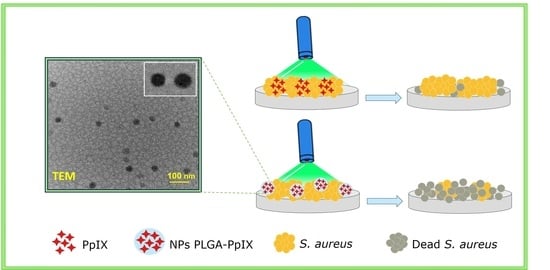
Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Photosensitizer Selection
2.3. Protoporphyrin-IX-Loaded PLGA Nanoparticles Synthesis and Characterization
2.4. In Vitro Biological Analyses
2.5. Statistical Analyses
3. Results
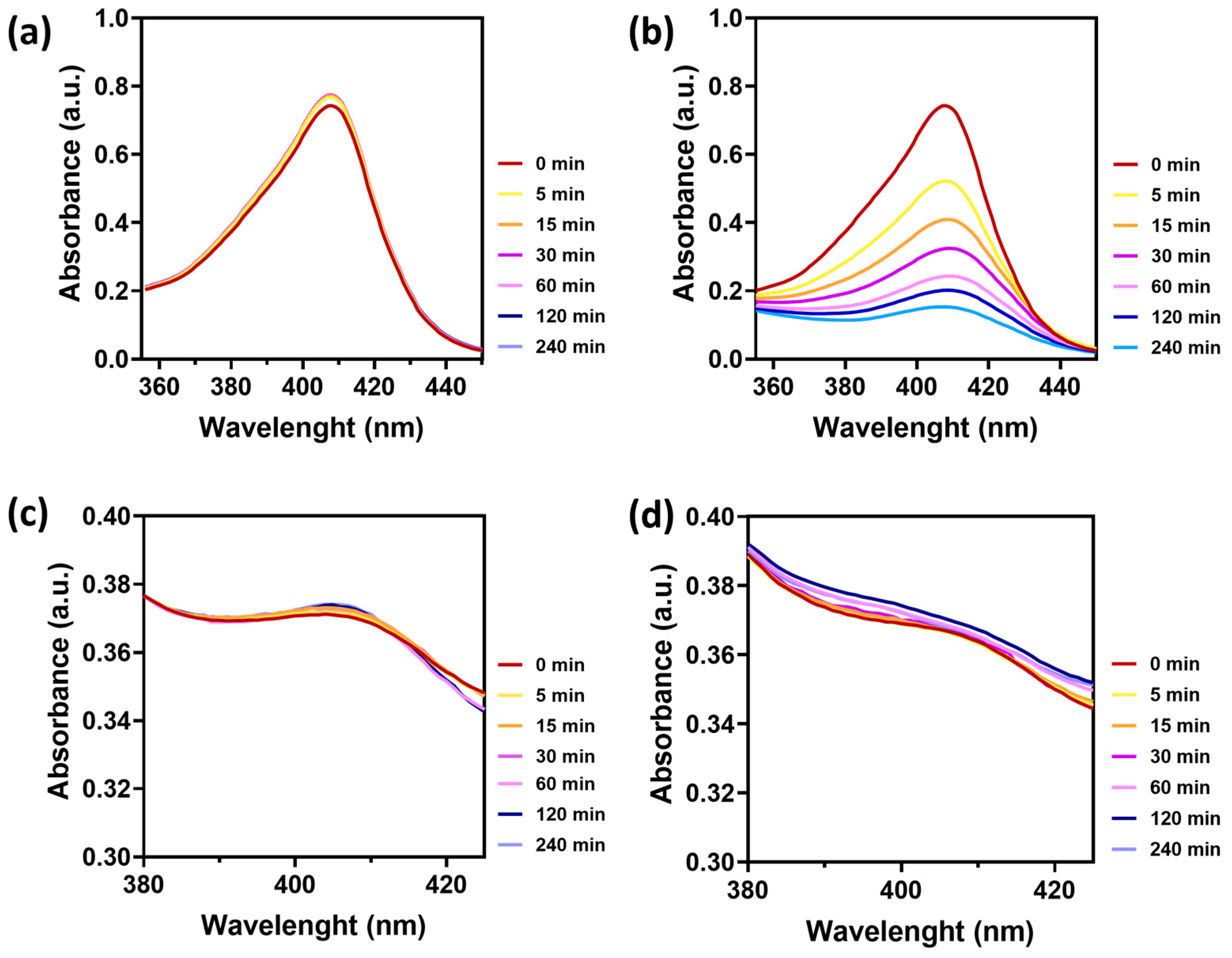
3.1. Photosensitizer Selection
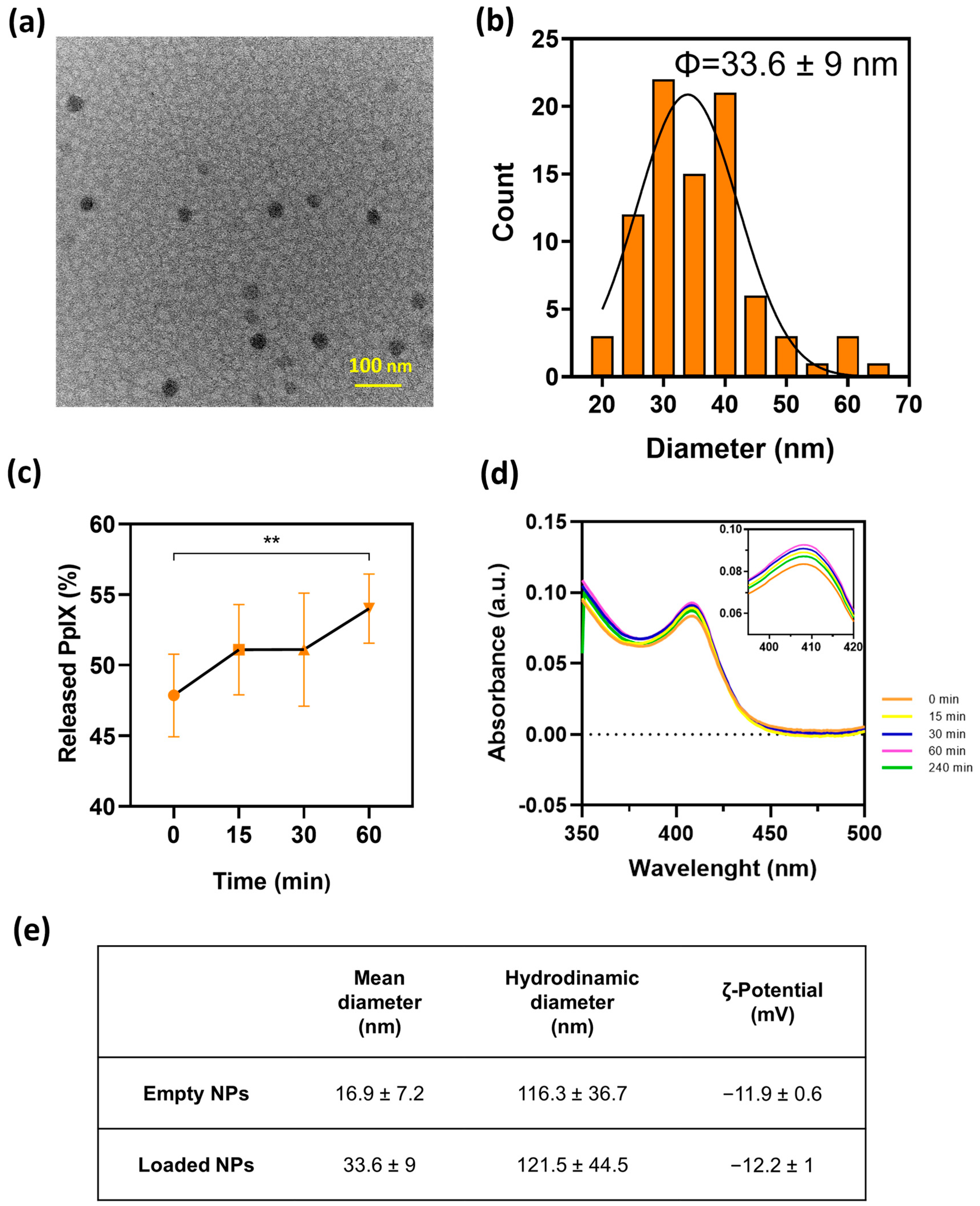
3.2. PpIX-Loaded PLGA Nanoparticles Synthesis and Characterization
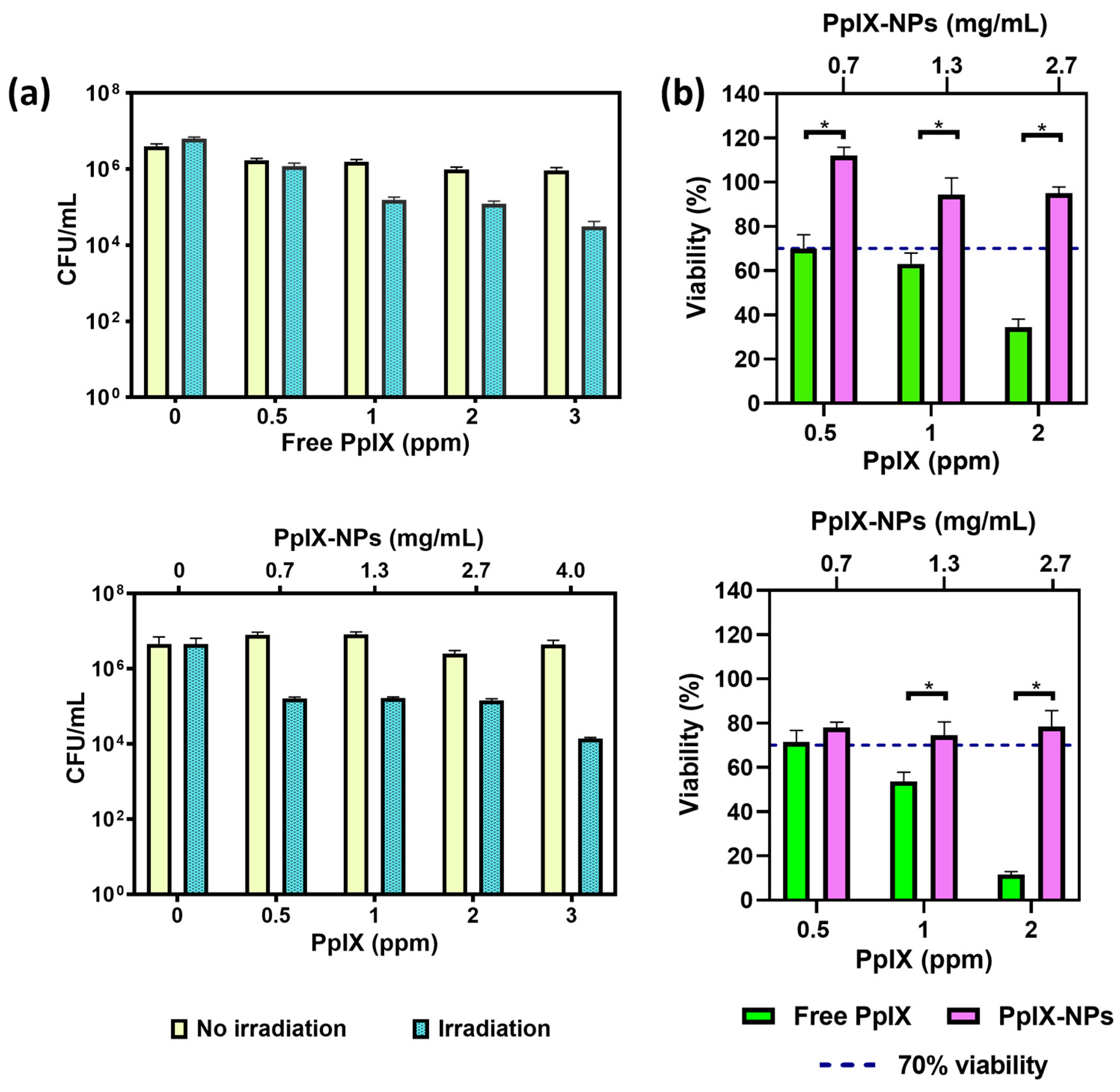
3.3. Biological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piksa, M.; Lian, C.; Samuel, I.C.; Pawlik, K.J.; Samuel, I.D.W.; Matczyszyn, K. The Role of the Light Source in Antimicrobial Photodynamic Therapy. Chem. Soc. Rev. 2023, 52, 1697–1722. [Google Scholar] [CrossRef] [PubMed]
- Do Prado-Silva, L.; Brancini, G.T.P.; Braga, G.Ú.L.; Liao, X.; Ding, T.; Sant’Ana, A.S. Antimicrobial Photodynamic Treatment (APDT) as an Innovative Technology to Control Spoilage and Pathogenic Microorganisms in Agri-Food Products: An Updated Review. Food Control 2022, 132, 108527. [Google Scholar] [CrossRef]
- Yan, E.; Kwek, G.; Qing, N.S.; Lingesh, S.; Xing, B. Antimicrobial Photodynamic Therapy for the Remote Eradication of Bacteria. ChemPlusChem 2023, 88, e202300009. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and In Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ogilby, P.R. Singlet Oxygen: There Is Indeed Something New under the Sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.C.; Lin, J.H.; Zhang, S.; Lou, C.W.; Li, T.T. Synergistic Antibacterial Strategy Based on Photodynamic Therapy: Progress and Perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Huo, J.; Jia, Q.; Huang, H.; Zhang, J.; Li, P.; Dong, X.; Huang, W. Emerging Photothermal-Derived Multimodal Synergistic Therapy in Combating Bacterial Infections. Chem. Soc. Rev. 2021, 50, 8762–8789. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Park, S.; Kim, Y.K.; Moon, M.; Park, J.; Lee, K.J.; Lee, S.; Kim, Y.P. Self-Luminescent Photodynamic Therapy Using Breast Cancer Targeted Proteins. Sci. Adv. 2020, 6, 3009–3020. [Google Scholar] [CrossRef]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated Photodynamic Therapy for Bacterial Infections. Adv. Healthc. Mater. 2019, 8, 1900608. [Google Scholar] [CrossRef]
- Qi, M.; Chi, M.; Sun, X.; Xie, X.; Weir, M.D.; Oates, T.W.; Zhou, Y.; Wang, L.; Bai, Y.; Xu, H.H.K. Novel Nanomaterial-Based Antibacterial Photodynamic Therapies to Combat Oral Bacterial Biofilms and Infectious Diseases. Int. J. Nanomed. 2019, 14, 6937–6956. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yang, G.; Wang, Y.; Jiang, H.; Fu, Y.; Yue, G.; Ju, R. Phototherapy-Based Combination Strategies for Bacterial Infection Treatment. Theranostics 2020, 10, 12241. [Google Scholar] [CrossRef] [PubMed]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomed. 2021, 16, 5831–5867. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Anderson, K.E.; Ma, X. Protoporphyrin IX: The Good, the Bad, and the Ugly. J. Pharmacol. Exp. Ther. 2016, 356, 267. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, E.; Chelakkot, V.S.; Licursi, M.; Rutihinda, S.G.; Som, J.; Derwish, L.; King, J.J.; Pongnopparat, T.; Mearow, K.; Larijani, M.; et al. Enhancement of Cancer-Specific Protoporphyrin IX Fluorescence by Targeting Oncogenic Ras/MEK Pathway. Theranostics 2018, 8, 2134. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Sumer, B.D.; Kessinger, C.W.; Dong, Y.; Huang, G.; Boothman, D.A.; Gao, J. Nanoscopic Micelle Delivery Improves the Photophysical Properties and Efficacy of Photodynamic Therapy of Protoporphyrin IX. J. Control. Release 2011, 151, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Moriyama, E.H.; Li, F.; Jarvi, M.T.; Allen, C.; Wilson, B.C. Diblock Copolymer Micelles Deliver Hydrophobic Protoporphyrin IX for Photodynamic Therapy. Photochem. Photobiol. 2007, 83, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Rwei, A.Y.; Alejo, T.; Wei, T.; Lopez-Franco, M.T.; Mendoza, G.; Sebastian, V.; Kohane, D.S.; Arruebo, M. Light-Emitting Photon-Upconversion Nanoparticles in the Generation of Transdermal Reactive-Oxygen Species. ACS Appl. Mater. Interfaces 2017, 9, 41737–41747. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.T. Recent Advances in Engineered Polymeric Materials for Efficient Photodynamic Inactivation of Bacterial Pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef]
- Koo, H.; Lee, H.; Lee, S.; Min, K.H.; Kim, M.S.; Lee, D.S.; Choi, Y.; Kwon, I.C.; Kim, K.; Jeong, S.Y. In Vivo Tumor Diagnosis and Photodynamic Therapy via Tumoral PH-Responsive Polymeric Micelles. Chem. Commun. 2010, 46, 5668–5670. [Google Scholar] [CrossRef]
- Rossi, L.M.; Silva, P.R.; Vono, L.L.R.; Fernandes, A.U.; Tada, D.B.; Baptista, M.S. Protoporphyrin IX Nanoparticle Carrier: Preparation, Optical Properties and Singlet Oxygen Generation. Langmuir 2008, 24, 12534–12538. [Google Scholar] [CrossRef]
- Liu, Y.; van der Mei, H.C.; Zhao, B.; Zhai, Y.; Cheng, T.; Li, Y.; Zhang, Z.; Busscher, H.J.; Ren, Y.; Shi, L. Eradication of Multidrug-Resistant Staphylococcal Infections by Light-Activatable Micellar Nanocarriers in a Murine Model. Adv. Funct. Mater. 2017, 27, 1701974. [Google Scholar] [CrossRef]
- Jia, H.R.; Zhu, Y.X.; Chen, Z.; Wu, F.G. Cholesterol-Assisted Bacterial Cell Surface Engineering for Photodynamic Inactivation of Gram-Positive and Gram-Negative Bacteria. ACS Appl. Mater. Interfaces 2017, 9, 15943–15951. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Li, Y.H.; Durrani, S.; Pang, A.P.; Gao, Y.; Wu, F.G.; Lin, F. Super-Stable Chitosan-Based Nanoparticles for Broad-Spectrum Antimicrobial Photodynamic Therapy. ACS Appl. Polym. Mater. 2022, 4, 425–434. [Google Scholar] [CrossRef]
- Choi, S.; Britigan, B.E.; Narayanasamy, P. Iron/Heme Metabolism-Targeted Gallium(III) Nanoparticles Are Active against Extracellular and Intracellular Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.L.; Del Ciampo, J.O.; Rossetti, F.C.; Bentley, M.V.L.B.; Pierre, M.B.R. Improved In Vitro and In Vivo Cutaneous Delivery of Protoporphyrin IX from PLGA-Based Nanoparticles. Photochem. Photobiol. 2013, 89, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.B.; da Silva, C.L.; Davanzo, N.N.; da Silva Souza, R.; Correa, R.J.; Tedesco, A.C.; Riemma Pierre, M.B. Protoporphyrin IX (PpIX) Loaded PLGA Nanoparticles for Topical Photodynamic Therapy of Melanoma Cells. Photodiagn. Photodyn. Ther. 2021, 35, 102317. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, D.; Sengupta, J.; Pink, D.; Raturi, A.; Chen, H.; Usmani, N.; Kumar, P.; Lewis, J.D.; Narain, R.; Moore, R.B. PEG-PLGA Nanospheres Loaded with Nanoscintillators and Photosensitizers for Radiation-Activated Photodynamic Therapy. Acta Biomater. 2020, 117, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H.; Wang, H. In Vitro Evaluation of 5-Aminolevulinic Acid (ALA) Loaded PLGA Nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef]
- de Andrade, L.R.; Primo, F.L.; da Silva, J.R.; Tedesco, A.C.; Lacava, Z.G.M. In Vitro Assessment of Anti-Tumorigenic Mechanisms and Efficacy of NanoALA, a Nanoformulation of Aminolevulic Acid Designed for Photodynamic Therapy of Cancer. Photodiagn. Photodyn. Ther. 2017, 20, 62–70. [Google Scholar] [CrossRef]
- Crow, J.P. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitrite In Vitro: Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Gerega, A.; Zolek, N.; Soltysinski, T.; Milej, D.; Sawosz, P.; Toczylowska, B.; Liebert, A. Wavelength-Resolved Measurements of Fluorescence Lifetime of Indocyanine Green. J. Biomed. Opt. 2011, 16, 067010. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation Kinetics of Indocyanine Green in Aqueous Solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Ma, L.W.; Iani, V.; Juzeniene, A. Influence of Light Exposure on the Kinetics of Protoporphyrin IX Formation in Normal Skin of Hairless Mice after Application of 5-Aminolevulinic Acid Methyl Ester. J. Investig. Dermatol. 2005, 125, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, X.; Wang, X.; Leung, A.W.; Xu, C.; Wang, P.; Liu, Q. Cytotoxic Effect of Protoporphyrin IX to Human Leukemia U937 Cells under Ultrasonic Irradiation. Cell. Physiol. Biochem. 2014, 33, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of Targeted Curcumin (CUR) Loaded PLGA Nanoparticles for In Vitro Photodynamic Therapy on Human Glioblastoma Cell Line. Photodiagn. Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-tetraphenylporphyrin Metal Complex-loaded Plga Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef] [PubMed]
- Löw, K.; Knobloch, T.; Wagner, S.; Wiehe, A.; Engel, A.; Langer, K.; von Briesen, H. Comparison of Intracellular Accumulation and Cytotoxicity of Free mTHPC and mTHPC-Loaded PLGA Nanoparticles in Human Colon Carcinoma Cells. Nanotechnology 2011, 22, 245102. [Google Scholar] [CrossRef]
- Bœuf-Muraille, G.; Rigaux, G.; Callewaert, M.; Zambrano, N.; Van Gulick, L.; Roullin, V.G.; Terryn, C.; Andry, M.C.; Chuburu, F.; Dukic, S.; et al. Evaluation of MTHPC-Loaded PLGA Nanoparticles for In Vitro Photodynamic Therapy on C6 Glioma Cell Line. Photodiagn. Photodyn. Ther. 2019, 25, 448–455. [Google Scholar] [CrossRef]
- Hongcharu, W.; Taylor, C.R.; Chang, Y.; Aghassi, D.; Suthamjariya, K.; Anderson, R.R. Topical ALA-Photodynamic Therapy for the Treatment of Acne Vulgaris. J. Investig. Dermatol. 2000, 115, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter pylori Accumulates Photoactive Porphyrins and Is Killed by Visible Light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 13 March 2024).
- Dellambra, E.; Dimri, G.P. Cellular Senescence and Skin Aging. In Skin Aging Handbook: An Integrated Approach to Biochemistry and Product Development; William Andrew Publishing: Norwich, NY, USA, 2009; pp. 129–148. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of Fibroblasts in Wound Healing and Tissue Remodeling on Earth and in Space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Miguel, J.; da Silva, D.B.; da Silva, G.C.C.; Corrêa, R.J.; de Oliveira Miguel, N.C.; Lione, V.D.O.F.; Riemma Pierre, M.B. Polymeric Nanoparticles Favor the In Vitro Dermal Accumulation of Protoporphyrin IX (PpIX) with Optimal Biocompatibility and Cellular Recovery in Culture of Healthy Dermal Fibroblasts after Photodynamic Therapy. J. Photochem. Photobiol. A Chem. 2020, 386, 112109. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing Malignant Melanoma Cells with Protoporphyrin IX-Loaded Polymersome-Mediated Photodynamic Therapy and Cold Atmospheric Plasma. Int. J. Nanomed. 2017, 12, 4117. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Zhang, K.; Li, X.; Liu, Q.; Wang, P. DNA Damage and Cell Cycle Arrest Induced by Protoporphyrin IX in Sarcoma 180 Cells. Cell Physiol. Biochem. 2013, 32, 778–788. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo, N.; Gamez, E.; Alejo, T.; Mendoza, G.; Arruebo, M. Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens. Materials 2024, 17, 1717. https://doi.org/10.3390/ma17081717
Izquierdo N, Gamez E, Alejo T, Mendoza G, Arruebo M. Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens. Materials. 2024; 17(8):1717. https://doi.org/10.3390/ma17081717
Chicago/Turabian StyleIzquierdo, Natalia, Enrique Gamez, Teresa Alejo, Gracia Mendoza, and Manuel Arruebo. 2024. "Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens" Materials 17, no. 8: 1717. https://doi.org/10.3390/ma17081717