Synthesis of Size-Adjustable CsPbBr3 Perovskite Quantum Dots for Potential Photoelectric Catalysis Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
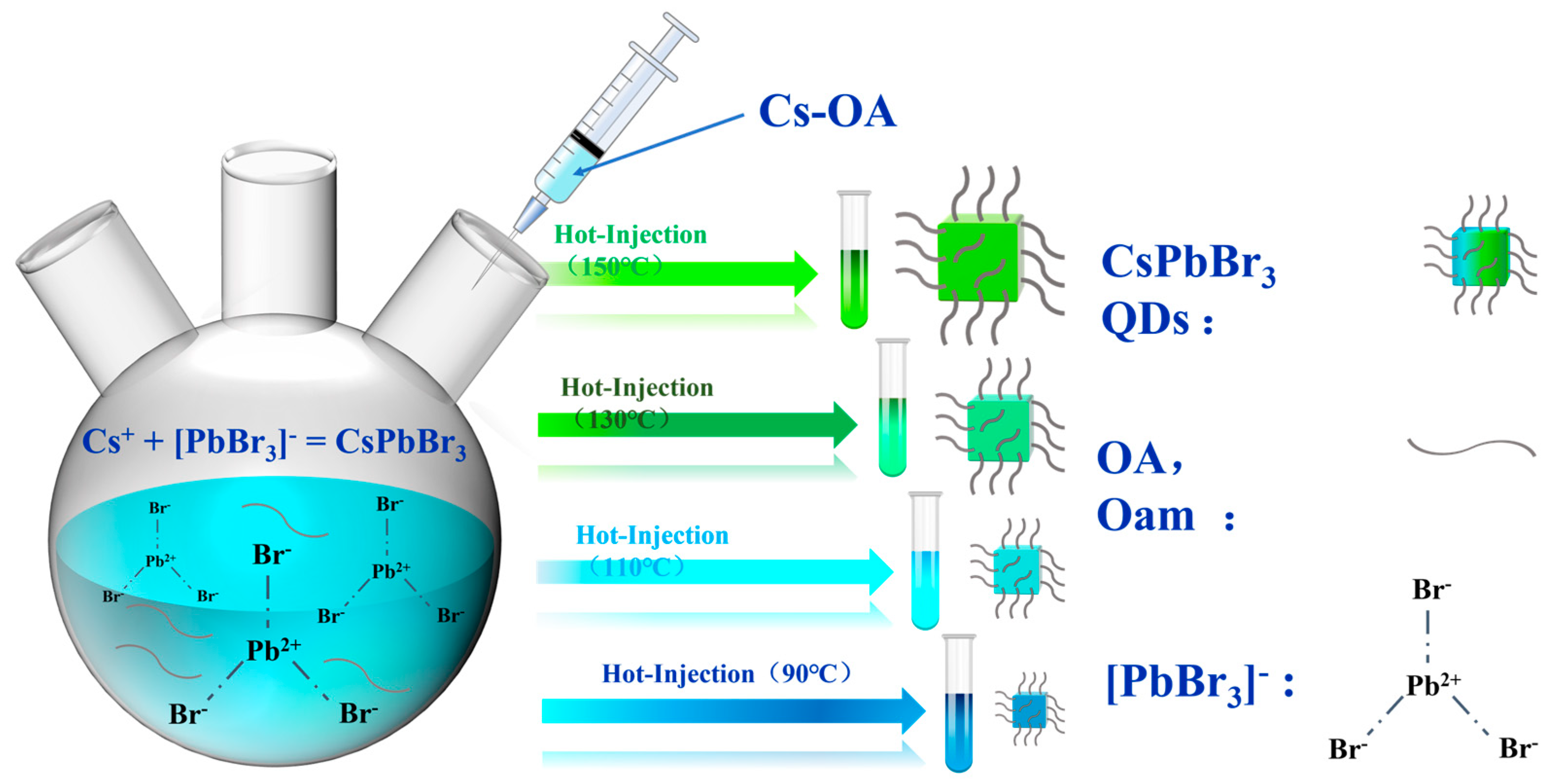
2.2.1. Preparation of CsOA Precursors
2.2.2. Synthesis of CsPbBr3 Quantum Dots (QDs)
2.2.3. Purification of CsPbBr3 Quantum Dots (QDs)
2.3. Characterization
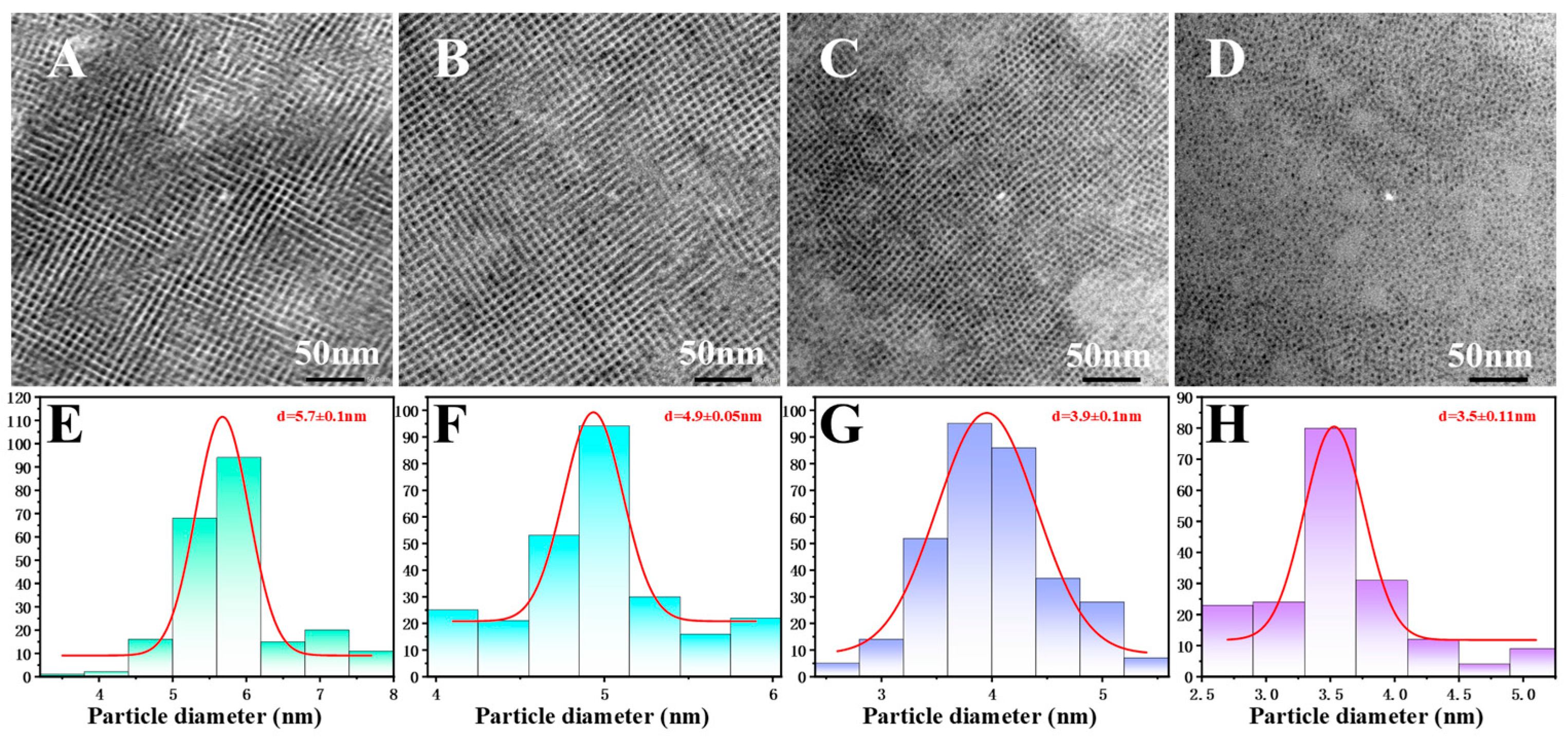
2.3.1. Characterization via Transmission Electron Microscopy (TEM)
2.3.2. Characterization via X-ray Diffraction (XRD)
2.3.3. X-ray Photoelectron Spectroscopy (XPS) Characterization
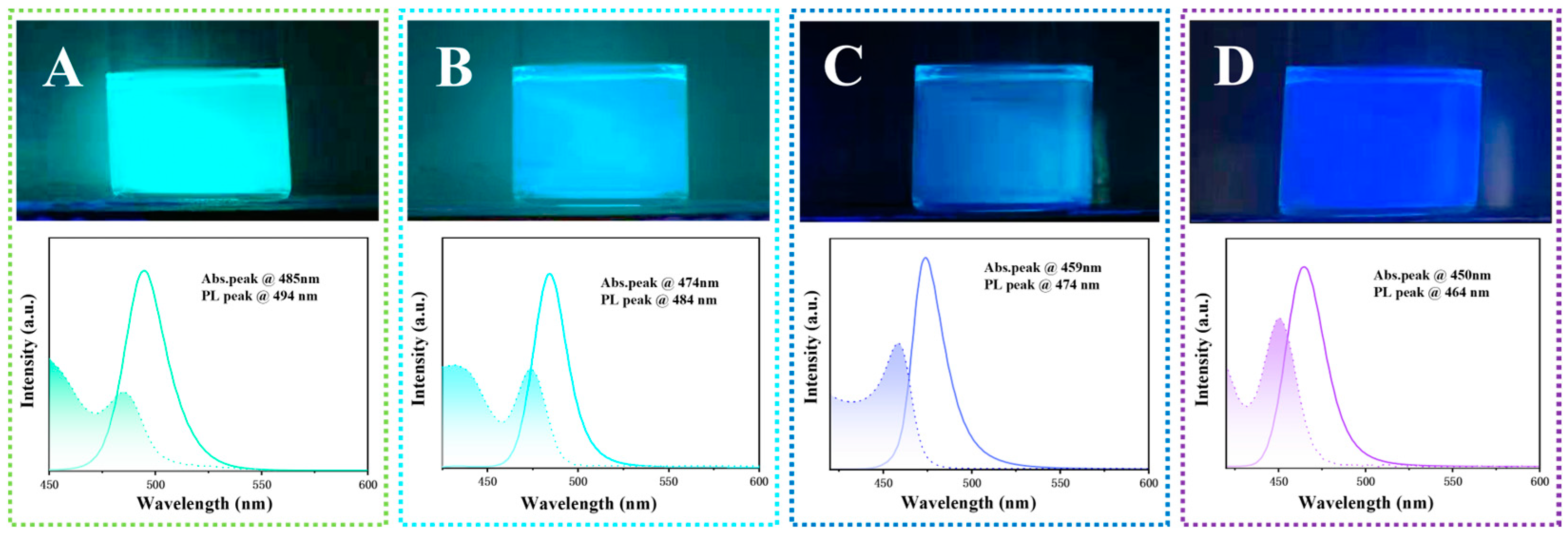
2.3.4. Fluorescence Spectrum Characterization
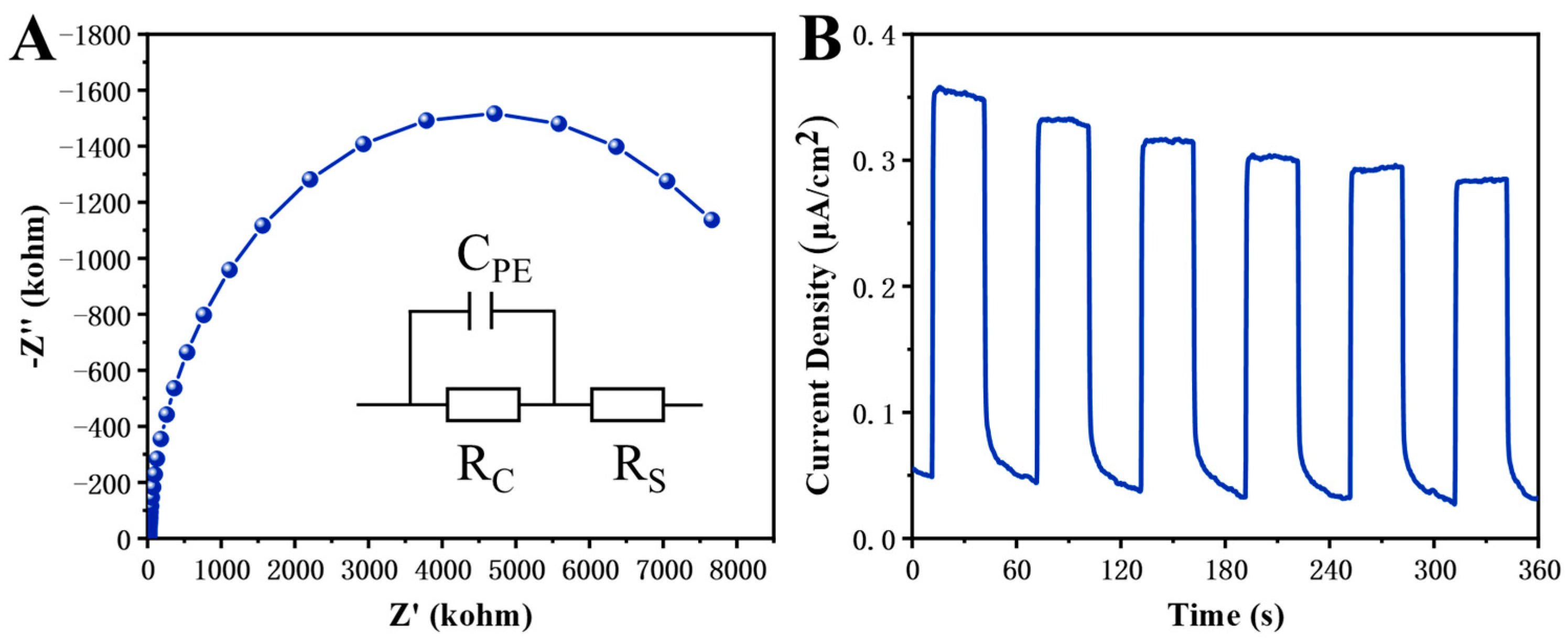
2.3.5. Electrochemistry (Photo) Measurements
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Hazarika, A.; Zhao, Q.; Ling, X.; Moot, T.; Ma, W.; Luther, J.M. Metal Halide Perovskites in Quantum Dot Solar Cells: Progress and Prospects. Joule 2020, 4, 1160–1185. [Google Scholar] [CrossRef]
- Solari, S.F.; Kumar, S.; Jagielski, J.; Shih, C.J. Monochromatic LEDs based on perovskite quantum dots: Opportunities and challenges. J. Soc. Inf. Disp. 2019, 27, 667–678. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, T.; Xu, T.; Ye, S.; Liu, R.; Sun, Z.; Tan, C.; Lv, X.; Yang, J.; et al. Unencapsulated CsPbClBr2 Film Photodetectors Grown by Thermal Vacuum Deposition Exhibit Exceptional Environmental Stability in High-Humidity Air. ACS Appl. Energy Mater. 2022, 5, 8709–8716. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Manna, L. What Defines a Halide Perovskite? ACS Energy Lett. 2020, 5, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ceratti, D.R.; Tenne, R.; Bartezzaghi, A.; Cremonesi, L.; Segev, L.; Kalchenko, V.; Oron, D.; Potenza, M.A.C.; Hodes, G.; Cahen, D. Self-Healing and Light-Soaking in MAPbI3: The Effect of H2O. Adv. Mater. 2022, 34, 2110239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, T.; Luo, X.; Tian, Y.; Lu, X.; Wu, K. Synthesis and Spectroscopy of Monodispersed, Quantum-Confined FAPbBr3 Perovskite Nanocrystals. Chem. Mater. 2019, 32, 549–556. [Google Scholar] [CrossRef]
- Saparov, B.; Mitzi, D.B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 2016, 116, 4558–4596. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Ahmed, T.; De, A.; Samanta, A. Tackling the Defects, Stability, and Photoluminescence of CsPbX3 Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 1610–1618. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, Y. Origins of p-Doping and Nonradiative Recombination in CsSnI3. Angew. Chem. Int. Ed. 2022, 61, 106800. [Google Scholar]
- Fakharuddin, A.; Gangishetty, M.K.; Abdi-Jalebi, M.; Chin, S.H.; bin Mohd Yusoff, A.R.; Congreve, D.N.; Tress, W.; Deschler, F.; Vasilopoulou, M.; Bolink, H.J. Perovskite light-emitting diodes. Nat. Electron. 2022, 5, 203–216. [Google Scholar] [CrossRef]
- Jing, H.; Peng, R.; Ma, R.M.; He, J.; Zhou, Y.; Yang, Z.; Li, C.Y.; Liu, Y.; Guo, X.; Zhu, Y.; et al. Flexible Ultrathin Single-Crystalline Perovskite Photodetector. Nano Lett. 2020, 20, 7144–7151. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Yang, D.; He, G.; Guo, D.; Yang, L.; Zheng, J.; Li, J.; Chen, J.; Ma, D. Photomultiplication-type perovskite photodetectors base on air-processed perovskite films. Org. Electron. 2023, 118, 106800. [Google Scholar] [CrossRef]
- Kannan, R.; Kim, A.R.; Eo, S.K.; Kang, S.H.; Yoo, D.J. Facile one-step synthesis of cerium oxide-carbon quantum dots/RGO nanohybrid catalyst and its enhanced photocatalytic activity. Ceram. Int. 2017, 43, 3072–3079. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Wang, X.; He, J.; Li, Y.; Liu, Y. Synthesis of Asymmetrical CsPbBr3/TiO2 Nanocrystals with Enhanced Stability and Photocatalytic Properties. Catalysts 2023, 13, 1048. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- Kagan, C.R.; Murray, C.B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotechnol. 2015, 10, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Halperin, W.P. Quantum size effects in metal particles. Rev. Mod. Phys. 1986, 58, 533–606. [Google Scholar] [CrossRef]
- Feldheim, L.; Keating, C.D. Self-assembly of single electron transistors and related devices. Chem. Soc. Rev. 1998, 27, 1–12. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K.; et al. Luminescent zero-dimensional organic metal halide hybrids with near-unity quantum efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef]
- Ioannou, D.; Griffin, D.K. Nanotechnology and molecular cytogenetics: The future has not yet arrived. Nano Rev. 2010, 1, 5117. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Yang, M.Z.; Chen, B.X.; Wang, X.D.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Pagliarulo, A.; Striccoli, M.; Calia, A.; Lettieri, M.; Colangiuli, D.; Curri, M.L.; Comparelli, R. Colloidal Nanocrystalline Semiconductor Materials as Photocatalysts for Environmental Protection of Architectural Stone. Crystals 2017, 7, 30. [Google Scholar] [CrossRef]
- Pincik, E.; Matsumoto, T. Progress in applied surface, interface and thin film science 2017. Appl. Surf. Sci. 2018, 461, 1–2. [Google Scholar] [CrossRef]
- Ball, P.; Garwin, L. Science at the atomic scale. Nature 1992, 355, 761–764. [Google Scholar] [CrossRef]
- Fu, P.; Shan, Q.; Shang, Y.; Song, J.; Zeng, H.; Ning, Z.; Gong, J. Perovskite nanocrystals: Synthesis, properties and applications. Sci. Bull. 2017, 62, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Endres, J.; Egger, D.A.; Kulbak, M.; Kerner, R.A.; Zhao, L.; Silver, S.H.; Hodes, G.; Rand, B.P.; Cahen, D.; Kronik, L.; et al. Valence and Conduction Band Densities of States of Metal Halide Perovskites: A Combined Experimental–Theoretical Study. J. Phys. Chem. Lett. 2016, 7, 2722–2729. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Li, Q.; Yang, K.; Guo, T.; Li, X.; Zeng, H. Emissions at Perovskite Quantum Dot/Film Interface with Halide Anion Exchange. ACS Photonics 2018, 5, 4504–4512. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Xu, J.; Li, S.; Cui, M.; Fu, X.; Liu, Y.; Liu, Y.; Wan, H.; Wei, K.; et al. Synthesis-on-substrate of quantum dot solids. Nature 2022, 612, 679–684. [Google Scholar] [CrossRef]
- Uddin, M.A.; Glover, J.D.; Park, S.M.; Pham, J.T.; Graham, K.R. Growth of Highly Stable and Luminescent CsPbX3 (X = Cl, Br, and I) Nanoplates via Ligand Mediated Anion Exchange of CsPbCl3 Nanocubes with AlX3. Chem. Mater. 2020, 32, 5217–5225. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; He, J.; Li, Y.; Liu, Y. Synthesis of All-Inorganic Halide Perovskite Nanocrystals for Potential Photoelectric Catalysis Applications. Catalysts 2023, 13, 1041. [Google Scholar] [CrossRef]
- Shekhirev, M.; Goza, J.; Teeter, J.D.; Lipatov, A.; Sinitskii, A. Synthesis of Cesium Lead Halide Perovskite Quantum Dots. J. Chem. Educ. 2017, 94, 1150–1156. [Google Scholar] [CrossRef]
- Chu, D.B.K.; Owen, J.S.; Peters, B. Nucleation and Growth Kinetics from LaMer Burst Data. J. Phys. Chem. A 2017, 121, 7511–7517. [Google Scholar] [CrossRef]
- Huang, X.; Guo, Q.; Yang, D.; Xiao, X.; Liu, X.; Xia, Z.; Fan, F.; Qiu, J.; Dong, G. Reversible 3D laser printing of perovskite quantum dots inside a transparent medium. Nat. Photonics 2020, 14, 82–88. [Google Scholar] [CrossRef]
- Dong, Y.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef]
- Rainò, G.; Becker, M.A.; Bodnarchuk, M.I.; Mahrt, R.F.; Kovalenko, M.V.; Stöferle, T. Superfluorescence from lead halide perovskite quantum dot superlattices. Nature 2018, 563, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Guan, W.; Xu, Y.; Wang, X.; Wu, L.; Chen, M.; Zhong, Q.; Xu, Y.; Li, Y.; Sham, T.-K.; et al. Construction of Single-Atom Platinum Catalysts Enabled by CsPbBr3 Nanocrystals. ACS Nano 2021, 15, 13129–13139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ye, F.; Sun, J.; Wang, Z.; Zhang, C.; Qian, J.; Zhang, X.; Choy, W.C.H.; Sun, X.W.; Wang, K.; et al. Efficient CsPbBr3 Nanoplatelet-Based Blue Light-Emitting Diodes Enabled by Engineered Surface Ligands. ACS Energy Lett. 2022, 7, 1137–1145. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.; Wang, H.; Zhan, Q.; Liu, Q.; Xia, Z. Postsynthetic Surface Trap Removal of CsPbX3 (X = Cl, Br, or I) Quantum Dots via a ZnX2/Hexane Solution toward an Enhanced Luminescence Quantum Yield. Chem. Mater. 2018, 30, 8546–8554. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.R.; Lee, T.H.; Lee, H.; Kang, H.; Park, S.; Jang, H.W.; Kim, S.Y. Enhanced Optical Properties and Stability of CsPbBr3 Nanocrystals Through Nickel Doping. Adv. Funct. Mater. 2021, 31, 2102770. [Google Scholar] [CrossRef]
- Liu, A.; Bi, C.; Li, J.; Zhang, M.; Cheng, C.; Binks, D.; Tian, J. High Color-Purity and Efficient Pure-Blue Perovskite Light-Emitting Diodes Based on Strongly Confined Monodispersed Quantum Dots. Nano Lett. 2023, 23, 2405–2411. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; He, J.; Wang, X.; Liu, Q.; Luo, X.; Wang, M.; Liu, J.; Liu, C.; Liu, Y. Synthesis of Size-Adjustable CsPbBr3 Perovskite Quantum Dots for Potential Photoelectric Catalysis Applications. Materials 2024, 17, 1607. https://doi.org/10.3390/ma17071607
Li H, He J, Wang X, Liu Q, Luo X, Wang M, Liu J, Liu C, Liu Y. Synthesis of Size-Adjustable CsPbBr3 Perovskite Quantum Dots for Potential Photoelectric Catalysis Applications. Materials. 2024; 17(7):1607. https://doi.org/10.3390/ma17071607
Chicago/Turabian StyleLi, Hang, Jiazhen He, Xiaoqian Wang, Qi Liu, Xuemin Luo, Mingwei Wang, Jinfeng Liu, Chengqi Liu, and Yong Liu. 2024. "Synthesis of Size-Adjustable CsPbBr3 Perovskite Quantum Dots for Potential Photoelectric Catalysis Applications" Materials 17, no. 7: 1607. https://doi.org/10.3390/ma17071607
APA StyleLi, H., He, J., Wang, X., Liu, Q., Luo, X., Wang, M., Liu, J., Liu, C., & Liu, Y. (2024). Synthesis of Size-Adjustable CsPbBr3 Perovskite Quantum Dots for Potential Photoelectric Catalysis Applications. Materials, 17(7), 1607. https://doi.org/10.3390/ma17071607





