Mechanical Properties of a Sustainable Low-Carbon Geopolymer Concrete Using a Pumice-Derived Sodium Silicate Solution
Abstract
:1. Introduction
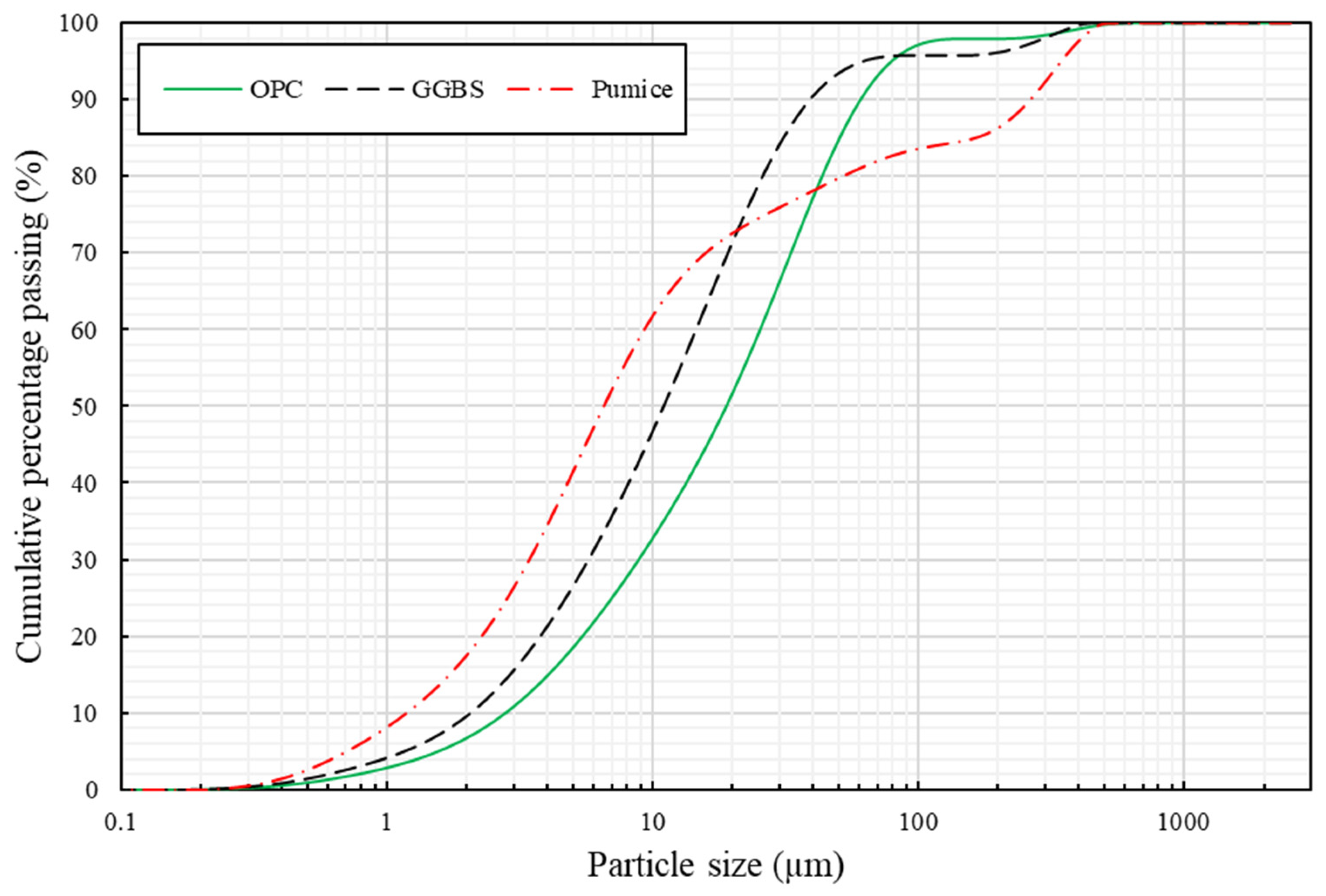
2. Materials
3. Methods
3.1. Mix Design
3.2. Test Alkaline Solutions’ Preparation
3.3. Concrete Specimens’ Preparation and Testing Methods
4. Results and Discussion
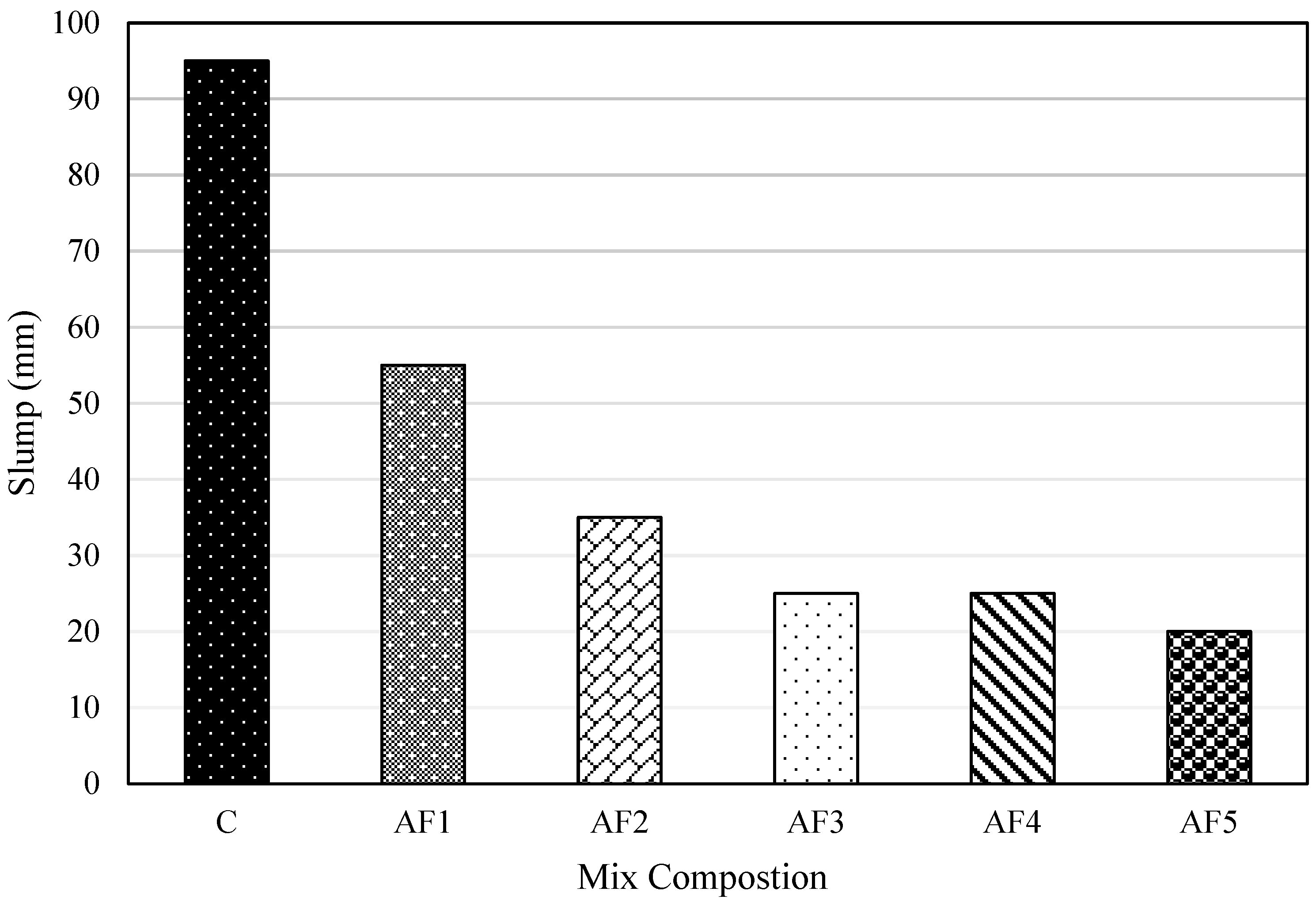
4.1. Consistency of Fresh Concrete
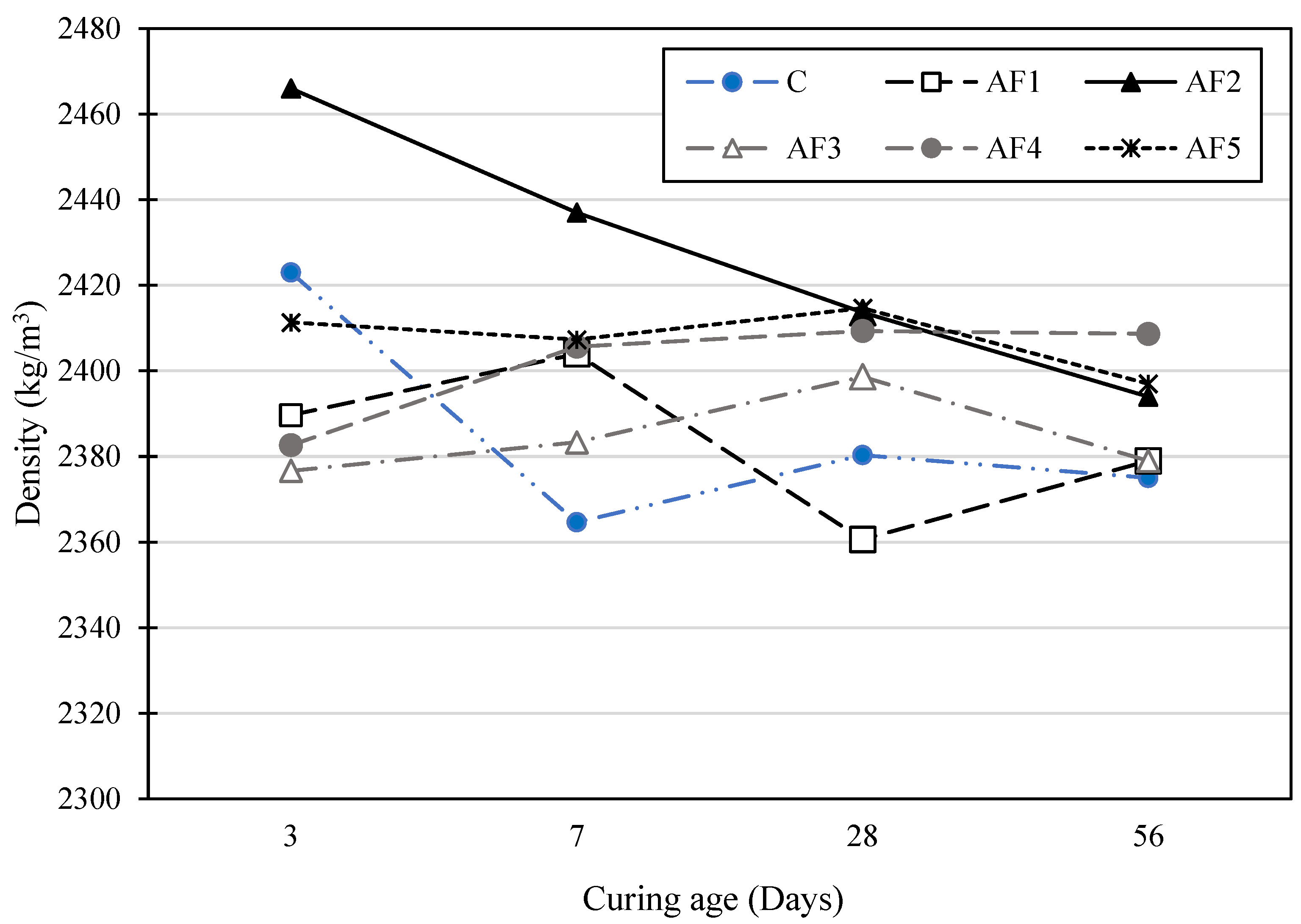
4.2. Density of Hardened Concrete
4.3. Concrete Strength Development
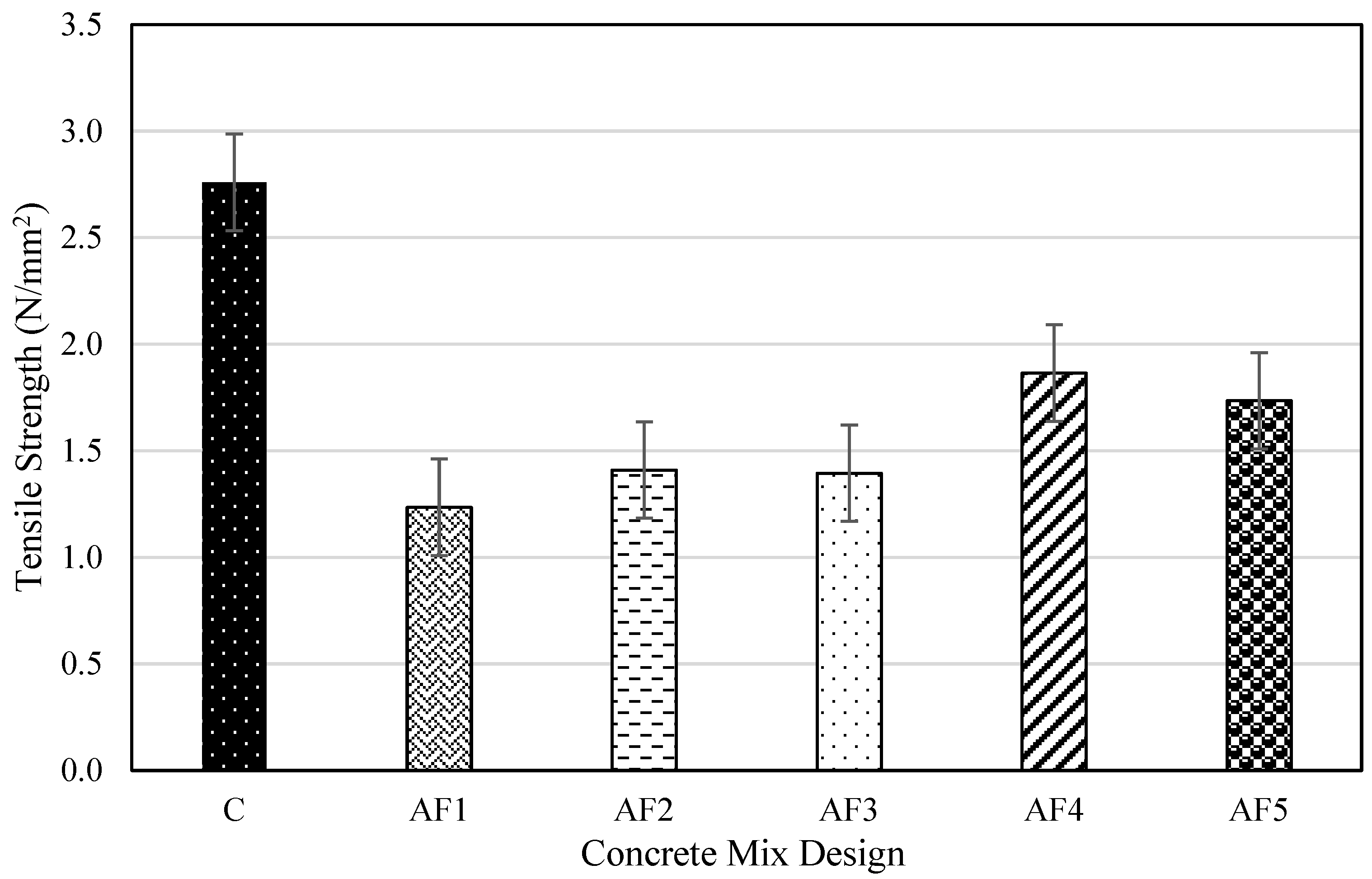
4.4. Tensile Splitting Strength of Concrete Mix Designs
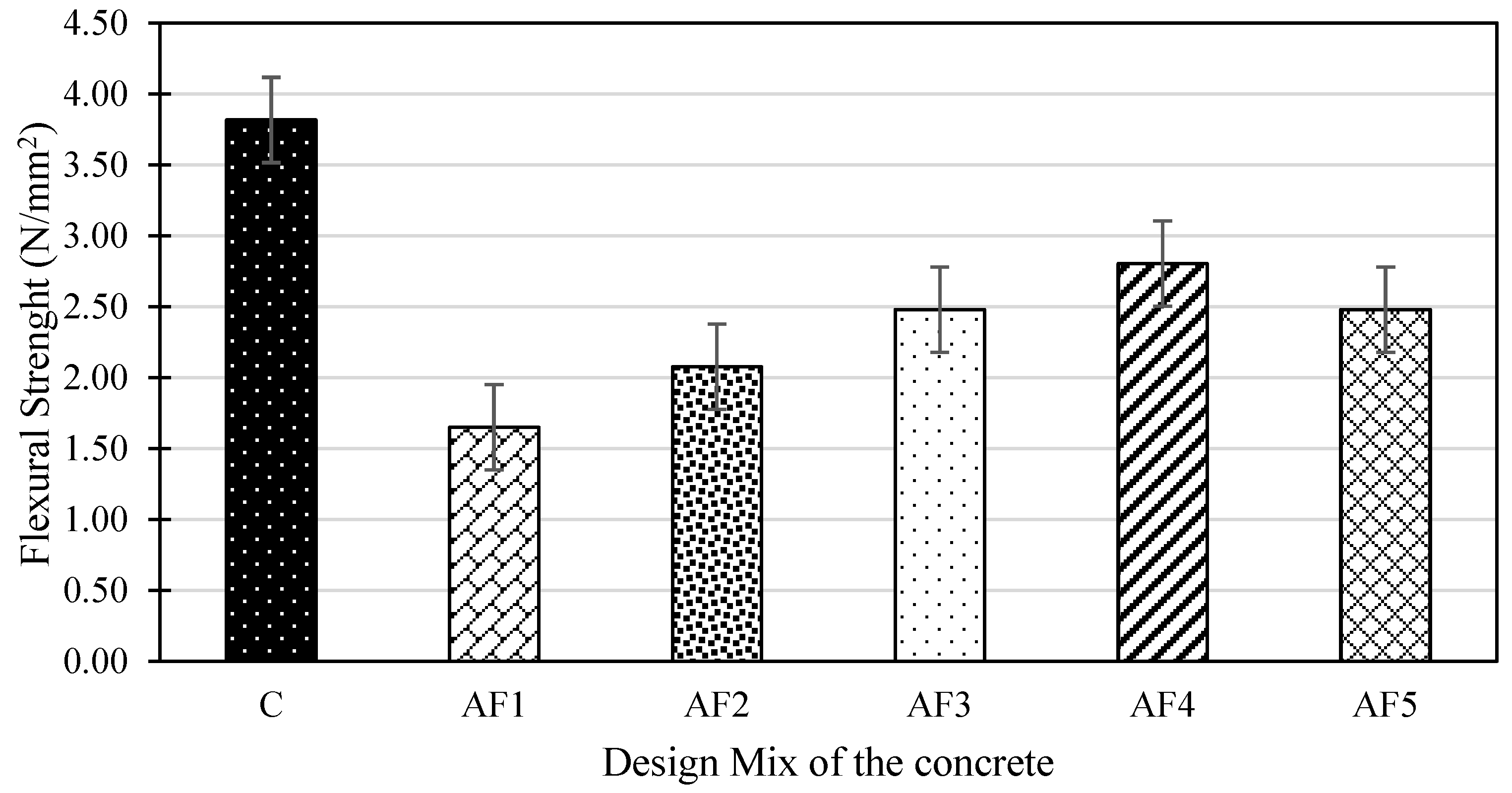
4.5. Flexural Strength of Concrete Mix Designs
5. Conclusions
- The workability of the geopolymer formulations was lower than that of the OPC concrete. For the geopolymer formulations, workability was reduced with an increasing A/P ratio. This confirms the poor workability of GGBS-based geopolymers. This could be addressed by blending GGBS with another precursor like fly ash, which can improve the workability with less water added.
- The hardened density of the concrete produced by the geopolymer was comparable and even slightly higher for most geopolymer formulations (AF2, AF3, AF4, AF5) on day 28 than the control PC concrete. This could be because of the dense and lower-porosity concrete formed by the activating solutions.
- The alkaline precursor ratio influenced the geopolymerisation and mechanical properties of the concrete. The GGBS-based geopolymer concrete improved in terms of mechanical properties, including compressive strength, tensile splitting strength, and flexural strength, with an increasing A/P ratio up to an A/P ratio of 0.4. It then reduced in performance after increasing the A/P ratio. It thus shows that the optimum design for the geopolymer formulation using the SSA derived from PP was at an A/P ratio of 0.4.
- The mechanical performance of the geopolymer concrete designs was lower than that of the control OPC concrete. The control concrete achieved a 28-day compressive strength of 29.4 MPa while the optimum geopolymer design recorded values of 18.8 MPa after 28 days and 22.4 MPa (19% increase) after 56 days, against OPC concrete that recorded 30.8 MPa on day 56 (5% increase). This shows that the dissolution effect of the extra water in the geopolymer mix might have affected the rate of geopolymerisation. The tensile splitting strength and the flexural strength results recorded for the geopolymer formulations, in contrast with the control concrete, observed a similar trend to compressive strength of all the mix designs studied.
- This study shows that the SSA-derived alkaline solution from pumice powder has the potential for developing geopolymer concrete even with lower results. This is because geopolymerisation is a complex process, and the resulting mechanical performance is influenced by all the parameters used in the concrete’s formulation. The AF4 mix could still be used in low-strength construction applications.
- Further research can be carried out to assess the durability of the developed concrete, coupled with a detailed carbon capture analysis/life cycle assessment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Provis, J.L.; Van Deventer, J.S.J. (Eds.) Geopolymers: Structures, Processing, Properties and Industrial Applications; Woodhead Publishing Ltd.: Cambridge, UK, 2009. [Google Scholar]
- Gunning, J.G. Concrete Technology (Level 4); Longman: London, UK, 1983. [Google Scholar]
- Oti, J.E.; Kinuthia, J.M.; Adeleke, B.O.; Billong, N. Durability of Concrete Containing PFA-GGBS By-Products. J. Civ. Eng. Constr. 2020, 9, 165–174. [Google Scholar] [CrossRef]
- Cement. IEA. Available online: https://www.iea.org/reports/cement (accessed on 15 November 2023).
- UratanI, J.M.; Griffiths, S. A Forward Looking Perspective on the Cement and Concrete Industry: Implications of Growth and Development in the Global South. Energy Res. Soc. Sci. 2023, 97, 102972. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A Critical Review on Application of Alkali Activated Slag as a Sustainable Composite Binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Heath, A.; Paine, K.; Goodhew, S.; Ramage, M. The Potential for Using Geopolymer Concrete in the UK. Proc. Inst. Civ. Eng. Constr. Mater. 2013, 166, 195–203. [Google Scholar] [CrossRef]
- Oti, J. Engineering Properties of Concrete made with Pulverised Fly Ash. Int. J. Mech. Prod. Eng. 2018, 6, 52–55. [Google Scholar]
- Zhou, X.; Slater, J.R.; Wavell, S.E.; Oladiran, O. Effects of PFA and GGBS on Early-Ages Engineering Properties of Portland Cement Systems. J. Adv. Concr. Technol. 2012, 10, 74–85. [Google Scholar] [CrossRef]
- Dhir, R.K.; McCarthy, M.J.; Magee, B.J. Impact of BS EN 450 PFA on Concrete Construction in the UK. Constr. Build. Mater. 1998, 12, 59–74. [Google Scholar] [CrossRef]
- Swamy, R.N. Cement Replacement Materials; Surrey University Press: Surrey, UK, 1986. [Google Scholar]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.k. Geopolymer Concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Kovalachuk, G. Directed Synthesis of Alkaline Aluminosilicate Minerals in a Geocement Matrix. Mater. Sci. 2007, 42, 53. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. (Eds.) Handbook of Akali-Activated Cements, Mortars and Concretes; Elservier: Amsterdam, The Netherlands; Woodhead Publishing Ltd.: Cambridge, UK, 2015. [Google Scholar]
- Aldred, J.; Day, J. Is Geopolymer Concrete a Suitable Alternative to Traditional Concrete. In Proceedings of the 37th Conference on Our World in Concrete and Structures, Singapore, 29–31 August 2012; Available online: https://earthfriendlyconcrete.com/wp-content/uploads/2022/01/jaldred_jday_geopolymer-concrete_singapore-2012.pdf (accessed on 15 November 2023).
- Chanh, N.V.; Trung, B.D.; Tuan, D.V. Recent Research Geopolymer Concrete. In Proceedings of the 3rd ACF International Conference-ACF/VCA, Ho Chi Minh City, Vietnam, 11–13 November 2008; Available online: https://www.researchgate.net/profile/Prem-Baboo/post/Is-Seawater-suitable-for-Geopolymer-concrete/attachment/59d636d679197b80779943c7/AS%3A390575271497729%401470131809520/download/A18.pdf (accessed on 15 November 2023).
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Singh, N.B.; Kumar, M.; Rai, S. Geopolymer cement and concrete: Properties. Mater. Today Proc. 2020, 29, 743–748. [Google Scholar] [CrossRef]
- Shobeiri, V.; Bennett, B.; Xie, T.; Visintin, P. A Comprehensive Assessment of the Global Warming Potential of Geopolymer Concrete. J. Clean. Prod. 2021, 297, 126669. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison Between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The Role of Inorganic Polymer Technology in the Development of ‘Green Concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Davidovits, J. Properties of Geopolymer Concrete. First International Conference on Alkaline Cements and Concrete. Sci. Inst. Bind. Mater. 1994, 1, 131–149. [Google Scholar]
- Provis, J.L.; Van Deventer, J.S. (Eds.) Alkali Activated Materials State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Zhang, H.-Y.; Liu, J.-C.; Wu, B. Mechanical Properties and Reaction Mechanism of One-Part Geopolymer Mortars. Constr. Build. Mater. 2021, 273, 121973. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 Emission Assessments of Alkali-Activated Concrete and Ordinary Portland Cement Concrete: A Comparative Analysis of Different Grades of Concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental Impact Assessment of Fly Ash and Silica Fume Based Geopolymer Concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-Term Durability Properties of Geopolymer Concrete: An In-depth Review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Jamieson, E.; McLellan, B.; Riessen, A.v.; Nikraz, H. Comparison of Embodied Energies of Ordinary Portland Cement with Bayer-Derived Geopolymer Products. J. Clean. Prod. 2015, 99, 112–118. [Google Scholar] [CrossRef]
- Billong, N.; Oti, J.; Kinuthia, J. Using Silica Fume Based Activator in Sustainable Geopolymer Binder for Building Application. Constr. Build. Mater. 2021, 275, 122177. [Google Scholar] [CrossRef]
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Ebailila, M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Hydroxide Solution. Materials 2023, 16, 2400. [Google Scholar] [CrossRef] [PubMed]
- Kamseu, E.; Moungam, L.M.B.; Cannio, M.; Billong, N.; Chaysuwan, D.; Melo, U.C.; Leonelli, C. Substitution of Sodium Silicate with Rice Husk Ash-NaOH Solution in Metakaolin Based Geopolymer Cement Concerning Reduction in Global Warming. J. Clean. Prod. 2017, 142, 3050–3060. [Google Scholar] [CrossRef]
- Rajan, H.S.; Kathirvel, P. Sustainable Development of Geopolymer Binder using Sodium Silicate Synthesized from Agricultural Waste. J. Clean. Prod. 2021, 286, 124959. [Google Scholar] [CrossRef]
- Figueiredo, R.A.M.; Brandão, P.R.G.; Soutsos, M.; Henriques, A.B.; Fourie, A.; Mazzinghy, D.B. Producing Sodium Silicate Powder from Iron Ore Tailings for use as an Activator in One-Part Geopolymer Binders. Mater. Lett. 2021, 288, 129333. [Google Scholar] [CrossRef]
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese Rice Husk Ash for the Production of Sodium Silicate as the Activator for Alkali-Activated Binders. J. Clean. Prod. 2018, 201, 272–286. [Google Scholar] [CrossRef]
- Fitzsimmons, C. How Is Pumice Formed? Available online: https://sciencing.com/pumice-formed-5232410.html (accessed on 15 November 2023).
- Rashad, A.M. An Overview of Pumice Stone as Cementitious Material-The Best Manual for Civil Engineer. Silicon 2021, 13, 551–572. [Google Scholar] [CrossRef]
- Kabay, N.; Mert, M.; Miyan, N.; Omur, T. Pumice as Precursor in Geopolymer Paste and Mortar. J. Civ. Eng. Constr. 2021, 10, 225–236. [Google Scholar] [CrossRef]
- Safari, Z.; Kurda, R.; Al-Hadad, B.; Mahmood, F.; Tapan, M. Mechanical Characteristics of Pumice-Based Geopolymer Paste. Resour. Conserv. Recycl. 2020, 162, 105055. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of Heat and Ambient Cured One-Part Geopolymer Mixes with Different Grades of Sodium Silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Gokçe, H.S.; Tuyan, M.; Nehdi, M.L. Alkali-Activated and Geopolymer Materials Developed Using Innovative Manufacturing Techniques: A Critical Review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials: State-of-The-Art Report (RILEM TC 224-AAM); Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- BS EN 197-1:2011; Cement. Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardisation: London, UK, 2011.
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I. Durability Assessment of Alkali Activated Slag (AAS) Concrete. Mater. Struct. 2012, 45, 1425–1437. [Google Scholar] [CrossRef]
- BS EN 15167-1:2006; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout—Part 1: Definitions, Specifications and Conformity Criteria. Certified Emergency Nurse Specialization: Oak Brook, IL, USA, 2006.
- Mat, M. GEOLOGYSCIENCE: Pumice. ed. 2023. Available online: https://geologyscience.com/rocks/igneous-rocks/extrusive-igneous-rocks/pumice/?amp (accessed on 7 September 2023).
- BS EN 12620:2002+A1:2008; Aggregates for Concrete. BSI Standards Limited: London, UK, 2008.
- EN 206:2013+A1:2016; Concrete—Specification, Performance, Production and Conformity. BSI Standards Limited: London, UK, 2016.
- Bernal, S.A.; Provis, J.L.; van Deventer, J.S.J. Impact of water content on the performance of alkali-activated slag concretes. In Durability of Concrete Structures, Proceedings of the ICDCS2018: 6th International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018; Basheer, P.A.M., Ed.; Whittles Publishing: Dunbeath, UK, 2018; pp. 143–148. [Google Scholar]
- BS EN 12350-2:2019; Testing Fresh Concrete. Part 1: Slump Test. BSI Standards Limited: London, UK, 2019.
- BS EN 12390-2:2019; Testing Hardened Concrete. Part 2: Making and Curing Specimens for Strength Tests. BSI Standards Limited: London, UK, 2019.
- BS EN 12390-2:2019; Testing Hardened Concrete. Making and Curing Specimens for Strength Tests. BSI Standards Limited: London, UK, 2019.
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and Heavy Metal Immobilization Behaviors of Slag Based Geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef] [PubMed]
- BS EN 12390-7:2019; Testing Hardened Concrete. Density of Hardened Concrete. BSI Standards Limited: London, UK, 2019.
- BS EN 12390-3:2019; Testing Hardened Concrete. Part 3: Compressive Strength of Test Specimens. BSI Standards Limited: London, UK, 2019.
- BS EN 12390-6:2009; Testing Hardened Concrete. Tensile Splitting Strength of Test Specimens. BSI Standards Limited: London, UK, 2009.
- BS EN 12390-5:2019; Testing Hardened Concrete. Flexural Strength of Test Specimens. BSI Standards Limited: London, UK, 2019.
- Bellum, R.R.; Muniraj, K.; Madduru, S.R.C. Exploration of Mechanical and Durability Characteristics of Fly Ash GGBFS Based Green Geopolymer Concrete. Appl. Sci. 2020, 2, 919. [Google Scholar] [CrossRef]
- Collins, F.G.; Sanjayan, J.G. Workability and Mechanical Properties of Alkali Activated Slag Concrete. Cem. Concr. Res. 1999, 29, 455–458. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on Setting, Workability and Early Strength Properties of Fly Ash Geopolymer Concrete Cured in Ambient Condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, H.; Zhang, Z.; Gao, X.; Zhang, C.; Wu, Q. Effect of Fly Ash Microsphere on the Rheology and Microstructure of Alkali Activated Fly Ash/Slag Pastes. Cem. Concr. Res. 2018, 109, 198–207. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and Mechanical Properties of Alkali-Activated Fly Ash-Slag Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Q.; Luo, T. Understanding the Workability of Alkali-Activated Phosphorus Slag Pastes: Effects of Alkali Dose and Silicate Modulus on Early-Age Hydration Reactions. Cem. Concr. Compos. 2022, 133, 104649. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Flexural Strength and Elastic Modulus of Ambient-Cured Blended Low-Calcium Fly Ash Geopolymer Concrete. Constr. Build. Mater. 2017, 130, 22–31. [Google Scholar] [CrossRef]
- BS EN 206:2013+A2:2021; Concrete Specification, Performance, Production and Conformity. BSI Standards Limited: London, UK, 2021.
- Vijai, K.; Kumutha, R.; Vishnuram, B.G. Effect of Types of Curing on Strength of Geopolymer Concrete. Int. J. Phys. Sci. 2010, 5, 1419–1423. [Google Scholar]
- Li, X.; Wang, Z.; Jiao, Z. Influence of Curing on the Strength Development of Calcium-Containing Geopolymer Mortar. Materials 2013, 6, 5069–5076. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.H.; Abdullah, M.M.A.B.; Salleh, M.A.A.M.; Azimi, E.A.; Chaiprapa, J.; Sandu, A.V. Strength Development of Solely Ground Granulated Blast Furnace Glag Geopolymers. Constr. Build. Mater. 2020, 250, 118720. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Saout, G.L.; Winnefeld, F. Influence of Slag Chemistry on the Hydration of Alkali-Activated Blast-Furnace Slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Thunuguntla, C.S.; Gunneswara Rao, T.D. Effect of Mix Design Parameters on Mechanical and Durability Properties of Alkali Activated Slag Concrete. Constr. Build. Mater. 2018, 193, 173–188. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, M.; Yang, K.; Yu, L.; Yang, C. Setting Behaviours and Early-Age Microstructures of Alkali-Activated Ground Granulated Blast Furnace Slag (GGBS) from Different Regions in China. Cem. Concr. Compos. 2020, 114, 103782. [Google Scholar] [CrossRef]
- Farhan, K.Z.; Johari, M.A.M.; Demirbog, R. Assessment of Important Parameters Involved in the Synthesis of Geopolymer Composites: A Review. Constr. Build. Mater. 2020, 264, 120276. [Google Scholar] [CrossRef]
- Sithole, N.T.; Mashifana, T. Geosynthesis of Building and Construction Materials through Alkaline Activation of Granulated Blast Furnace Slag. Constr. Build. Mater. 2020, 264, 120712. [Google Scholar] [CrossRef]
- Özdal, M.; Karakoç, M.B.; Özcan, A. Investigation of the Properties of Two Different Slag-Based Geopolymer Concretes Exposed to Freeze–Thaw Cycles. Struct. Concr. J. 2021, 22, E332–E340. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymerisation Kinetics. 2. Reaction Kinetic Modelling. Chem. Eng. Sci. 2007, 62, 2318–2329. [Google Scholar] [CrossRef]
- Cui, Y.-l.; Wang, D.; Wang, Y.; Sun, R.; Rui, Y. Effects of the n(H2O: Na2Oeq) Ratio on the Geopolymerization Process and Microstructures of Fly Ash-Based Geopolymers. J. Non-Cryst. Solids 2019, 511, 19–28. [Google Scholar] [CrossRef]
- Jeong, Y.; Oh, J.E.; Jun, Y.; Park, J.; Ha, J.-h.; Sohn, S.G. Influence of Four Additional Activators on Hydrated-Lime [Ca(OH)2] Activated Ground Granulated Blast-Furnace Slag. Cem. Concr. Compos. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Shilar, F.A.; Ganachari, S.V.; Patil, V.B.; Khan, T.; Dawood Abdul Khadar, M.Y.S. Molarity activity effect on mechanical and microstructure properties of geopolymer concrete: A review. Case Stud. Constr. Mater. 2022, 16, e01014. [Google Scholar] [CrossRef]
- Mohamed, O.; Khattab, R.; Alzo’ub, A.K. Factors Affecting Compressive Strength Development in Alkali-activated Slag Concrete. IOP Conf. Ser. Mater. Sci. Eng. 2019, 603, 042037. [Google Scholar] [CrossRef]
- Rangan, B.V. Geopolymer concrete for environmental protection. Indian Concr. J. 2014, 88, 41–59. [Google Scholar]
- Asghar, R.; Khan, M.A.; Alyousef, R.; Javed, M.F.; Ali, M. Promoting the Green Construction: Scientometric Review on the Mechanical and Structural Performance of Geopolymer Concrete. Constr. Build. Mater. 2023, 368, 130502. [Google Scholar] [CrossRef]
- Thomas, R.J.; Peethamparan, S. Alkali-Activated Concrete: Engineering Properties and Stress–Strain Behavior. Constr. Build. Mater. 2015, 93, 49–56. [Google Scholar] [CrossRef]
- Vora, P.R.; Dave, U.V. Parametric Studies on Compressive Strength of Geopolymer Concrete. Procedia Eng. 2013, 51, 210–219. [Google Scholar] [CrossRef]
- Gaedicke, C.; Torres, A.; Huynh, K.C.; Marines, A. A Method to Correlate Splitting Tensile Strength and Compressive Strength of Pervious Concrete Cylinders and Cores. Constr. Build. Mater. 2016, 125, 271–278. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shariq, M.; Mahdi, F. Structural behavior of reinforced geopolymer concrete beams—A review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]

| Oxides | Compositions (%) | ||
|---|---|---|---|
| OPC | GGBS | Pumice | |
| CaO | 61.49 | 37.99 | 0.8 |
| MgO | 3.54 | 8.78 | 0.1 |
| SiO2 | 18.84 | 35.54 | 76.2 |
| Al2O3 | 4.77 | 11.46 | 13.5 |
| Na2O | 0.02 | 0.37 | 1.6 |
| P2O5 | 0.1 | 0.02 | 1.8 |
| Fe2O3 | 2.87 | 0.42 | 1.1 |
| Mn2O3 | 0.05 | 0.43 | - |
| K2O | 0.57 | 0.43 | - |
| TiO2 | 0.26 | 0.7 | 0.2 |
| V2O5 | 0.06 | 0.04 | - |
| BaO | 0.05 | 0.09 | - |
| SO3 | 3.12 | 1.54 | - |
| Loss on ignition | 4.3 | 2 | - |
| Other Properties | Pumice |
|---|---|
| Chemical Name | Amorphous Aluminium Silicate |
| Hardness (MOHS) | 6 |
| pH | 7.2 |
| Radioactivity | None |
| Loss on Ignition (LOI) | 0.05 |
| Softening Point | 900 °C |
| Water-Soluble Substances | 0.0015 |
| Acid-Soluble Substances | 0.029 |
| Reactivity | Inert |
| Appearance | White powder (GE brightness of 84) |
| Properties | Fine Aggregate (Sand) | Coarse Aggregate (10 mm) | Coarse Aggregate (20 mm) |
|---|---|---|---|
| Shape index (%) | - | 12 | 32 |
| Impact value | - | 18 | 12 |
| Saturated density (kg/m3) | 2820 | 2680 | 1330 |
| Dry density (kg/m3) | 2710 | 2570 | 1420 |
| Water absorption (%) | 0.85 | 1.5 | 12.8 |
| Flakiness index (%) | - | 23 | 37 |
| Mix Code | Elaborated Abbreviation | Concrete Binder | W (L) | Aggregates (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC (kg) | Geopolymer Binder | FA | Coarse Aggregate | ||||||||
| GGBS (kg) | A/P | SS:SH | Activator (mL) | ||||||||
| Ratio | SS | SH | 10 mm | 20 mm | |||||||
| C | OPC (Control 1) | 8.9 | - | - | - | - | - | 3.7 | 7.9 | 8.8 | 17.8 |
| AF1 | AF1-AP0.1-1SSA:SH | - | 8.1 | 0.1 | 01:01 | 275 | 275 | 3.2 | 7.9 | 8.8 | 17.8 |
| AF2 | AF2-AP0.2-1SSA:SH | - | 7.4 | 0.2 | 01:01 | 505 | 505 | 2.7 | 7.9 | 8.8 | 17.8 |
| AF3 | AF3-AP0.3-1SSA:SH | - | 6.8 | 0.3 | 01:01 | 699 | 699 | 2.3 | 7.9 | 8.8 | 17.8 |
| AF4 | AF4-AP0.4-1SSA:SH | - | 6.3 | 0.4 | 01:01 | 865 | 865 | 2.0 | 7.9 | 8.8 | 17.8 |
| AF5 | AF5-AP0.5-1SSA:SH | - | 5.9 | 0.5 | 01:01 | 1010 | 1010 | 1.7 | 7.9 | 8.8 | 17.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oti, J.; Adeleke, B.O.; Anowie, F.X.; Kinuthia, J.M.; Ekwulo, E. Mechanical Properties of a Sustainable Low-Carbon Geopolymer Concrete Using a Pumice-Derived Sodium Silicate Solution. Materials 2024, 17, 1792. https://doi.org/10.3390/ma17081792
Oti J, Adeleke BO, Anowie FX, Kinuthia JM, Ekwulo E. Mechanical Properties of a Sustainable Low-Carbon Geopolymer Concrete Using a Pumice-Derived Sodium Silicate Solution. Materials. 2024; 17(8):1792. https://doi.org/10.3390/ma17081792
Chicago/Turabian StyleOti, Jonathan, Blessing O. Adeleke, Francis X. Anowie, John M. Kinuthia, and Emma Ekwulo. 2024. "Mechanical Properties of a Sustainable Low-Carbon Geopolymer Concrete Using a Pumice-Derived Sodium Silicate Solution" Materials 17, no. 8: 1792. https://doi.org/10.3390/ma17081792








