar] [CrossRef]
- Grzesik, Z.; Smola, G.; Miszczak, M.; Stygar, M.; Dabrowa, J.; Zajusz, M.; Swierczek, K.; Danielewski, M. Defect structure and transport properties of (Co,Cr,Fe,Mn,Ni)3O4 spinelstructured high entropy oxide. J. Eur. Ceram. Soc. 2019, 40, 835–839. [Google Scholar] [CrossRef]
- Wang, B.; Yao, J.; Wang, J.H.; Chang, A. Spinel-type high-entropy (Co0.2Mn0.2Fe0.2Zn0.2Ti0.2)3O4 oxides constructed from disordered cations and oxygen vacancies. J. Alloys Compd. 2022, 897, 163188. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, X.; Lan, X.X.; Hu, R. A Spinel (FeNiCrMnMgAl)3O4 high entropy oxide as a cycling stable anode material for Li-ion batteries. Processes 2021, 10, 49. [Google Scholar] [CrossRef]
- Patra, J.; Nguyen, T.X.; Tsai, C.C.; Clemens, O.; Li, J.; Pal, P.; Chan, W.K.; Lee, C.H.; Chen, H.Y.T.; Ting, J.M.; et al. Effects of elemental modulation on phase purity and electrochemical properties of Co-free high-entropy spinel oxide anodes for lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2110992. [Google Scholar] [CrossRef]
- Su, L.; Ren, J.K.; Lu, T.; Chen, K.X.; Ouyang, J.W.; Zhang, Y.; Zhu, X.Y.; Wang, L.Y.; Min, H.H.; Luo, W.; et al. Deciphering structural origins of highly reversible lithium storage in high entropy oxides with in situ transmission electron microscopy. Adv. Mater 2023, 35, 2205751. [Google Scholar] [CrossRef]
- Nowak, M.; Walczak, K.; Milewska, A.; Plotek, J.; Budziak, J.; Molenda, J. Electrochemical performance of different high-entropy cathode materials for Na-ion batteries. J. Alloys Compd. 2023, 968, 172316. [Google Scholar] [CrossRef]
- Beere, H.K.; Saray, N.S.; Reddy, X.Z.; Kulkarni, P.; Samanta, K.; Jung, H.Y.; Ghosh, D. Compositionally complex ball-in-ball type metal oxide anode via laser-induced fast fabrication for binder-free high-capacity Li-ion batteries. ACS Nano 2024, 80, 110325. [Google Scholar] [CrossRef]
- Bano, A.; Noked, M.; Major, D.T. Theoretical insights into high-entropy Ni-Rich layered oxide cathodes for low-strain li-ion batteries. Chem. Mater. 2023, 35, 8426–8439. [Google Scholar] [CrossRef]
- Brandt, T.G.; Tuokkola, A.R.; Yu, M.J.; Laine, R.M. Liquid-feed flame spray pyrolysis enabled synthesis of Co- and Cr-free, high-entropy spinel oxides as Li-ion anodes. Chem. Eng. J. 2023, 474, 145495. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, S.Y.; Kuo, C.H.; Huang, S.C.; Lin, M.H.; Li, C.H.; Chen, H.Y.T.; Wang, C.C.; Liao, Y.F.; Lin, C.C. In operando synchrotron X-ray studies of a novel spinel (Ni0.2Co0.2Mn0.2Fe0.2Ti0.2)3O4 high-entropy oxide for energy storage applications. J. Mater. Chem. A 2020, 8, 21756–21770. [Google Scholar] [CrossRef]
- Lokcu, E.; Anik, M. Investigating the structural and lithium storage properties of high-entropy oxides in the Mg-Co-Ni-Cu-Zn-O system. Scr. Mater. 2024, 240, 115840. [Google Scholar] [CrossRef]
- Ritter, T.G.; Goncalves, J.M.; Stoyanov, S.; Ghorbani, A.; Shokuhfar, T.; Shahbazian, Y.R. AlTiMgLiO medium entropy oxide additive for PEO-based solid polymer electrolytes in lithium ion batteries. J. Energy Storage 2023, 72, 108491. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Patra, J.; Tsai, C.C.; Xuan, W.Y.; Chen, H.Y.T.; Dyer, M.S.; Clemens, O.; Li, J.; Majumder, S.B.; Chang, J.K.; et al. Secondary-phase-induced charge-discharge performance enhancement of Co-free high entropy spinel oxide electrodes for Li-ion batteries. Adv. Funct. Mater. 2023, 33, 2300509. [Google Scholar] [CrossRef]
- Huang, C.Y.; Huang, C.W.; Wu, M.C.; Patra, J.; Nguyen, T.; Chang, M.T.; Clemens, O.; Ting, J.M.; Chang, J.K.; Wu, W.W. Atomic-scale investigation of lithium/delithiation mechanism in high-entropy spinel oxide with superior electrochemical performance. Chem. Eng. J. 2021, 420, 129839. [Google Scholar] [CrossRef]
- Manojkumar, S.; Lang, C.H.; Wu, S.H.; Chang, J.K.; Rajan, J. Systematic study of Co-free LiNi0.9Mn0.07Al0.03O2 Ni-rich cathode materials to realize high-energy density Li-ion batteries. J. Colloid Interface Sci. 2024, 661, 1070–1081. [Google Scholar] [CrossRef]
- Kondo, T.; Matsumura, K.; Rozier, P.; Simon, P.; Machida, K.; Takeda, S.; Ishimoto, S.; Tamamitsu, K.; Iwama, E.; Naoi, W.; et al. Enhancing the phase stability of γ-phase Li3VO4 for high-performance hybrid supercapacitors: Investigating influential factors and mechanistic insights. Chem. Mater. 2024, 36, 2495–2507. [Google Scholar] [CrossRef]


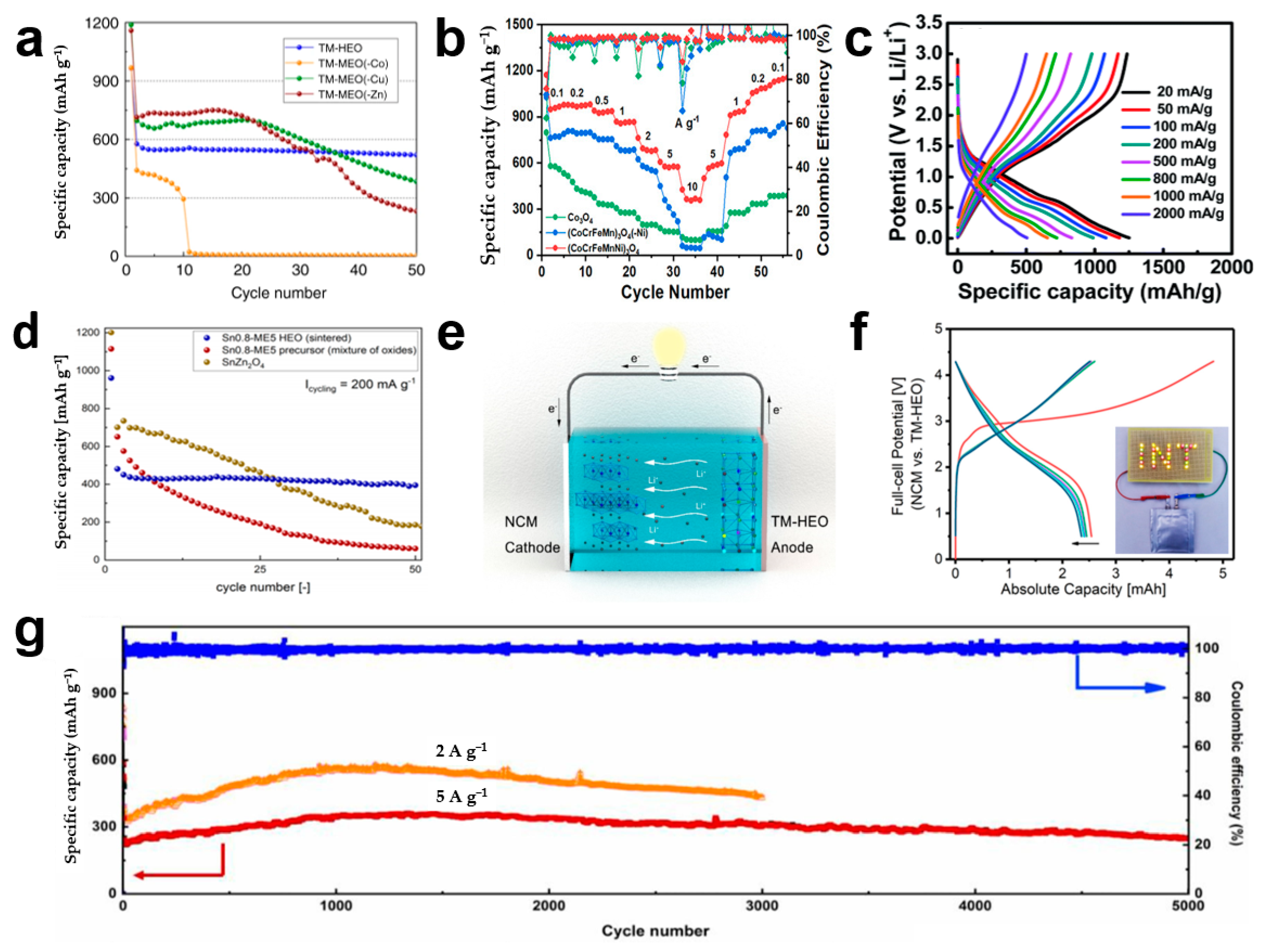

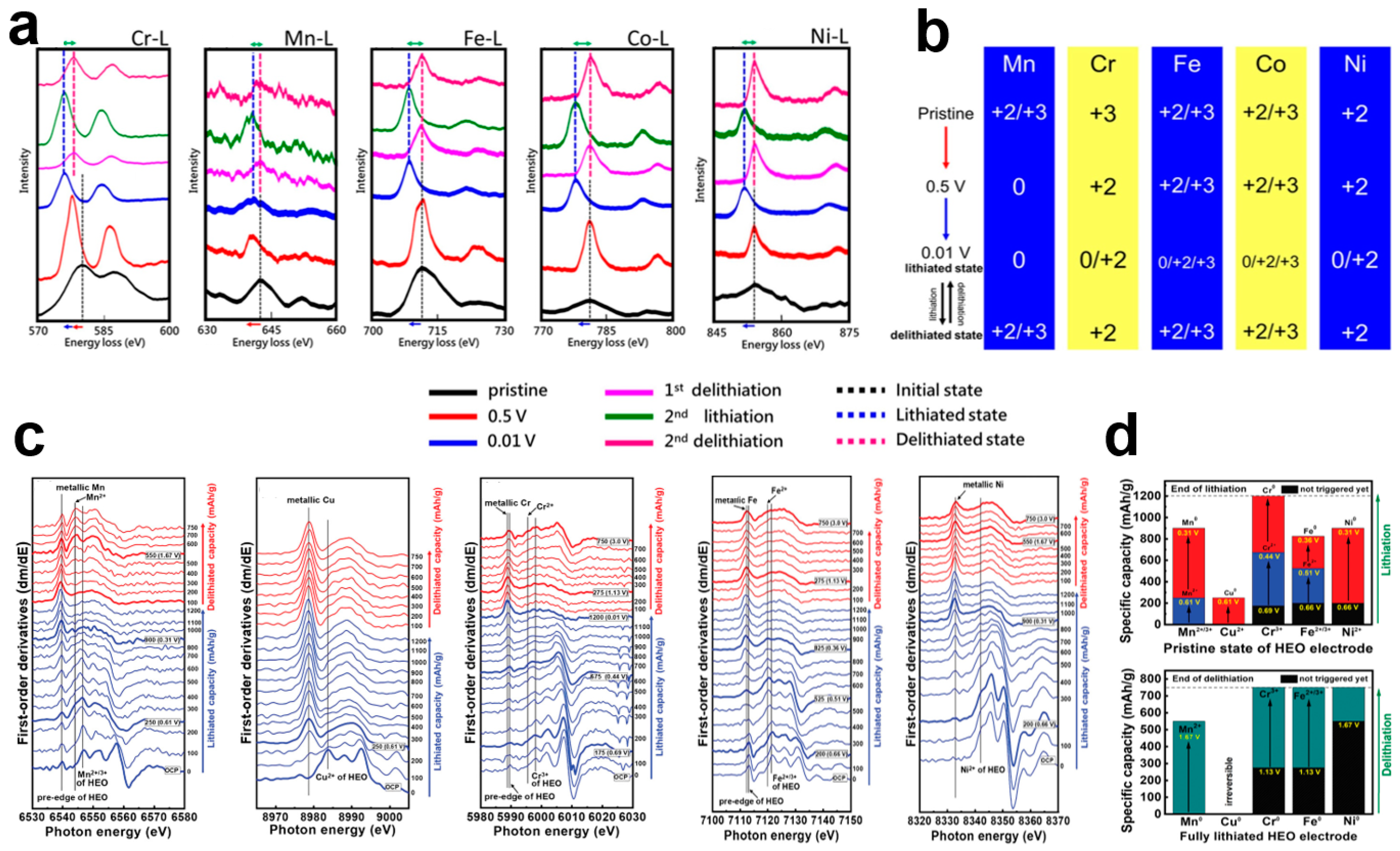
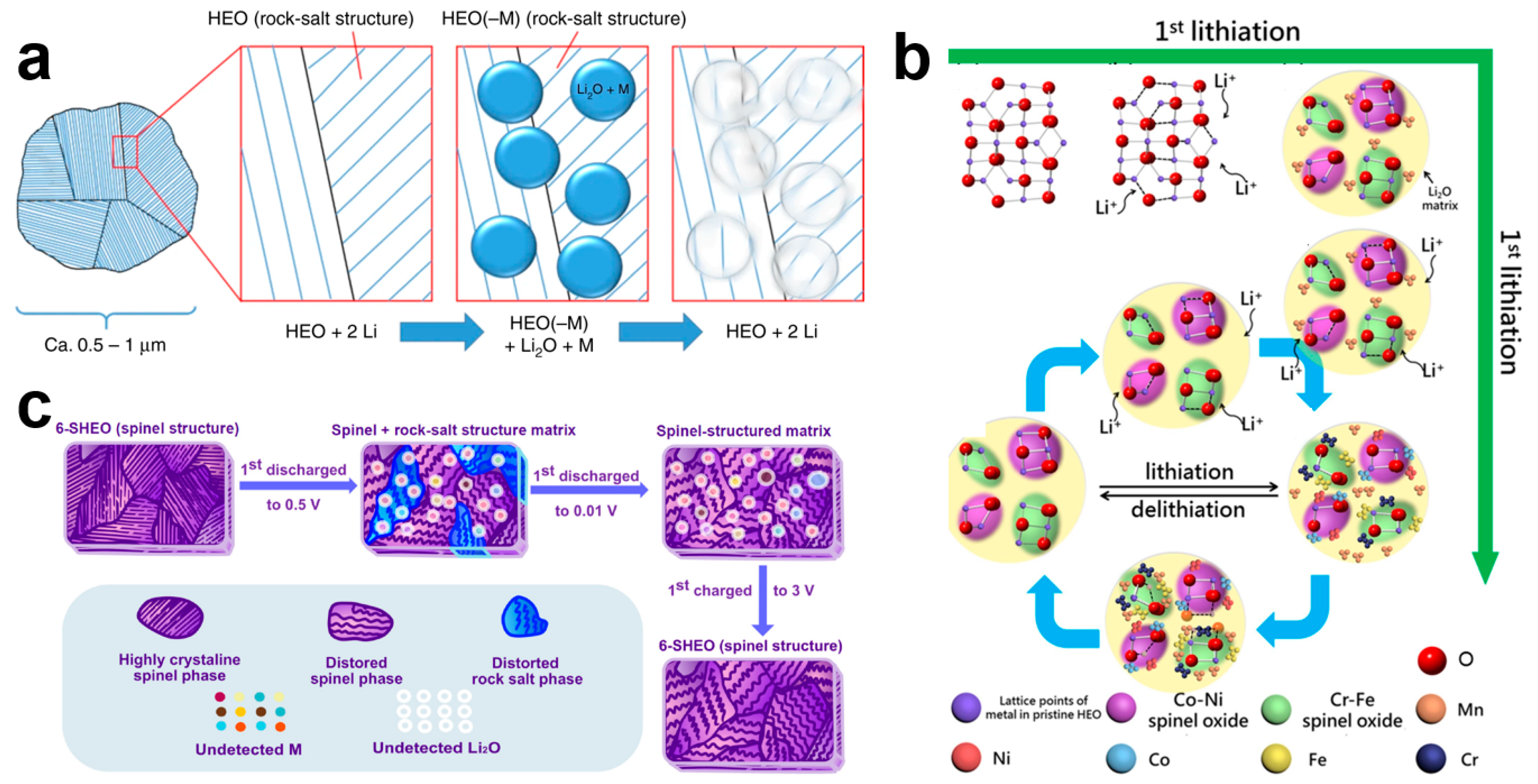
| Composition | Preparation Method | Pore Size | Specific Surface Area (m2 g−1) | Ref. |
|---|---|---|---|---|
| (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 | Solid-state | 3–20 nm | 12.31 | [35] |
| (FeCoNiCrMnCuLi)3O4 | Solid-state | 10–100 nm | 1.674 | [70] |
| (CrMnFeNiCu)3O4 | Wet chemical | 2–18 nm | 13.66 | [71] |
| (Al0.2CoCrFeMnNi)0.58O4−δ | Wet chemical | 2–20 nm | 44.86 | [54] |
| (La0.2Y0.2Gd0.2Ce0.2Sm0.2)Zr2O7 | Wet chemical | 2–100 μm | - | [72] |
| (Ce0.2Zr0.2Ti0.2Sn0.2Ga0.2)O2−δ | Wet chemical | 20–30 μm | - | [73] |
| (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O | Wet chemical | 10–145 nm | 43.54 | [68] |
| La(Co0.2Al0.2Fe0.2Mn0.2Cu0.2)O3 | Wet chemical | 6–20 nm | 20.71 | [74] |
| Composition | Structure | Method | Current Density (mA g−1) | Cycle Number | Reversible Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O | Rock salt | NSP | 200 | 500 | 590 | [38] |
| Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O | Rock salt | SRM | 100 | 300 | 920 | [57] |
| (MgCoNiCuZn)O | Rock salt | SRM | 100 | 300 | 920 | [90] |
| (MgCoNiCuZn)O | Rock salt | SCS | C/5 | 100 | 350 | [91] |
| (MgCoNiCuZn)O | Rock salt | NSP | 200 | 600 | 330 | [92] |
| (MgCoNiCuZn)O | Rock salt | SRM | 120 | 200 | 350 | [93] |
| (MgCoNiCuZnLi)O | Rock salt | Molten salt | 1000 | 100 | 610 | [94] |
| (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 | Spinel | SRM | 2000 | 800 | 272 | [35] |
| (FeCoNiCrMn)3O4 | Spinel | SRM | 500 | 300 | 402 | [21] |
| (CoCrFeMnNi)3O4 | Spinel | SCS | 2000 | 200 | 500 | [95] |
| (FeCoNiCrMn)3O4 | Spinel | SRM | 2000 | 1200 | 596.5 | [96] |
| (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4 | Spinel | SRM | 2000 | 3000 | 244 | [97] |
| (FeNiCrMnMgAl)3O4 | Spinel | SCS | 200 | 200 | 657 | [98] |
| (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 | Spinel | SCS | 100 | 50 | 980 | [87] |
| (FeCoNiCrMnCuLi)3O4 | Spinel | SRM | 500 | 100 | 522.1 | [70] |
| (CrNiMnFeCu)3O4 | Spinel | SCS | 500 | 400 | 685 | [99] |
| (Co0.2Ni0.2Mn0.2Zn0.2Fe0.2)3O3.2 | Spinel | SCS | 100 | 200 | 625 | [100] |
| [(NaBi)0.2(LiLa)0.2(CeK)0.2Ca0.2Sr0.2]TiO3 | Perovskite | SRM | 100 | 50 | 85 | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Tian, Y.; Luo, C.; Zhao, W.; Qin, C.; Wang, Z. Porous High-Entropy Oxide Anode Materials for Li-Ion Batteries: Preparation, Characterization, and Applications. Materials 2024, 17, 1542. https://doi.org/10.3390/ma17071542
Dong L, Tian Y, Luo C, Zhao W, Qin C, Wang Z. Porous High-Entropy Oxide Anode Materials for Li-Ion Batteries: Preparation, Characterization, and Applications. Materials. 2024; 17(7):1542. https://doi.org/10.3390/ma17071542
Chicago/Turabian StyleDong, Lishan, Yihe Tian, Chang Luo, Weimin Zhao, Chunling Qin, and Zhifeng Wang. 2024. "Porous High-Entropy Oxide Anode Materials for Li-Ion Batteries: Preparation, Characterization, and Applications" Materials 17, no. 7: 1542. https://doi.org/10.3390/ma17071542






