Research on Sol-Gel Synthesis of Low-Temperature Na2O-B2O3-SiO2 Vitrified Bonds and Preparation of High-Strength Stacked Abrasives Using the Molding and Crushing Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Selection of Component Points
2.1.2. Preparation of Vitrified Bond Powder by Sol-Gel Method
2.1.3. Preparation of Vitrified Bond Powders by Dry Mixing Method
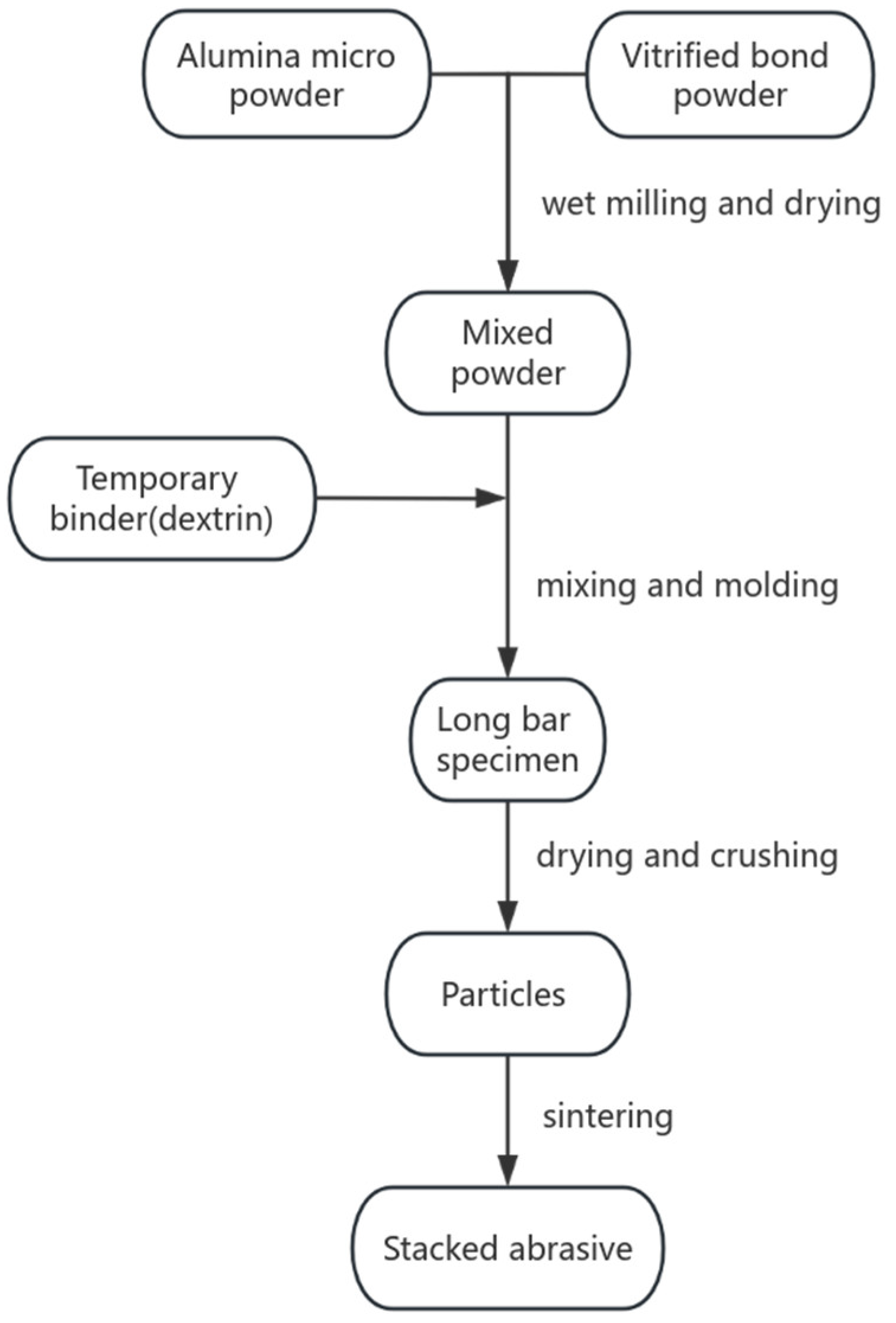
2.1.4. Preparation of Stacked Abrasives by Molding and Crushing Method
2.2. Sample Preparation
3. Results and Discussion
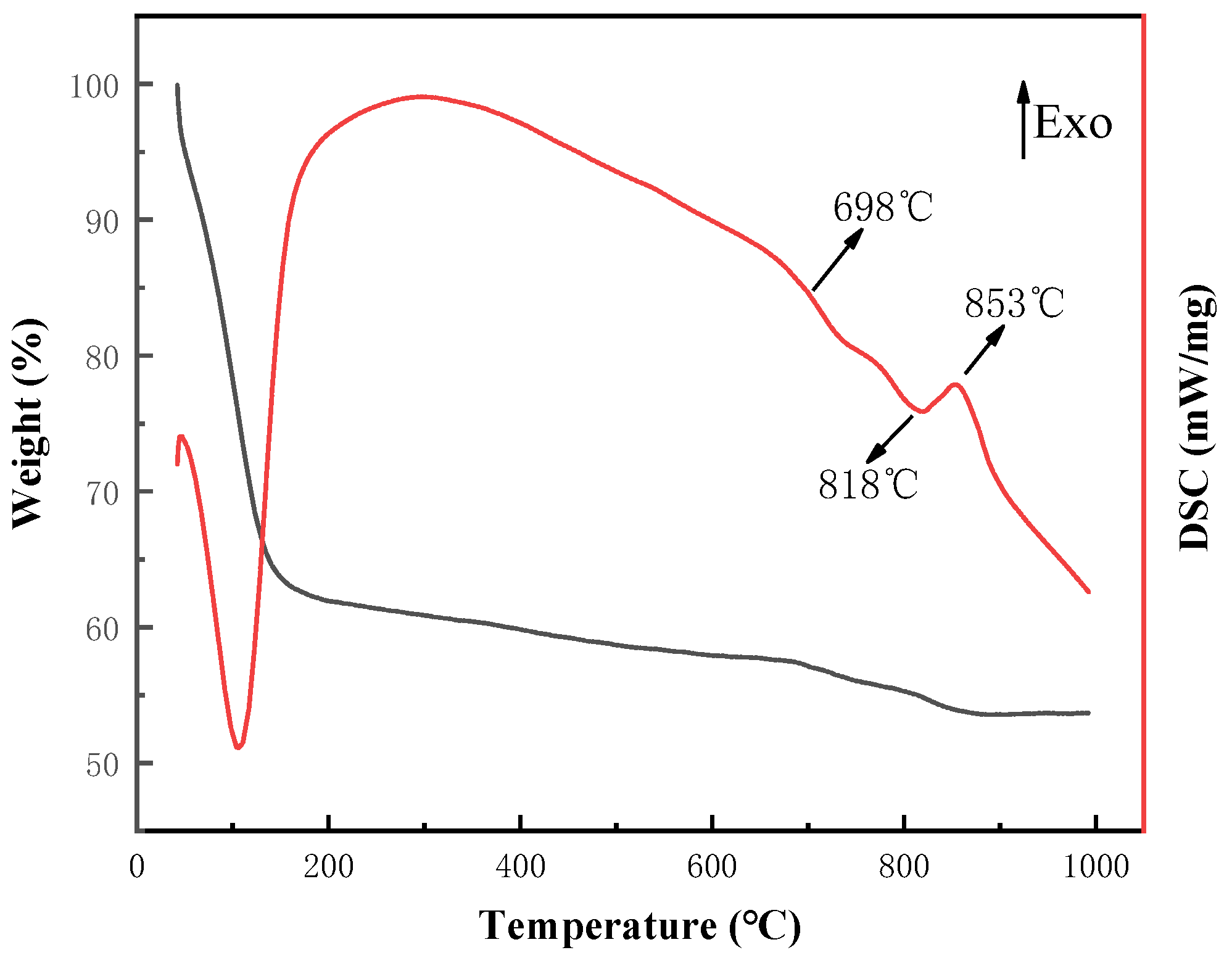
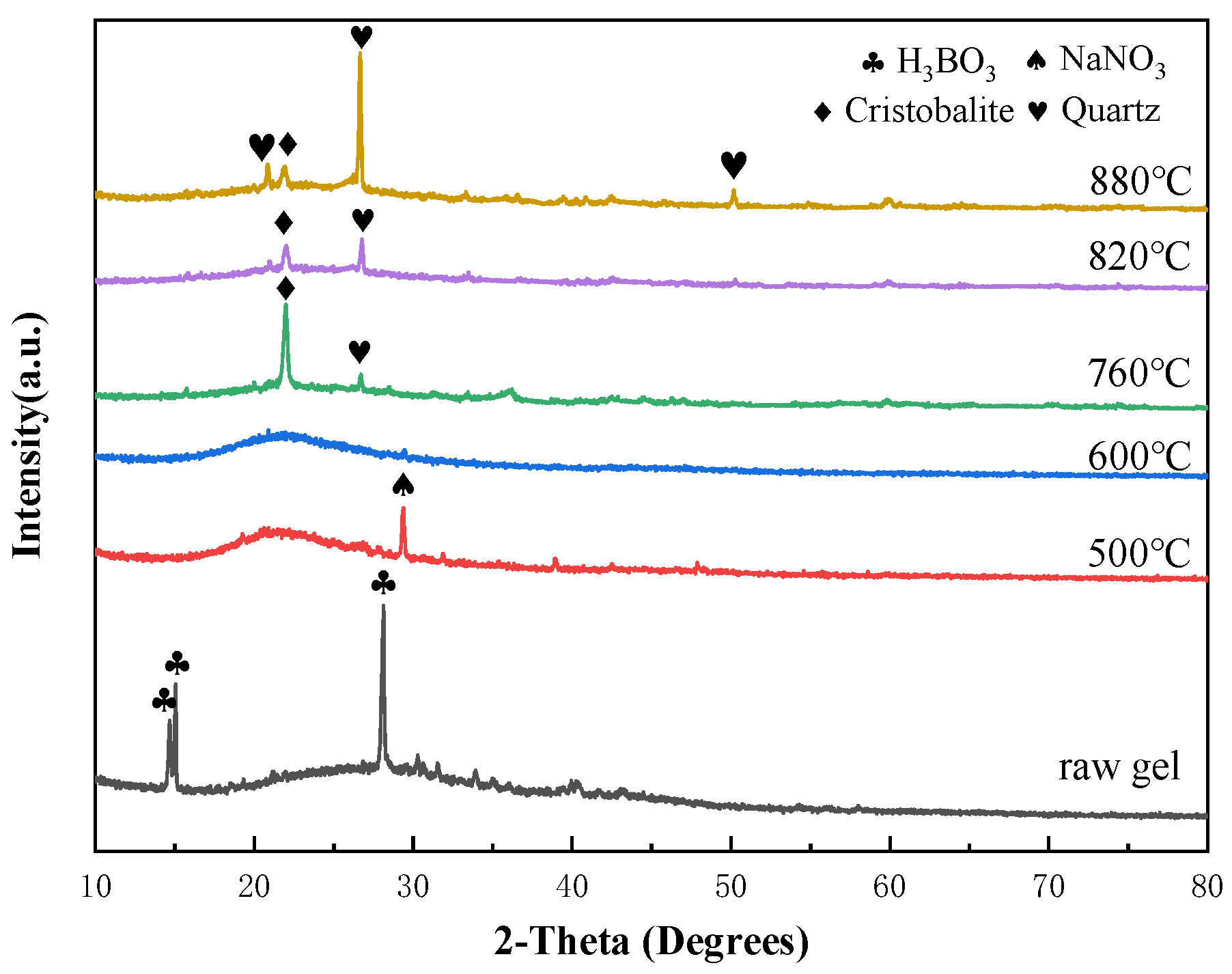
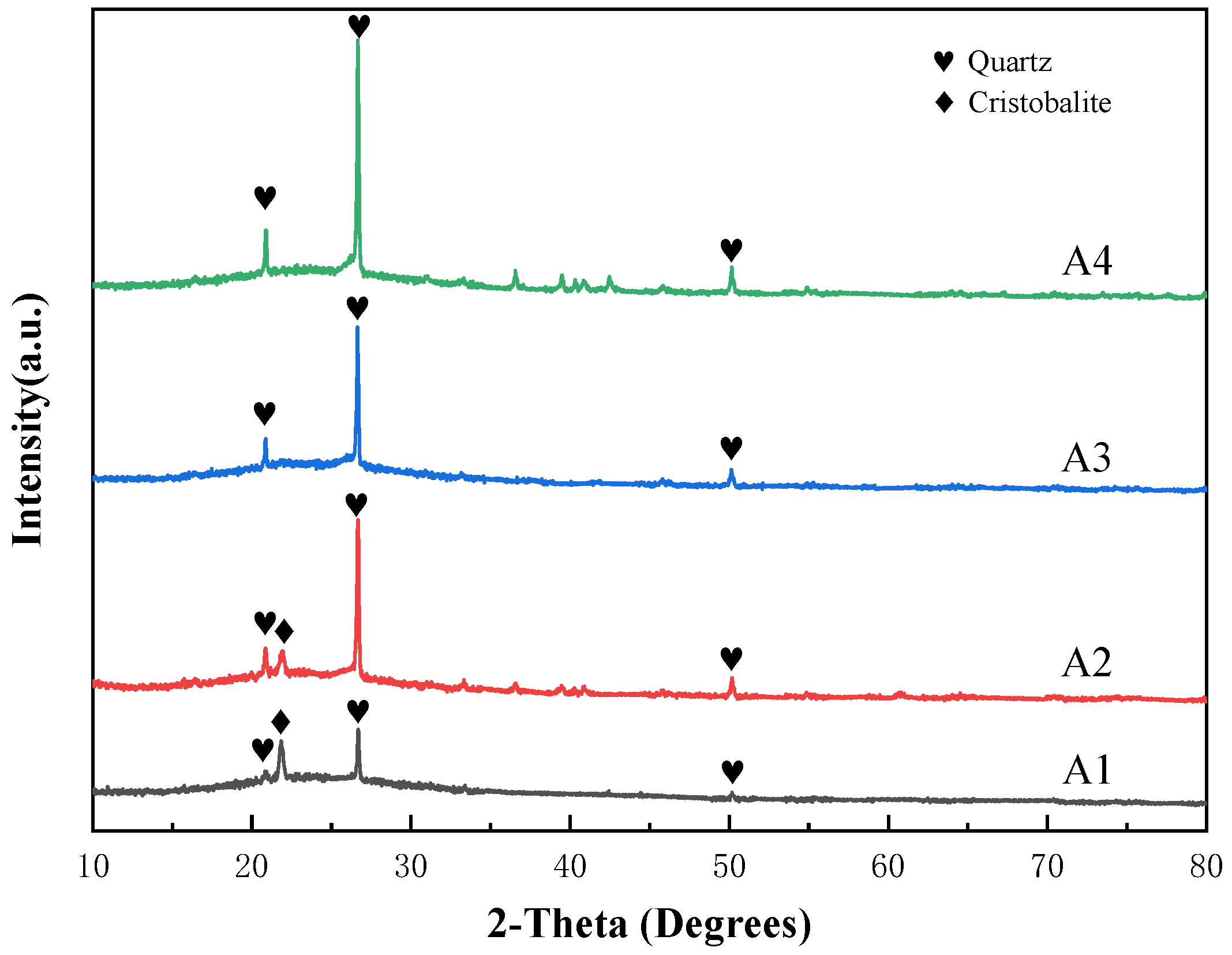
3.1. Thermal Analysis and Phase Analysis of Bond Powder Prepared by Sol-Gel Method
3.2. Single Particle Compressive Strength of Corundum Stacked Abrasives
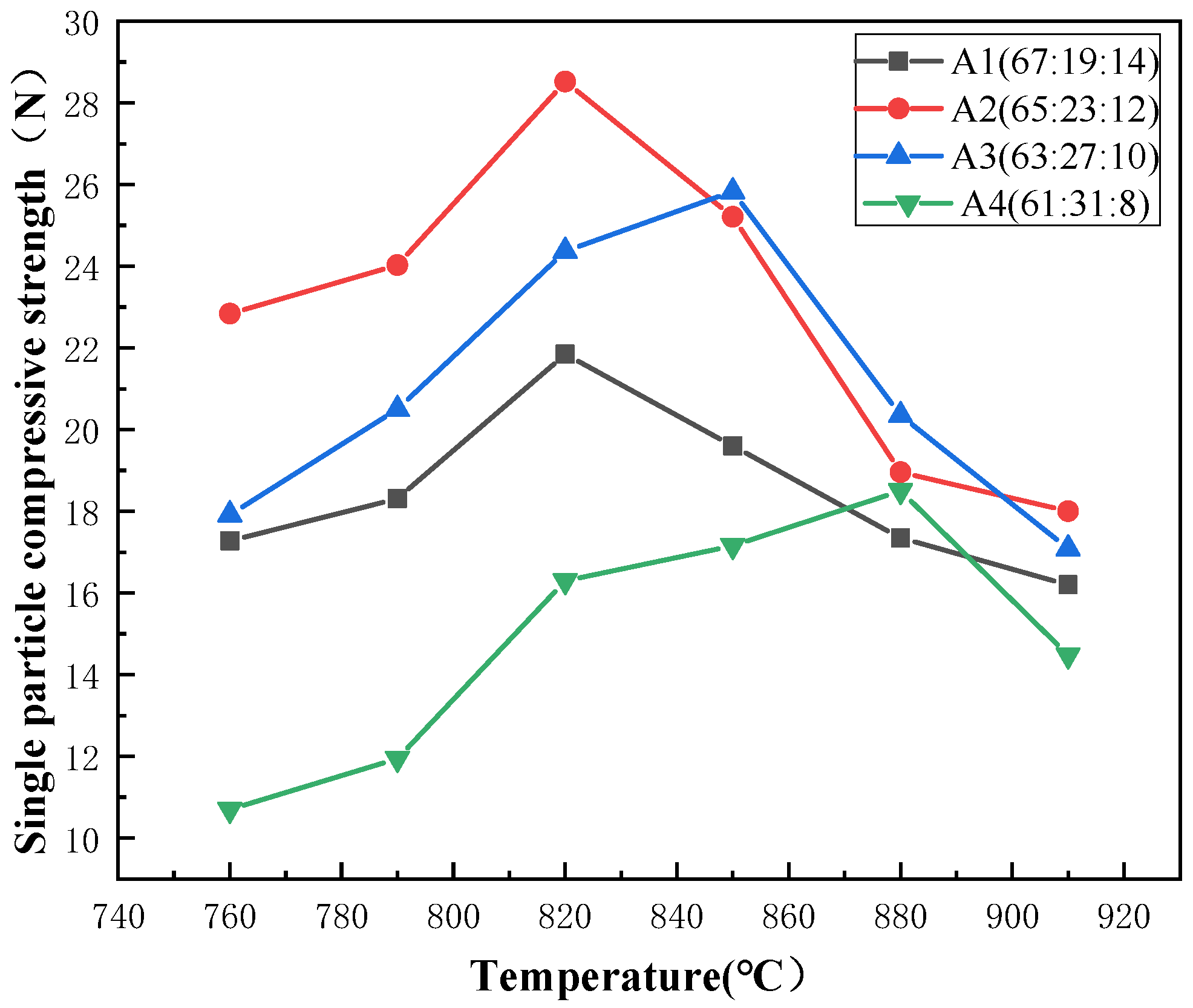
3.2.1. Effect of Oxide Component Ratios of Vitrified Bonds on the Single Particle Compressive Strength of Corundum Stacked Abrasives
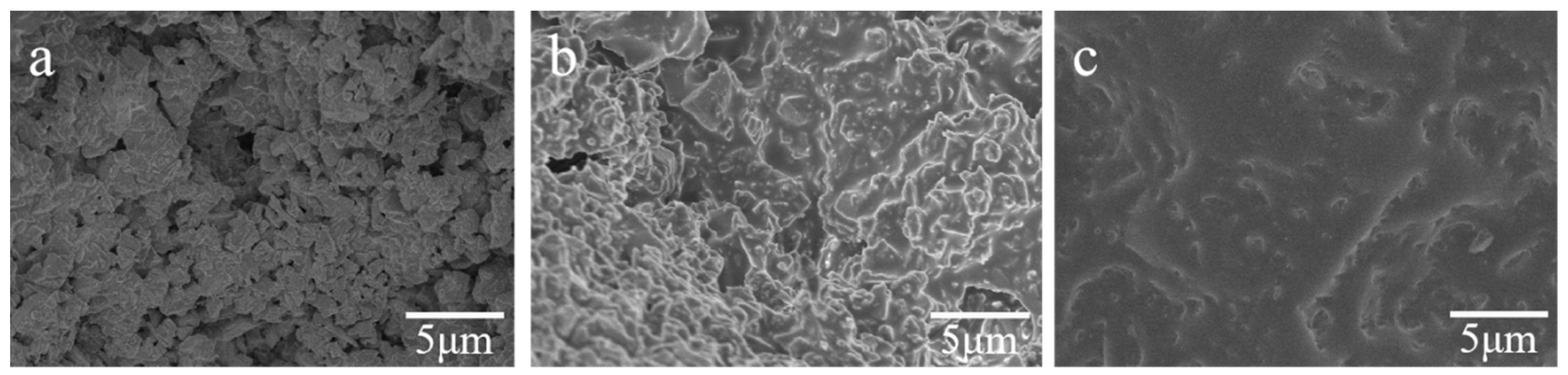
3.2.2. Microstructure of Stacked Abrasives at Different Sintering Temperatures
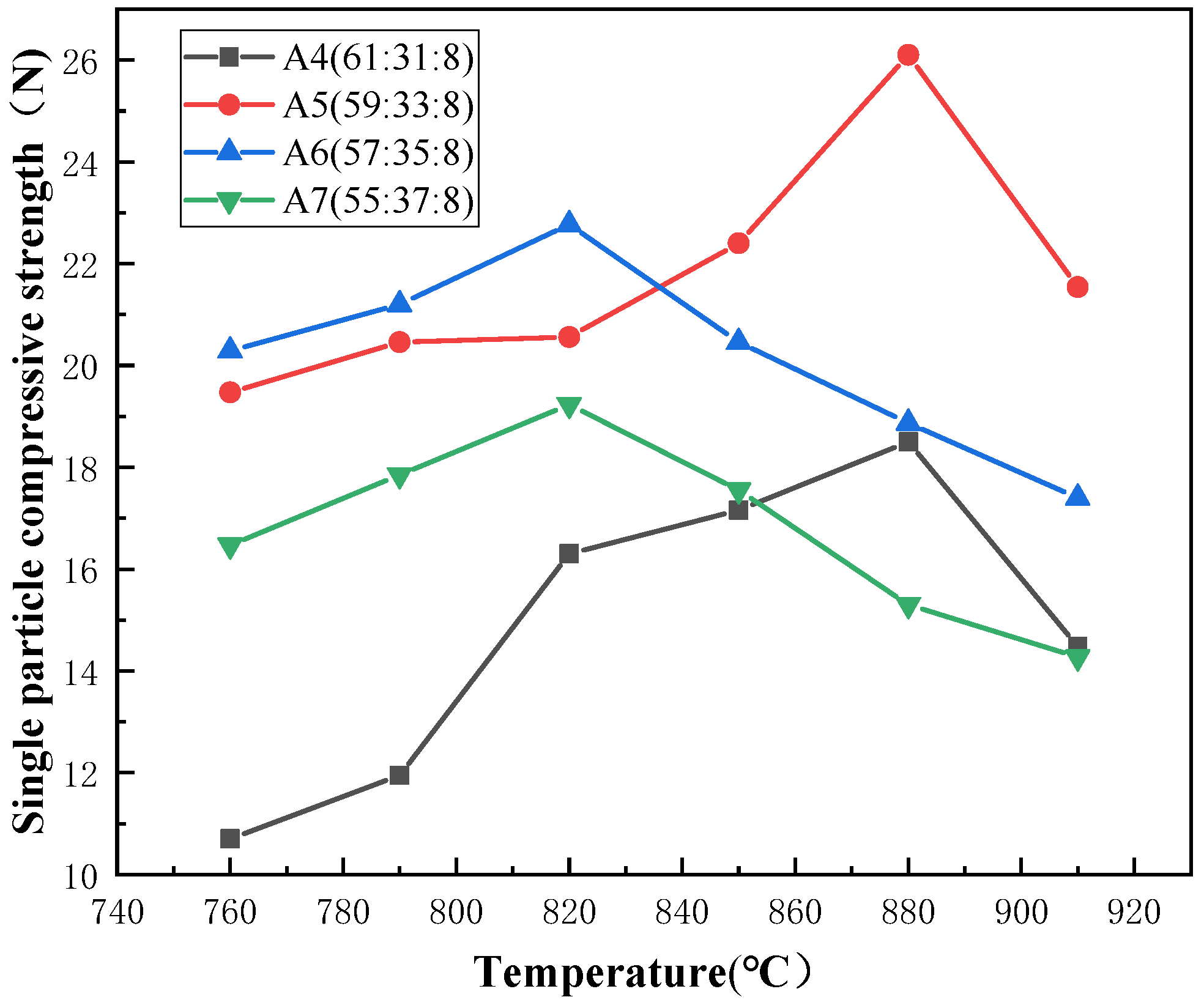
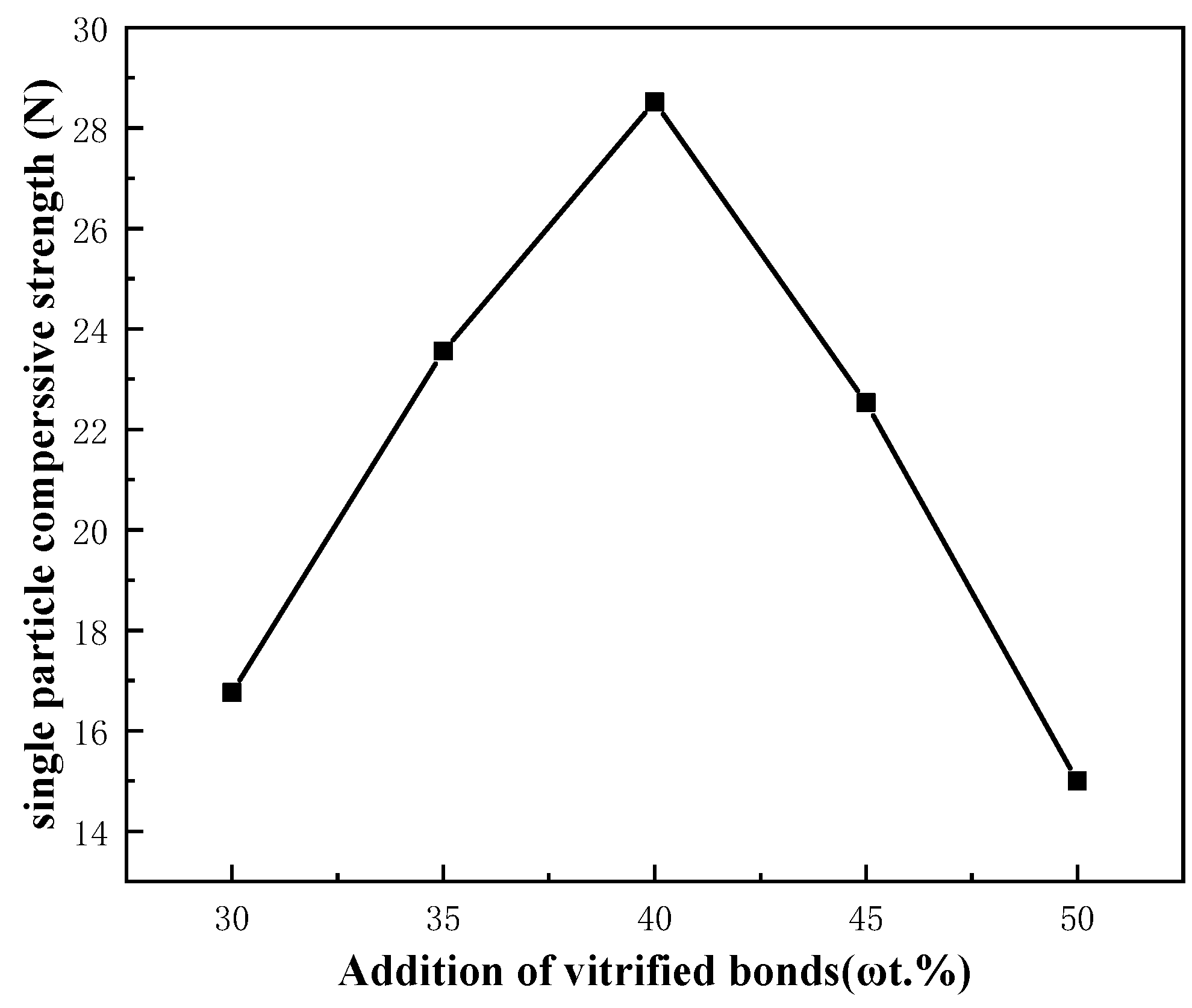
3.2.3. Stacked Abrasives under Different Additions of Vitrified Bonds
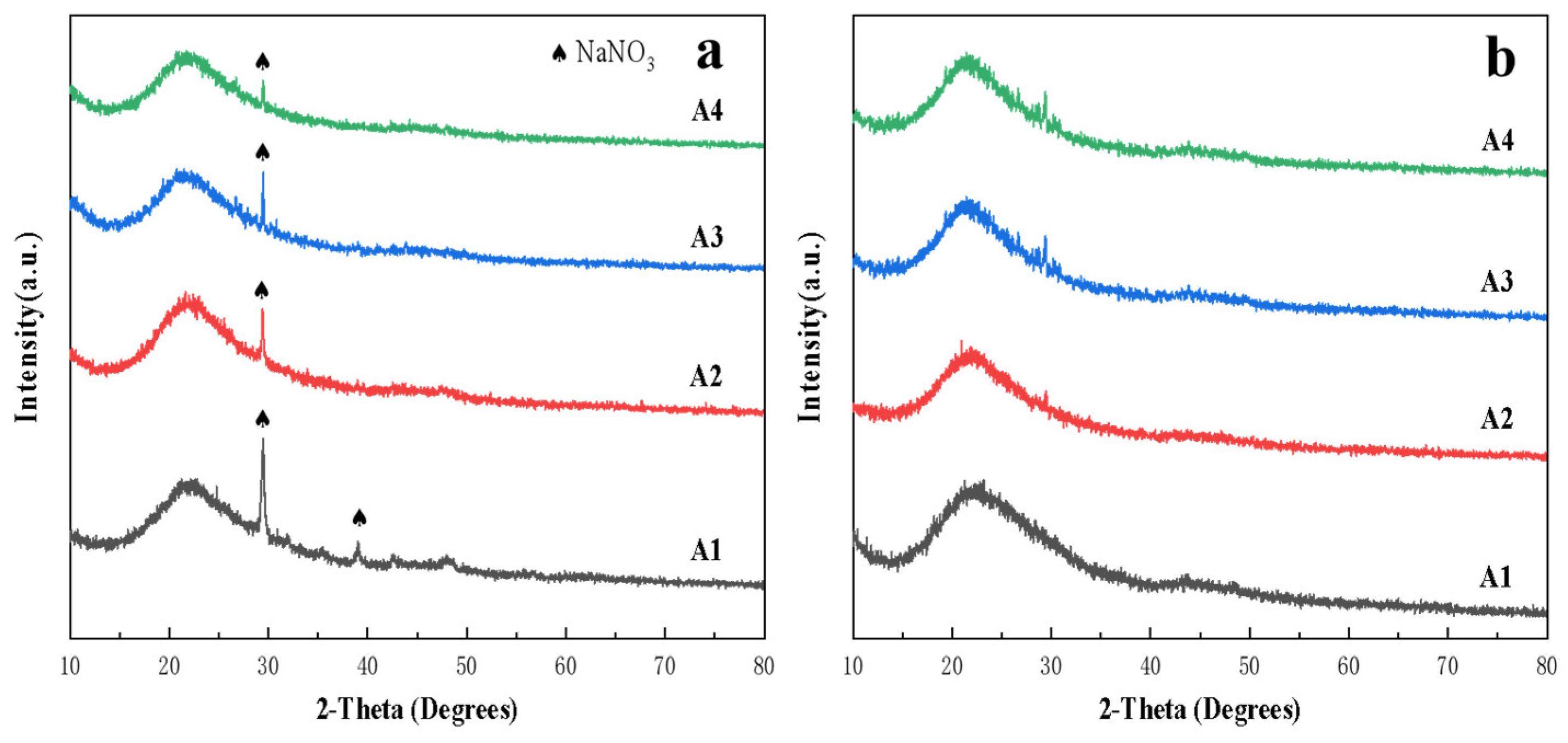
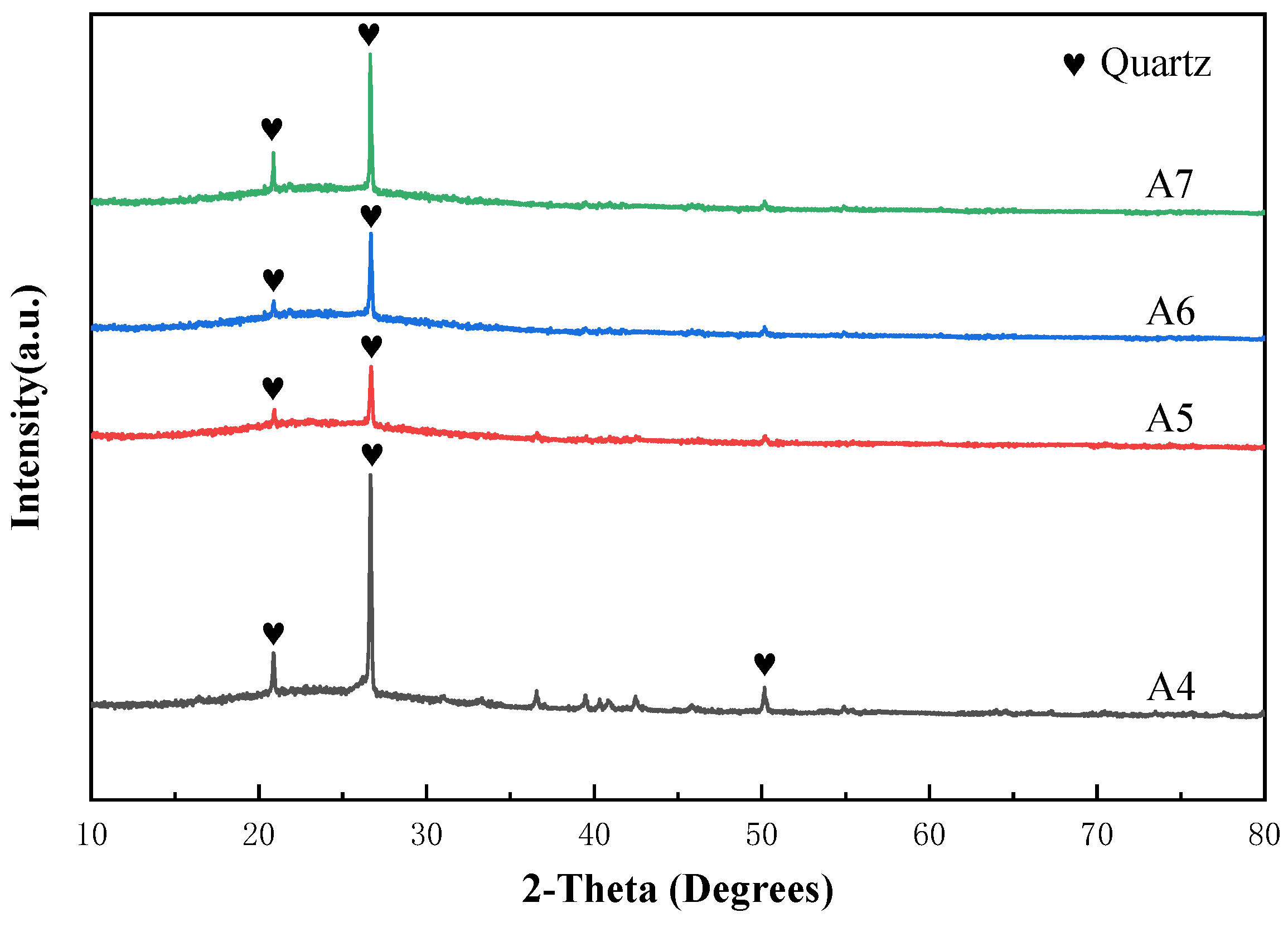
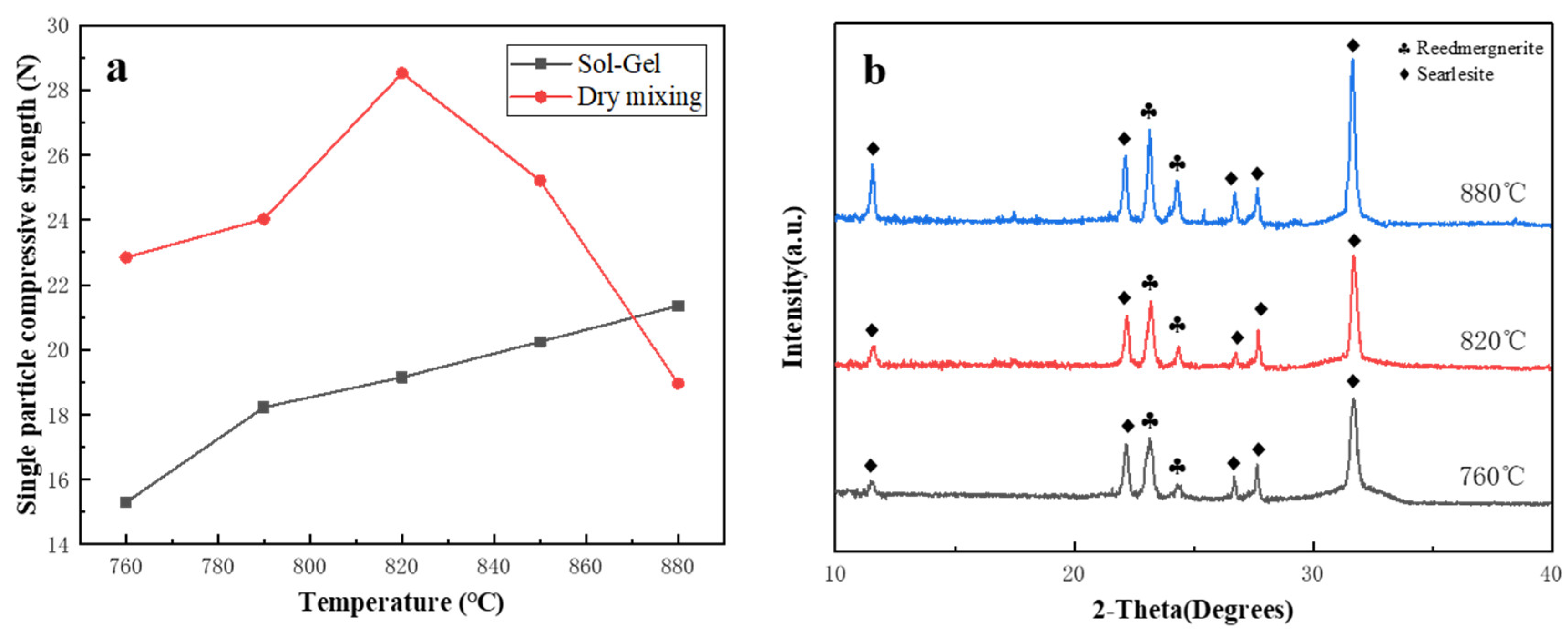
3.3. Stacked Abrasives Prepared by Dry Mixing Method
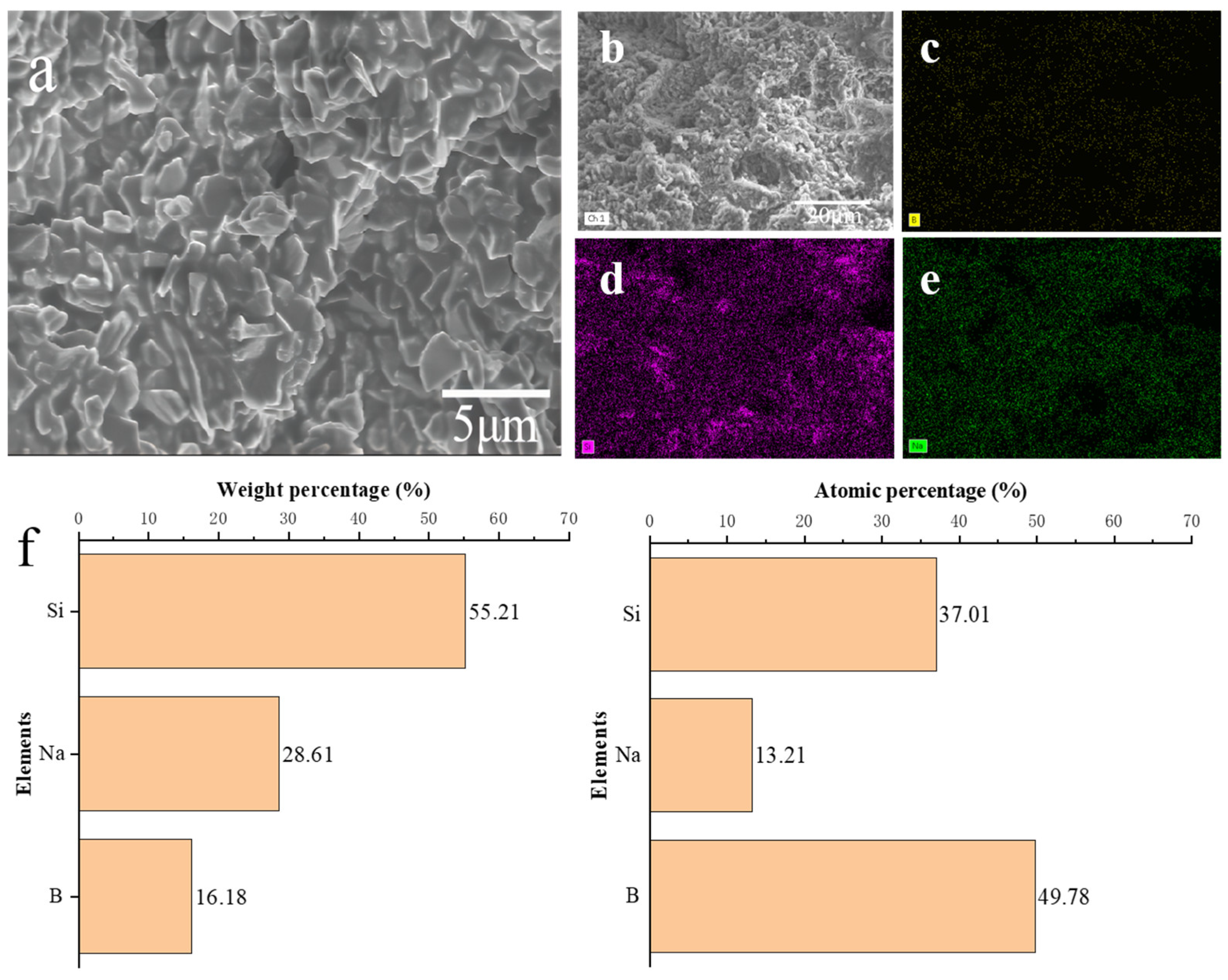
3.4. Compressive Strength and Microstructure of Diamond Stacked Abrasives
4. Conclusions
- The heat treatment temperature of the ternary system vitrified bond powder prepared by the sol-gel method should be set at 600 °C to ensure complete decomposition of NaNO3.
- When the composition ratio of the ceramic bond is A2: n(SiO2):n(B2O3):n(Na2O) = 65:23:12, the sintering temperature is 820 °C, and the amount of bond added is 40%, the prepared corundum stacked abrasives can reach the maximum single grain compressive strength which is 28.56 N, and the diamond stacked abrasives can reach the single grain compressive strength of 28.14 N.
- Vitrified bonds were prepared using the dry mixing method at the same composition point A2. However, within the temperature range of 760 °C to 880 °C, it was found to be merely a process of the crystallization of sodium borosilicate, and the strength of the agglomerated abrasives sintered at low temperatures was far inferior to that of sol-gel vitrified bonds stacked abrasives.
- This paper selects the most basic ternary oxide system as the vitrified bond. Therefore, it is possible to continue using the sol-gel method to add some intermediate oxides such as ZnO and Al2O3 to the vitrified bond with A2. This addition aims to further reduce the sintering temperature of the stacked abrasives while increasing the compressive strength. Furthermore, the effect of the addition ratio of intermediate oxides on the thermal expansion coefficient of the ceramic binder can be discussed in order to achieve the most suitable match with the abrasive micro-powder, thereby preparing stacked abrasives with excellent performance for application.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Xiao, G.; Chen, B.; Zhuo, X.; Zhao, Z.; Huang, Y. Influence mechanism of abrasive belt wear on fatigue resistance of TC17 grinding surface. Eng. Fail. Anal. 2022, 141, 106644. [Google Scholar] [CrossRef]
- Klocke, F.; Soo, S.L.; Karpuschewski, B.; Webster, J.A.; Novovic, D.; Elfizy, A.; Axinte, D.A.; Tönissen, S. Abrasive machining of advanced aerospace alloys and composites. CIRP Ann. 2015, 64, 581–604. [Google Scholar] [CrossRef]
- Caggiano, A.; Teti, R. CBN Grinding Performance Improvement in Aircraft Engine Components Manufacture. Procedia CIRP 2013, 9, 109–114. [Google Scholar] [CrossRef]
- Zheng, G.; Zhao, J.; Gao, Z.; Cao, Q. Cutting performance and wear mechanisms of Sialon–Si3N4 graded nano-composite ceramic cutting tools. Int. J. Adv. Manuf. Technol. 2012, 58, 19–28. [Google Scholar] [CrossRef]
- Thakare, M.R.; Wharton, J.A.; Wood, R.J.K.; Menger, C. Effect of abrasive particle size and the influence of microstructure on the wear mechanisms in wear-resistant materials. Wear 2012, 276–277, 16–28. [Google Scholar] [CrossRef]
- Kopac, J.; Krajnik, P. High-performance grinding—A review. J. Mater. Process. Technol. 2006, 175, 278–284. [Google Scholar] [CrossRef]
- Bai, F.; Liao, Y.; Xuan, C.; Zhang, F. Preparation of vitrified bond diamond wheel based on Bi2O3-B2O3 glass system and its grinding performance on monocrystalline silicon. Diam. Abras. Eng. 2023, 43, 432. [Google Scholar] [CrossRef]
- Ren, L.; Wang, N.; Wang, X.; Li, X.; Li, Y.; Zhang, G.; Lei, X. Modeling and analysis of material removal depth contour for curved-surfaces abrasive belt grinding. J. Mater. Process. Technol. 2023, 316, 117945. [Google Scholar] [CrossRef]
- da Silva, W.M.; Costa, H.L.; de Mello, J.D.B. Transitions in abrasive wear mechanisms: Effect of the superimposition of interactions. Wear 2011, 271, 977–986. [Google Scholar] [CrossRef]
- Kong, S.; Liu, Y.; Sun, Y. Investigation on preparation of composite vitrified bonding ultrafine diamond grinding wheel and machining of PcBN inserts. Ceram. Int. 2023, 49, 4631–4640. [Google Scholar] [CrossRef]
- Guo, B.; Kuang, H.; Liu, X.; Jiang, H.; Tu, R.; Yang, M.; Zhang, S. Exploring the influences of BaO amount on the wettability and mechanical behavior of vitrified bond diamond composites. Materials 2024, 17, 339. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xiao, B.; Wang, S. Effects of Al2O3 addition on sintering behaviour, microstructure, and physical properties of Al2O3/vitrified bond CBN composite. Ceram. Int. 2023, 49, 9173–9184. [Google Scholar] [CrossRef]
- Xia, P.; Jiang, R.; Li, Z.; Zhu, Y.; Zhai, C.; Feng, D.; Sun, P. Effect of Y2O3 on the properties of vitrified bond and vitrified diamond composites. Compos. B Eng. 2014, 67, 515–520. [Google Scholar] [CrossRef]
- Forbrigger, C.; Bauer, R.; Warkentin, A. A review of state-of-the-art vitrified bond grinding wheel grooving processes. Int. J. Adv. Manuf. Technol. 2017, 90, 2207–2216. [Google Scholar] [CrossRef]
- Wu, H.; Xu, Q.; Zhou, H.; Guan, X.; Shi, X.; Li, G. Research progress of vitrified bond CBN grinding wheel. Diam. Abras. Eng. 2023, 43, 455. [Google Scholar] [CrossRef]
- Tanaka, T.; Esaki, S.; Nishida, K.; Nakajima, T.; Ueno, K. Development and application of porous vitrified-bonded wheel with ultra-fine diamond abrasives. Key Eng. Mater. 2009, 257-258, 251–256. [Google Scholar] [CrossRef]
- Loh, Z.W.; Mohd Zaid, M.H.; Awang Kechik, M.M.; Fen, Y.W.; Yaakob, Y.; Hilmi Mayzan, M.Z.; Liza, S.; Cheong, W.M. Effect of CaF2/P2O5 ratios on physical and mechanical properties of novel CaO–Na2O–B2O3–SiO2 glasses. J. Phys. Chem. Solids 2022, 171, 110991. [Google Scholar] [CrossRef]
- Passaro, J.; Bifulco, A.; Calabrese, E.; Imparato, C.; Raimondo, M.; Pantani, R.; Aronne, A.; Guadagno, L. Hybrid hemp particles as functional fillers for the manufacturing of hydrophobic and anti-icing epoxy composite coatings. ACS Omega 2023, 8, 23596–23606. [Google Scholar] [CrossRef]
- Kessler, V.G.; Seisenbaeva, G.A. Molecular mechanisms of the metal oxide sol-gel process and their application in approaches to thermodynamically challenging complex oxide materials. J. Sol-Gel Sci. Technol. 2023, 107, 190–200. [Google Scholar] [CrossRef]
- Rex, A.; dos Santos, J.H.Z. The use of sol–gel processes in the development of supported catalysts. J. Sol-Gel Sci. Technol. 2023, 105, 30–49. [Google Scholar] [CrossRef]
- Huang, B.; Li, C.; Zhang, Y.; Ding, W.; Yang, M.; Yang, Y.; Zhai, H.; Xu, X.; Wang, D.; Debnath, S.; et al. Advances in fabrication of ceramic corundum abrasives based on sol–gel process. Chin. J. Aeronaut. 2021, 34, 1–17. [Google Scholar] [CrossRef]
- Silva, A.A.C.; Gomes, T.I.; Martins, B.D.P.; Garcia, R.B.R.; Cividanes, L.D.S.; Kawachi, E.Y. New insights in adhesive properties of hybrid epoxy-silane coatings for aluminum substrates: Effect of composition and preparation methods. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3105–3115. [Google Scholar] [CrossRef]
- Nitu; Fopase, R.; Pandey, L.M.; Srinivasan, A. Effect of systematic substitution of Na2O for SiO2 on devitrification and bioactivity of sol-gel derived 69.5SiO2-24.5CaO-6P2O5 ceramics. Mater. Chem. Phys. 2024, 313, 128731. [Google Scholar] [CrossRef]
- Sun, D.L.; Wu, A.Y.; Yin, S.T. Structure, Properties, and impedance spectroscopy of CaCu3Ti4O12 ceramics prepared by sol-gel process. J. Am. Ceram. Soc. 2008, 91, 169–173. [Google Scholar] [CrossRef]
- Fiume, E.; Migneco, C.; Verné, E.; Baino, F. Comparison between bioactive sol-gel and melt-derived glasses/glass-ceramics based on the multicomponent SiO2–P2O5–CaO–MgO–Na2O–K2O system. Materials 2020, 13, 540. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiume, E.; Miola, M.; Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 2018, 15, 841–860. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.-P.; Chen, S.-P.; Wan, L.; Li, J.-W.; Liao, M.-Y. Variations in structure and properties of vitrified bonds and vitrified diamond composites prepared by sol-gel and melting methods at different sintering temperature. Ceram. Int. 2020, 46, 21202–21210. [Google Scholar] [CrossRef]
- Ju, L.; Long, W.; Qiu, H.; Chuang, C. Preparation and properties of spherical diamond stacked abrasives by inverse microemulsion method. J. Funct. Mater. 2018, 49, 10140. [Google Scholar]
- Koroleva, O.N.; Shabunina, L.A.; Bykov, V.N. Structure of borosilicate glass according to raman spectroscopy data. Glass Ceram. 2011, 67, 340–342. [Google Scholar] [CrossRef]
- Furukawa, T.; White, W.B. Raman spectroscopic investigation of sodium borosilicate glass structure. J. Mater. Sci. 1981, 16, 2689–2700. [Google Scholar] [CrossRef]
- Karasu, B.; Demirel, İ.; Aydın, S.; Dalkıran, M.; Lik, B. Past and present approaches to borosilicate glasses. El-Cezeri 2020, 7, 940–969. [Google Scholar] [CrossRef]
- Zhang, A.J.; Li, Z.H.; Li, Z.C.; Zhu, Y.M. Preparation of microcrystalline CBN abrasive. Key Eng. Mater. 2009, 368–372, 933–935. [Google Scholar] [CrossRef]
- Cao, D.; Fang, W.-J.; Wan, L.; Liu, X.-P.; Li, J.-W.; Hong, Q.; Hu, W.-D.; Han, K. Influence of holding time on the interfacial solid solution and mechanical properties of agglomerated white fused alumina abrasives. Ceram. Int. 2022, 48, 9468–9476. [Google Scholar] [CrossRef]

| Sample Composition Points | SiO2, % | B2O3, % | Na2O, % |
|---|---|---|---|
| A1 | 67 | 19 | 14 |
| A2 | 65 | 23 | 12 |
| A3 | 63 | 27 | 10 |
| A4 | 61 | 31 | 8 |
| A5 | 59 | 33 | 8 |
| A6 | 57 | 35 | 8 |
| A7 | 55 | 37 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Liang, L.; Li, Z.; Zhu, Y. Research on Sol-Gel Synthesis of Low-Temperature Na2O-B2O3-SiO2 Vitrified Bonds and Preparation of High-Strength Stacked Abrasives Using the Molding and Crushing Method. Materials 2024, 17, 1799. https://doi.org/10.3390/ma17081799
Wang P, Liang L, Li Z, Zhu Y. Research on Sol-Gel Synthesis of Low-Temperature Na2O-B2O3-SiO2 Vitrified Bonds and Preparation of High-Strength Stacked Abrasives Using the Molding and Crushing Method. Materials. 2024; 17(8):1799. https://doi.org/10.3390/ma17081799
Chicago/Turabian StyleWang, Pei, Lingrui Liang, Zhihong Li, and Yumei Zhu. 2024. "Research on Sol-Gel Synthesis of Low-Temperature Na2O-B2O3-SiO2 Vitrified Bonds and Preparation of High-Strength Stacked Abrasives Using the Molding and Crushing Method" Materials 17, no. 8: 1799. https://doi.org/10.3390/ma17081799
APA StyleWang, P., Liang, L., Li, Z., & Zhu, Y. (2024). Research on Sol-Gel Synthesis of Low-Temperature Na2O-B2O3-SiO2 Vitrified Bonds and Preparation of High-Strength Stacked Abrasives Using the Molding and Crushing Method. Materials, 17(8), 1799. https://doi.org/10.3390/ma17081799





