Synthesis of BiOX-Red Mud/Granulated Blast Furnace Slag Geopolymer Microspheres for Photocatalytic Degradation of Formaldehyde
Abstract
:1. Introduction
2. Experimental Method
2.1. Raw Materials
2.2. Characterization Methods
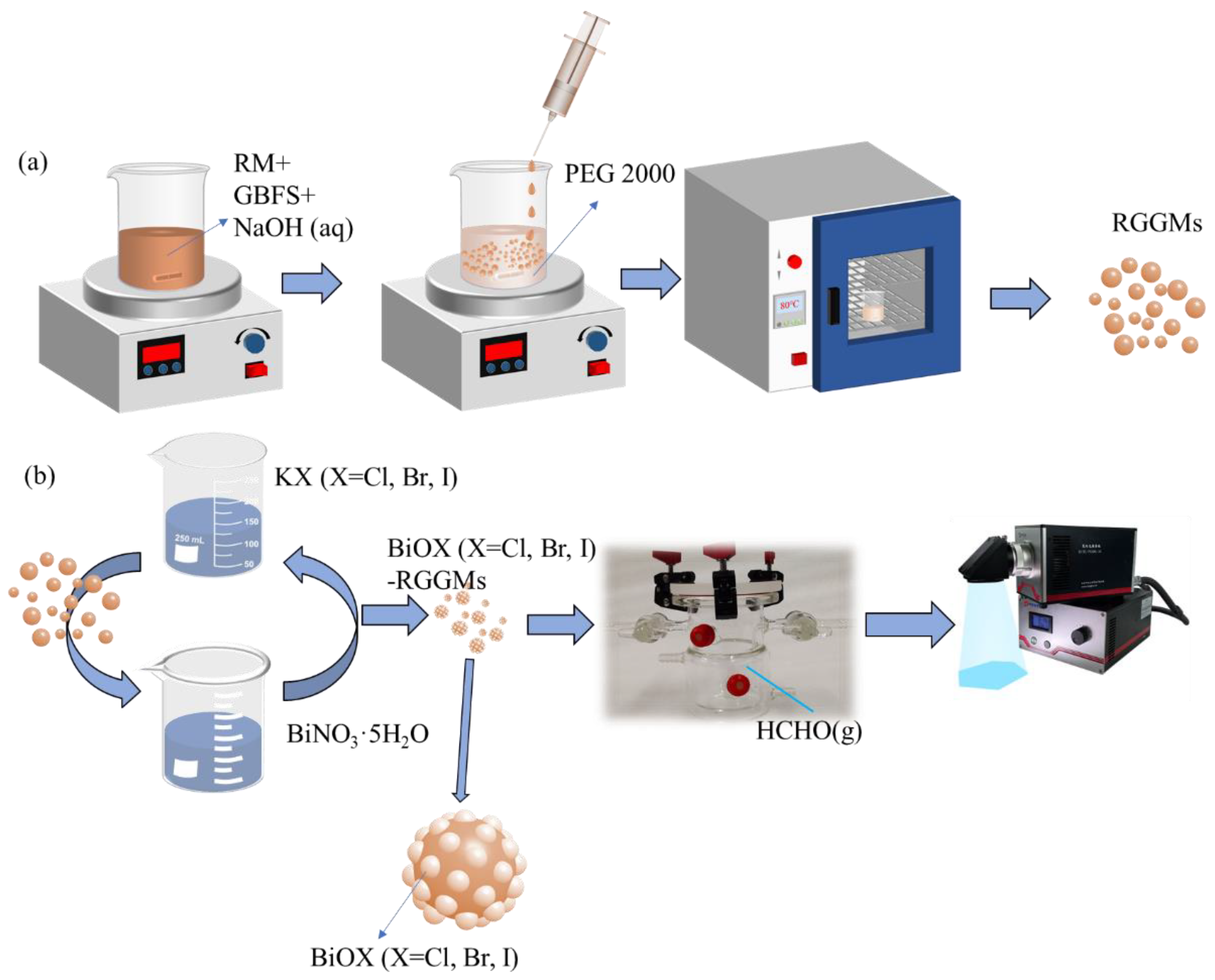
2.3. Preparation of RGGMs and BiOX (X = Cl, Br, I)-RGGMs
2.4. Photocatalytic Performance Evaluation
3. Results and Discussion
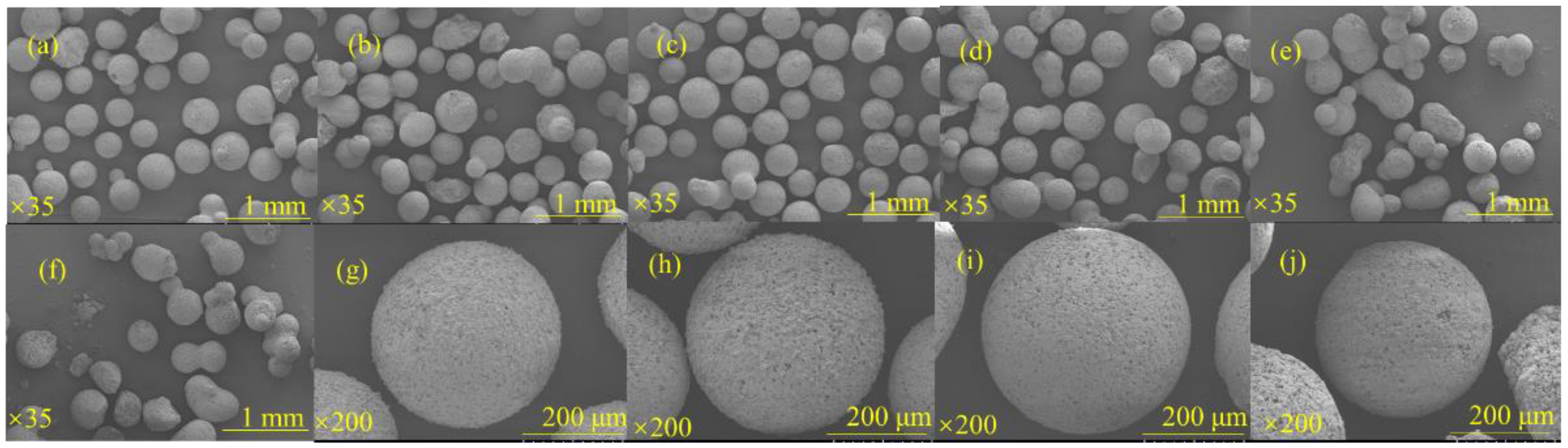
3.1. Synthesis and Microscopic Morphology of RGGMs
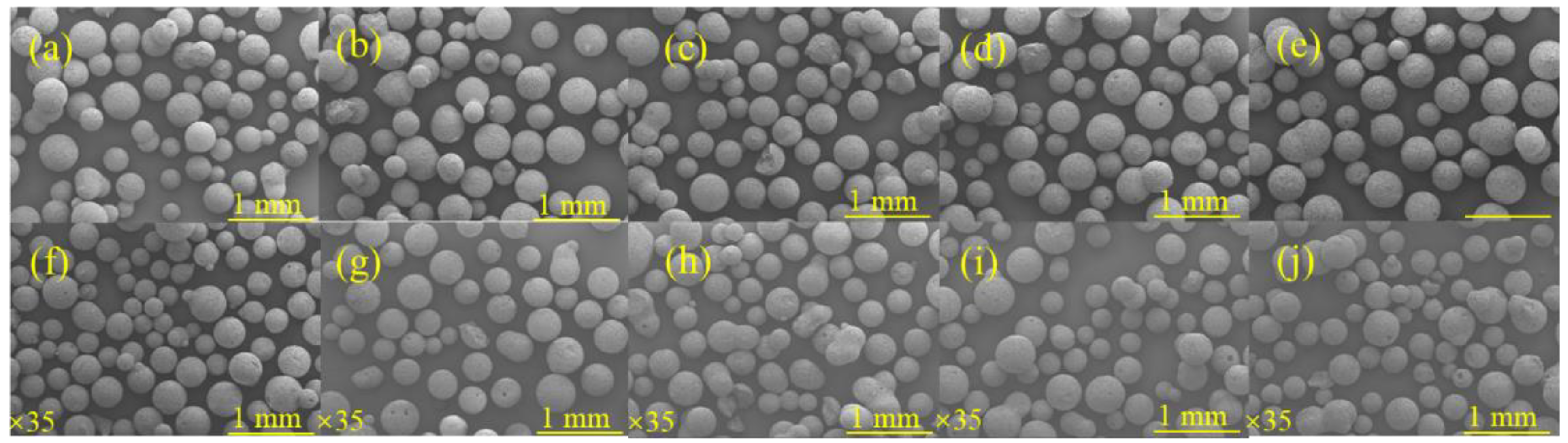
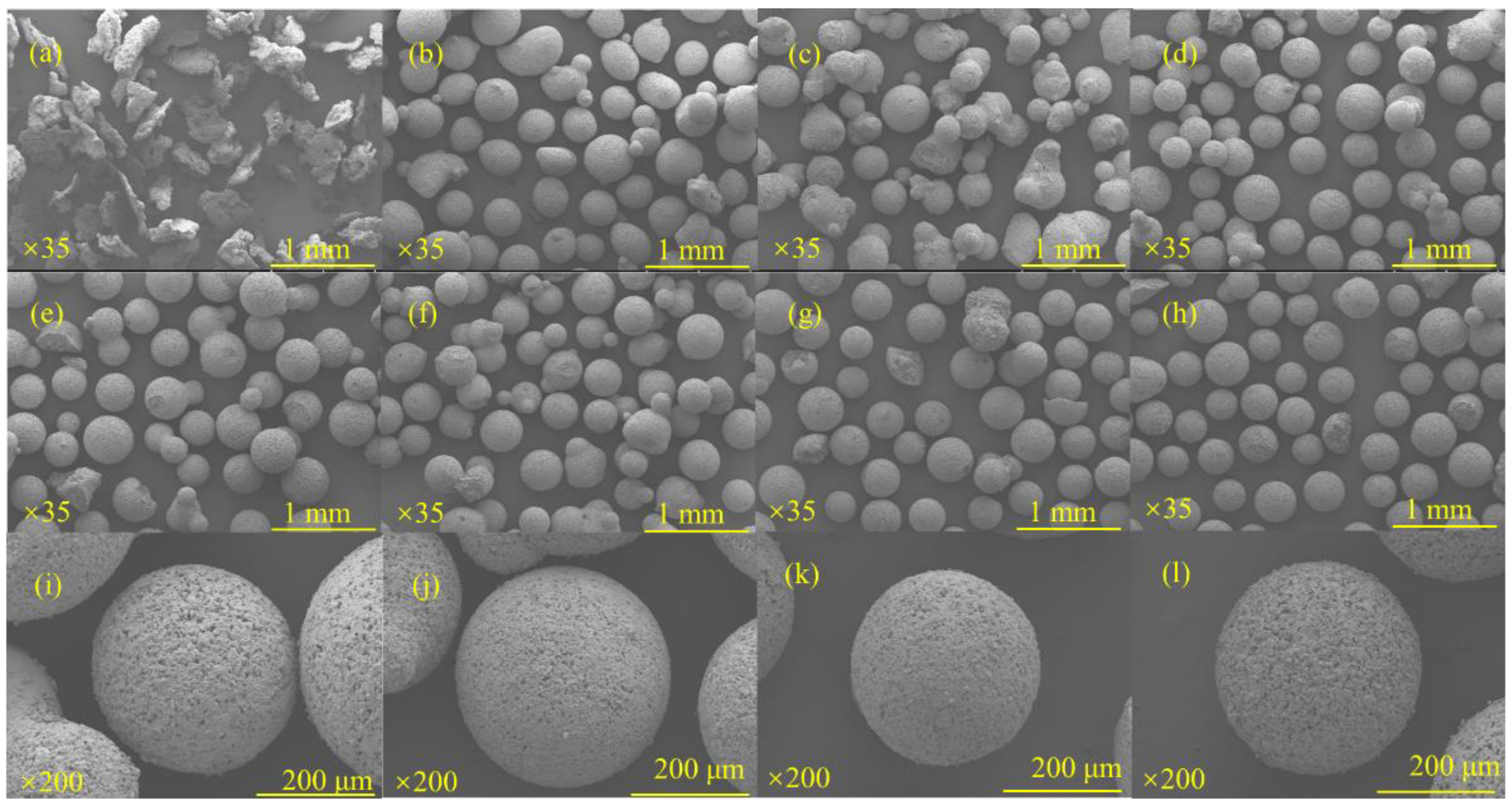
3.1.1. Effect of Red Mud Dosing on the Sphericity of Microspheres
3.1.2. Effect of NaOH Concentration on the Sphericity of Microspheres
3.1.3. Effect of Liquid–Solid Ratio on the Sphericity of Microspheres
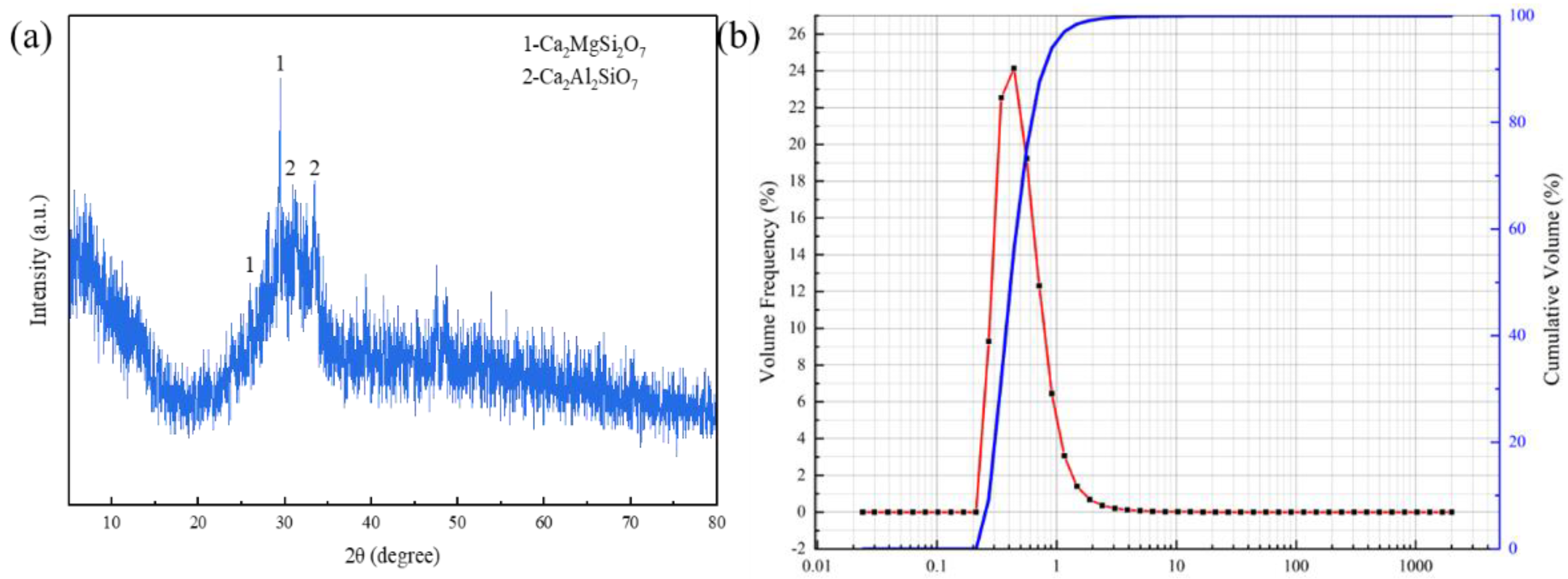
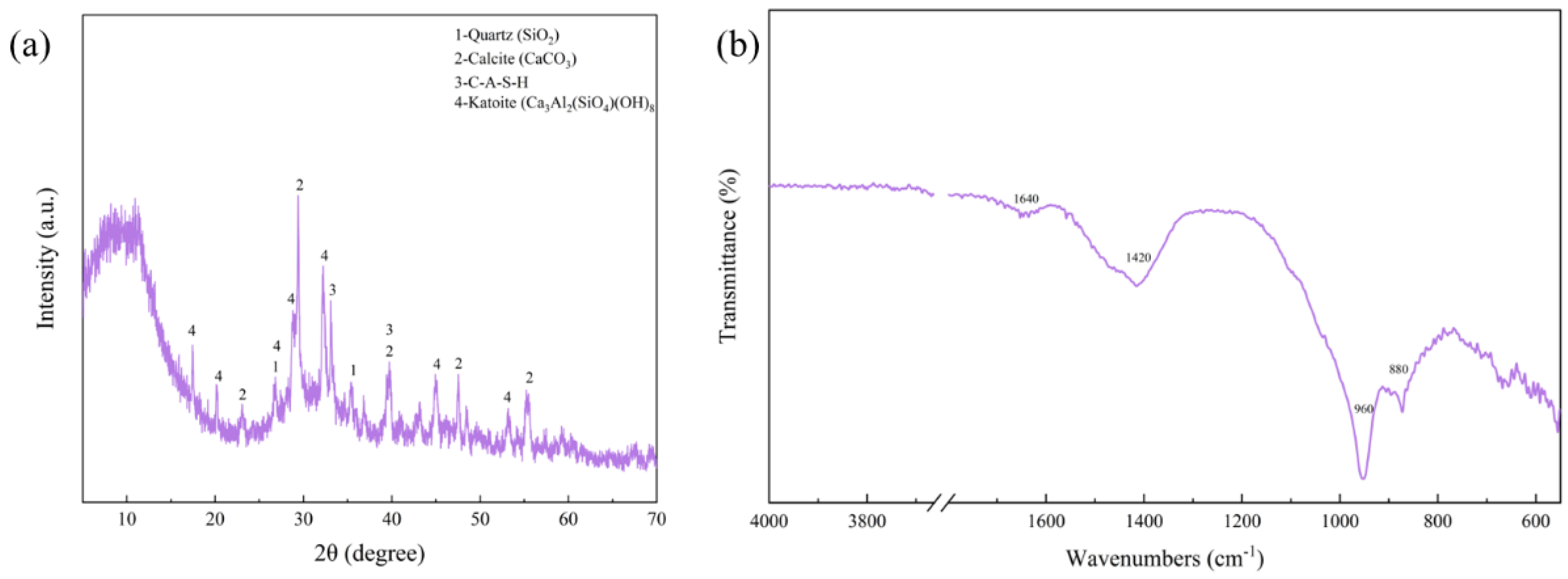
3.2. Mineral Composition Analysis of RGGMs
3.3. HCHO Removal Performance of BiOX (X = Cl, Br, I)-RGGMs
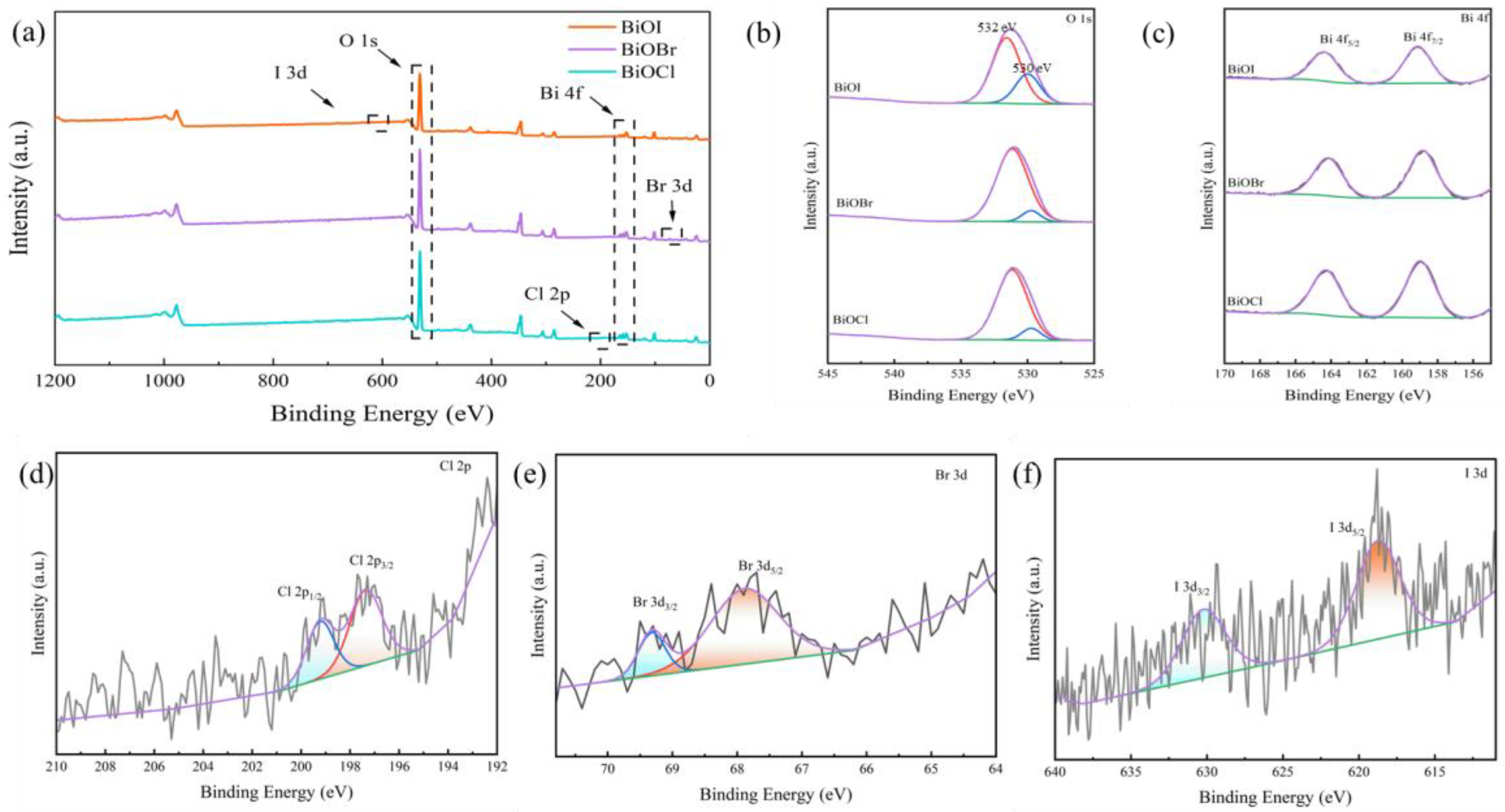
3.3.1. Loading of BiOX (X = Cl, Br, I)
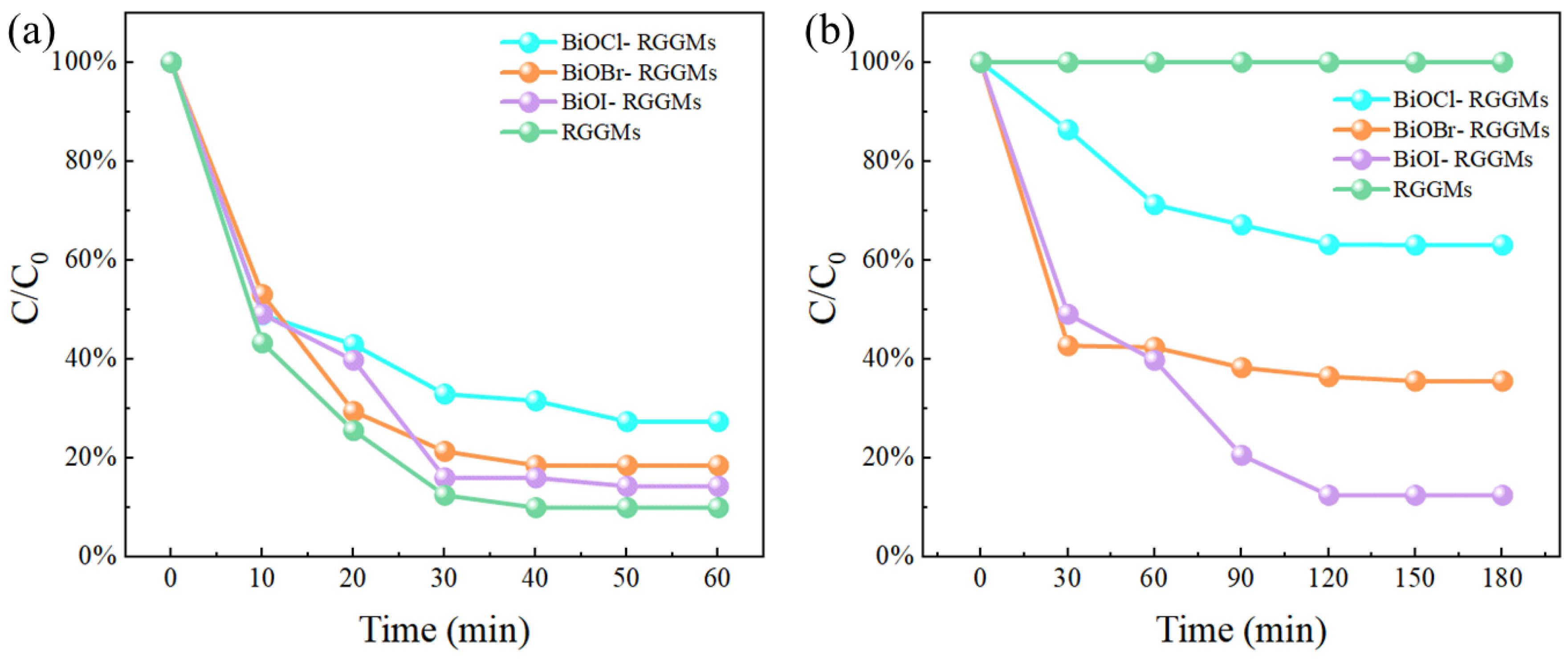
3.3.2. HCHO Removal Performance
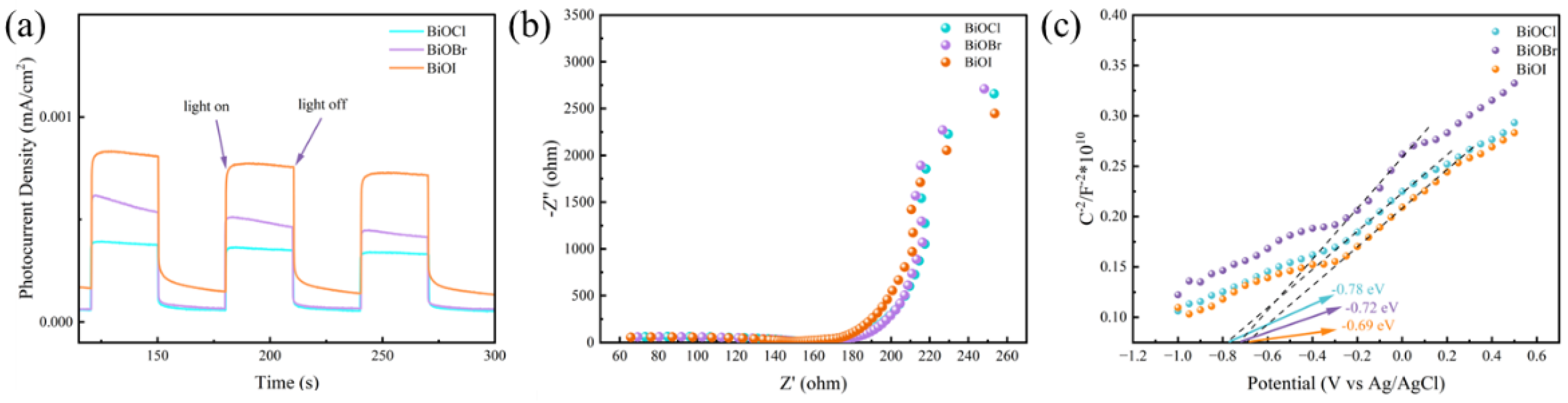
3.4. Optoelectronic Properties of BiOX (X = Cl, Br, I)-RGGMs
4. Conclusions
- (1)
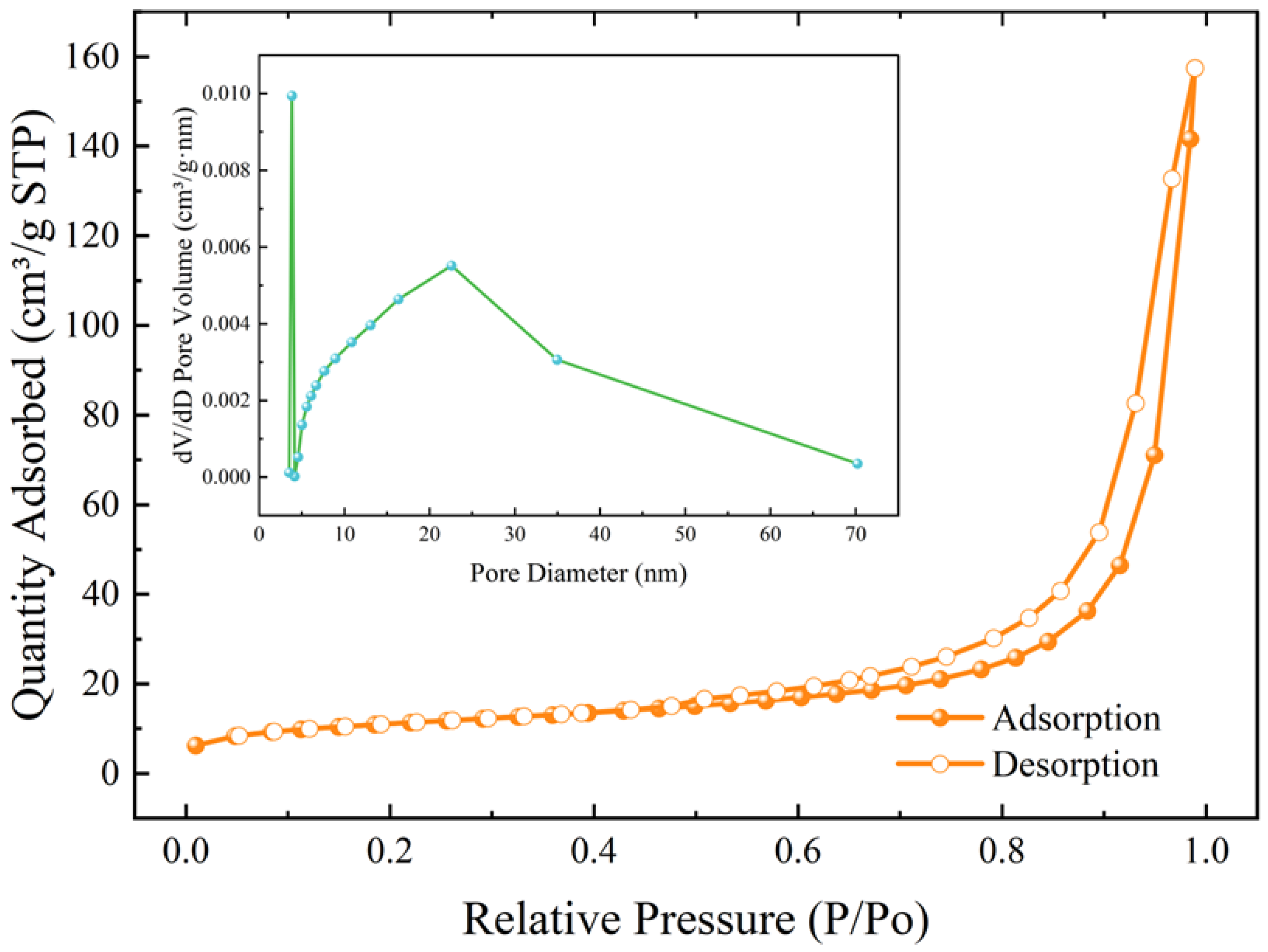
- The microspheres had the best sphericity at 50% addition of red mud, 6 mol NaOH solution concentration and 30 mL addition. The main mineral compositions in the microspheres were CaCO3 and C-A-S-H. The specific surface area was up to 38.80 m2/g, which was mainly mesoporous.
- (2)
- After loading BiOX (X = Cl, Br, I), BiOX (X = Cl, Br, I)-RGGMs showed good adsorption and degradation of formaldehyde gas. Among them, BiOI-RGGMs showed the best performance, and the degradation rate of 87.46% could be achieved at about 2 h, followed by BiOBr-RGGMs and BiOCl-RGGMs. It can be found by SEM, TEM and XPS that BiOX (X = Cl, Br, I) has been successfully loaded onto the surface of the microspheres and the difference in the performance of BiOX (X = Cl, Br, I)-RGGMs for the photocatalytic degradation of formaldehyde is in accordance with the results of their photoelectric tests.
- (3)
- BiOX (X = Cl, Br, I)-RGGMs are simple to prepare, inexpensive, easy to recycle and reuse, and have good application prospects in degrading indoor formaldehyde gas. The performance of BiOX (X = Cl, Br, I)-RGGMs in degrading other pollutants deserves further investigation in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert, B. Indoor Formaldehyde Removal by Catalytic Oxidation, Adsorption and Nanofibrous Membranes: A Review. Environ. Chem. Lett. 2021, 19, 2551–2579. [Google Scholar] [CrossRef]
- Almaie, S.; Vatanpour, V.; Rasoulifard, M.H.; Koyuncu, I. Volatile Organic Compounds (VOCs) Removal by Photocatalysts: A Review. Chemosphere 2022, 306, 135655. [Google Scholar] [CrossRef] [PubMed]
- Kuk, S.K.; Ji, S.M.; Kang, S.; Yang, D.S.; Kwon, H.J.; Koo, M.S.; Oh, S.; Lee, H.C. Singlet-Oxygen-Driven Photocatalytic Degradation of Gaseous Formaldehyde and Its Mechanistic Study. Appl. Catal. B Environ. 2023, 328, 122463. [Google Scholar] [CrossRef]
- Enesca, A.; Sisman, V. Indoor Air Photocatalytic Decontamination by UV–Vis Activated CuS/SnO2/WO3 Heterostructure. Catalysts 2022, 12, 728. [Google Scholar] [CrossRef]
- Carter, E.M.; Katz, L.E.; Speitel, G.E.; Ramirez, D. Gas-Phase Formaldehyde Adsorption Isotherm Studies on Activated Carbon: Correlations of Adsorption Capacity to Surface Functional Group Density. Environ. Sci. Technol. 2011, 45, 6498–6503. [Google Scholar] [CrossRef] [PubMed]
- Nhan, N.V.H.; Tu, L.N.Q.; Loc, B.T.; Vinh, D.C.; Phuong, N.T.T.; Duong, N.T.H.; Van Dung, N.; Mai, T.T.T.; Long, N.Q. Microwave-Assisted Synthesis of Carbon Nanodots/TiO2 Composite with Enhanced Photocatalytic Oxidation of VOCs in a Continuous Flow Reaction. Top. Catal. 2023. [Google Scholar] [CrossRef]
- Narindri Rara Winayu, B.; Tsai, Y.-Y.; Chu, H. Enhancement of Formaldehyde Removal by Graphene, S, and N Doping on TiO2 Nanocomposite Photocatalyst. J. Phys. Chem. Solids 2022, 170, 110961. [Google Scholar] [CrossRef]
- Bathla, A.; Kukkar, D.; Heynderickx, P.M.; Younis, S.A.; Kim, K.-H. Removal of Gaseous Formaldehyde by Portable Photocatalytic Air Purifier Equipped with Bimetallic Pt@Cu-TiO2 Filter. Chem. Eng. J. 2023, 469, 143718. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Q.; Ma, Y.; Zhu, R.; Fan, Y.; Huang, J.; Niu, H.; Dong, Y.; Li, D.; Zhang, Y.; et al. Highly Efficient Photocatalytic Hydrogen Evolution and Simultaneous Formaldehyde Degradation over Z-Scheme ZnIn2S4-NiO/BiVO4 Hierarchical Heterojunction under Visible Light Irradiation. Chem. Eng. J. 2021, 423, 130164. [Google Scholar] [CrossRef]
- Yuan, F.; Li, C.; Yang, R.; Tan, Y.; Ma, R.; Zhang, X.; Zheng, S.; Sun, Z. High-Efficient Mineralization of Formaldehyde by Three-Dimensional “PIZZA”-like Bismuth Molybdate-Titania/Diatomite Composite. J. Colloid Interface Sci. 2022, 624, 713–724. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zheng, L.; Lin, G.L.; Ni, L.F.; Song, X.C. Synthesis and Photocatalytic Activity of BiOCl/Diatomite Composite Photocatalysts: Natural Porous Diatomite as Photocatalyst Support and Dominant Facets Regulator. Adv. Powder Technol. 2020, 31, 339–350. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, Z.; Ren, Y.; Wang, Y.; Zhang, W. Preparation, Characterization and Application of Red Mud, Fly Ash and Desulfurized Gypsum Based Eco-Friendly Road Base Materials. J. Clean. Prod. 2021, 284, 124777. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The Composition, Recycling and Utilisation of Bayer Red Mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Wu, P.; Liu, X.; Zhang, Z.; Wei, C.; Wang, J.; Gu, J. The Harmless and Value-Added Utilization of Red Mud: Recovering Iron from Red Mud by Pyrometallurgy and Preparing Cementitious Materials with Its Tailings. J. Ind. Eng. Chem. 2024, 132, 50–65. [Google Scholar] [CrossRef]
- Jovičević-Klug, M.; Souza Filho, I.R.; Springer, H.; Adam, C.; Raabe, D. Green Steel from Red Mud through Climate-Neutral Hydrogen Plasma Reduction. Nature 2024, 625, 703–709. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, Y.; Li, Z.; Li, Y.; Ren, Y. Binary Reaction Behaviors of Red Mud Based Cementitious material: Hydration Characteristics and Na+ Utilization. J. Hazard. Mater. 2021, 410, 124592. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Fořt, J.; Klapiszewska, I.; Thomas, M.; Klapiszewski, Ł.; Černý, R. A Literature Review of the Latest Trends and Perspectives Regarding Alkali-Activated Materials in Terms of Sustainable Development. J. Mater. Res. Technol. 2023, 25, 5394–5425. [Google Scholar] [CrossRef]
- Babisk, M.P.; Amaral, L.F.; Ribeiro, L.D.S.; Vieira, C.M.F.; Prado, U.S.D.; Gadioli, M.C.B.; Oliveira, M.S.; Luz, F.S.D.; Monteiro, S.N.; Garcia Filho, F.D.C. Evaluation and Application of Sintered Red Mud and Its Incorporated Clay Ceramics as Materials for Building Construction. J. Mater. Res. Technol. 2020, 9, 2186–2195. [Google Scholar] [CrossRef]
- Maihatchi Ahamed, A.; Pons, M.-N.; Ricoux, Q.; Goettmann, F.; Lapicque, F. Production of Electrolytic Iron from Red Mud in Alkaline Media. J. Environ. Manag. 2020, 266, 110547. [Google Scholar] [CrossRef]
- Habibi, H.; Mokmeli, M.; Shakibania, S.; Pirouzan, D.; Pourkarimi, Z. Separation and Recovery of Titanium and Scandium from the Red Mud. Sep. Purif. Technol. 2023, 317, 123882. [Google Scholar] [CrossRef]
- Wu, P.; Liu, X.; Zhang, Z.; Wei, C. Properties of Red Mud-Filled and Modified Resin Composites. Constr. Build. Mater. 2023, 409, 133984. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X. Application Status and Development Trend of Red Mud-Based Photocatalytic Materials for Degrading Organic Pollutants in Water. J. Eng. Sci. 2021, 43, 22–32. [Google Scholar]
- Zakira, U.; Zheng, K.; Xie, N.; Birgisson, B. Development of High-Strength Geopolymers from Red Mud and Blast Furnace Slag. J. Clean. Prod. 2023, 383, 135439. [Google Scholar] [CrossRef]
- Abadel, A.A.; Alghamdi, H.; Alharbi, Y.R.; Alamri, M.; Khawaji, M.; Abdulaziz, M.A.M.; Nehdi, M.L. Investigation of Alkali-Activated Slag-Based Composite Incorporating Dehydrated Cement Powder and Red Mud. Materials 2023, 16, 1551. [Google Scholar] [CrossRef]
- Singh, V.; Bano, S.; Chauhan, V.B.; Pal, P.; Kumar, A.; Srivastava, J.B. Red Mud as a Sustainable Road Construction Material: An Experimental Investigation. Constr. Build. Mater. 2024, 411, 134549. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, Z.; Zhang, X.; Zhang, Y.; Chen, X.; Zeng, G.; Xu, H. Highly Efficient Catalytic Ozonation Degradation of Levofloxacin by Facile Hydrogenation-Modified Red Mud Wastes. Environ. Pollut. 2023, 334, 122149. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Q.; Fan, X.; Zheng, J.; Liu, Y.; Ma, Y.; He, J. Enhanced Degradation of Sulfamethoxazole by Activation of Peroxodisulfate with Red Mud Modified Biochar: Synergistic Effect between Adsorption and Nonradical Activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Oprčkal, P.; Mladenovič, A.; Zupančič, N.; Ščančar, J.; Milačič, R.; Zalar Serjun, V. Remediation of Contaminated Soil by Red Mud and Paper Ash. J. Clean. Prod. 2020, 256, 120440. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Wu, D.; Yang, S.; Fang, H.; Sun, S. Mechanochemical Treatment with Red Mud Added for Heavy Metals Solidification in Municipal Solid Waste Incineration Fly Ash. J. Clean. Prod. 2023, 398, 136642. [Google Scholar] [CrossRef]
- Belviso, C.; Lucini, P.; Mancinelli, M.; Abdolrahimi, M.; Martucci, A.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Sturini, M. Lead, Zinc, Nickel and Chromium Ions Removal from Polluted Waters Using Zeolite Formed from Bauxite, Obsidian and Their Combination with Red Mud: Behaviour and Mechanisms. J. Clean. Prod. 2023, 415, 137814. [Google Scholar] [CrossRef]
- Ayala, J.; Fernández, B. Treatment from Abandoned Mine Landfill Leachates. Adsorption Technology. J. Mater. Res. Technol. 2019, 8, 2732–2740. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Y.; Dai, W.; Yang, H.; Jiang, L.; Li, K.; Jin, W. Preparation and Properties of Porous Concrete Based on Geopolymer of Red Mud and Yellow River Sediment. Materials 2024, 17, 923. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, S.; Zhu, H.; Qian, W.; Wang, P.; Yang, R.; Zhang, J.; Cen, Q.; Liu, Z.; Ning, P. Enhanced NOx Absorption in Flue Gas by Wet Oxidation of Red Mud and Phosphorus Sludge. J. Hazard. Mater. 2024, 465, 133075. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wang, K.; Wei, H.; Wei, D.; Wei, X.; Wei, B.; Shao, L.; Fujita, T.; Cui, X. Efficient Preparation of Red Mud-Based Geopolymer Microspheres (RM@GMs) and Adsorption of Fluoride Ions in Wastewater. J. Hazard. Mater. 2023, 442, 130027. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lei, H.; Muhammad, Y.; Chen, F.; Gao, F.; Wei, Y.; Fujita, T. Controlled Preparation of Cerium Oxide Loaded Slag-Based Geopolymer Microspheres (CeO2@SGMs) for the Adsorptive Removal and Solidification of F− from Acidic Waste-Water. J. Hazard. Mater. 2020, 400, 123199. [Google Scholar] [CrossRef]
- Chen, F.; Wang, K.; Shao, L.; Muhammad, Y.; Wei, Y.; Gao, F.; Wang, X.; Cui, X. Synthesis of Fe2O3-Modified Porous Geopolymer Microspheres for Highly Selective Adsorption and Solidification of F− from Waste-Water. Compos. Part B Eng. 2019, 178, 107497. [Google Scholar] [CrossRef]
- Thakur, N.; Armstrong, D.W. Arsenic Sequestration by Iron Oxide Coated Geopolymer Microspheres. J. Clean. Prod. 2021, 291, 125931. [Google Scholar] [CrossRef]
- Su, Q.; Yang, S.; He, Y.; Qin, Z.; Cui, X. Prepared Self-Growing Supported Nickel Catalyst by Recovering Ni (Ⅱ) from Metal Wastewater Using Geopolymer Microspheres. J. Hazard. Mater. 2020, 389, 121919. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.; Wang, K.; Muhammad, Y.; Chen, S.; Dong, D.; Wei, Y.; Fujita, T. Preparation and Conversion Mechanism of Different Geopolymer-Based Zeolite Microspheres and Their Adsorption Properties for Pb2+. Sep. Purif. Technol. 2022, 282, 119971. [Google Scholar] [CrossRef]
- Luo, K.; Ye, J.; Zhang, W.; Chen, J.; Yan, F.; Li, G.; Ren, X.; Li, J. Leaching Kinetics and Reactivity Regulation of Red Mud in an NaOH Solution. Constr. Build. Mater. 2024, 421, 135750. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A.; Blanco-Varela, M.T. Pore Solution in Alkali-Activated Slag Cement Pastes. Relation to the Composition and Structure of Calcium Silicate Hydrate. Cem. Concr. Res. 2004, 34, 139–148. [Google Scholar] [CrossRef]
- Maria Calvino, M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Pipitone, C.; Giannici, F. Beeswax/Halloysite Microparticles Embedded within a Geopolymeric Layer for the Protective Coating of Steel. Mater. Lett. 2023, 330, 133257. [Google Scholar] [CrossRef]
- Werling, N.; Schwaiger, R.; Dathe, F.; Dehn, F.; Emmerich, K. Micromechanical Properties of Geopolymers with Different Calcined Clay Precursors. Appl. Clay Sci. 2024, 250, 107259. [Google Scholar] [CrossRef]
- Song, S.; Zhang, N.; Yuan, J.; Zhang, Y. New Attempt to Produce Red Mud-Iron Tailing Based Alkali-Activated Mortar: Performance and Microstructural Characteristics. J. Build. Eng. 2021, 43, 103222. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lisuzzo, L.; Cavallaro, G.; Lazzara, G.; Milioto, S. Halloysite Based Geopolymers Filled with Wax Microparticles as Sustainable Building Materials with Enhanced Thermo-Mechanical Performances. J. Environ. Chem. Eng. 2022, 10, 108594. [Google Scholar] [CrossRef]
- Brahmi, A.; Ziani, S.; AitAli, S.; Benkhaoula, B.N.; Yu, Y.; Ahouari, H.; Khireddine, H.; Luukkonen, T. Porous Metakaolin Geopolymer as a Reactive Binder for Hydroxyapatite Adsorbent Granules in Dye Removal. Hybrid Adv. 2024, 5, 100134. [Google Scholar] [CrossRef]
- Dong, D.; Wang, K.; Yi, M.; Liang, Y.; Muhammad, Y.; Wei, E.; Wei, Y.; Fujita, T. Preparation of TiO2 Photocatalyst Microspheres by Geopolymer Technology for the Degradation of Tetracycline. J. Clean. Prod. 2022, 339, 130734. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Xu, W.; Wang, H.; Zhou, J.; Chen, Q.; Chen, J.; Chen, D. Oxygen Vacancies Mediated Flower-like BiOX Microspheres for Photocatalytic Purification of Methyl Mercaptan Odor: Significant Distinction Induced by Halogen Elements. Chem. Eng. J. 2023, 471, 144658. [Google Scholar] [CrossRef]
- Shiferraw, B.T.; Baye, A.F.; Kim, H. Mechanistic Insights into the Deprotonation of Methanol by Facile Synthesized 3D Flower-like BiOX (X= Cl, Br, I) Catalysts for Rapid Hydrogen Generation from NaBH4 Methanolysis: The ‘X’ Factor. Int. J. Hydrog. Energy 2023, S0360319923042635. [Google Scholar] [CrossRef]
- Aracena, A.; Rezende, M.C.; García, M.; Muñoz-Becerra, K.; Wrighton-Araneda, K.; Valdebenito, C.; Celis, F.; Vásquez, O. Alkylated Benzodithienoquinolizinium Salts as Possible Non-Fullerene Organic N-Type Semiconductors: An Experimental and Theoretical Study. Materials 2021, 14, 6239. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, Y.; Jia, S.; Qing, Y.; Li, L.; Chen, Y.; Wu, Y. Synthesis of Cu-Doped TiO2 on Wood Substrate with Highly Efficient Photocatalytic Performance and Outstanding Recyclability for Formaldehyde Degradation. Molecules 2023, 28, 972. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Lin, Z.; Huang, H.; Wang, H.; Meng, M.; Liu, Y.; Zhou, Y. The Co3O4/CN-x%CeO2 (x = 0, 3, 6) Composites for Photocatalytic Degradation of HCHO under Visible-Light Irradiation: Performance and Characterization. Res. Chem. Intermed. 2023, 49, 1179–1196. [Google Scholar] [CrossRef]
- Wang, X.; Hong, S.; Lian, H.; Zhan, X.; Cheng, M.; Huang, Z.; Manzo, M.; Cai, L.; Nadda, A.; Le, Q.V.; et al. Photocatalytic Degradation of Surface-Coated Tourmaline-Titanium Dioxide for Self-Cleaning of Formaldehyde Emitted from Furniture. J. Hazard. Mater. 2021, 420, 126565. [Google Scholar] [CrossRef]
- Di, Y.; Zhang, X.; Wang, X.; Zheng, S. Construction of BiOCl/Clinoptilolite Composite Photocatalyst for Boosting Formaldehyde Removal. Materials 2021, 14, 6469. [Google Scholar] [CrossRef]




| Chemical Composition (wt%) | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | Na2O | MgO | Others |
|---|---|---|---|---|---|---|---|---|
| Red mud | 42.99 | 22.20 | 10.40 | 9.89 | 3.75 | 3.46 | 1.76 | 5.55 |
| Granulated blast furnace slag | 42.76 | 24.85 | 15.61 | 0.36 | 2.64 | 0.55 | 7.78 | 5.45 |
| Atomic% | C | O | Na | Mg | Al | Si | Ca | Fe |
|---|---|---|---|---|---|---|---|---|
| Figure 7a | 17.05 | 66.22 | - | 0.85 | 0.46 | 0.96 | 14.46 | - |
| Figure 7b | 12.80 | 69.31 | 1.23 | 1.55 | 2.71 | 5.21 | 6.57 | 0.62 |
| Atomic% | BiOCl | BiOBr | BiOI |
|---|---|---|---|
| C | 17.28 | 16.34 | 13.03 |
| O | 62.68 | 64.76 | 64.19 |
| Na | 0.38 | 0.27 | 0.75 |
| Al | 3.29 | 3.32 | 3.20 |
| Si | 7.15 | 5.81 | 7.13 |
| Ca | 8.01 | 8.80 | 9.29 |
| Fe | 0.60 | 0.60 | 0.66 |
| Bi | 0.60 | 0.11 | 0.30 |
| Materials | Lighting Time | Degradation | References |
|---|---|---|---|
| Cu–TiO2/wood composites | 2 h | 85.59% | [51] |
| Co3O4/CN-x%CeO2 (x = 0, 3, 6) composites | 9 h | 91.5% | [52] |
| Tourmaline-titanium dioxide composite | 10 h | 92.2% | [53] |
| BiOCl/clinoptilolite composite photocatalyst | 2 h | 87.7% | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Zhang, N.; Wang, Y.; Wang, Y.; Zhang, J.; Cai, Q.; Zhang, Y. Synthesis of BiOX-Red Mud/Granulated Blast Furnace Slag Geopolymer Microspheres for Photocatalytic Degradation of Formaldehyde. Materials 2024, 17, 1585. https://doi.org/10.3390/ma17071585
Lu P, Zhang N, Wang Y, Wang Y, Zhang J, Cai Q, Zhang Y. Synthesis of BiOX-Red Mud/Granulated Blast Furnace Slag Geopolymer Microspheres for Photocatalytic Degradation of Formaldehyde. Materials. 2024; 17(7):1585. https://doi.org/10.3390/ma17071585
Chicago/Turabian StyleLu, Ping, Na Zhang, Ying Wang, Yidi Wang, Jiale Zhang, Qingyi Cai, and Yihe Zhang. 2024. "Synthesis of BiOX-Red Mud/Granulated Blast Furnace Slag Geopolymer Microspheres for Photocatalytic Degradation of Formaldehyde" Materials 17, no. 7: 1585. https://doi.org/10.3390/ma17071585





