The Preparation of an Ultrafine Copper Powder by the Hydrogen Reduction of an Ultrafine Copper Oxide Powder and Reduction Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ultrafine Copper Powders
2.3. Characterization
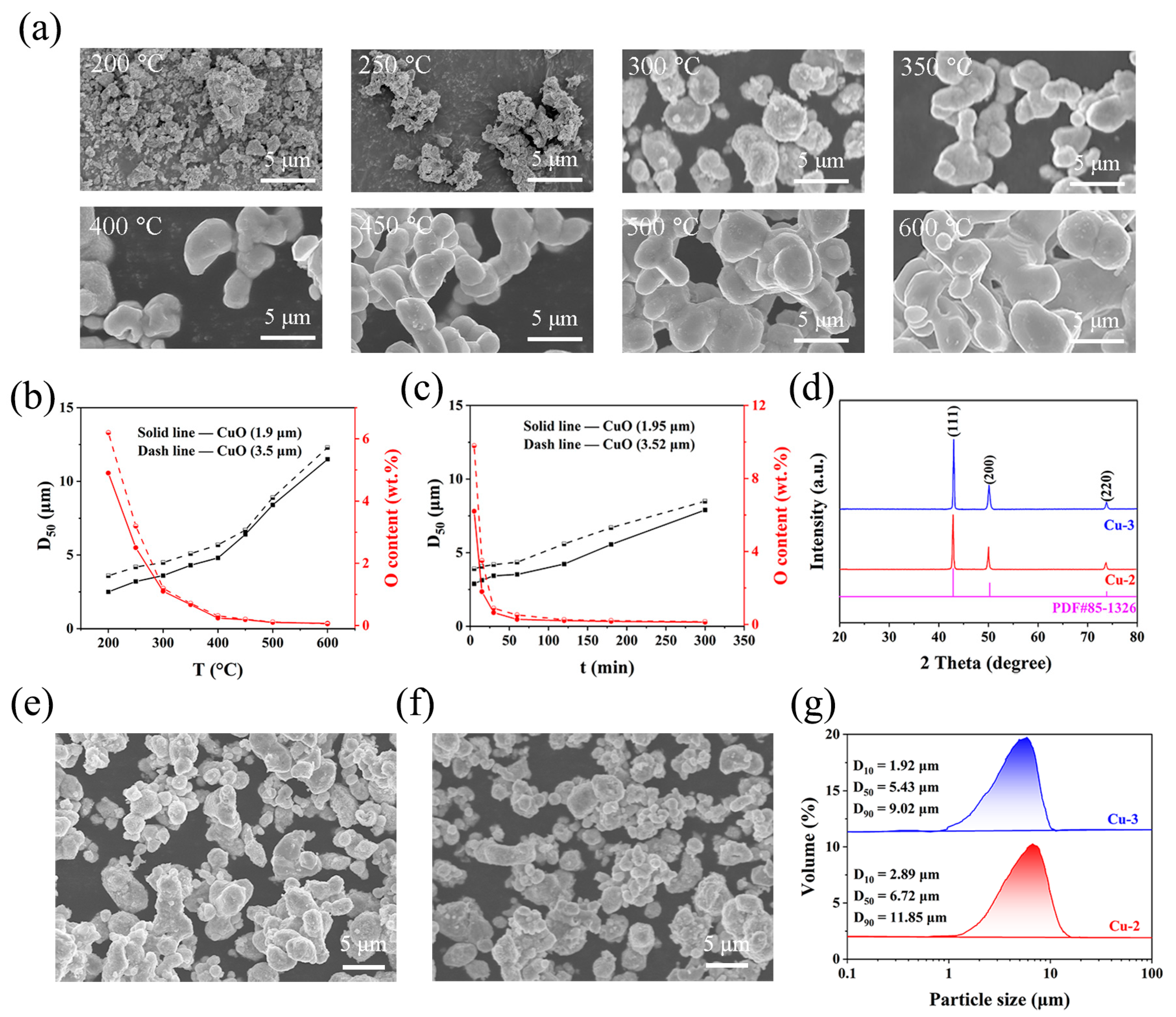
3. Results
3.1. Evolution of Physicochemical Properties of CuO Powders in the Air-Jet Milling Process
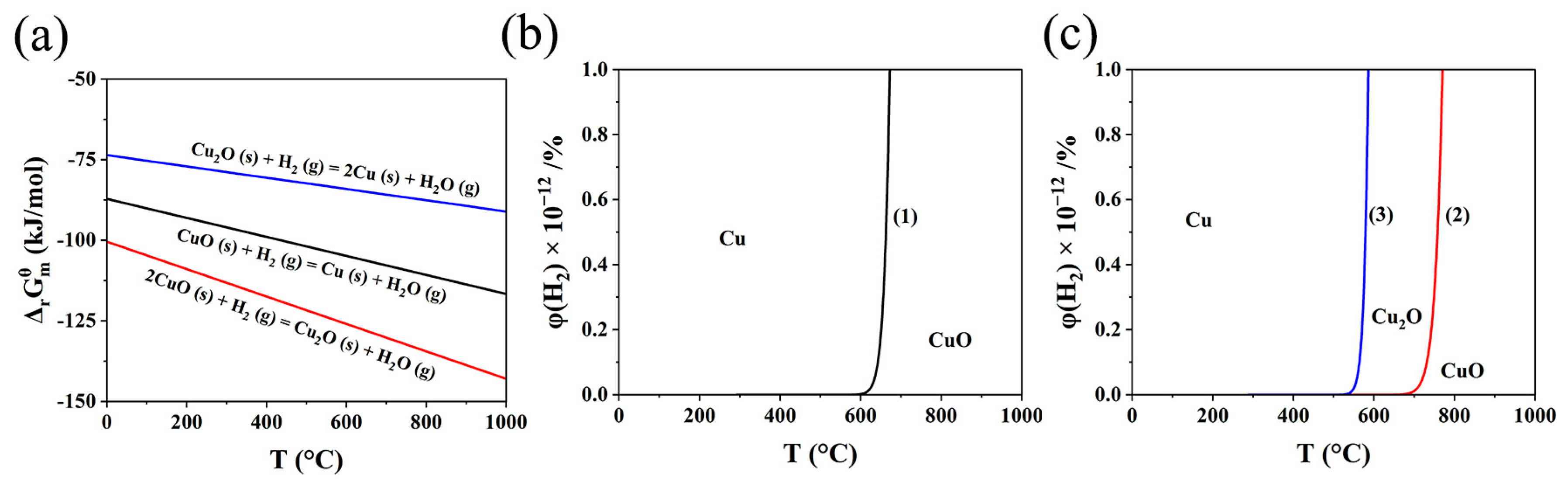
3.2. Thermodynamics of Reaction between CuO and H2
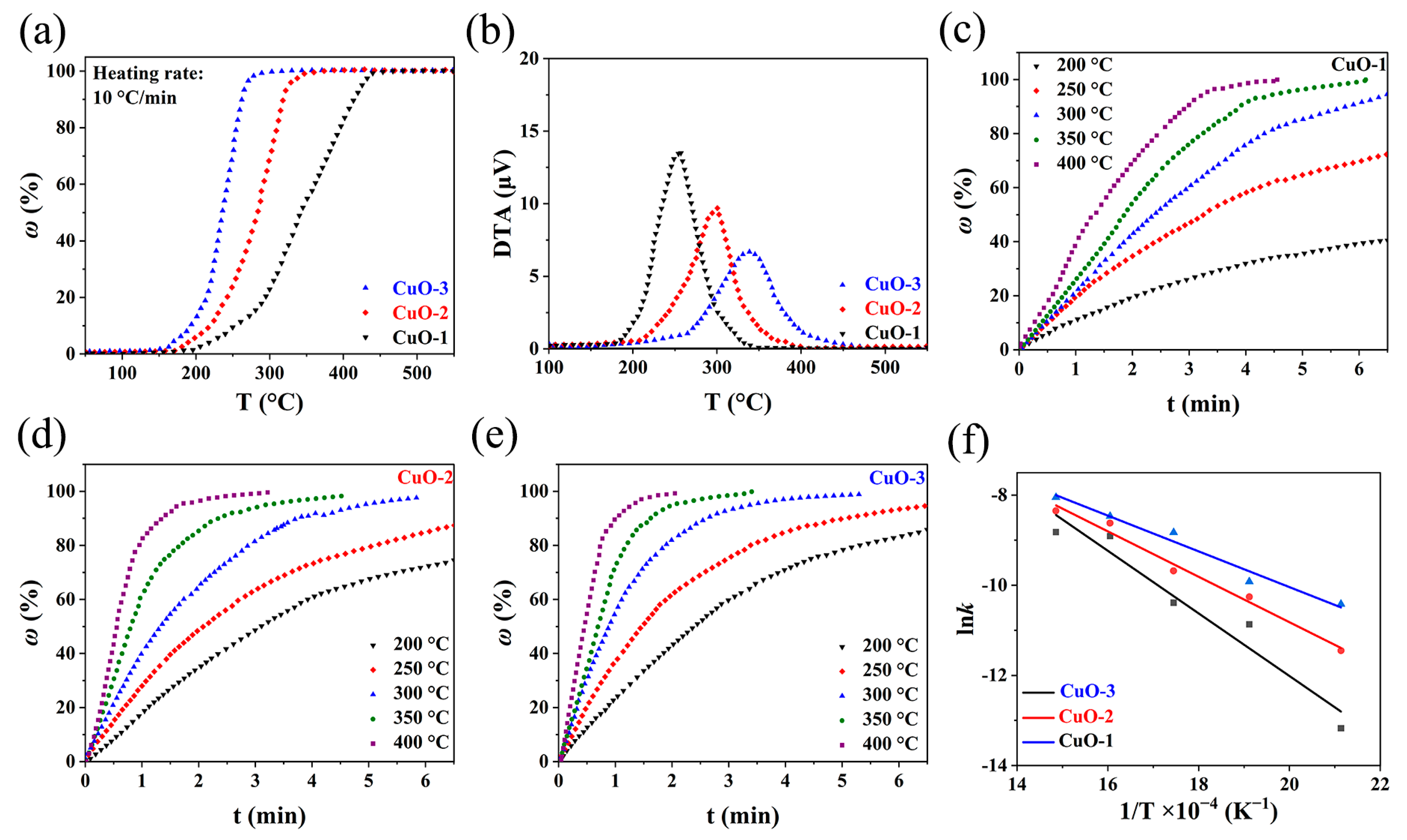
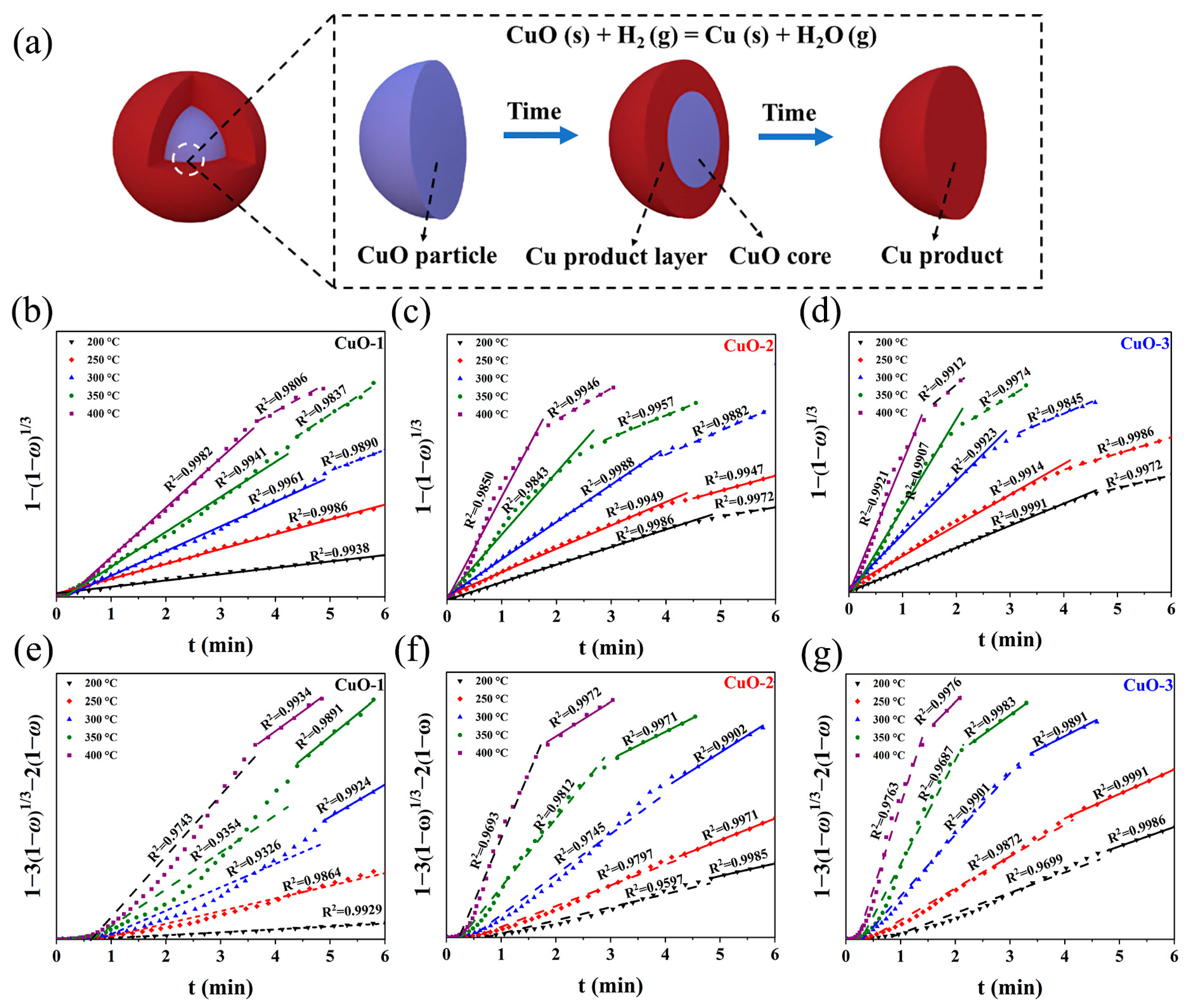
3.3. Kinetics of Reaction between CuO and H2
3.4. Effect of Technological Parameter on Properties of Ultrafine Copper Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanov, Y.F.; Osmonoliev, M.N.; Sedoi, V.S.; Arkhipov, V.A.; Bondarchuk, S.S.; Vorozhtsov, A.B.; Korotkikh, A.G.; Kuznetsov, V.T. Productions of Ultra-Fine Powders and Their Use in High Energetic Compositions. Propellants Explos. Pyrotech. 2003, 28, 319–333. [Google Scholar] [CrossRef]
- Biçer, M.; Şişman, İ. Controlled synthesis of copper nano/microstructures using ascorbic acid in aqueous CTAB solution. Powder Technol. 2010, 198, 279–284. [Google Scholar] [CrossRef]
- Wu, S.; Qin, H.; Li, P. Preparation of fine copper powders and their application in BME-MLCC. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2006, 13, 250–255. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, S.; Sheng, Y.; Tsao, H. Reduction-assisted sintering of micron-sized copper powders at low temperature by ethanol vapor. RSC Adv. 2015, 5, 53275–53279. [Google Scholar] [CrossRef]
- Bidulsky, R.; Gobber, F.S.; Bidulska, J.; Ceroni, M.; Kvackaj, T.; Grande, M.A. Coated Metal Powders for Laser Powder Bed Fusion (L-PBF) Processing: A Review. Metals 2021, 11, 1831. [Google Scholar] [CrossRef]
- Zein El Abedin, S.; Saad, A.Y.; Farag, H.K.; Borisenko, N.; Liu, Q.X.; Endres, F. Electrodeposition of selenium, indium and copper in an air- and water-stable ionic liquid at variable temperatures. Electrochim. Acta 2007, 52, 2746–2754. [Google Scholar] [CrossRef]
- Ding, J.; Tsuzuki, T.; McCormick, P.G.; Street, R. Ultrafine Cu particles prepared by mechanochemical process. J. Alloys Compd. 1996, 234, L1–L3. [Google Scholar] [CrossRef]
- Vitulli, G.; Bernini, M.; Bertozzi, S.; Pitzalis, E.; Salvadori, P.; Coluccia, S.; Martra, G. Nanoscale Copper Particles Derived from Solvated Cu Atoms in the Activation of Molecular Oxygen. Chem. Mater. 2002, 14, 1183–1186. [Google Scholar] [CrossRef]
- Rosenband, V.; Gany, A. Preparation of nickel and copper submicrometer particles by pyrolysis of their formates. J. Mater. Process. Technol. 2004, 153–154, 1058–1061. [Google Scholar] [CrossRef]
- Huang, H.H.; Yan, F.Q.; Kek, Y.M.; Chew, C.H.; Xu, G.Q.; Ji, W.; Oh, P.S.; Tang, S.H. Synthesis, Characterization, and Nonlinear Optical Properties of Copper Nanoparticles. Langmuir 1997, 13, 172–175. [Google Scholar] [CrossRef]
- Sun, J.; Jing, Y.; Jia, Y.; Tillard, M.; Belin, C. Mechanism of preparing ultrafine copper powder by polyol process. Mater. Lett. 2005, 59, 3933–3936. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorobyov, A.M.; Lyakhov, N.Z. A novel method to prepare copper microspheres via chemical reduction route. J. Mater. Res. Technol. 2021, 13, 1254–1265. [Google Scholar] [CrossRef]
- Songping, W.; Shuyuan, M. Preparation of micron size copper powder with chemical reduction method. Mater. Lett. 2006, 60, 2438–2442. [Google Scholar] [CrossRef]
- Magdassi, S.; Grouchko, M.; Kamyshny, A. Copper Nanoparticles for Printed Electronics: Routes Towards Achieving Oxidation Stability. Materials 2010, 3, 4626–4638. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, K. Preparation of spherical ultrafine copper powder via hydrogen reduction-densification of Mg(OH)2-coated Cu2O powder. Int. J. Miner. Metall. Mater. 2012, 19, 1063–1068. [Google Scholar] [CrossRef]
- Pelletiers, T.W., II; Daye, W.K. Production of Copper and Copper Alloy Powders. In ASM Handbook Volume 7: Powder Metallurgy; ASM International: Novelty, OH, USA, 2015; pp. 544–554. [Google Scholar]
- Klar, E.; Berry, D.F. Copper powder metallurgy products. In ASM Handbook Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM Handbook Committee: Novelty, OH, USA, 1990. [Google Scholar]
- Dhokey, N.B.; Walunj, M.G.; Chaudhari, U.C. Influence of water pressure and apex angle on prediction of particle size for atomization of copper powder. Adv. Powder Technol. 2014, 25, 795–800. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.; Li, F.; Feng, C.; Liu, Z.; Zhou, Y. Development and progress on hydrogen metallurgy. Int. J. Miner. Metall. Mater. 2020, 27, 713–723. [Google Scholar] [CrossRef]
- Balac, P. Mechanical activation in technology of metals extraction. Metall 2000, 54, 190–195. [Google Scholar]
- James, S.L.; Friščić, T. Mechanochemistry. Chem. Soc. Rev. 2013, 42, 7494–7496. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Fernández-Bertran, J.F. Mechanochemistry: An overview. Pure Appl. Chem. 1999, 71, 581–586. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, C.; Ren, C.; Tang, J.; Gao, L.; Omran, M.; Chen, G. The effect of mechanical activation duration on the structure and physical properties of titanium slag. Mater. Today Commun. 2023, 35, 106200. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Altin, E.; Mallembakam, M.R.; Peukert, W.; Forssberg, E. Microstructural characterization of hematite during wet and dry millings using Rietveld and XRD line profile analyses. Powder Technol. 2008, 186, 9–21. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. Reduction kinetics of mechanically activated hematite concentrate with hydrogen gas using nonisothermal methods. Thermochim. Acta 2007, 454, 69–77. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. Effects of mechanical activation on the reduction behavior of hematite concentrate. Int. J. Miner. Process. 2007, 82, 96–105. [Google Scholar] [CrossRef]
- Ding, J.; Tsuzuki, T.; McCormick, P.G.; Street, R. Structure and magnetic properties of ultrafine Fe powders by mechanochemical processing. J. Magn. Magn. Mater. 1996, 162, 271–276. [Google Scholar] [CrossRef]
- Ding, J.; Tsuzuki, T.; McCormick, P.G.; Street, R. Ultrafine Co and Ni particles prepared by mechanochemical processing. J. Phys. D Appl. Phys. 1996, 29, 2365. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146. [Google Scholar] [CrossRef]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Lee Black, D.; McQuay, M.Q.; Bonin, M.P. Laser-based techniques for particle-size measurement: A review of sizing methods and their industrial applications. Prog. Energy Combust. Sci. 1996, 22, 267–306. [Google Scholar] [CrossRef]
- Tromans, D.; Meech, J.A. Enhanced dissolution of minerals: Stored energy, amorphism and mechanical activation. Miner. Eng. 2001, 14, 1359–1377. [Google Scholar] [CrossRef]
- Tahmasebi, R.; Shamanian, M.; Abbasi, M.H.; Panjepour, M. Effect of iron on mechanical activation and structural evolution of hematite–graphite mixture. J. Alloys Compd. 2009, 472, 334–342. [Google Scholar] [CrossRef]
- Alex, T.C.; Kumar, R.; Roy, S.K.; Mehrotra, S.P. Anomalous reduction in surface area during mechanical activation of boehmite synthesized by thermal decomposition of gibbsite. Powder Technol. 2011, 208, 128–136. [Google Scholar] [CrossRef]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int. J. Hydrog. Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef]
- Li, J.; Mayer, J.W.; Tu, K.N. Nucleation and growth of Cu2O in the reduction of CuO thin films. Phys. Rev. B 1992, 45, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances; Wiley Online Library: New York, NY, USA, 1989; Volume 304. [Google Scholar]
- Jelić, D.; Tomić-Tucaković, B.; Mentus, S. A kinetic study of copper(II) oxide powder reduction with hydrogen, based on thermogravimetry. Thermochim. Acta 2011, 521, 211–217. [Google Scholar] [CrossRef]
- Kim, J.Y.; Rodriguez, J.A.; Hanson, J.C.; Frenkel, A.I.; Lee, P.L. Reduction of CuO and Cu2O with H2:H embedding and kinetic effects in the formation of suboxides. J. Am. Chem. Soc. 2003, 125, 10684–10692. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Moslim, N.A.M.; Sivakumar, R.; Rezan, S.A.; Udayakumar, S.; Ibrahim, N.; Baharun, N.; Fauzi, M.N.A.; Kasim, S.R.; Mohamed, A.R. Isothermal and non-isothermal kinetic modelling of carbothermal reduction of Titanium Dioxide-Iron (II) Oxide (TiO2-Fe2O3) composite with natural gas. Mater. Today Proc. 2019, 17, 655–663. [Google Scholar] [CrossRef]
- Homma, S.; Ogata, S.; Koga, J.; Matsumoto, S. Gas–solid reaction model for a shrinking spherical particle with unreacted shrinking core. Chem. Eng. Sci. 2005, 60, 4971–4980. [Google Scholar] [CrossRef]
- Metolina, P.; Ribeiro, T.R.; Guardani, R. Hydrogen-based direct reduction of industrial iron ore pellets: Statistically designed experiments and computational simulation. Int. J. Miner. Metall. Mater. 2022, 29, 1908–1921. [Google Scholar] [CrossRef]
- Melchiori, T.; Canu, P. Improving the Quantitative Description of Reacting Porous Solids: Critical Analysis of the Shrinking Core Model by Comparison to the Generalized Grain Model. Ind. Eng. Chem. Res. 2014, 53, 8980–8995. [Google Scholar] [CrossRef]
- Coble, R.L. Effects of Particle-Size Distribution in Initial-Stage Sintering. J. Am. Ceram. Soc. 1973, 56, 461–466. [Google Scholar] [CrossRef]
- Ledford, C.; Rock, C.; Carriere, P.; Frigola, P.; Gamzina, D.; Horn, T. Characteristics and Processing of Hydrogen-Treated Copper Powders for EB-PBF Additive Manufacturing. Appl. Sci. 2019, 9, 3993. [Google Scholar] [CrossRef]
- Wong-Ángel, W.D.; Téllez-Jurado, L.; Chávez-Alcalá, J.F.; Chavira-Martínez, E.; Verduzco-Cedeño, V.F. Effect of copper on the mechanical properties of alloys formed by powder metallurgy. Mater. Des. 2014, 58, 12–18. [Google Scholar] [CrossRef]
- Ojha, N.K.; Zyryanov, G.V.; Majee, A.; Charushin, V.N.; Chupakhin, O.N.; Santra, S. Copper nanoparticles as inexpensive and efficient catalyst: A valuable contribution in organic synthesis. Coord. Chem. Rev. 2017, 353, 1–57. [Google Scholar] [CrossRef]

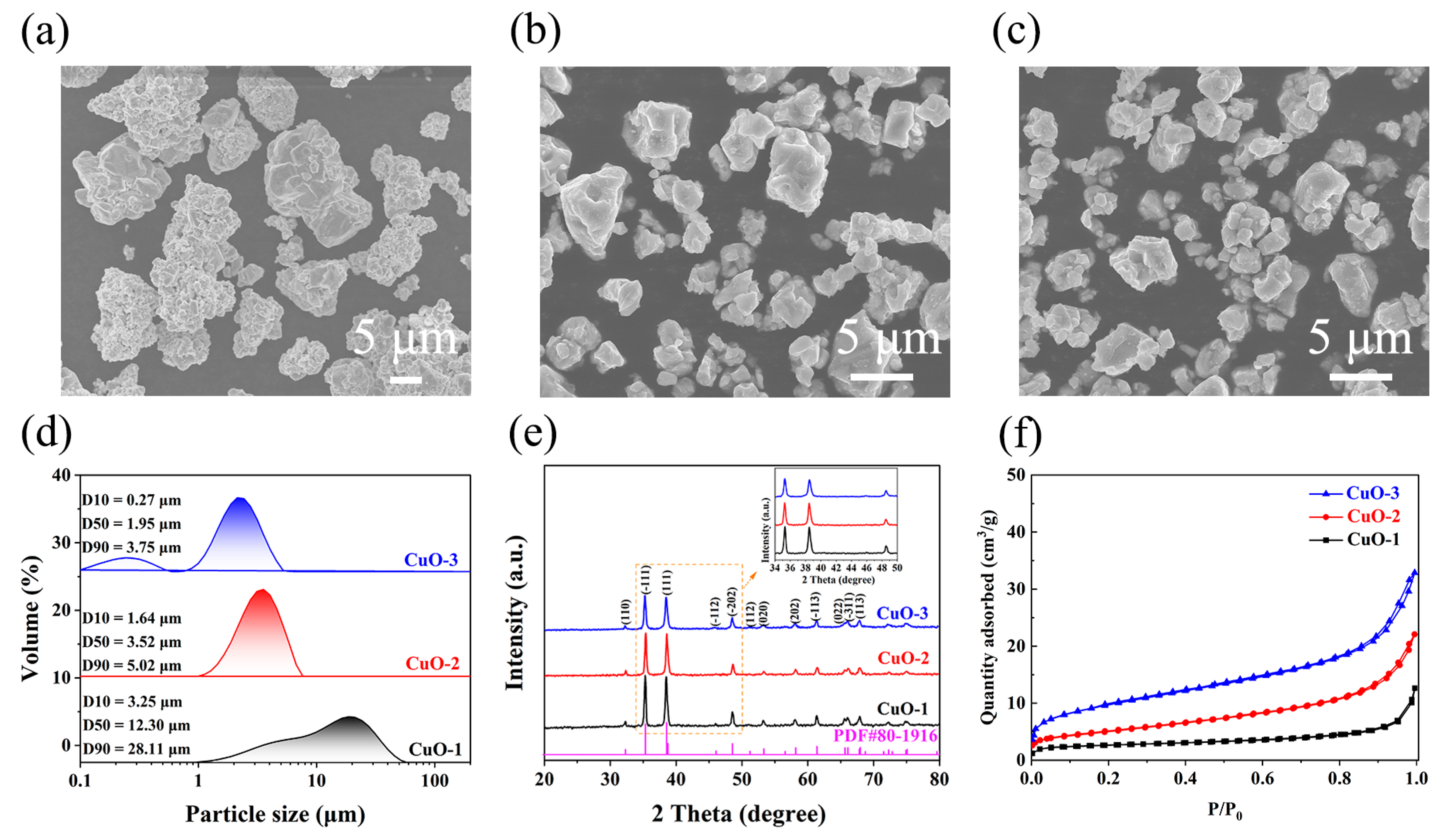
| Sample | D50 (μm) | d (nm) | ε (%) | SSABET (m2/g) | ρa (g/cm3) | E (kJ/mol) |
|---|---|---|---|---|---|---|
| CuO-1 | 12.30 | 104 | 0.03 | 0.51 | 3.47 | 57.74 |
| CuO-2 | 3.52 | 78 | 0.45 | 1.74 | 1.42 | 41.88 |
| CuO-3 | 1.95 | 69 | 0.71 | 2.47 | 1.16 | 32.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Pang, J.; Han, W.; Luo, L.; Cheng, X.; Zhao, Z.; Lv, C.; Liu, J. The Preparation of an Ultrafine Copper Powder by the Hydrogen Reduction of an Ultrafine Copper Oxide Powder and Reduction Kinetics. Materials 2024, 17, 1613. https://doi.org/10.3390/ma17071613
Li S, Pang J, Han W, Luo L, Cheng X, Zhao Z, Lv C, Liu J. The Preparation of an Ultrafine Copper Powder by the Hydrogen Reduction of an Ultrafine Copper Oxide Powder and Reduction Kinetics. Materials. 2024; 17(7):1613. https://doi.org/10.3390/ma17071613
Chicago/Turabian StyleLi, Shiwen, Jianming Pang, Wei Han, Lingen Luo, Xiaoyu Cheng, Zhimin Zhao, Chaoran Lv, and Jue Liu. 2024. "The Preparation of an Ultrafine Copper Powder by the Hydrogen Reduction of an Ultrafine Copper Oxide Powder and Reduction Kinetics" Materials 17, no. 7: 1613. https://doi.org/10.3390/ma17071613
APA StyleLi, S., Pang, J., Han, W., Luo, L., Cheng, X., Zhao, Z., Lv, C., & Liu, J. (2024). The Preparation of an Ultrafine Copper Powder by the Hydrogen Reduction of an Ultrafine Copper Oxide Powder and Reduction Kinetics. Materials, 17(7), 1613. https://doi.org/10.3390/ma17071613





