Preparation and Characterization of Bismaleimide-Resin-Based Composite Materials
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Experimental Procedureheng
2.3. Characterization
- δ—bending strength in megapascals (MPa);
- F—the maximum load that the specimen can withstand or that can reach the specified deflection value, in Newton (N);
- L—span between the two support points of the specimen, in millimeters (mm);
- b—width of the specimen in millimeters (mm);
- h—thickness of the specimen in millimeters (mm).
3. Results
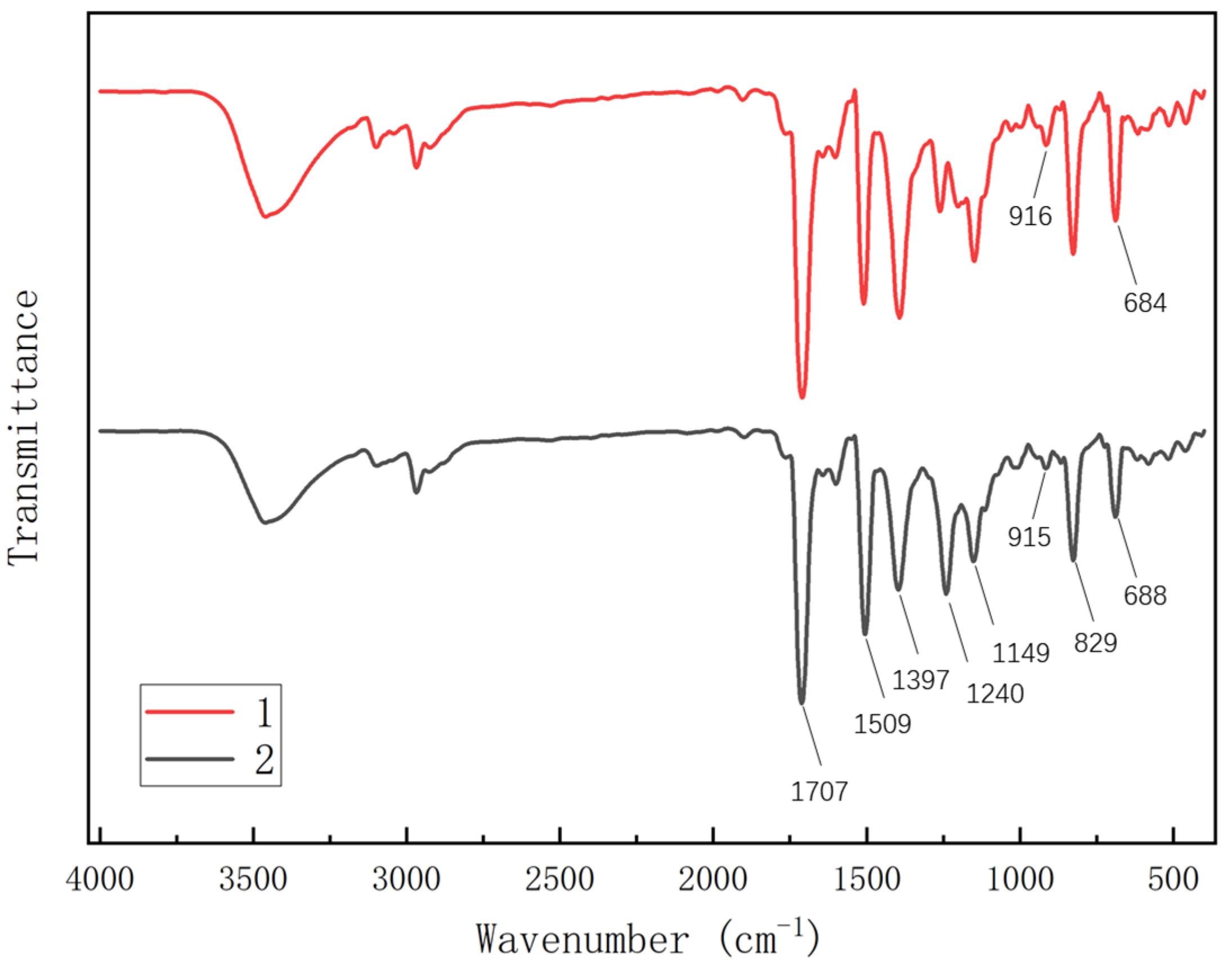
3.1. Modification and Structural Analysis
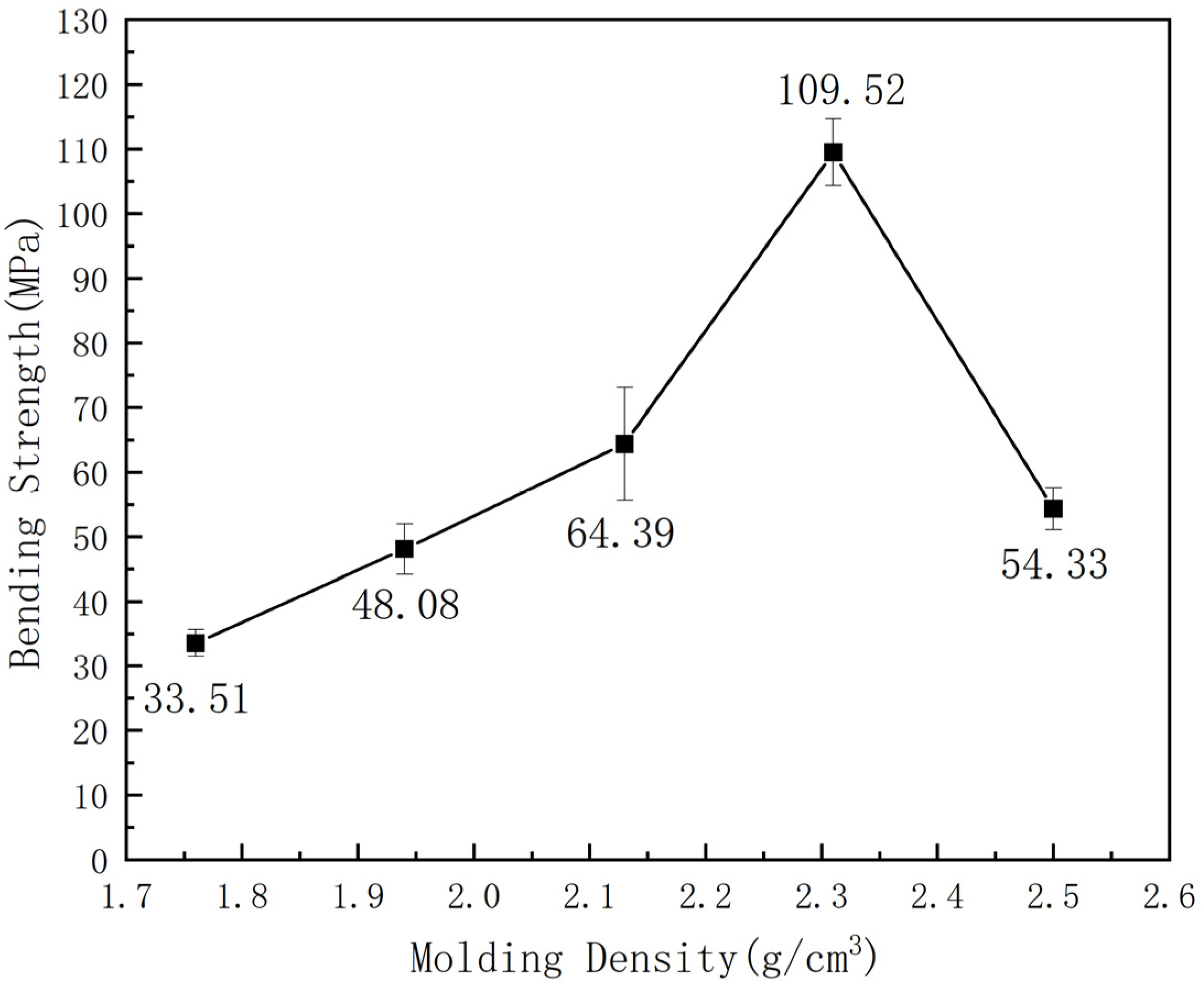
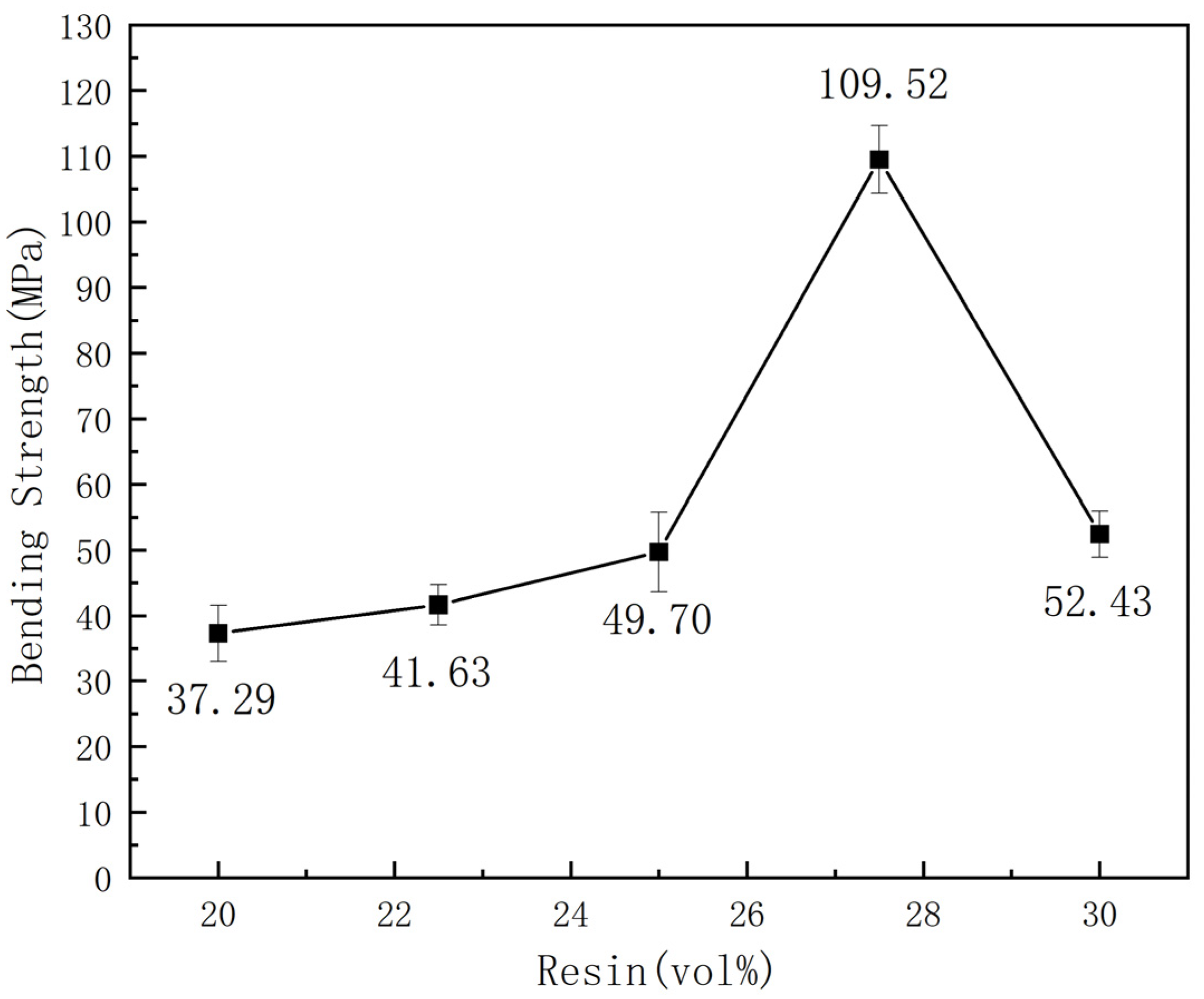
3.2. Impact of Various Processing Parameters on Composite Mechanical Properties
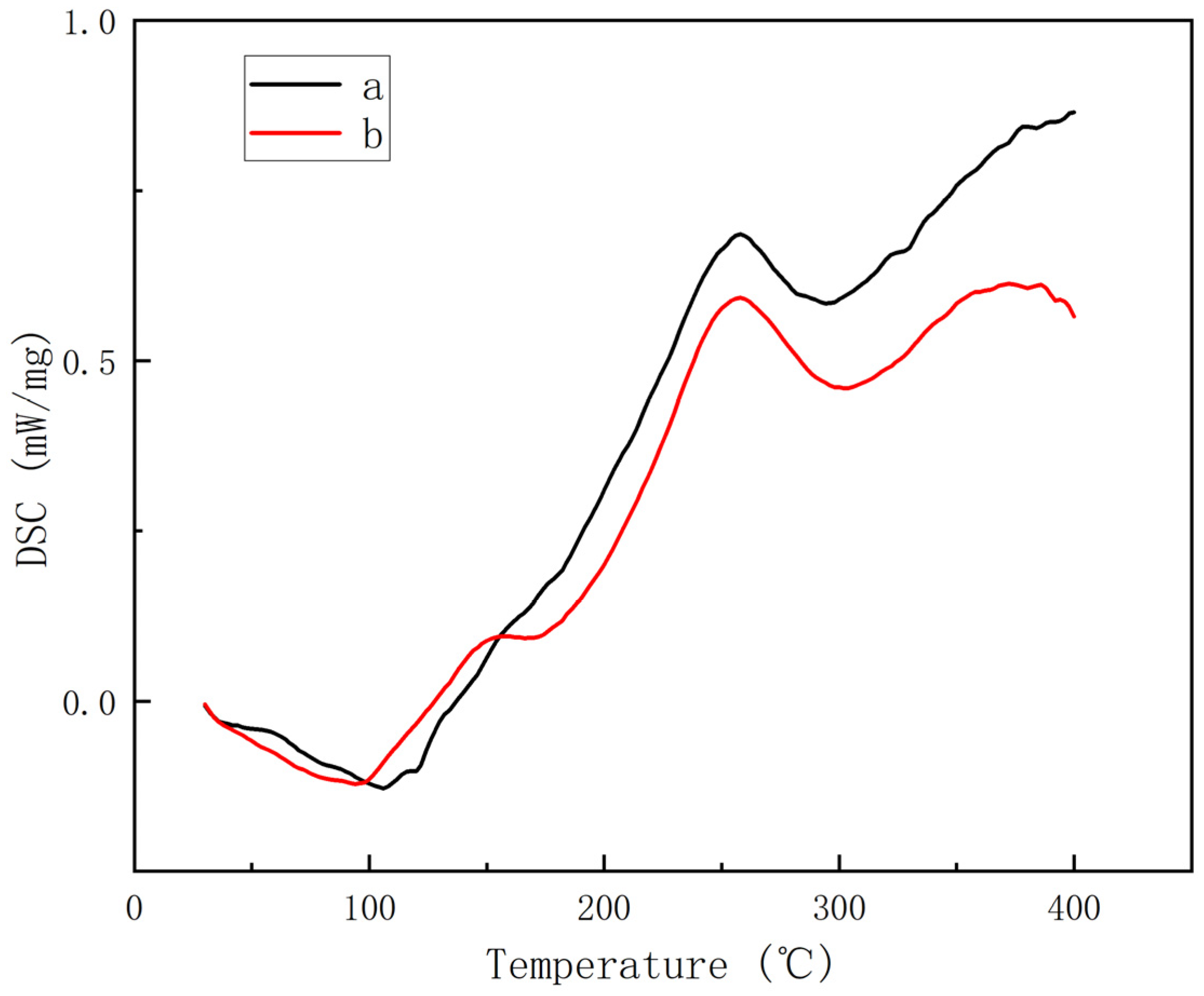
3.3. Mechanical Properties and Thermal Stability Analysis of Composite Materials
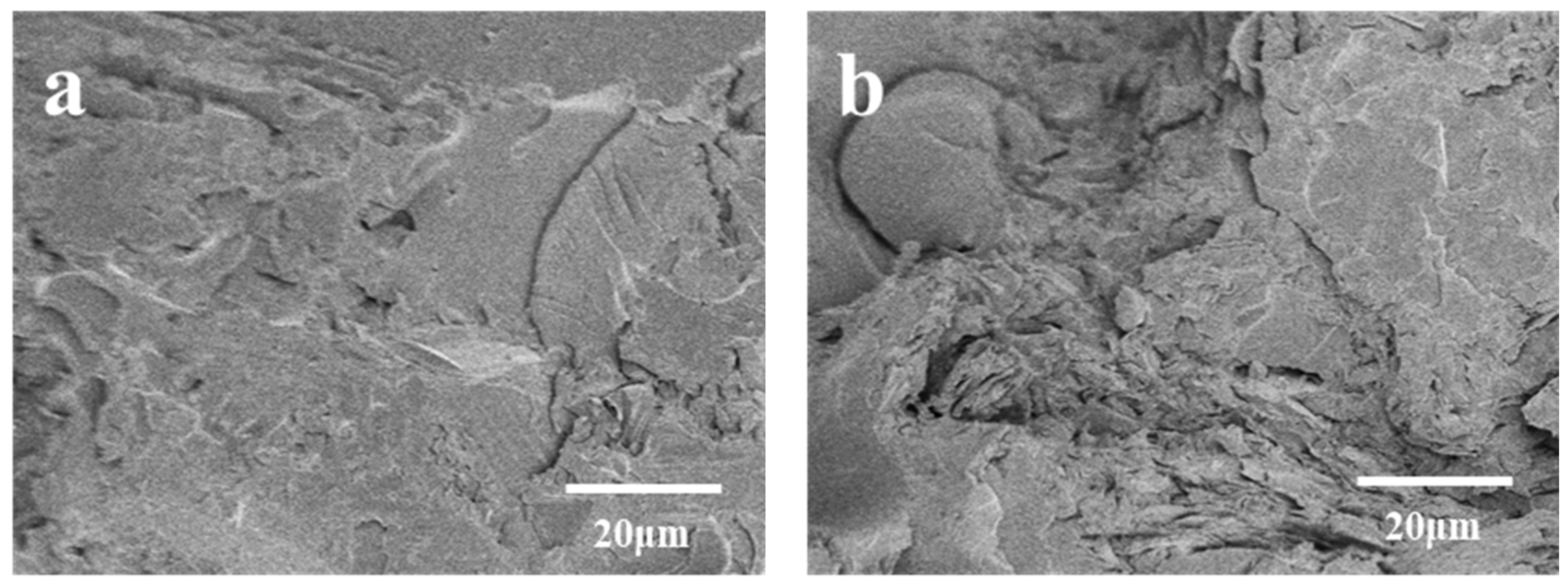
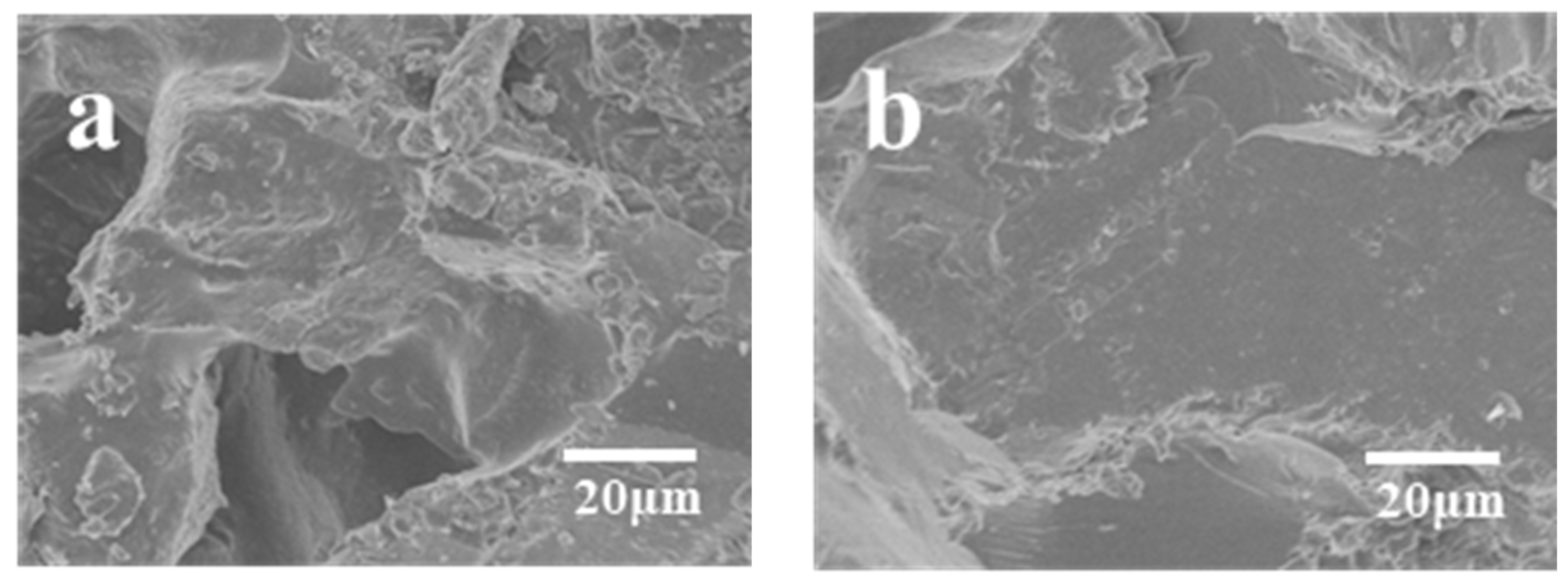
3.4. Composite Fracture Surface Analysis
4. Conclusions
- Various process parameters exert a profound influence on the mechanical properties of composite materials. Notably, differing molding densities significantly impact the overall mechanical reinforcement of these materials. Insufficient molding density may result in inadequate material density, consequently diminishing its mechanical resistance, whereas excessive molding density can induce an uneven or excessively dense internal arrangement of composite materials, leading to incomplete curing or uneven distribution, thus impairing mechanical properties. Hence, optimal resin content plays a pivotal role in enhancing the overall mechanical properties of composite materials while excessive resin content may induce deformation and pore formation, contributing to a decline in mechanical properties.
- The glass transition temperature (Tg) of the modified BMI system exceeds 270 °C, with the temperature at 5% weight loss surpassing 400 °C. Moreover, the maximum degradation rate temperature is lower than that observed before modification, suggesting minimal reduction in the heat resistance of the BMI system following the introduction of ether bonds.
- The incorporation of ether bonds led to a substantial enhancement in the mechanical properties of BDM/DAPPA/BMIX/SiC composites. Without high-temperature heat treatment, the modified BMI/SiC composites attained a bending strength of 109.52 MPa, marking a 24% increase compared to their pre-modification counterparts. Furthermore, the microstructure of the composite material, devoid of high-temperature heat treatment, exhibited negligible voids or cracks, underscoring the robust bonding between the resin and silicon carbide particles at typical operating temperatures, ensuring prolonged utility.
- A comparative analysis of BDM/DAPPA/SiC composite materials and BDM/DAPBA/BMIX/SiC composite materials following high-temperature heat treatment revealed the superior performance of the former. High-temperature heat treatment at 270 °C, 300 °C, 350 °C, and 400 °C resulted in enhanced mechanical properties compared to unmodified composite materials. Notably, at 270 °C and 350 °C, the bending strengths were 98.28 MPa and 65.69 MPa, respectively, representing 24% and 26% increases over unmodified composite materials. Furthermore, the thermal stability of the modified composite material exhibited minimal deterioration post-modification, ensuring the retention of satisfactory mechanical properties.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Q.; Wang, B.; Zhang, C.; Liang, Z. Functionalized Carbon-Nanotube Sheet/Bismaleimide Nanocomposites: Mechanical and Electrical Performance Beyond Carbon-Fiber Composites. Small 2010, 6, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, X.; Weng, L.; Sun, X.; Zhu, P.; Wang, Q. High toughness and excellent electrical performance bismaleimide resin modified by hyperbranched unsaturated polyester of flexible aliphatic side chains. High Perform. Polym. 2021, 33, 695–703. [Google Scholar] [CrossRef]
- Sandeep Singh, K.; Parvesh, A.; Sarbjit, S.; Sundeep Kumar, A.; Parveen, S.; Anil, K. Machine Learning-Based Erosion Behavior of Silicon Carbide Reinforced Polymer Composites. Silicon 2020, 13, 1113–1119. [Google Scholar] [CrossRef]
- Saikiran, C.; Palli, S.; Bondala, R.; Sharma, R.C.; Sreeramulu, D.; Dontikurti, R. Experimental Investigation on Thermal Behaviour of Silicon Carbide (SiC) based Polymer Composites under Hygrothermal Conditions. Int. J. Veh. Struct. Syst. 2023, 15, 222–226. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, L.; Tang, Y.; Zhang, X.; Zheng, K.; Tian, X. Enhanced thermal conductivity of silicon carbide nanowires (SiCw)/epoxy resin composite with segregated structure. Compos. Part A Appl. Sci. Manuf. 2019, 116, 98–105. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Q.; Xu, W.; Cai, W.; Inoue, Y.; Zhu, Y. Effect of carbon nanotube length on thermal, electrical and mechanical properties of CNT/bismaleimide composites. Carbon 2013, 53, 145–152. [Google Scholar] [CrossRef]
- Song, S.A.; Lee, Y.; Kim, Y.S.; Kim, S.S. Mechanical and thermal properties of carbon foam derived from phenolic foam reinforced with composite particles. Compos. Struct. 2017, 173, 1–8. [Google Scholar] [CrossRef]
- Liu, P.; Qu, C.; Wang, D.; Zhao, M.; Liu, C. High-performance bismaleimide resins with low cure temperature for resin transfer molding process. High Perform. Polym. 2017, 29, 298–304. [Google Scholar] [CrossRef]
- Iredale, R.J.; Ward, C.; Hamerton, I. Modern advances in bismaleimide resin technology: A 21st century perspective on the chemistry of addition polyimides. Prog. Polym. Sci. 2017, 69, 1–21. [Google Scholar] [CrossRef]
- Radue, M.S.; Varshney, V.; Baur, J.W.; Roy, A.K.; Odegard, G.M. Molecular Modeling of Cross-Linked Polymers with Complex Cure Pathways: A Case Study of Bismaleimide Resins. Macromolecules 2018, 51, 1830–1840. [Google Scholar] [CrossRef]
- Iyer, N.P.; Arunkumar, N. Review on Fiber reinforced/modified Bismaleimide resin composites for Aircraft Structure Application. IOP Conf. Ser. Mater. Sci. Eng. 2020, 923, 012051. [Google Scholar] [CrossRef]
- Chandran, M.S.; Niranjana, S.; Nair, C.P.R. Maleimide based Alder-ene thermosets: Recent advances. In Handbook of Thermoset Plastics; William Andrew Publishing: Norwich, NY, USA, 2022; pp. 619–657. [Google Scholar] [CrossRef]
- Xia, Z.; Shuilai, Q.; Linxin, H.; Xin, W.; Yulu, Z.; Fukai, C.; Bibo, W.; Lei, S.; Yuan, H. Synthesis of star-shaped allyl phosphazene small molecules for enhancing fire safety and toughness of high performance BMI resin. Chem. Eng. J. 2021, 425, 130655. [Google Scholar] [CrossRef]
- Zou, Q.; Xiao, F.; Gu, S.Q.; Li, J.; Zhang, D.J.; Liu, Y.F.; Chen, X.B. Toughening of Bismaleimide Resin Based on the Self-Assembly of Flexible Aliphatic Side Chains. Ind. Eng. Chem. Res. 2019, 58, 16526–16531. [Google Scholar] [CrossRef]
- Chozhan, C.K.; Chandramohan, A.; Alagar, M. Cyclohexane and phosphorus based benzoxazine-bismaleimide hybrid polymer matrices: Thermal and morphological properties. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 686–696. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Wu, Q.; Wang, S.; Huang, G.; Wu, J. Strong and tough self-healing elastomers enabled by dual reversible networks formed by ionic interactions and dynamic covalent bonds. Polymer 2018, 157, 172–179. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Liang, G.; Gu, A. Achieving ultrahigh glass transition temperature, halogen-free and phosphorus-free intrinsic flame retardancy for bismaleimide resin through building network with diallyloxydiphenyldisulfide. Polymer 2020, 203, 122769. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Bao, Y.; Wei, W.; Li, X.; Liu, X. Bismaleimide resins modified by an allyl ether of bio-based resveratrol with excellent halogen-free and phosphorus-free intrinsic flame retardancy and ultrahigh glass transition temperature. Polym. Degrad. Stab. 2021, 193, 109717. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Liu, J.; Zhou, M.; Cai, W.; Wang, J.; Chu, F.; Xing, W.; Song, L.; Hu, Y. Construction of porous g-C3N4@PPZ tubes for high performance BMI resin with enhanced fire safety and toughness. Chem. Eng. J. 2020, 401, 126094. [Google Scholar] [CrossRef]
- Kamiyama, S.; Hirano, Y.; Okada, T.; Ogasawara, T. Lightning strike damage behavior of carbon fiber reinforced epoxy, bismaleimide, and polyetheretherketone composites. Compos. Sci. Technol. 2018, 161, 107–114. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, R.; Chen, P.; Yu, Q.; Wang, J.; Jia, C. Preparation and properties of modified bismaleimide resins based on phthalide-containing monomer. J. Appl. Polym. Sci. 2013, 130, 1084–1091. [Google Scholar] [CrossRef]
- Neda, M.; Okinaga, K.; Shibata, M. High-performance bio-based thermosetting resins based on bismaleimide and allyl-etherified eugenol derivatives. Mater. Chem. Phys. 2014, 148, 319–327. [Google Scholar] [CrossRef]
- Devi, K.A.; Nair, C.P.R.; Ninan, K.N. Diallyl bisphenol A—Novolac epoxy system cocured with bisphenol-a-bismaleimide—Cure and thermal properties. J. Appl. Polym. Sci. 2007, 106, 1192–1200. [Google Scholar] [CrossRef]
- Yuan, L.; Ma, X.; Gu, A.; Yan, H.; Liang, G.; Wang, W.; Wu, J. A novel organic rectorite modified bismaleimide/diallylbisphenol A system. Polym. Adv. Technol. 2009, 20, 826–833. [Google Scholar] [CrossRef]
- Hu, H.; Li, C.; Wang, C.; Shu, R.; Shen, Y. Curing kinetics, thermal and erosive wear characteristics of bismaleimide blends modified by polyaryletherketone. High Perform. Polym. 2023, 35, 126–141. [Google Scholar] [CrossRef]
- Sipaut, C.S.; Padavettan, V.; Rahman, I.A.; Mansa, R.F.; Dayou, J.; Jafarzadeh, M. An optimized preparation of bismaleimide-diamine co-polymer matrices. Polym. Adv. Technol. 2014, 25, 673–683. [Google Scholar] [CrossRef]
- Yu, F.-E.; Lee, H.-Y.; Hsu, J.-M.; Pan, J.-P.; Wang, T.-H.; Chern, C.-S. Kinetics of polymerization of N,N′-bismaleimide-4, 4′-diphenylmethane with barbituric acid. J. Taiwan Inst. Chem. Eng. 2014, 45, 2820–2826. [Google Scholar] [CrossRef]
- Xiong, X.; Chen, P.; Zhu, N.; Yu, Q.; Zhang, J.; Wang, B. Synthesis and Properties of a Novel Bismaleimide Resin Containing 1,3,4-Oxadiazole Moiety and the Blend Systems Thereof With Epoxy Resin. Polym. Eng. Sci. 2011, 51, 1599–1606. [Google Scholar] [CrossRef]
- Hao, D.; Akatsu, T.; Kamochi, N.; Inada, M.; Shiraishi, A. Near-zero sintering shrinkage in pottery with wollastonite addition. J. Eur. Ceram. Soc. 2023, 43, 700–707. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Wan, J.; Su, H. Calculation on the permittivity of the wollastonite ceramic matrix. Solid State Sci. 2012, 14, 1467–1470. [Google Scholar] [CrossRef]
- Ursache, O.; Gaina, C.; Gaina, V.; Musteata, V.E. High performance bismaleimide resins modified by novel allyl compounds based on polytriazoles. J. Polym. Res. 2012, 19, 9968. [Google Scholar] [CrossRef]
- Chandran, M.S.; Sanil, K.; Sunitha, K.; Mathew, D.; Rao, V.L.; Nair, C.P.R. Alder-ene polymers derived from allyl aralkyl phenolic resin and bismaleimides: Carbon fiber composites properties. Polym. Adv. Technol. 2016, 27, 984–992. [Google Scholar] [CrossRef]
- Ian, H.; Brendan, J.H.; Jewell, S.L.; Purvi, P. Studying the co-reaction of propenyl-substituted cyanate ester-bismaleimide blends using model compounds. React. Funct. Polym. 2012, 72, 279–286. [Google Scholar] [CrossRef]
- Wang, Y.; Kou, K.; Zhuo, L.; Chen, H.; Zhang, Y.; Wu, G. Thermal, mechanical and dielectric properties of BMI modified by the Bis allyl benzoxazine. J. Polym. Res. 2015, 22, 51. [Google Scholar] [CrossRef]
- Jena, R.K.; Yue, C.Y.; Sk, M.M.; Ghosh, K. A novel high performance bismaleimide/diallyl bisphenol A (BMI/DBA)-epoxy interpenetrating network resin for rigid riser application. RSC Adv. 2015, 5, 79888–79897. [Google Scholar] [CrossRef]
- Chen, K.-L.; Zhang, T.; Cui, Y.; Wang, Z.-Y. Progress of hyperbranched polymers (HBPs) as modifiers in epoxy resins. Cailiao Gongcheng-J. Mater. Eng. 2019, 47, 11–18. [Google Scholar] [CrossRef]
- Crawford, A.O.; Cavalli, G.; Howlin, B.J.; Hamerton, I. Investigation of structure property relationships in liquid processible, solvent free, thermally stable bismaleimide-triazine (BT) resins. React. Funct. Polym. 2016, 102, 110–118. [Google Scholar] [CrossRef]
- Babkin, A.V.; Erdni-Goryaev, E.M.; Solopchenko, A.V.; Kepman, A.V.; Avdeev, V.V. Mechanical and thermal properties of modified bismaleimide matrices toughened by polyetherimides and polyimide. Polym. Adv. Technol. 2016, 27, 774–780. [Google Scholar] [CrossRef]
- Cheng, Y.; Xia, B.; Fang, C.; Yang, L. Structure, mechanical and thermal properties of BMI/E-44/CNTs ternary composites via amination method. J. Mater. Sci. Technol. 2017, 33, 1187–1194. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Liu, L.; Qu, C.; Liu, C.; Yang, H. Vinyl-terminated butadiene acrylonitrile improves the toughness, processing window, and thermal stability of bismaleimide resin. High Perform. Polym. 2017, 29, 1199–1208. [Google Scholar] [CrossRef]
- Li, W.; Xue, F.; Li, Q. Modification of bismaleimide resin by using γ-aminopropyl triethoxysilane functionalised graphene oxide. Plast. Rubber Compos. 2018, 47, 187–191. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Yue, Y.; Ji, W.; Ren, R. Enhanced mechanical and thermal properties of bismaleimide composites with covalent functionalized graphene oxide. RSC Adv. 2016, 6, 54410–54417. [Google Scholar] [CrossRef]
- Schmitt, B.J.; Kirste, R.G.; Jelenič, J. Untersuchungen zur thermodynamik und konformation von makromolekülen in polymermischungen in der nähe von entmischungspunkten durch neutronenbeugung. Die Makromol. Chem. Macromol. Chem. Phys. 1980, 181, 1655–1672. [Google Scholar] [CrossRef]
- Sipaut, C.S.; Mansa, R.F.; Padavettan, V.; Rahman, I.A.; Dayou, J.; Jafarzadeh, M. The Effect of Surface Modification of Silica Nanoparticles on the Morphological and Mechanical Properties of Bismaleimide/Diamine Matrices. Adv. Polym. Technol. 2015, 34, 21492. [Google Scholar] [CrossRef]
- Alexander, R.; Murthy, T.S.R.C.; Ravikanth, K.V.; Prakash, J.; Mahata, T.; Bakshi, S.R.; Krishnan, M.; Dasgupta, K. Effect of graphene nano-platelet reinforcement on the mechanical properties of hot pressed boron carbide based composite. Ceram. Int. 2018, 44, 9830–9838. [Google Scholar] [CrossRef]





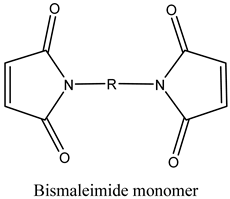
| Monomers | |
|---|---|
 | 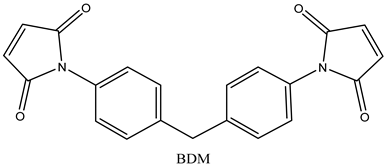 |
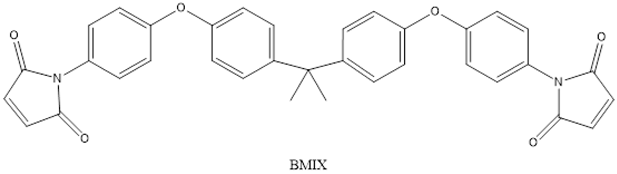 | |
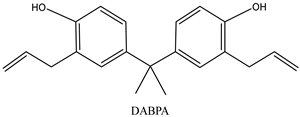 |  |
| Samples | Weight Loss Temperature (°C) | Maximum Degradation Rate Temperature (°C) | ||
|---|---|---|---|---|
| 5% | 15% | 30% | ||
| BDM/DABPA | 416 | 431 | 456 | 420 |
| BDM/DABPA/BMIX | 409 | 432 | 463 | 469 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Wang, P.; Li, Z.; Zhu, Y. Preparation and Characterization of Bismaleimide-Resin-Based Composite Materials. Materials 2024, 17, 1727. https://doi.org/10.3390/ma17081727
Liang L, Wang P, Li Z, Zhu Y. Preparation and Characterization of Bismaleimide-Resin-Based Composite Materials. Materials. 2024; 17(8):1727. https://doi.org/10.3390/ma17081727
Chicago/Turabian StyleLiang, Lingrui, Pei Wang, Zhihong Li, and Yumei Zhu. 2024. "Preparation and Characterization of Bismaleimide-Resin-Based Composite Materials" Materials 17, no. 8: 1727. https://doi.org/10.3390/ma17081727
APA StyleLiang, L., Wang, P., Li, Z., & Zhu, Y. (2024). Preparation and Characterization of Bismaleimide-Resin-Based Composite Materials. Materials, 17(8), 1727. https://doi.org/10.3390/ma17081727





