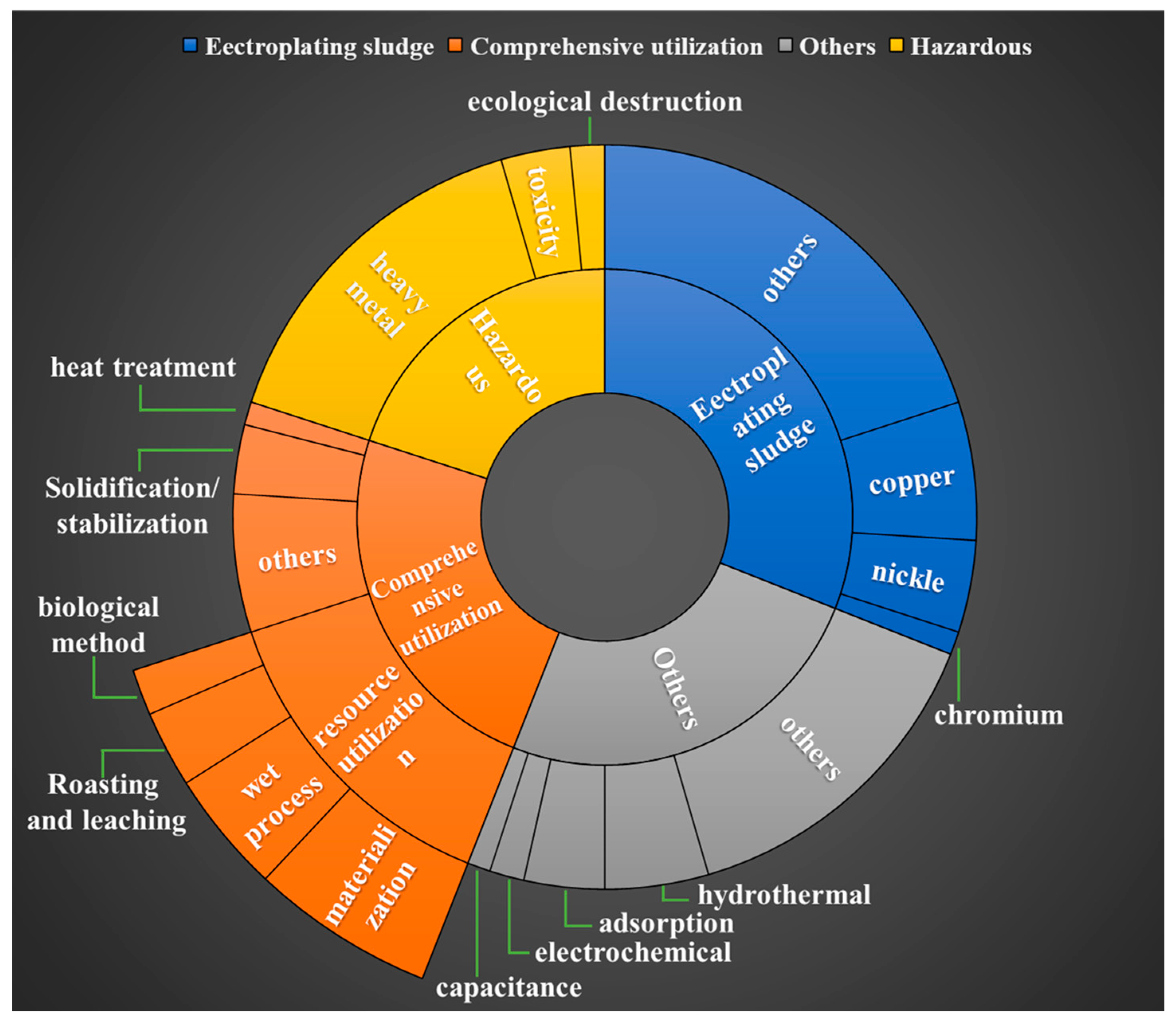
Approaches for the Treatment and Resource Utilization of Electroplating Sludge
Abstract
:1. Introduction
2. Methodology Adopted for Review
3. The Nature of Electroplating Sludge
3.1. Source, Composition, and Classification of Electroplating Sludge
3.2. Hazards of Electroplating Sludge
4. Electroplating Sludge Disposal Methods
4.1. Solidification/Stabilization Treatment
4.2. Heat Treatment
5. Electroplating Sludge Resource Utilization
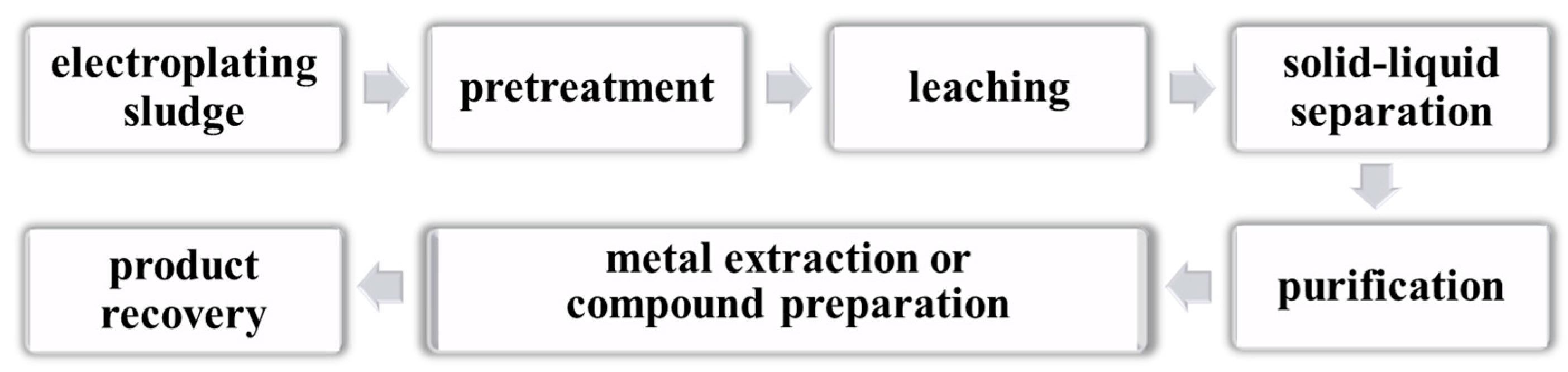
5.1. Hydrometallurgical Treatment
5.1.1. Acid Leaching Method
5.1.2. Ammonia Leaching Method
5.2. Pyrometallurgical Treatment
5.2.1. Roasting and Leaching Methods
5.2.2. Smelting Method
5.3. Biological Treatment
5.3.1. Biological Leaching
5.3.2. Biological Adsorption
5.3.3. Sludge Composting
5.4. Materialization Treatment
5.5. Technical Comparison
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magalhães, J.M.; Silva, J.E.; Castro, F.P.; Labrincha, J.A. Physical and chemical characterisation of metal finishing industrial wastes. J. Environ. Manag. 2005, 75, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Huang, Q.; Zhou, J.; Wu, Z.; Deng, H.; Liu, X.; Lin, Z. One-step extraction of high-purity CuCl2·2H2O from copper-containing electroplating sludge based on the directional phase conversion. J. Hazard. Mater. 2021, 413, 125469. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Zhang, Z. Approaches for electroplating sludge treatment and disposal technology: Reduction, pretreatment and reuse. J. Environ. Manag. 2024, 349, 119535. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Su, T.; Zhu, S.; Chen, Y.; Yu, Y.; Xie, X.; Yang, J.; Huo, M.; Bian, D. Stepwise extraction of Fe, Al, Ca, and Zn: A green route to recycle raw electroplating sludge. J. Environ. Manag. 2021, 300, 113700. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Chen, Y.; Chen, Y.; Zhu, S.; Liu, J.; Ren, H.; Su, T.; Huo, M. Efficient separation of impurities Fe/Al/Ca and recovery of Zn from electroplating sludge using glucose as reductant. Sci. Total Environ. 2023, 896, 165202. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.; Hua, W.; Jianhang, H. Co-treatment of electroplating sludge, copper slag, and spent cathode carbon for recovering and solidifying heavy metals. J. Hazard. Mater. 2021, 417, 126020. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, V.; Borzenko, O.; Kaliuzhna, I. Vertical Migration of Copper and Zinc Ions in Soils Polluted by Electroplating Sludge. CLEAN Soil Air Water 2021, 49, 2100087. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Cai, X.; Zheng, T.; Zhou, S.; Chen, Y.; Yuan, Y. Recycling electroplating sludge to produce sustainable electrocatalysts for the efficient conversion of carbon dioxide in a microbial electrolysis cell. Electrochim. Acta 2016, 222, 177–184. [Google Scholar] [CrossRef]
- Yu, J.-x.; Li, H.-x.; Zhou, R.-y.; Li, X.-d.; Wu, H.-j.; Xiao, C.-q.; Chi, R.-A. Surface ion imprinted bagasse for selective removal of Cu (II) from the leaching solution of electroplating sludge. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128394. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.K.; Agrawal, M.; Marshall, F.M. Health risk assessment of heavy metals via dietary intake of foodstuffs from the wastewater irrigated site of a dry tropical area of India. Food Chem. Toxicol. 2010, 48, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Huyen, P.T.; Dang, T.D.; Tung, M.T.; Huyen, N.T.T.; Green, T.A.; Roy, S. Electrochemical copper recovery from galvanic sludge. Hydrometallurgy 2016, 164, 295–303. [Google Scholar] [CrossRef]
- Lee, J.-C.; Pandey, B.D. Bio-processing of solid wastes and secondary resources for metal extraction—A review. Waste Manag. 2012, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.T.; Au, W.Y.; van Ewijk, S.; Roy, A.; Stegemann, J.A. Elemental and mineralogical composition of metal-bearing neutralisation sludges, and zinc speciation—A review. J. Hazard. Mater. 2021, 416, 125676. [Google Scholar] [CrossRef]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Su, J.; Jin, G.; Li, C.; Zhu, X.; Dou, Y.; Li, Y.; Wang, X.; Wang, K.; Gu, Q. Ultrasonic preparation of nano-nickel/activated carbon composite using spent electroless nickel plating bath and application in degradation of 2,6-dichlorophenol. J. Environ. Sci. 2014, 26, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, I.S.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. Development of Coloured Stoneware Bodies through the Incorporation of Industrial Cr/Ni Electroplating Sludge. Sustainability 2021, 13, 1999. [Google Scholar] [CrossRef]
- Tian, Q.; Dong, B.; Guo, X.; Xu, Z.; Wang, Q.; Li, D.; Yu, D. Comparative atmospheric leaching characteristics of scandium in two different types of laterite nickel ore from Indonesia. Miner. Eng. 2021, 173, 107212. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Liu, X.; Li, X.; Zheng, J.; Gao, H.; Li, L.; Xu, W.; Wang, S.; Xie, M.; et al. Effective separation and recovery of Zn, Cu, and Cr from electroplating sludge based on differential phase transformation induced by chlorinating roasting. Sci. Total Environ. 2022, 820, 153260. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.R.G.; de Melo Franco, J. Production of concrete paving blocks (CPB) utilising electroplating residues—Evaluation of mechanical and micro-structural properties. Can. J. Chem. Eng. 2012, 90, 1092–1101. [Google Scholar] [CrossRef]
- Bian, R.; Su, T.; Gao, Y.; Chen, Y.; Zhu, S.; Liu, C.; Wang, X.; Qu, Z.; Zhang, Y.; Zhang, H. Enrichment and recycling of Zn from electroplating wastewater as zinc phosphate via coupled coagulation and hydrothermal route. Arab. J. Chem. 2022, 15, 103664. [Google Scholar] [CrossRef]
- Benvenuti, T.; Krapf, R.S.; Rodrigues, M.A.S.; Bernardes, A.M.; Zoppas-Ferreira, J. Recovery of nickel and water from nickel electroplating wastewater by electrodialysis. Sep. Purif. Technol. 2014, 129, 106–112. [Google Scholar] [CrossRef]
- Hou, Z.; Meng, H.; Shao, X.; Wang, X.; Tahir, M.U.; Ahmad, S.; Yang, C.; Su, X.; Zhang, L. Synthesis of amorphous hollow Ni(HCO3)2 nanostructures with excellent supercapacitor performance from nickel-containing electroplating sludge. J. Environ. Chem. Eng. 2022, 10, 106906. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Shao, L.-M.; He, P.-J. Preparation of a metal-phosphate/chromium oxide nanocomposite from Cr(III)-containing electroplating sludge and its optical properties as a nanopigment. Process Saf. Environ. Prot. 2015, 98, 261–267. [Google Scholar] [CrossRef]
- Matović, L.; Vujasin, R.; Kumrić, K.; Krstić, S.; Wu, Y.-N.; Kabtamu, D.M.; Devečerski, A. Designing of technological scheme for conversion of Cr-rich electroplating sludge into the black ceramic pigments of consistent composition, following the principles of circular economy. J. Environ. Chem. Eng. 2021, 9, 105038. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Wu, Y.-N.; Chen, Q.; Zheng, L.; Otake, K.-I.; Matović, L.; Li, F. Facile Upcycling of Hazardous Cr-Containing Electroplating Sludge into Value-Added Metal–Organic Frameworks for Efficient Adsorptive Desulfurization. ACS Sustain. Chem. Eng. 2020, 8, 12443–12452. [Google Scholar] [CrossRef]
- Heng, W.; Yong, Y.; Jianhang, H.; Hua, W. A novel method for effective solidifying chromium and preparing crude stainless steel from multi-metallic electroplating sludge. J. Hazard. Mater. 2024, 465, 133068. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, T.; Wu, J.; Li, H.; Chu, S.; Zhu, X.; Zhang, L.; Lu, J.; Ivanets, A.; Davronbek, B.; et al. Preparation of copper-based catalysts from electroplating sludge by ultrasound treatment and their antibiotic degradation performance. Environ. Res. 2023, 216, 114567. [Google Scholar] [CrossRef]
- Jandova, J.; Štefanová, T.Á.; Niemczyková, R. Recovery of Cu-concentrates from waste galvanic copper sludges. Hydrometallurgy 2000, 57, 77–84. [Google Scholar] [CrossRef]
- Stefanova, R.Y. Sorption of metal ions from aqueous solutions by thermally activated electroplating sludge. J. Environ. Sci. Health Part A 2000, 35, 593–607. [Google Scholar] [CrossRef]
- Weng, C.; Sun, X.; Han, B.; Ye, X.; Zhong, Z.; Li, W.; Liu, W.; Deng, H.; Lin, Z. Targeted conversion of Ni in electroplating sludge to nickel ferrite nanomaterial with stable lithium storage performance. J. Hazard. Mater. 2020, 393, 122296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, L.; Huang, M.; Liu, Y.; Xu, J.; Xu, Z.; Lei, Y. Treating waste with waste: Metals recovery from electroplating sludge using spent cathode carbon combustion dust and copper refining slag. Sci. Total Environ. 2022, 838, 156453. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, W.; Zhang, L.; Cheng, H.; Wang, Y.; Tang, R.; Zhou, H. Bioleaching of Copper-Containing Electroplating Sludge. J. Environ. Manag. 2021, 285, 112133. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ge, J.; Wu, Z.; Lin, J.; Zhu, Z.; Yang, Q.; Liu, X. One-step extraction of CuCl2 from Cu-Ni mixed electroplating sludge by chlorination-mineralization surface-interface phase change modulation. Surf. Interfaces 2023, 37, 102535. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Chou, W.-S.; Chen, W.-S.; Chang, F.-C.; Shen, Y.-H.; Chang, J.-E.; Tsai, M.-S. Recovery of cupric oxide from copper-containing wastewater sludge by acid leaching and ammonia purification process. Desalination Water Treat. 2012, 47, 120–129. [Google Scholar] [CrossRef]
- Nair, A.; Juwarkar, A.A.; Devotta, S. Study of speciation of metals in an industrial sludge and evaluation of metal chelators for their removal. J. Hazard. Mater. 2008, 152, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Zheng, J.; Song, Y.; Shi, Z.; Lin, Z.; Chai, L. Different Pathways for Cr(III) Oxidation: Implications for Cr(VI) Reoccurrence in Reduced Chromite Ore Processing Residue. Environ. Sci. Technol. 2020, 54, 11971–11979. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wu, Y.; Hu, L.; Zhang, W.; Mao, L. Evaluating physical-mechanical properties and long periods environmental risk of fired clay bricks incorporated with electroplating sludge. Constr. Build. Mater. 2019, 227, 116716. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; Mao, L.; Wu, Q. Use of electroplating sludge in production of fired clay bricks: Characterization and environmental risk evaluation. Constr. Build. Mater. 2018, 159, 27–36. [Google Scholar] [CrossRef]
- Liu, Y.; Khan, A.; Wang, Z.; Chen, Y.; Zhu, S.; Sun, T.; Liang, D.; Yu, H. Upcycling of Electroplating Sludge to Prepare Erdite-Bearing Nanorods for the Adsorption of Heavy Metals from Electroplating Wastewater Effluent. Water 2020, 12, 1027. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Yan, B.; Wu, J.; Kong, D.; Romanovski, V.; Ivanets, A.; Li, H.; Chu, S.; Su, X. Removal of chromium from electroplating sludge by roasting-acid leaching and catalytic degradation of antibiotics by its residue. J. Environ. Chem. Eng. 2024, 12, 111754. [Google Scholar] [CrossRef]
- Wang, X.; Ding, C.; Long, H.; Wu, Y.; Jiang, F.; Chang, R.; Xue, S.; Wu, J.; Cheng, K. A novel approach to treating nickel-containing electroplating sludge by solidification with basic metallurgical solid waste. J. Mater. Res. Technol. 2023, 27, 3644–3654. [Google Scholar] [CrossRef]
- Babel, S.; del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Chang, J.-E.; Lu, H.-C.; Chiang, L.-C. Reuse of heavy metal-containing sludges in cement production. Cem. Concr. Res. 2005, 35, 2110–2115. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, H.; Mao, L.; Hashmi, M.Z.; Xu, F.; Tang, X. Stabilization/solidification of chromium-bearing electroplating sludge with alkali-activated slag binders. Chemosphere 2020, 240, 124885. [Google Scholar] [CrossRef] [PubMed]
- Orescanin, V.; Mikulic, N.; Mikelic, I.L.; Posedi, M.; Kampic, S.; Medunic, G. The bulk composition and leaching properties of electroplating sludge prior/following the solidification/stabilization by calcium oxide. J. Environ. Sci. Health Part A 2009, 44, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Yang, X.; Dong, S.; Zhou, J.; Sun, Y.; Xu, Y.; Liu, Q. Stabilization of chromium-bearing electroplating sludge with MSWI fly ash-based Friedel matrices. J. Hazard. Mater. 2009, 165, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Asavapisit, S.; Chotklang, D. Solidification of electroplating sludge using alkali-activated pulverized fuel ash as cementitious binder. Cem. Concr. Res. 2004, 34, 349–353. [Google Scholar] [CrossRef]
- Mao, L.; Gao, B.; Deng, N.; Zhai, J.; Zhao, Y.; Li, Q.; Cui, H. The role of temperature on Cr(VI) formation and reduction during heating of chromium-containing sludge in the presence of CaO. Chemosphere 2015, 138, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, K.; Kikukawa, N. Plasma in-flight treatment of electroplating sludge. Vacuum 2000, 59, 244–251. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Qian, C.; Cui, Y.; Shi, G.; Cheng, J.; Li, X.; Xin, B. Heat treatment-enhanced bioleaching of new electroplating sludge containing high concentration of CuS and its mechanisms. Sep. Purif. Technol. 2023, 307, 12276. [Google Scholar] [CrossRef]
- Sim, H.H.; Rafatullah, M.; Alosaimi, A.M.; Hussein, M.A. Copper, chromium and nickel recovery from electroplating sludge using acid digestion method. Int. J. Environ. Anal. Chem. 2022, 104, 1144–1158. [Google Scholar] [CrossRef]
- Deng, J.; Feng, X.; Qiu, X. Extraction of heavy metal from sewage sludge using ultrasound-assisted nitric acid. Chem. Eng. J. 2009, 152, 177–182. [Google Scholar] [CrossRef]
- Silva, J.; Soares, D.; Paiva, A.; Labrincha, J.; Castro, F. Leaching behaviour of a galvanic sludge in sulphuric acid and ammoniacal media. J. Hazard. Mater. 2005, 121, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, F.; Ma, Y.; Cai, T.; Li, H.; Huang, Z.; Yuan, G. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. J. Hazard. Mater. 2010, 178, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Deng, C.; Liu, T.; Xue, D.; Gong, J.; Tan, R.; Mi, X.; Gong, B.; Wang, Z.; Liu, C.; et al. Selective recovery of copper from electroplating sludge by integrated EDTA mixed with citric acid leaching and electrodeposition. Sep. Purif. Technol. 2022, 301, 121917. [Google Scholar] [CrossRef]
- Shi, C.; Zuo, X.; Yan, B. Selective recovery of nickel from stainless steel pickling sludge with NH3-(NH4)2CO3 leaching system. Environ. Technol. 2022, 44, 3249–3262. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, W.; Zhu, H.; Xu, J.; Li, G.; Fu, D.; Luo, L. Highly Selective Copper and Nickel Separation and Recovery from Electroplating Sludge in Light Industry. Pol. J. Environ. Stud. 2015, 24, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.; Bernardes, A. Galvanic sludge metals recovery by pyrometallurgical and hydrometallurgical treatment. J. Hazard. Mater. 2006, 131, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.D.; dos Santos, V.S.; Bernardes, A.M. Metals recovery from galvanic sludge by sulfate roasting and thiosulfate leaching. Miner. Eng. 2014, 60, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Wang, J.; Huang, Q.; Xin, Y.; Xin, B. Screening Bioleaching Systems and Operational Conditions for Optimal Ni Recovery from Dry Electroplating Sludge and Exploration of the Leaching Mechanisms Involved. Geomicrobiol. J. 2015, 33, 179–184. [Google Scholar] [CrossRef]
- Sathyavathi, S.; Manjula, A.; Rajendhran, J.; Gunasekaran, P. Extracellular synthesis and characterization of nickel oxide nanoparticles from Microbacterium sp. MRS-1 towards bioremediation of nickel electroplating industrial effluent. Bioresour. Technol. 2014, 165, 270–273. [Google Scholar] [CrossRef]
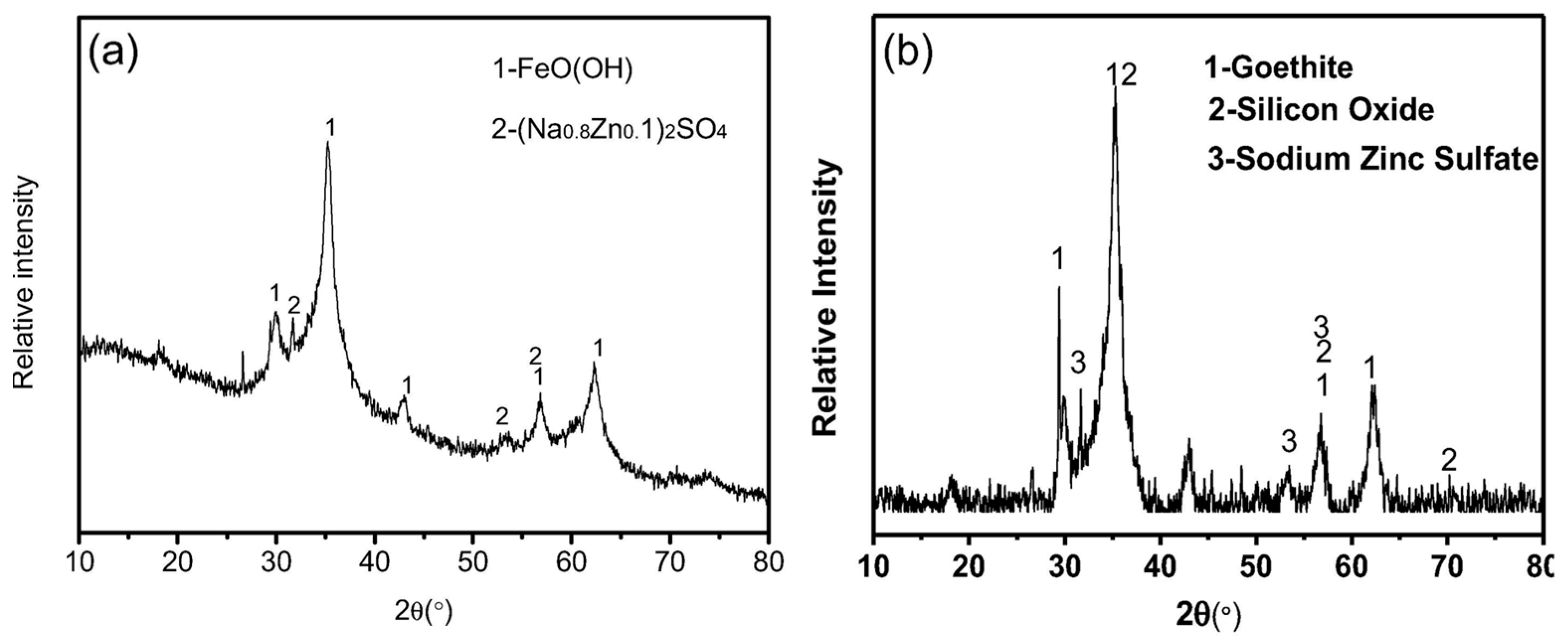
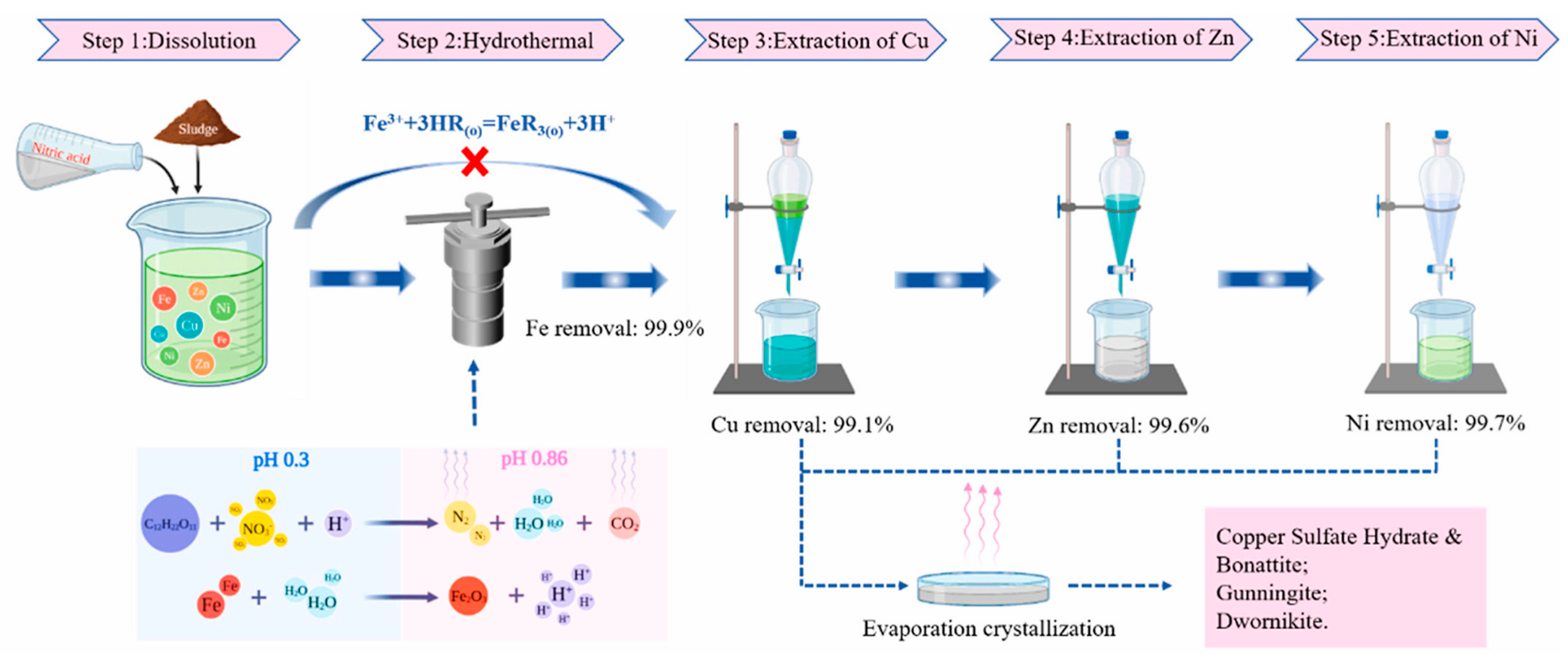
- Yuxin, Z.; Ting, S.; Hongyu, C.; Ying, Z.; Zhi, G.; Suiyi, Z.; Xinfeng, X.; Hong, Z.; Yidi, G.; Yang, H. Stepwise recycling of Fe, Cu, Zn and Ni from real electroplating sludge via coupled acidic leaching and hydrothermal and extraction routes. Environ. Res. 2023, 216, 114462. [Google Scholar] [CrossRef]
- Li, P.P.; Peng, C.S.; Li, F.M.; Song, S.X.; Juan, A.O. Copper and Nickel Recovery from Electroplating Sludge by the Process of Acid-leaching and Electro-depositing. Int. J. Environ. Res. 2011, 5, 797–804. [Google Scholar]
- Lee, J.-c.; Kim, E.-y.; Kim, J.-H.; Kim, W.; Kim, B.-S.; Pandey, B.D. Recycling of WC–Co hardmetal sludge by a new hydrometallurgical route. Int. J. Refract. Met. Hard Mater. 2011, 29, 365–371. [Google Scholar] [CrossRef]
- Salhi, R. Recovery of nickel and copper from metal fInishing hydroxide sludges by ammoniacal leaching. Miner. Process. Extr. Metall. 2013, 119, 147–152. [Google Scholar] [CrossRef]
- Tian, L.; Chen, L.; Gong, A.; Wu, X.; Cao, C.; Liu, D.; Chen, Z.-Q.; Xu, Z.-F.; Liu, Y. Separation and Extraction of Valuable Metals from Electroplating Sludge by Carbothermal Reduction and Low-Carbon Reduction Refining. Jom 2019, 72, 782–789. [Google Scholar] [CrossRef]
- Tian, B.; Cui, Y.; Qin, Z.; Wen, L.; Li, Z.; Chu, H.; Xin, B. Indirect bioleaching recovery of valuable metals from electroplating sludge and optimization of various parameters using response surface methodology (RSM). J. Environ. Manag. 2022, 312, 114927. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, J.; Wang, D.; Zhou, L. Enhancing sewage sludge dewaterability by bioleaching approach with comparison to other physical and chemical conditioning methods. J. Environ. Sci. 2012, 24, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yin, H.; Mai, B.; Peng, H.; Qin, H.; He, B.; Zhang, N. Biosorption of chromium from aqueous solution and electroplating wastewater using mixture of Candida lipolytica and dewatered sewage sludge. Bioresour. Technol. 2010, 101, 3893–3902. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Qin, W.; Zhang, Y.; Liu, Y.; Lyu, Q.; Chen, G.; Feng, Z.; Ji, G.; Yan, Z. The inoculation of thermophilic heterotrophic nitrifiers improved the efficiency and reduced ammonia emission during sewage sludge composting. Chem. Eng. J. 2024, 479, 147237. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Li, S.; Bai, Z.; Ma, L. Advanced composting technologies promotes environmental benefits and eco-efficiency: A life cycle assessment. Bioresour. Technol. 2022, 346, 126576. [Google Scholar] [CrossRef] [PubMed]
- González, I.; Robledo-Mahón, T.; Silva-Castro, G.A.; Rodríguez-Calvo, A.; Gutiérrez, M.C.; Martín, M.Á.; Chica, A.F.; Calvo, C. Evolution of the composting process with semi-permeable film technology at industrial scale. J. Clean. Prod. 2016, 115, 245–254. [Google Scholar] [CrossRef]
- Kuroda, K.; Katahira, T.; Yamada, M.; Uezono, I.; Nakamura, N.; Yamaguchi, T.; Yamauchi, M. Co-composting of sewage sludge with plant biomass, and analysis of microbiome relevant to plant growth promotion. Bioresour. Technol. Rep. 2023, 22, 101401. [Google Scholar] [CrossRef]
- Feng, X.; Wu, Z.; Chen, X. Removal of metal ions from electroplating effluent by EDI process and recycle of purified water. Sep. Purif. Technol. 2007, 57, 257–263. [Google Scholar] [CrossRef]
- Chen, D.; Hou, J.; Yao, L.-h.; Jin, H.-m.; Qian, G.-R.; Xu, Z.P. Ferrite materials prepared from two industrial wastes: Electroplating sludge and spent pickle liquor. Sep. Purif. Technol. 2010, 75, 210–217. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.-Z.; Zhu, H.-J.; Liu, Z.-Z.; Xu, Y.-F.; Liu, Q.; Qian, G.-R. Ferrite process of electroplating sludge and enrichment of copper by hydrothermal reaction. Sep. Purif. Technol. 2008, 62, 297–303. [Google Scholar] [CrossRef]
- Guan, X.; Huang, M.; Yang, L.; Wang, G.; Guan, X. Facial design and synthesis of CoSx/Ni-Co LDH nanocages with rhombic dodecahedral structure for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 151–162. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Y.; Zhai, D.; Qian, G. Electroplating sludge-derived spinel catalysts for NO removal via NH3 selective catalysis reduction. Appl. Surf. Sci. 2020, 528, 146969. [Google Scholar] [CrossRef]
- Zang, X.; Dai, Z.; Guo, J.; Dong, Q.; Yang, J.; Huang, W.; Dong, X. Controllable synthesis of triangular Ni(HCO3)2 nanosheets for supercapacitor. Nano Res. 2016, 9, 1358–1365. [Google Scholar] [CrossRef]
- Hou, Z.; Liu, T.; Usman Tahir, M.; Ahmad, S.; Shao, X.; Yang, C.; He, B.; Su, X. Facile conversion of nickel-containing electroplating sludge into nickel-based multilevel nano-material for high-performance pseudocapacitors. Appl. Surf. Sci. 2021, 538, 147978. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, H.; Zhong, G.; Yan, X.; Su, X.; Lin, Z. Synthesis of NiFeAl LDHs from electroplating sludge and Their excellent supercapacitor performance. J. Hazard. Mater. 2021, 404, 124113. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-J.; Kang, J.-K.; Lee, C.-G.; Bae, S.; Park, S.-J. Use of thermally activated Fenton sludge for Cd removal in zinc smelter wastewater: Mechanism and feasibility of Cd removal. Environ. Pollut. 2023, 334, 122166. [Google Scholar] [CrossRef] [PubMed]
- Ract, P.G.; Espinosa, D.C.R.; Tenório, J.A.S. Determination of Cu and Ni incorporation ratios in Portland cement clinker. Waste Manag. 2003, 23, 281–285. [Google Scholar] [CrossRef] [PubMed]

| Metal | Minimum | Maximum | Metal Price per Ton ($) * |
|---|---|---|---|
| Cu | 0.0003 | 60.2 | 8736 |
| Zn | 0.0003 | 55.7 | 3529 |
| Ni | <0.0005 | 36.0 | 26,560 |
| Sn | 0.0007 | 3.76 | 25,207 |
| Pb | <0.0002 | 16.2 | 2106 |
| Al | 0.00007 | 35.4 | 2569 |
| Cr | <0.001 | 28.5 | 9530–10,221 |
| Types of Sludge | Plating | w/% | Processing Method | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Cr | Fe | Ni | Cu | Zn | Sn | |||
| Separate sludge | Ni | 0.69 | 1.55 | 96.2 | Nickel-based composite nanomaterials [22] | ||||
| Cr | 13.8 | 9.77 | 1.42 | 0.33 | Chromium oxide nanocomposites [23] | ||||
| 62.9 | 11.8 | 3.58 | 2.6 | 6.5 | Pigment synthesis [24] | ||||
| 9.0 | 6.3 | 12.0 | 4.00 | 3.0 | 6.0 | Metal–organic framework [25] | |||
| 3.94 | 7.52 | 6.13 | 5.14 | Crude stainless [26] | |||||
| Cu | 16.1 | 16.1 | 49.9 | 0.11 | 2.91 | Catalyst [27] | |||
| 5.7 | 0.1 | 1.5 | 0.3 | 3.8 | 1.2 | Smelting [28] | |||
| Sn | 24.4 | 4.0 | Sn@C Nanorodzn | ||||||
| Zn | 12.6 | 8.7 | Flocculant [20] | ||||||
| Mixed sludge | Cr-Ni-Zn | 3.74 | 2.94 | 13.6 | 4.94 | 1.18 | 7.49 | Sorbent [29] | |
| Cu-Cr | 12.44 | 3.91 | 7.55 | 6.02 | 5.13 | 12.44 | Solidify [6] | ||
| Cu-Cr-Ni | 13.3 | 2.06 | 5.57 | 6.09 | 3.09 | Ferrite [30] | |||
| 4.22 | 7.27 | 6.51 | Oxidation roasting, water immersion [31] | ||||||
| 15.38 | 0.09 | 12.87 | 0.41 | 12.94 | 0.04 | Bioleaching [32] | |||
| Cu-Ni | 18.62 | 3.01 | 5.51 | 6.93 | 1.6 | Chlorination–mineralization–volatilization [33] | |||
| Treatment Approaches | Reagents and Operations | Efficiency |
|---|---|---|
| Acid leaching | H2SO4, added NaOH until the pH of 7 and 14 | 98.99% Cu, 99.27% Ni and 97.22% Cr [52] |
| HNO3, HCl, hydrothermally | 99.8% Fe, 96.6% Al, and 98.7% Zn [20] | |
| HNO3 0-0.65 ppm, sonication 0–20 min, | 82.2% Zn, 87.3% Pb and 9.5% Cu [53] | |
| H2SO4 100 g/L, liquid-to-solid ratio 5:1, particle size <1 mm, digestion time 1 h | 88.6% Cu, 98.0% Ni and 99.2% Zn [54] | |
| H2O2, H2SO4, ultrasonic, two-stage acid leaching | 97.42% Cu, 98.46% Ni, 98.63% Zn, 98.32% Cr and 100% Fe [55] | |
| EDTA 70 mM, citric acid 10 mM, electrodeposition | 82.21% Cu [56] | |
| Ammonia leaching | NH3-(NH4)2CO3, liquid-to-solid ratio 23.7:1, NH3·H2O 5.10 mol/L, 28.28 min | 98.04% Ni [57] |
| NH3 20%, pH 10.8–11.2, 90 min | 70% Cu, 50% Zn and Ni [58] | |
| Roasting and leaching | chlorination roasting, (NH4)2CO3, H2O2, 60 °C | 99% Zn, 98% Cu and 96% Cr [59] |
| sulfating roasting, galvanic sludge/pyrite ratio 1:0.4, roasting 90 min, 550 °C | 60% Zn, 43% Ni and 50% Cu [54] | |
| sulfate roasting, sulfate roasting, sludge/sulfur 1:0.4, 550 °C 90 min, water leaching 15 min | 80% Ag, 63% Cu and 73% Zn [60] | |
| Bioleaching | Sulfobacillus acidophilus, Acidithiobacillus caldus | 94.7%Cd, 99%Cr, 98.8%Cu, 97.4%Mn, 98.9%Ni and 78.7%Zn [32] |
| Acidthiobacillus thiooxidans, Acidithiobacillus ferrooxidans, pH = 1.0, 30 °C, S = 0.8 g/L | 100%Ni [61] | |
| Nickel-resistant bacteria, 0.8% NaCl, 30 °C | 95%Ni [62] |
| Characteristic | Acid Leaching | Ammonia Leaching |
|---|---|---|
| Inhibition of Fe and Cr | Inefficient | Effective |
| Selectivity | Low | High |
| Leaching Efficiency | Varies depending on the metal being leached | Generally high |
| Environmental Impact | Acid pollution | Ammonia leak |
| Equipment | Corrosive to equipment | Requires well-sealed equipment |
| Health and Safety Risks | Acid fumes, wastewater | Ammonia gas, wastewater |
| Recycling of Leaching Agents | Challenging | Feasible |
| Pyrometallurgical | Hydrometallurgical | Pyrometallurgical + Hydrometallurgical | Materialization | Biological | |
|---|---|---|---|---|---|
| Equipment investment | large | lesser | large | lesser | small |
| Production capacity | large | small | lesser | large | small |
| Environmental protection | flue gas, pollution, less wastewater | waste gas, acid, and alkali wastewater | flue gas and wastewater | exhaust gas | no waste |
| Running cost | high | high | high | high | low |
| Types of metals recovered | single | various | various | none | none |
| Floor space | large | large | large | lesser | small |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Wang, H.; Liu, X.; Zhang, Z.; Liu, Y. Approaches for the Treatment and Resource Utilization of Electroplating Sludge. Materials 2024, 17, 1707. https://doi.org/10.3390/ma17071707
Guo S, Wang H, Liu X, Zhang Z, Liu Y. Approaches for the Treatment and Resource Utilization of Electroplating Sludge. Materials. 2024; 17(7):1707. https://doi.org/10.3390/ma17071707
Chicago/Turabian StyleGuo, Song, Huimin Wang, Xiaoming Liu, Zengqi Zhang, and Yu Liu. 2024. "Approaches for the Treatment and Resource Utilization of Electroplating Sludge" Materials 17, no. 7: 1707. https://doi.org/10.3390/ma17071707
APA StyleGuo, S., Wang, H., Liu, X., Zhang, Z., & Liu, Y. (2024). Approaches for the Treatment and Resource Utilization of Electroplating Sludge. Materials, 17(7), 1707. https://doi.org/10.3390/ma17071707









