Solvothermal Guided V2O5 Microspherical Nanoparticles Constructing High-Performance Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Experimental Section
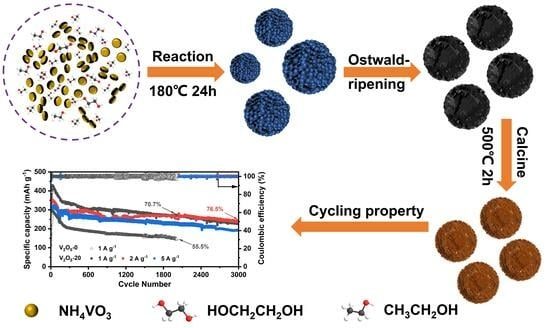
2.1. Synthesis of V2O5 Microspheres
2.2. Electrochemical Measurement
3. Results and Discussion
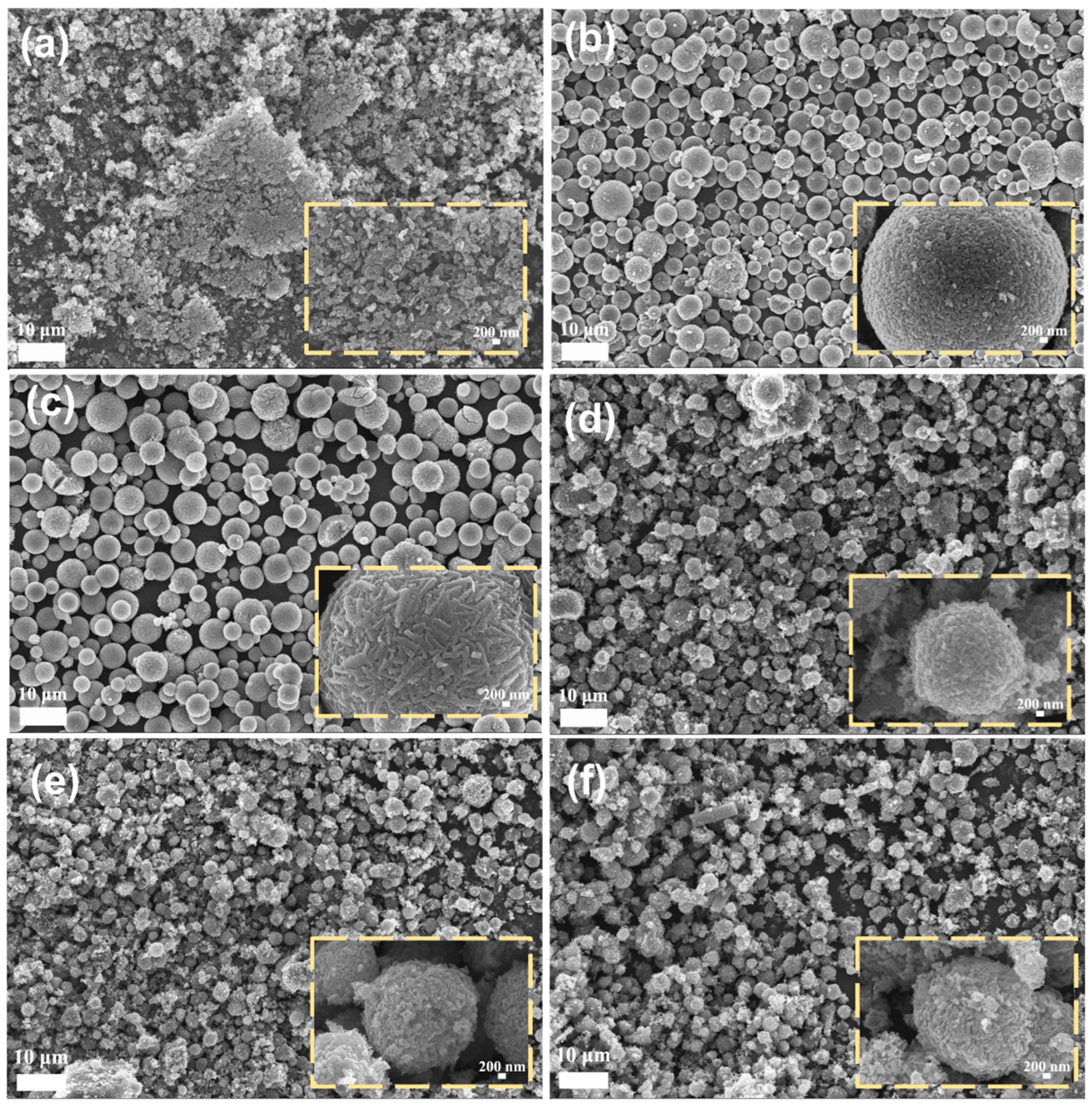
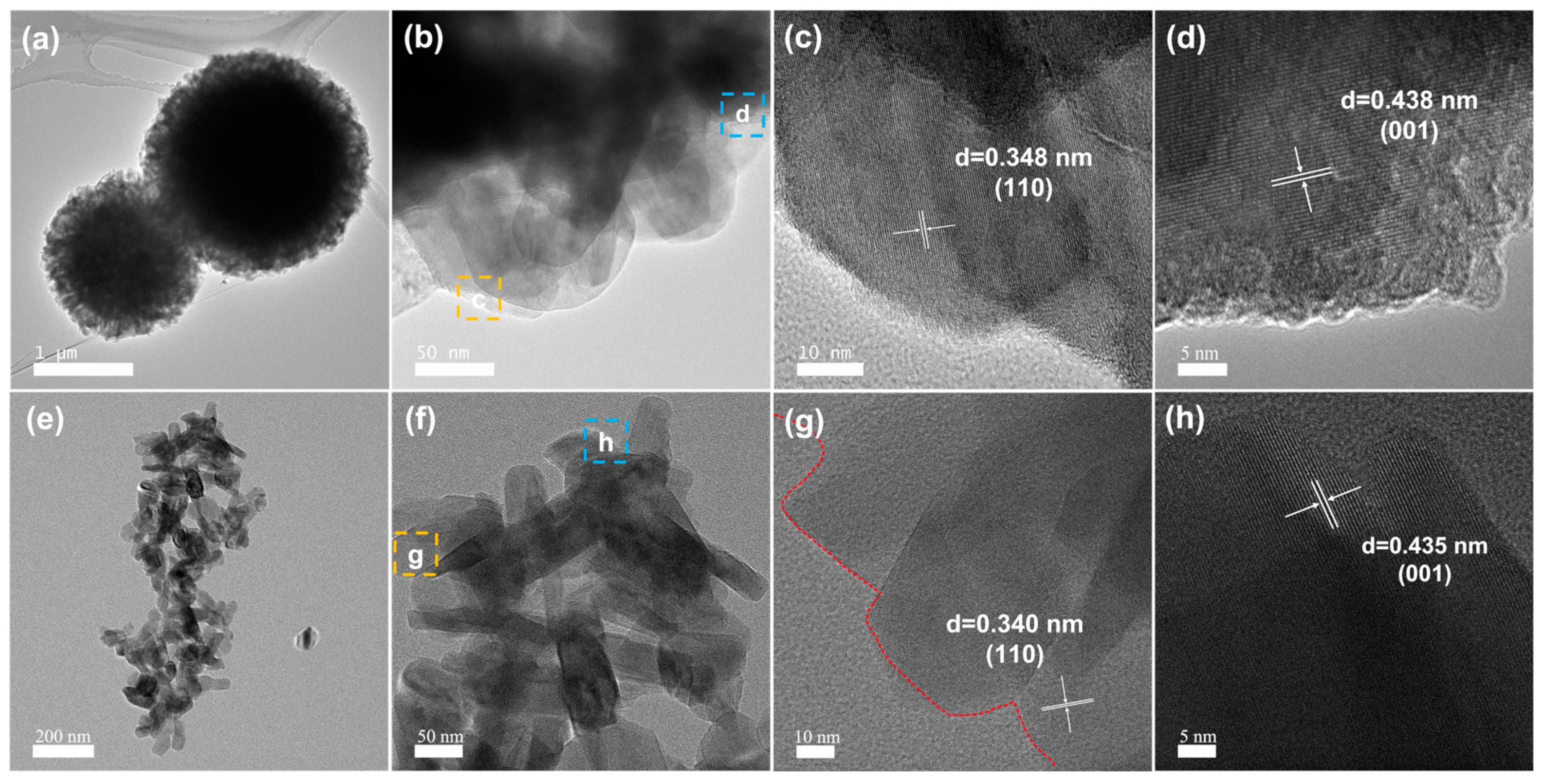
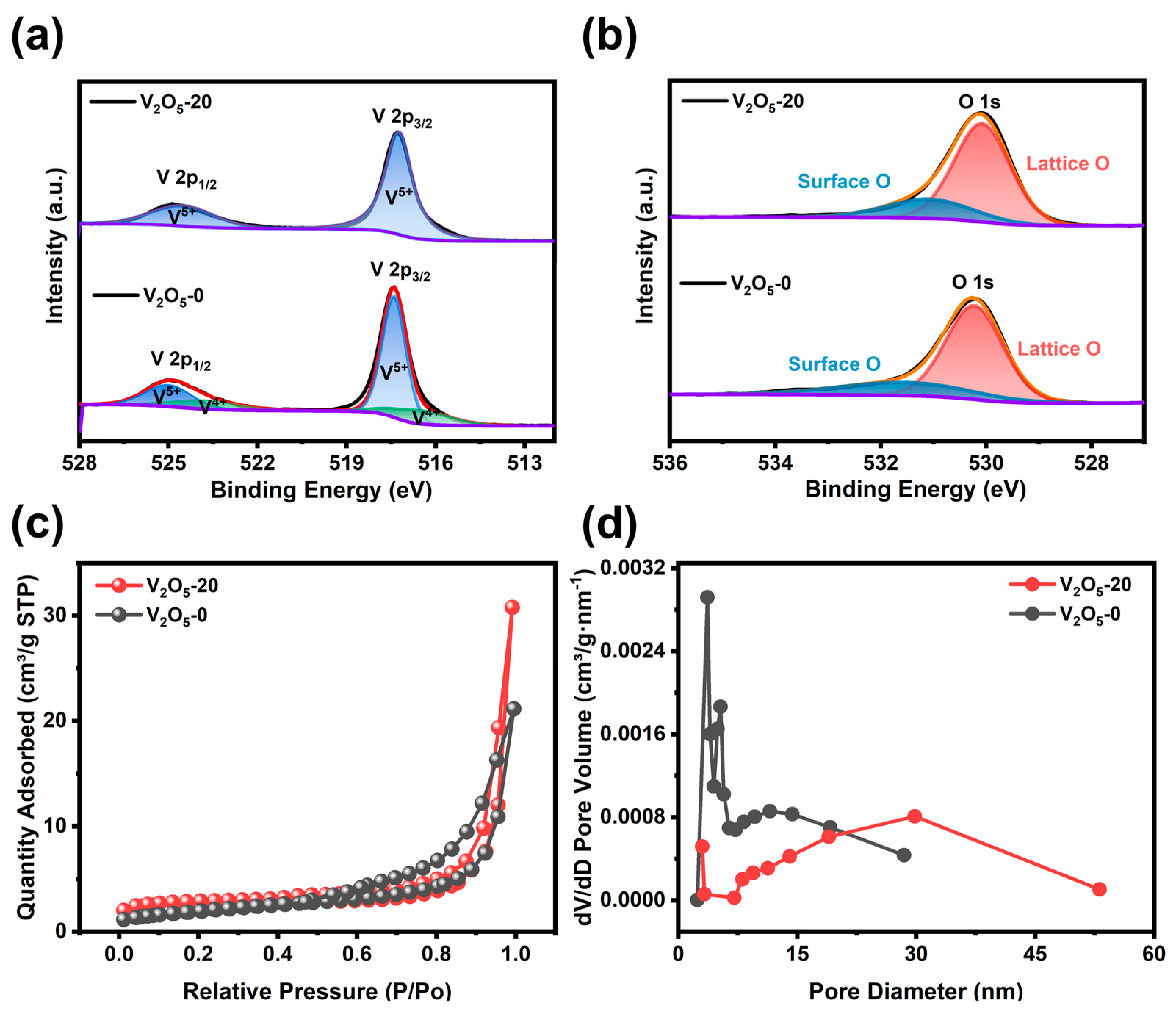
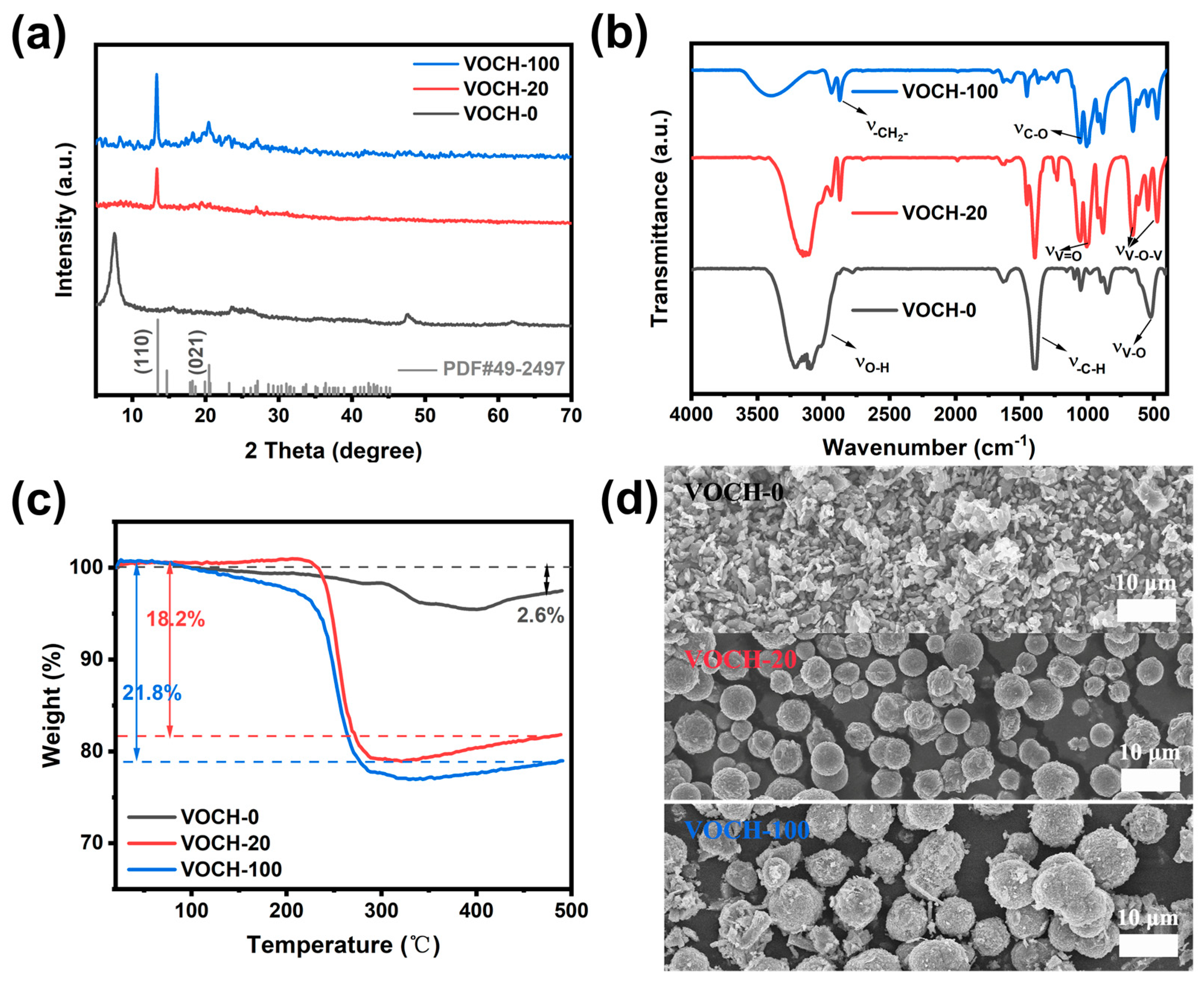
3.1. Structure and Morphology
3.2. Mechanistic Analysis of Microsphere Formation
3.3. Electrochemical Performance
3.3.1. Influence of Electrode Materials on Zinc-Ion Batteries
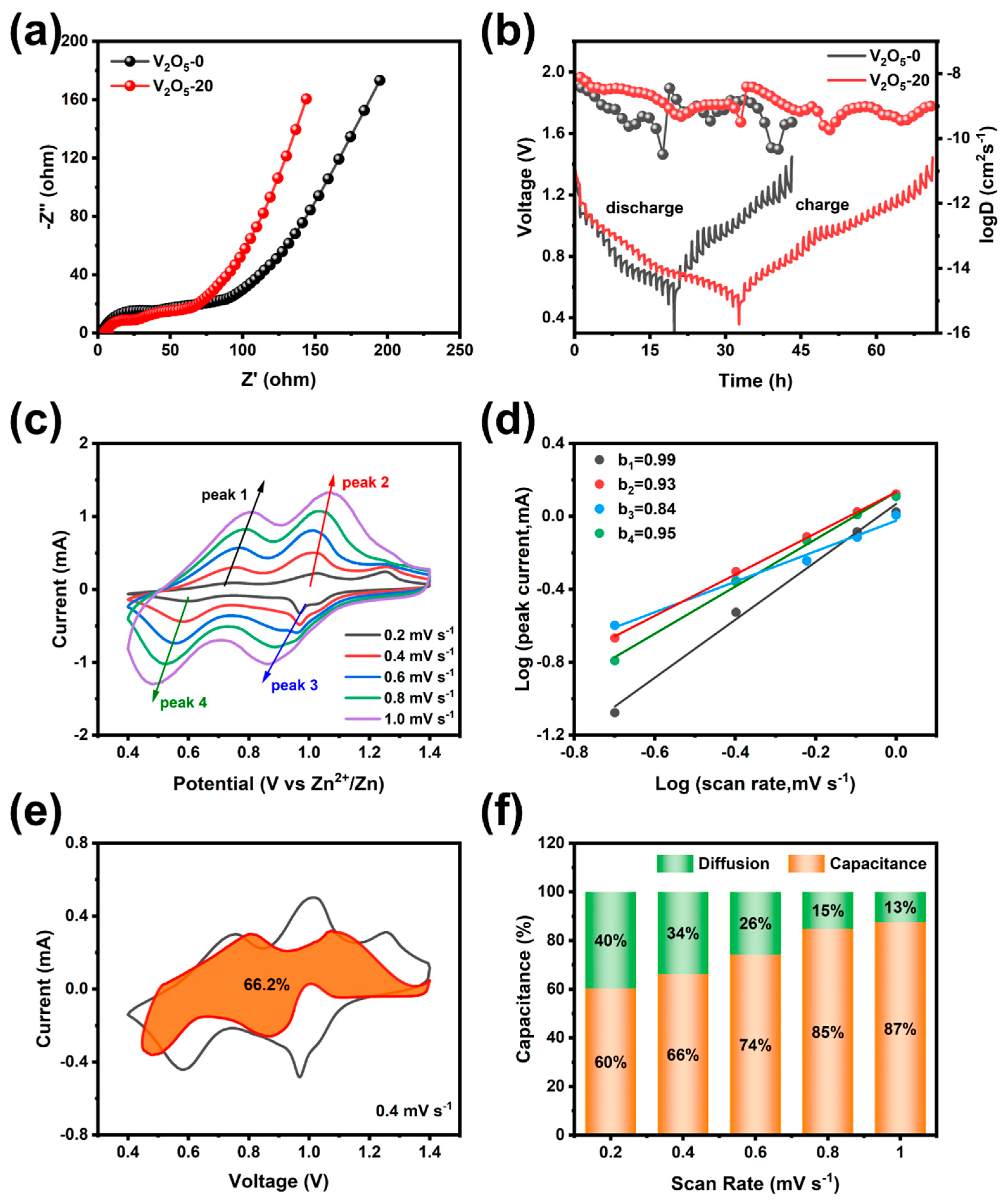
3.3.2. The Reaction Kinetics of V2O5 Microspheres
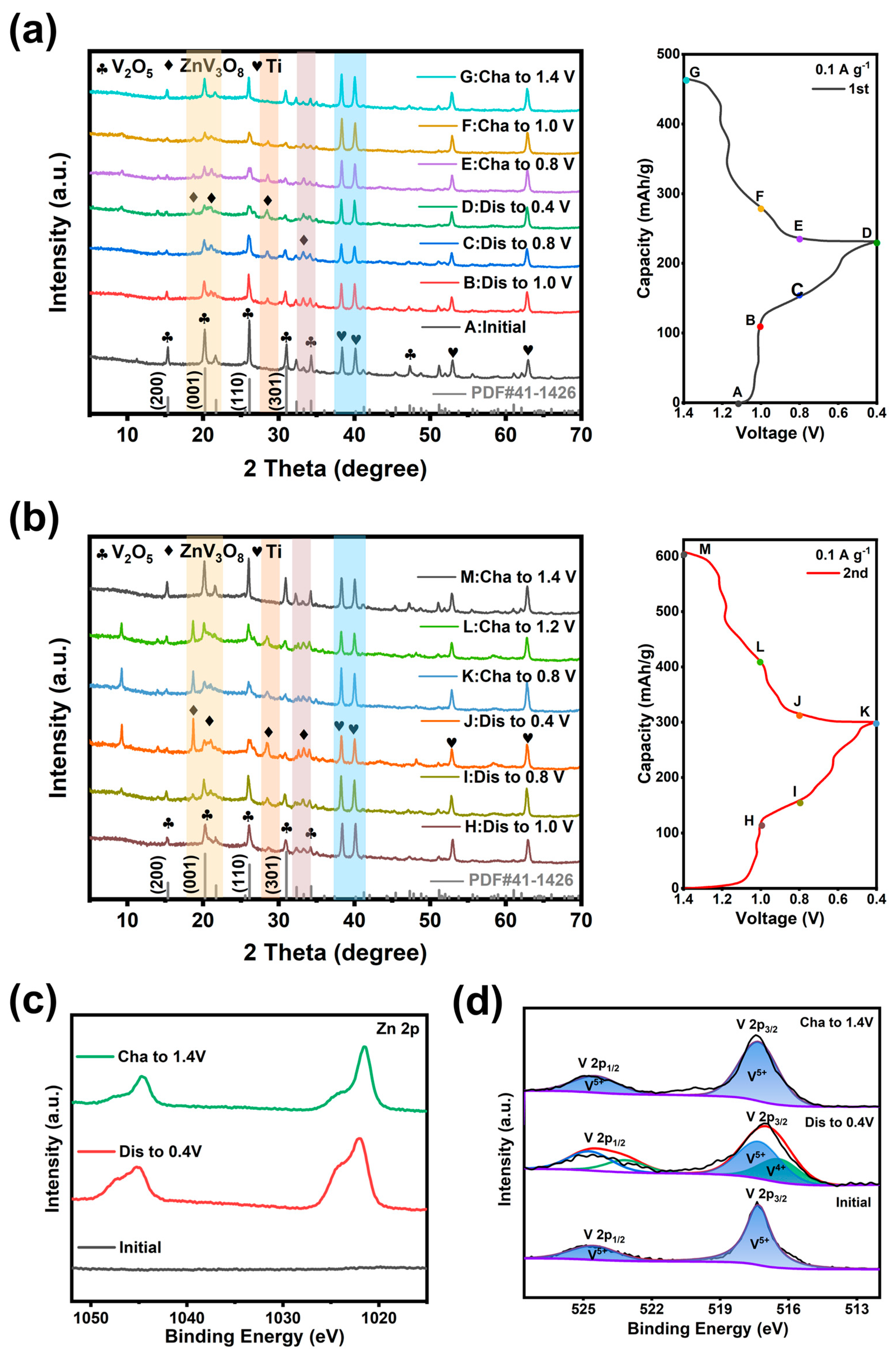
3.3.3. Investigation of the Energy Storage Mechanism of V2O5 Microspheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by Side Battery Technologies with Lithium-Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089. [Google Scholar] [CrossRef]
- Lionetto, F.; Arianpouya, N.; Bozzini, B.; Maffezzoli, A.; Nematollahi, M.; Mele, C. Advances in Zinc-Ion Structural Batteries. J. Energy Storage 2024, 84, 110849. [Google Scholar] [CrossRef]
- Purusottam Reddy, B.; Reddy Prasad, P.; Mallikarjuna, K.; Chandra Sekhar, M.; Lee, Y.-W.; Suh, Y.; Park, S.-H. Mn–Co Prussian Blue Analogue Cubic Frames for Efficient Aqueous Zn Ion Batteries. Microporous Mesoporous Mater. 2023, 362, 112793. [Google Scholar] [CrossRef]
- Wang, J.; Szabo, L.; Madhav, D.; Ferreira, I.; Vandeginste, V. Recent Progress in Interfacial Engineering Strategies for Mn-Based Oxide Cathodes in Aqueous Zinc-Ion Batteries: Mechanisms, Modifications, and Performance Enhancement. Energy Storage Mater. 2023, 63, 103015. [Google Scholar] [CrossRef]
- Saha, P.; Ali, A.; Nayem, S.M.A.; Shaheen Shah, S.; Aziz, M.A.; Saleh Ahammad, A.J. Vanadium-Based Cathodic Materials of Aqueous Zn-Ion Battery for Superior-Performance with Prolonged-Life Cycle. Chem. Rec. 2024, 24, e202200310. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, N.; Yeşilot, S.; Sariyer, S.; Ghosh, A.; Kılıç, A.; Sel, O.; Demir-Cakan, R. A Small Inorganic-Organic Material Based on Anthraquinone-Decorated Cyclophosphazene as Cathode for Aqueous Electrolyte Zinc-Ion Batteries. Mater. Today Energy 2023, 33, 101280. [Google Scholar] [CrossRef]
- Tuttle, M.R.; Walter, C.; Brackman, E.; Moore, C.E.; Espe, M.; Rasik, C.; Adams, P.; Zhang, S. Redox-Active Zinc Thiolates for Low-Cost Aqueous Rechargeable Zn-Ion Batteries. Chem. Sci. 2021, 12, 15253–15262. [Google Scholar] [CrossRef]
- Lewis, C.-E.M.; Fernando, J.F.S.; Siriwardena, D.P.; Firestein, K.L.; Zhang, C.; Golberg, D.V. Carbon-Integrated Vanadium Oxide Hydrate as a High-Performance Cathode Material for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4159–4169. [Google Scholar] [CrossRef]
- Deka Boruah, B.; Mathieson, A.; Park, S.K.; Zhang, X.; Wen, B.; Tan, L.; Boies, A.; De Volder, M. Vanadium Dioxide Cathodes for High-Rate Photo-Rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100115. [Google Scholar] [CrossRef]
- Jiang, H.; Gong, W.; Zhang, Y.; Liu, X.; Waqar, M.; Sun, J.; Liu, Y.; Dong, X.; Meng, C.; Pan, Z.; et al. Quench-Tailored Al-Doped V2O5 Nanomaterials for Efficient Aqueous Zinc-Ion Batteries. J. Energy Chem. 2022, 70, 52–58. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Kim, M.; Lee, J.; Kim, K.J.; Choi, J.W. Corrosion as the Origin of Limited Lifetime of Vanadium Oxide-Based Aqueous Zinc Ion Batteries. Nat. Commun. 2022, 13, 2371. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.I.; Sharlaev, A.S.; Berezina, O.Y.; Tolstopjatova, E.G.; Fu, L.; Kondratiev, V.V. Electrospun V2O5 Nanofibers as High-Capacity Cathode Materials for Zinc-Ion Batteries. Mater. Lett. 2022, 308, 131212. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D.D.; Chan, S.H. Hierarchical 3D Micro-/Nano-V2O5 (Vanadium Pentoxide) Spheres as Cathode Materials for High-Energy and High-Power Lithium Ion-Batteries. Energy 2014, 76, 607–613. [Google Scholar] [CrossRef]
- Hu, P.; Zhu, T.; Ma, J.; Cai, C.; Hu, G.; Wang, X.; Liu, Z.; Zhou, L.; Mai, L. Porous V2O5 Microspheres: A High-Capacity Cathode Material for Aqueous Zinc–Ion Batteries. Chem. Commun. 2019, 55, 8486–8489. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, H.; Li, M.; Zheng, Y.; Zhou, M.; Gui, H.; Xiang, Y.; Xu, C.; Li, X.; Wang, R. High-Performance Aqueous Zinc-Ion Batteries Enabled by Binder-Free and Ultrathin V2O5– x @Graphene Aerogels with Intercalation Pseudocapacitance. ACS Appl. Mater. Interfaces 2022, 14, 53677–53689. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, M.; Kirubasankar, B.; Shi, M.; Velayutham, S.; Wang, B.; Angaiah, S.; Yan, C. Morphology Restrained Growth of V2O5 by the Oxidation of V-MXenes as a Fast Diffusion Controlled Cathode Material for Aqueous Zinc Ion Batteries. Chem. Commun. 2020, 56, 6412–6415. [Google Scholar] [CrossRef] [PubMed]
- Roex, E.; Boschini, F.; Delaval, V.; Schrijnemakers, A.; Cloots, R.; Mahmoud, A. Spray-Dried V2O5 as Cathode Material for High-Performance Aqueous Zinc-Ion Batteries. J. Electroanal. Chem. 2023, 929, 117133. [Google Scholar] [CrossRef]
- Muthukumar, K.; Rajendran, S.; Sekar, A.; Chen, Y.; Li, J. Synthesis of V2O5 Nanoribbon–Reduced Graphene Oxide Hybrids as Stable Aqueous Zinc-Ion Battery Cathodes via Divalent Transition Metal Cation-Mediated Coprecipitation. ACS Sustain. Chem. Eng. 2023, 11, 2670–2679. [Google Scholar] [CrossRef]
- Gao, W.; Michalička, J.; Pumera, M. Hierarchical Atomic Layer Deposited V2O5 on 3D Printed Nanocarbon Electrodes for High-Performance Aqueous Zinc-Ion Batteries. Small 2022, 18, 2105572. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Zheng, Q.; Li, X. Porous V2O5 Yolk–Shell Microspheres for Zinc Ion Battery Cathodes: Activation Responsible for Enhanced Capacity and Rate Performance. J. Mater. Chem. A 2020, 8, 5186–5193. [Google Scholar] [CrossRef]
- Qin, H.; Chen, L.; Wang, L.; Chen, X.; Yang, Z. V2O5 Hollow Spheres as High Rate and Long Life Cathode for Aqueous Rechargeable Zinc Ion Batteries. Electrochim. Acta 2019, 306, 307–316. [Google Scholar] [CrossRef]
- Minart, G.; Croguennec, L.; Weill, F.; Labrugère-Sarroste, C.; Olchowka, J. Increasing Tap Density of Carbon-Coated Na3V2(PO4)2F3 via Mechanical Grinding: Good or Bad Idea? ACS Appl. Energy Mater. 2024. [Google Scholar] [CrossRef]
- Jo, C.; Yu, J.H.; Kim, H.J.; Hwang, J.; Kim, J.; Jung, H.; Myung, S. Sulfurized Carbon Composite with Unprecedentedly High Tap Density for Sodium Storage. Adv. Energy Mater. 2022, 12, 2102836. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.H. Polyhedral TiO2 Particle-Based Cathode for Li-S Batteries with High Volumetric Capacity and High Performance in Lean Electrolyte. Chem. Eng. J. 2020, 399, 125670. [Google Scholar] [CrossRef]
- Hsuan Chiang, T.; Chen, T.-M. Synthesis and Characterization of Single-Crystalline Vanadium Pentoxide by the Low-Temperature of Glycothermal Method. Mater. Lett. 2015, 157, 205–208. [Google Scholar] [CrossRef]
- Tao, B.; Zhang, Y.; Han, D.; Li, Y.; Yan, Z. Synthesis of Corundum-Type In2O3 Porous Spheres and Their Photocatalytic Properties. J. Mater. Chem. A 2014, 2, 5455–5461. [Google Scholar] [CrossRef]
- Guo, R.; Bao, Y.; Kang, Q.; Liu, C.; Zhang, W.; Zhu, Q. Solvent-Controlled Synthesis and Photocatalytic Activity of Hollow TiO2 Microspheres Prepared by the Solvothermal Method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127931. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N.; Prasanna, B.P.; Nandan, K.R.; Yogesh Kumar, K.; Prasad, S.B.B. Polyaniline/V2O5 Composites for Anticorrosion and Electromagnetic Interference Shielding. Mater. Chem. Phys. 2021, 259, 124059. [Google Scholar] [CrossRef]
- Pinna, N.; Willinger, M.; Weiss, K.; Urban, J.; Schlögl, R. Local Structure of Nanoscopic Materials: V2O5 Nanorods and Nanowires. Nano Lett. 2003, 3, 1131–1134. [Google Scholar] [CrossRef]
- Pan, G.X.; Xia, X.H.; Cao, F.; Chen, J.; Zhang, Y.J. Carbon Cloth Supported Vanadium Pentaoxide Nanoflake Arrays as High-Performance Cathodes for Lithium Ion Batteries. Electrochim. Acta 2014, 149, 349–354. [Google Scholar] [CrossRef]
- Ding, Y.; Peng, Y.; Chen, W.; Niu, Y.; Wu, S.; Zhang, X.; Hu, L. V-MOF Derived Porous V2O5 Nanoplates for High Performance Aqueous Zinc Ion Battery. Appl. Surf. Sci. 2019, 493, 368–374. [Google Scholar] [CrossRef]
- Vincent, M.; Avvaru, V.S.; Haranczyk, M.; Etacheri, V. High-Performance Mg−Li Hybrid Batteries Based on Pseudocapacitive Anatase Ti1- x Co x O 2- y Nanosheet Cathodes. ChemSusChem 2022, 15, e202102562. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Sai Avvaru, V.; Haranczyk, M.; Etacheri, V. Fast-Charging and Long-Lasting Mg-Na Hybrid Batteries Based on Extremely Pseudocapacitive Bronze TiO2 Nanosheet Cathodes. Chem. Eng. J. 2022, 433, 133810. [Google Scholar] [CrossRef]
- Jiang, L.; Qu, Y.; Ren, Z.; Yu, P.; Zhao, D.; Zhou, W.; Wang, L.; Fu, H. In Situ Carbon-Coated Yolk–Shell V2O3 Microspheres for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 1595–1601. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tang, X.; Wang, H. Solvothermal Synthesis and Self-Assembling Mechanism of Micro-Nano Spherical LiFePO4 with High Tap Density. RSC Adv. 2016, 6, 75602–75608. [Google Scholar] [CrossRef]
- Shubhashish, S.; Amin, A.S.; Dang, Y.; Karasik, S.J.; Khanna, H.S.; Suib, S.L. Synthesis of Highly Porous Metal Oxide Nanoparticles for Adsorption Applications. ACS Appl. Nano Mater. 2022, 5, 7078–7091. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M. Enhanced Ethanol Electro-Oxidation Reaction on Carbon Supported Pd-Metal Oxide Electrocatalysts. J. Colloid Interface Sci. 2017, 505, 230–240. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile Preparation of Porous NiTiO3 Nanorods with Enhanced Visible-Light-Driven Photocatalytic Performance. J. Mater. Chem. 2012, 22, 16471. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Ma, X.; Gong, J. Ethylene Glycol: Properties, Synthesis, and Applications. Chem. Soc. Rev. 2012, 41, 4218. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Cui, F.; Meng, J.; Chen, H.; Zeng, X. Enhanced Rate and Cycling Performances of Hollow V2O5 Nanospheres for Aqueous Zinc Ion Battery Cathode. Appl. Surf. Sci. 2020, 507, 145137. [Google Scholar] [CrossRef]
- Chen, H.; Qin, H.; Chen, L.; Wu, J.; Yang, Z. V2O5@CNTs as Cathode of Aqueous Zinc Ion Battery with High Rate and High Stability. J. Alloys Compd. 2020, 842, 155912. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, P.; Liu, H.; Wu, X.; Zhi, C. Tetragonal VO2 Hollow Nanospheres as Robust Cathode Material for Aqueous Zinc Ion Batteries. Mater. Today Energy 2020, 17, 100431. [Google Scholar] [CrossRef]
- Lesel, B.K.; Ko, J.S.; Dunn, B.; Tolbert, S.H. Mesoporous Li xMn2O4 Thin Film Cathodes for Lithium-Ion Pseudocapacitors. ACS Nano 2016, 10, 7572–7581. [Google Scholar] [CrossRef] [PubMed]
- Maity, C.K.; Goswami, N.; Verma, K.; Sahoo, S.; Nayak, G.C. A Facile Synthesis of Boron Nitride Supported Zinc Cobalt Sulfide Nano Hybrid as High-Performance Pseudocapacitive Electrode Material for Asymmetric Supercapacitors. J. Energy Storage 2020, 32, 101993. [Google Scholar] [CrossRef]
- Pavithra, S.; Sakunthala, A.; Kumar, P.S.; Kathirvel, V. Synthesis and Electrochemical Properties of Novel ZnV3O8 by Rheological Phase Reaction Method for High Performance Zinc Based Aqueous Supercapacitor. J. Energy Storage 2023, 69, 107977. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Yan, K.; Sun, Y.; Chen, Y.; Tang, Y.; Pan, J.; Wan, P. Solvothermal Guided V2O5 Microspherical Nanoparticles Constructing High-Performance Aqueous Zinc-Ion Batteries. Materials 2024, 17, 1660. https://doi.org/10.3390/ma17071660
Jia X, Yan K, Sun Y, Chen Y, Tang Y, Pan J, Wan P. Solvothermal Guided V2O5 Microspherical Nanoparticles Constructing High-Performance Aqueous Zinc-Ion Batteries. Materials. 2024; 17(7):1660. https://doi.org/10.3390/ma17071660
Chicago/Turabian StyleJia, Xianghui, Kaixi Yan, Yanzhi Sun, Yongmei Chen, Yang Tang, Junqing Pan, and Pingyu Wan. 2024. "Solvothermal Guided V2O5 Microspherical Nanoparticles Constructing High-Performance Aqueous Zinc-Ion Batteries" Materials 17, no. 7: 1660. https://doi.org/10.3390/ma17071660
APA StyleJia, X., Yan, K., Sun, Y., Chen, Y., Tang, Y., Pan, J., & Wan, P. (2024). Solvothermal Guided V2O5 Microspherical Nanoparticles Constructing High-Performance Aqueous Zinc-Ion Batteries. Materials, 17(7), 1660. https://doi.org/10.3390/ma17071660