Abstract
The sustainable microwave (MW) synthesis of hydroxyapatite (HAp) from decarbonized eggshells was investigated. Decarbonization of eggshells, as a natural source of calcium carbonate (CaCO3), was carried out in the current study at ambient conditions to reduce the footprint of CO2 emissions on our environment where either calcination or acidic direct treatments of eggshells produce CO2 emissions, which is a major cause for global warming. Eggshell decarbonization was carried out via the chemical reaction with sodium hydroxide (NaOH) alkaline solution in order to convert eggshell waste into calcium hydroxide (Ca(OH)2) and simultaneously store CO2 as a sodium carbonate (Na2CO3) by-product which is an essential material in many industrial sectors. The produced Ca(OH)2 was mixed with ammonium dihydrogen phosphate (NH4H2PO4) reagent at pH~11 before being subjected to MW irradiation at 2.45 GHz frequency for 5 min using 800 Watts to prepare HAp. The prepared Nano-HAp was characterized using X-ray diffraction (XRD) where the crystal size was ~28 nm using the Scherrer equation. The elongated rod-like nano-HAp crystals were characterized using scanning electron microscopy (SEM) equipped with dispersive energy X-ray spectroscopy (EDS), Fourier transform infrared spectroscopy (FTIR), and transmission electron microscopy (TEM). MW synthesis of decarbonized eggshells is considered as a sustainable and environmentally friendly route to produce promising bioceramics such as nano-HAp. Concurrently, decarbonization of eggshells offers the ability to store CO2 as a high value-added Na2CO3 material.
1. Introduction
Waste management and sustainability should be considered in all humankind activities due to their associated environmental and economic impacts. A variety of natural waste materials should be utilized more effectively via sustainable waste management routes. While it may seem that some of these waste materials are useless at certain stages, their unexploited potential application is being revealed later. Nowadays, more attention is given toward waste management in order to convert generated wastes into useful materials to avoid its associated environmental side effects. On the other side, sustainable development will be greatly enhanced via converting those wastes into high value-added products. Eggshell waste is considered as one of the most common food wastes. In 2021, it was reported that around 250,000 tons of eggshells was formed annually all over the world [1]. This worldwide reported number is expected to increase dramatically in the near future where the consumption trend of chicken eggs as relatively affordable sources of protein is going to dramatically increase its waste quantity. The significant challenges associated with that massive waste generation will be directly affecting the environment and as a result the economy. Eggshell waste material usually turns into landfill or to a low value-added material. Eggshells are mainly calcite (calcium carbonate) with about 94 wt.%. Other components of eggshells, in wt.%, could be magnesium carbonate (1%), calcium phosphate (1%) and organic debris (4%) [2]. Remarkably, eggshell waste is considered as a hazardous waste according to European Union regulations [3]. Therefore, it is of great interest to convert eggshell waste into valuable bioceramic materials such as HAp for different applications [4]. The utilization of eggshells to produce HAp bioceramics materials was investigated extensively over the years using different routes [5,6,7] via either direct calcination, acidic or alkaline treatments of eggshells without decarbonization. It is expected that such utilization will not only reduce such waste but will also convert it into a useful HAp material.
HAp is one of the most important biomaterials due to its similar structure to human bones and teeth as well as its unique ability to be integrated with tissues. It is an attractive material for many biomedical applications such as bone substitute materials in orthopedics and dentistry due to its excellent biocompatibility, bioactivity and osteoconduction properties [8,9,10,11]. HAp is an inorganic component found naturally in human hard tissues and also in other sources such as fish bones, coral, eggshells, chicken bones, etc. [12]. Stoichiometric HAp has the molecular formula of Ca5(PO4)3OH, which has a hexagonal dipyramidal crystal structure with a space group P63/m as shown in Figure 1 with the lattice parameters and unit cell volume shown in Table 1, where its unit cell has two formula units, so it is usually written as Ca10(PO4)6(OH)2 [13]. In the shown HAp unit cell, four Ca atoms (blue and white) are surrounded by nine O atoms from the phosphate moieties, while the other six Ca atoms (blue only) are surrounded by the other six O atoms from the phosphate moieties. HAp can include other traces such as phosphite ions (PO33−), chloride ions (Cl−), fluoride ions (F−) and hydroxyl ions (OH−) depending on its source [8].
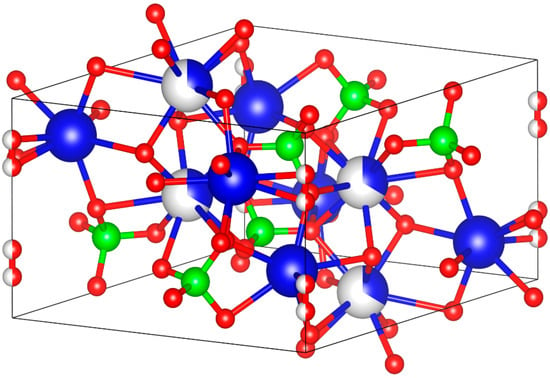
Figure 1.
Hydroxyapatite unit cell (Ca: blue; P: green; O: red).

Table 1.
Crystal structure and lattice parameters of HAp [13].
Microwave (MW) energy had been used as a promising processing tool for many materials because of its several advantages when compared to the commonly used traditional processing techniques [14,15]. MW is a powerful and significantly different tool to process several different materials with different applications where in most cases an improvement in the materials performance and properties were observed [16,17,18,19,20,21,22]. MW processing can lead to a short processing time, less energy consumption, selective heating and self-limiting reactions.
The circular economy is based on sustainability, recyclability and benefiting from waste materials via converting them into high value-added materials in a closed loop where the “3R” principles (reduce, reuse and recycle) are perfectly implemented. Based on that, waste should be turned into new products which consequently will lead to a significant increase in the sustainable economic development. Hence, industrial-scale conversion of eggshells waste into HAp material is expected to yield a higher economic value when compared with the expenses associated with the regular removal processes of such waste [23]. At the same time, this conversion will decrease in the danger of pathogen spread and will reduce the dumping costs. Hence, this approach will not only enhance the environmental profits but also will help greatly to achieve the ultimate goal of sustainable future and life.
The primary goals of this work are to decarbonize eggshell waste under ambient conditions via alkaline treatment with high conversion yield and to convert the resulting decarbonized eggshells precursor (Ca(OH)2) into a high value-added HAp material using microwave processing. Consequently, the 3R concept can be effectively implemented for a better sustainable future and life. To the best of our knowledge, almost all the reported previous studies to produce HAp from eggshells waste using different methods had never used the decarbonizations route for eggshells. Furthermore, this new proposed eggshell decarbonization route is going to safely contain and store CO2 as a valuable sodium carbonate byproduct, soda ash, that can be used in different useful industrial sectors which makes the whole process more environmentally and economically appealing.
2. Materials and Methods
Eggshell waste material was collected from KFUPM food court restaurant where it was subjected to tap water washing several times before it was later dried at 110 °C for 3 h in an oven. The dried collected eggshells waste was then crushed in an electric motor blender for 30 min before it was sieved and passed through a 100-mesh sieve (~149 µm) to give a homogenous powder. The resulting prepared powder was then used for the decarbonization route where specific amounts of eggshell powder were mixed with sodium hydroxide pellets (from Sigma Aldrich, Burlington, MA, USA) and distilled water as shown in Table 2 to achieve a maximum conversion percentage of ~96% of eggshell waste into calcium hydroxide (Ca(OH)2) and sodium carbonate (Na2CO3), as the resulting materials from this decarbonization process, as per the following chemical reaction:
(Eggshell) CaCO3(s) + 2NaOH(aq) + xH2O → Ca(OH)2 + Na2CO3·xH2O (x = 0 or 1)

Table 2.
Used eggshell decarbonization parameters with 96% conversion.
The previous parameters, shown in Table 2, were adapted from the study carried out by Hanein et al. [24] where the decarbonization of commercial CaCO3 at atmospheric temperatures and pressures were investigated and described in more detail in which different starting proportions of CaCO3, NaOH and H2O were studied to achieve different sufficient conversion percentages.
In the current study, the decarbonization reaction was accomplished under ambient conditions, using Table 2 parameters, with continuous mixing using a magnetic stirrer in a 100 mL Teflon beaker for ~10 min. A vacuum filtration system was used to separate the products via different steps. The unreacted excess NaOH was recovered using methanol alcohol from the other compounds. Later, with water preferential dissolution, the other two resulting precipitates (Ca (OH)2 and Na2CO3) were obtained and dried separately as shown in Figure 2. The obtained dried sodium carbonate, soda ash, was then stored in a closed container for future usage while the other obtained dried calcium hydroxide powder was used as the calcium source precursor for the HAp synthesis using microwave processing.
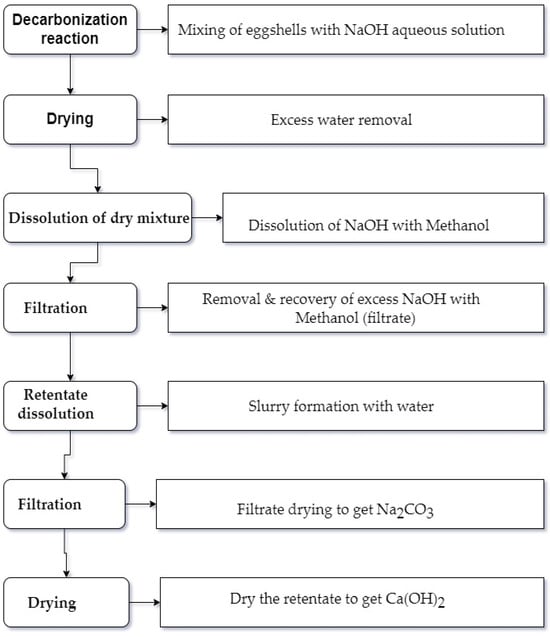
Figure 2.
Schematic chart for eggshell decarbonization process to obtain Na2CO3 and Ca(OH)2.
Mixing of 300 mL Ca(OH)2 solution obtained from the ambient condition decarbonization process along with 100 mL solution of ammonium dihydrogen phosphate (NH4H2PO4) reagent (Sigma Aldrich), as the phosphate source, was carried out drop wise with continuous stirring to maintain the resulting mixture pH at around 11 using the Orion Star A215 pH meter. The concentration of Ca2+ and PO4−3 in the mixture was 0.7775 and 0.4656 M, respectively, which correspond to the Ca/P molar ratio of 1.67, as the theoretical molar ratio of HAp, according to the following reaction:
10Ca(OH)2 + 6NH4H2PO4 → Ca10(PO4)6(OH)2 + 6NH4OH + 12H2O
Later, the resulting mixture was transferred into a 4-neck flask and directly heated using the MW synthesis unit, Sineo MAS-II Plus (Sineo Microwave Chemistry Technology Co., Ltd., Shanghai, China), where the mixture was exposed to MW radiation at 2.45 GHz for 5 min using 800 Watt. The temperature was controlled at around 95 °C with continuous stirring during MW exposure time, as shown in Figure 3. After cooling, the reaction mixture was collected, filtered through vacuum filtration system, and washed several times with distilled water before being dried in a conventional oven at 110 °C for 3 h. The obtained dried Hap powder was characterized using X-ray diffraction (XRD) with Rigaku Miniflex-II Mini-X-ray Diffraction machine (Rigaku, Tokyo, Japan) at 2° min−1 rate using Cu Kα source with 0.15406 nm wavelength, where a 10 mA current and 30 kV voltage were used. Furthermore, the two resulting precipitates, Ca (OH)2 and Na2CO3, were also characterized using XRD to confirm the successful completion of the decarbonization process under ambient conditions. FTIR analysis was performed on a Smart iTR NICOLET iS10 FTIR machine (Thermo Fisher Scientific, Waltham, MA, USA). Wavenumber 4000 cm−1 to 400 cm−1 was scanned with a resolution of 4 cm−1 and 16 scans. Furthermore, the HAp powder was also characterized using a Field emission scanning electron microscopy (FE-SEM) model (FEI Quanta 250) equipped with an EDS detector (150 mm2 Oxford detector) with accelerating voltage 200 V–30 kV, where the investigated powders were platinum-coated using a Quorum coating machine model Q150R (Quorum Technologies, Lewes, UK). A JEM-2100F field emission electron microscope (FE TEM) (JEOL, Tokyo, Japan) with 200 kV was used to investigate the obtained HAp powder where the powder was dispersed in 2-propanol/water and sonicated before testing.
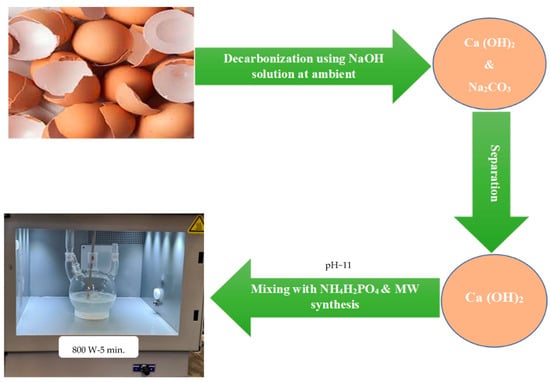
Figure 3.
Microwave synthesis setup of HAp from decarbonized eggshell.
3. Results
Figure 4 shows the XRD pattern of eggshell powder after being cleaned, crushed and sieved. The XRD peaks were indexed and identified as CaCO3, as expected, where the peaks match with the Joint Committee on Powder Diffraction Standards (JCPDS) file No. 05-0586 [25]. The crystallite size of the eggshell powder was calculated to be around 61 nm for (104) planes using the Scherrer equation as shown below:
where is the full-width at half-maximum (FWHM) of the diffraction peak in radians, L is crystalline size in (Å), k = 0.9, θ is the Bragg angle, and λ = 1.5406 Å.
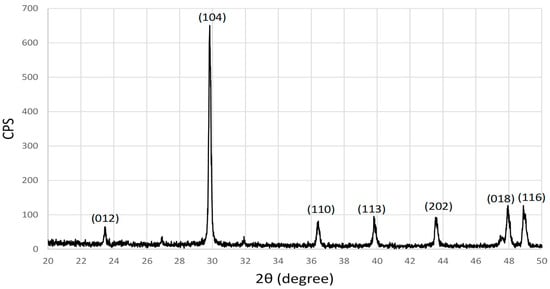
Figure 4.
XRD of the eggshell powder.
The XRD of the sodium carbonate by-product that was formed after the decarbonization process of eggshell is shown in Figure 5. The XRD analysis shows that the major peaks are matching very well with sodium carbonate compounds where a mixture of sodium carbonate (NaCO3) and hydrate sodium carbonate (Na2CO3·H2O) are formed [26]. The detected sodium carbonate (NaCO3) by-product is donated by the letter (a) in the XRD pattern with a calculated crystal size of around 48 nm for the (002) plane. It is identified as a monoclinic crystal system with space group (P21/n) where its peaks are indexed according to the card # c11-1130 of JCPDS—International Center for Diffraction Data (ICDD) [27]. On the other hand, the hydrate sodium carbonate (Na2CO3·H2O) is donated by the letter (b) in the graph with a crystal size of 42 nm for the (420) plane. Moreover, it is indexed as an orthorhombic-crystal-structure monoclinic crystal system with a space group (Pca21). Its peaks are indexed according to the card # c08-0448 of JCPDS data [28]. This confirms the successful decarbonization of eggshells without any release of carbon dioxide and simultaneously storing CO2 as useful soda ash material.
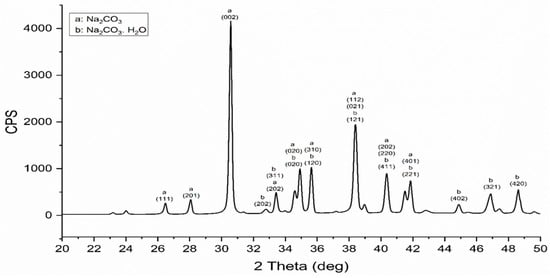
Figure 5.
XRD of sodium carbonate mixture by-products resulted from the decarbonization process.
On the other hand, the XRD pattern of the resulting calcium hydroxide by-product, Ca(OH)2, that was also formed as a results of the decarbonization process of the eggshell is shown in Figure 6. This formed calcium hydroxide (Ca(OH)2) by-product is identified as a hexagonal crystal system with space group (P-3m1) with calculated crystal size of around 15 nm for (011) plane. The detected peaks are indexed according to JCPDS file number (01-073-5492) and agree with previous work [29]. This proves the successful formation of the Ca(OH)2 by-product from the decarbonization process of eggshell waste that will be used as the main source of calcium for HAp synthesis using MW.
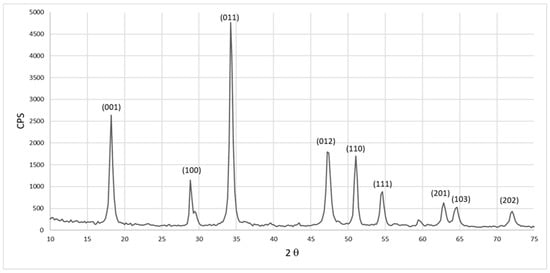
Figure 6.
XRD of calcium hydroxide by-product resulted from the decarbonization process.
The XRD pattern of microwave-synthesized HAp powder using decarbonized eggshells is shown in Figure 7, where the 2θ scan range was between 20° to 55°. The existence of the unique intense peak at 2θ = 31.75° for the (211) plane is identified, which is a characteristic peak for the apatite formation, in addition to the other remaining peaks that are related to the HAp structure. So, the XRD pattern indicates the successful formation of a single pure crystalline hexagonal HAp phase where it is indexed and identified in accordance with JCPDS file number (09–0432) and matches well with the literature [19,30,31]. The calculated crystal size for synthesized HAp was found to be around 28 nm, using the Scherrer equation on the (002) plane. So, MW synthesis of the decarbonized eggshell had successfully produced pure nano-sized HAp as revealed by the XRD pattern.
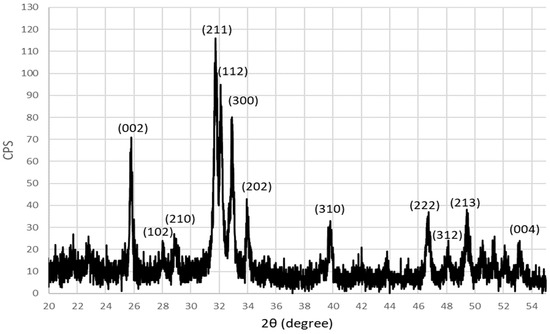
Figure 7.
XRD pattern of microwave-synthesized HAp using decarbonized eggshell.
The FTIR spectra of the synthesized HAp is shown in Figure 8. The absorption bands at around 1430 and 867 cm−1 are assigned to the presence of carbonate ions in the HAp sample which is due to the reaction of atmospheric carbon dioxide with HAp during synthesis, while the unique [PO4]−3 stretching mode is observed at 1022 cm−1. Furthermore, the other bending modes of [PO4]−3 is observed at around 565 and 585 cm−1. The peaks around 1640 and 3430 cm−1 are due to the vibration modes of the H2O molecule which matches what was reported in the literature [8,32,33,34].
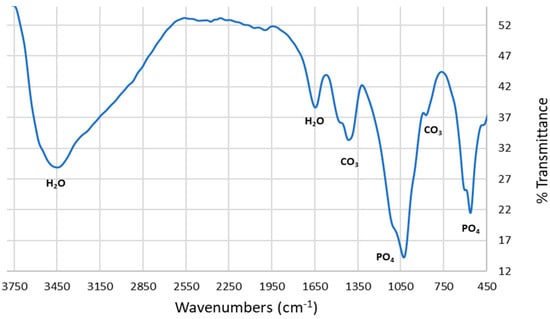
Figure 8.
FTIR spectrum of microwave-synthesized HAp using decarbonized eggshell.
SEM micrographs of the MW-synthesized HAp from the decarbonized eggshell are shown in Figure 9. The morphology of the synthesized HAp appears to be an agglomeration of individual particles with rod/needle-like shapes in the nano-size range as predicated from the Sheerer equation using the XRD pattern. The individual particles’ exact shape could not be easily recognized from SEM micrographs due to their tiny nano-size. The EDX analysis is shown in Figure 9d where the peaks of calcium, phosphorous and oxygen indicate the synthesis of HAP with the exact Ca/P ratio of 1.67 as designed in the synthesis step.
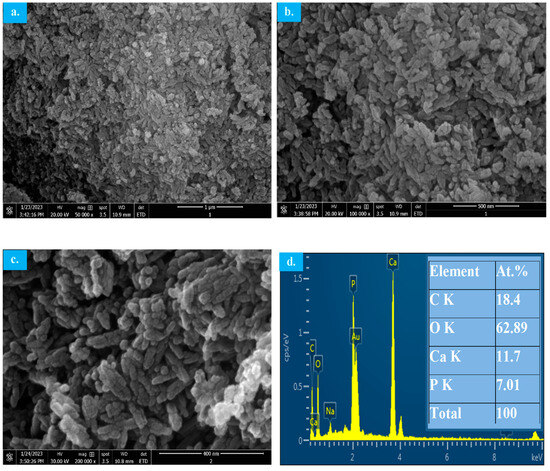
Figure 9.
SEM-EDX micrographs of MW-synthesized HAp using decarbonized eggshell. (a–c) SEM micrographs; (d) EDX.
TEM images of the MW-synthesized HAp are shown in Figure 10. The figure reveals a highly crystalline apatite structure without the presence of any other crystalline phases that is consistent with the XRD and FTIR analyses. TEM images clearly show the HAp particle shape as it is now much easier to define as highly agglomerated and elongated nano-rods/needles with an average size of around 25 ± 5 nm. The selected-area electron diffraction (SAED) pattern (Figure 10e) exhibits concentric rings that represent the presence of the polycrystalline structure of MW-synthesized HAp which is in agreement with the XRD pattern [8].
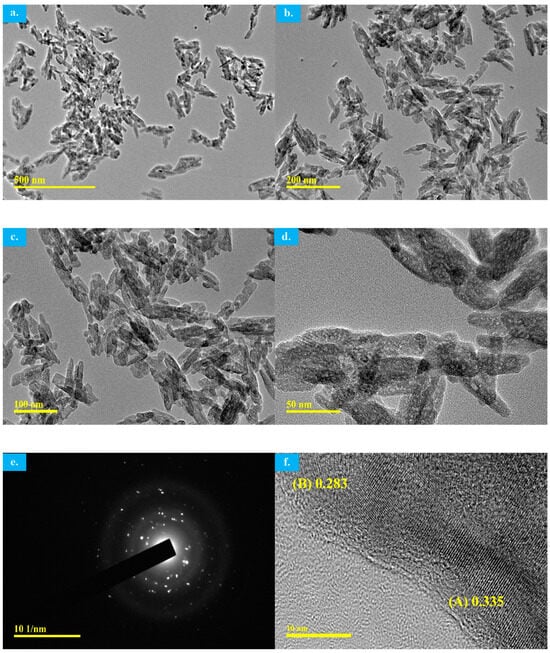
Figure 10.
(a–f) TEM images of the MW-synthesized HAp using decarbonized eggshell.
The interplanar lattice fringe spacing of the MW synthesized HAp using decarbonized eggshell were measured as in Figure 10f. At point A, the d spacing is calculated to be 0.335 which corresponds to the (0002) plane, the middle basal plane of the HAp hexagonal unit cell. This is actually in closer agreement with an earlier study investigating an apatite hexagonal cell structure where the d-spacing value of 0.34 nm for the same plane was reported [35]. Furthermore, the d spacings for the (002) plane of the crystalline particles was reported to be 0.34 nm in another investigation for a needle-shaped apatite crystal structure [36]. Point B in Figure 10f shows a measured value of 0.283 nm that closely corresponds to the figure of the lattice plane, which is also in close agreement with the same earlier study [35] where they reported d-spacings of 0.281 nm for that same lattice plane of a hexagonal apatite cell. Furthermore, the d spacing between lattice fringes of needle-shaped apatite crystal plate particles at (211) or (112) planes, the two major XRD peaks for HAp, were reported to be in the range of 0.27–0.29 nm [36] which is a closer match to the measured point B value in Figure 10f of the MW-synthesized nano-sized HAp.
4. Discussion
4.1. Decarbonization of Eggshells Waste
First, if eggshell waste has been treated via either direct calcination or direct acidic treatment, as in most of the reported works [1,3,37], to produce HAp materials from such waste, there will be a release of greenhouse emission gas (CO2) from both routes. These emissions will have a direct negative effect on the environment where the amount of greenhouse emissions is going to increase. Detailed calculations for the amount of CO2 emissions from both methods are provided below.
4.1.1. Direct Calcination of Eggshells
The detailed equation with molar mass calculation for that calcination route is shown below where it can be concluded that if around 100 g of eggshells, CaCO3, will be calcined, it will produce around 56.1 g of calcium oxide (CaO), which will be used as the Ca precursor for HAp preparation. On the other hand, the amount of CO2 emissions released from that direct calcination of eggshells method will be around 44 g.
CaCO3 (s) = CaO (s) + CO2 (g)
(100.1 g) (56.1 g) (44 g)
4.1.2. Acidic Treatment of Eggshells
Furthermore, almost a similar amount of CO2 emissions will be released to the environment if eggshells are directly treated with acids such as hydrochloric acid (HCl), as an example and as per the equation below, assuming an excess of HCl where the reaction will run until the CaCO3 is totally used up. So, the acidic treatment route of eggshells will also produce around 44.0 g (around 22.414 L) of CO2, if around 100.1 g of eggshells powder is treated with acids.
CaCO3 (s) + 2HCl (aq) = CaCl2 (s) + CO2 (g) + H2O (aq)
(100.1 g) (72.9 g) (111.0 g) (44.0 g) (18.0 g).
In conclusion, assuming using any of the two above discussed methods, if one (1) ton/day of eggshells is processed to produce HAp via either calcination or acidic treatment, CO2 gas emissions of around 440,000 g/day (440 kg/day) will be released to our environment. Consequently, both routes of HAp production will have a direct negative impact on our environment and will increase the greenhouse emissions. Its negative effects are similar to consuming around 1 barrel of oil/day, or similar to burning around 187.4 L of gasoline, or similar to burning around 220 Kg of coal/day. These previous terms and metrics were determined according to the calculations estimated using the “Greenhouse Gas Equivalencies Calculator” managed by the Environmental Protection Agency (EPA), Washington, DC, USA [38]. This online calculator tool transforms emissions data into easily comprehensible terms. With the use of this calculator, you may convert intangible data into easily understood metrics. This calculator could be very helpful when explaining a given greenhouse gas reduction plan, reduction objectives, or other actions focused on lowering greenhouse gas emissions. It is based on solid calculations and conversion factors from scientific work, data and research. As an example of those greenhouse amount calculations for one of the previously mentioned metrics, which is a barrel of oil consumed, the CO2 emissions/barrel of crude oil are calculated by the calculation example below via the multiplying of the average heat content from 1 barrel times the carbon coefficient times the fraction oxidized times the ratio (44/12) of the molecular weight of CO2 to that of carbon.
where the average heat content of crude oil is 5.80 million British thermal units (mmbtu) per barrel [39]. The average carbon coefficient of crude oil is 20.31 kg carbon per mmbtu [39]. The fraction oxidized is assumed to be 100 percent [40].
Ex: 5.80 mmbtu/barrel × 20.31 kg C/mmbtu × 44 kg CO2/12 kg C × 1 metric ton/1000 kg = 0.43 metric tons CO2/barrel
4.1.3. Decarbonization of Eggshells with NaOH
In the current work, at room temperature, the decarbonization of eggshells is carried out using NaOH to obtain Ca(OH)2, which will be used later as the calcium precursor for the HAp preparation. Furthermore, calcium carbonate by-products are also formed from that decarbonization process where the CO2 emissions are not going to occur and are actually sequestered due to the formation of that by-product, as shown in the two equations below:
10CaCO3(s) + 20NaOH(aq) + xH2O = 10Ca(OH)2 (s) + 10Na2CO3·xH2O (x = 0 or 1) (s)
(1000.9 g) (799.9 g) (740.9 g) (1059.9 g)
10Ca(OH)2 (s) + 6NH4H2PO4 (s) = Ca10(PO4)6(OH)2 (s) + 6NH4OH (aq) + 12H2O (aq)
(740.9 g) (690.2 g) (1004.6 g) (210.3 g) (216.2 g)
According to the molar mass calculation of the above equations, if around 1 kg of eggshell waste is decarbonized by the addition of 799.9 g of sodium hydroxide, it will produce 740.9 g of calcium hydroxide and 1059.9 g of sodium carbonate Na2CO3. The formation of Na2CO3 is going to store and sequestrate around 440 Kg of CO2 emissions from being released to the environment. On the other hand, that formed amount (740.9 g) of calcium hydroxide will be used to synthesize around 1 kg of the value-added HAp product after the addition of ammonium dihydrogen phosphate using microwave energy processing.
Consequently, this decarbonization route will have a positive effect on the environment and is considered as a sustainable and green synthesis route where if only one (1) ton of HAp is produced/day from that decarbonized eggshells waste route, its positive environmental effect is equivalent to carbon sequestered by around 7.3 tree seedlings grown for 10 years based on that 1 ton/day calculations.
Furthermore, if we extrapolate this HAp production process using this decarbonization route for a year (i.e., 1 ton of HAp for 365 days), this will be equivalent to carbon sequestered by around 2656 tree seedlings grown for 10 years, or similar to avoiding CO2 emissions from around 68,406 L of gasoline consumed, or similar to avoiding greenhouse emissions from around 80,283 Kg of coal burned, or similar to avoiding greenhouse emissions resulting from consuming around 372 barrels of oil. These previously discussed positive effects on the environment will be added to the expected economic gain via converting this hazard eggshell waste into high value-added products such as hydroxyapatite and soda ash that are actually used in many applications and fields.
4.2. Microwave-Assisted Synthesis of HAp from Decarbonized Eggshells
As discussed before, the decarbonized eggshell by-product Ca(OH)2, which is the calcium source, was effectively reacted with NH4H2PO4 to create nano-HAp via MW synthesis. In less time, such as 5 min only, a single phase of pure nano-sized HAp biomaterial with a crystal size of 28 nm was produced. XRD, FTIR and electron microscopy (SEM-EDX and TEM) were used to analyze the synthesized nano-HAp. XRD examination revealed the production of HAP and provided the distinctive apatite peak for 2θ values between 31.9 and 32.2. Furthermore, the FTIR spectra’s peaks show that this HAp MW-synthesized sample contains hydroxyl (OH−), phosphate (PO43−) and carbonate (CO32−) groups, indicating that the HAp was synthesized successfully. An aggregation of individual particles with rod-like particles was seen using SEM and TEM, and they were recognized as part of the distinct polycrystalline hexagonal crystal structure of HAp. With its distinct Ca/P ratio of 1.67, EDX data have verified the synthesis of HAp. FE-TEM was used to measure and identify the d-spacings of the principal lattice planes in the hexagonal HAp lattice cell and its particles. In general, the crystal size, structure and the morphology of the crystals of the MW-synthesized Hap from the decarbonized eggshell source is comparable with what was reported in the literature [1,19,30], confirming the successful preparation of HAp from that decarbonized eggshell waste.
5. Conclusions
The decarbonization process of eggshell waste was achieved successfully with a high conversion percentage using the alkaline sodium hydroxide solution treatment resulting in two (2) valuable by-products. The first one was calcium hydroxide that was used as a calcium source precursor for nano-HAp preparation using MW synthesis, and the second one was soda ash (Na2CO3). The decarbonization process will not only transfer this waste into valuable biomaterial such as nano-HAp but also will store and trap carbon dioxide as a major global warming and climate change factor into a useful by-product, soda ash (Na2CO3). In fact, soda ash global reserves are really limited and not geographically widespread, while it is being used in vast industrial activities such as ceramics, glasses, detergents and chemicals industries. This process of eggshell decarbonization is economically and environmentally appealing since almost 1 mole of CaCO3 is going to produce an almost equal amount of soda ash and the Ca(OH)2 precursor that is going to be converted later into nano-sized HAp material, as a promising biomaterial material. The decarbonization process is vital for such eggshells hazard waste, as defined by European union regulation where it will satisfy the “3R” concept of circular economy and sustainability demand for better human life. This decarbonization route is having several positive environmental metrics and effects on lowering the greenhouse emissions for better green and sustainable recycling of eggshell waste. Furthermore, MW synthesis was successfully used to prepare nano-HAp via the reaction of the decarbonized eggshell by-product, Ca(OH)2 with NH4H2PO4. A single phase of pure nano-sized HAp biomaterial with a crystal size of 28 nm was prepared in a shorter time (5 min). The prepared nano-HAp was characterized using XRD, FTIR and electron microscopy (SEM-EDX and TEM), where an agglomeration of individual particles with rod-like particles were observed and identified with their unique polycrystalline hexagonal crystal structure of HAp. EDX data had also confirmed the formation of HAp with its unique Ca/P ratio of 1.67. The d-spacings of the major lattice planes in the hexagonal HAp lattice cell and its particles were measured and identified using FE-TEM.
Funding
This research was funded by Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum and Minerals (KFUPM), grant number “INAM 2302”, and “The APC was funded by KFUPM”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
I would like to thank the Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum and Minerals (KFUPM) for their great support. I acknowledge the support given from the Interdisciplinary Research Center for Advanced Materials (IRC-AM) during this project. I am also grateful to the Mechanical Engineering Department for their kind support.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Pu’Ad, N.M.; Alipal, J.; Abdullah, H.; Idris, M.; Lee, T. Synthesis of eggshell derived hydroxyapatite via chemical precipitation and calcination method. Mater. Today Proc. 2021, 42, 172–177. [Google Scholar]
- Wu, S.C.; Tsou, H.K.; Hsu, H.C.; Hsu, S.K.; Liou, S.P.; Ho, W.F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Quina, M.J.; Soares, M.A.; Quinta-Ferreira, R. Applications of industrial eggshell as a valuable anthropogenic resource. Resour. Conserv. Recycl. 2017, 123, 176–186. [Google Scholar] [CrossRef]
- Legeros, R.Z.; Legeros, J.P. 16-Hydroxyapatite. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 367–394. [Google Scholar]
- Shi, D.; Tong, H.; Lv, M.; Luo, D.; Wang, P.; Xu, X.; Han, Z. Optimization of hydrothermal synthesis of hydroxyapatite from chicken eggshell waste for effective adsorption of aqueous Pb(II). Environ. Sci. Pollut. Res. 2021, 28, 58189–58205. [Google Scholar] [CrossRef]
- Laohavisuti, N.; Boonchom, B.; Boonmee, W.; Chaiseeda, K.; Seesanong, S. Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci. Rep. 2021, 11, 15143. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Iordache, F.; Trusca, R.; Ciocan, L.T.; Ficai, A.; Andronescu, E. Nano-hydroxyapatite vs. Xenografts: Synthesis, characterization, and in vitro behavior. Nanomaterials 2021, 11, 2289. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Devaraj, M.; Govindan, S.K.; Kattimani, V.S.; Kreedapathy, G.E. Eggshell derived mesoporous biphasic calcium phosphate for biomedical applications using rapid thermal processing. Int. J. Appl. Ceram. Technol. 2019, 16, 1932–1943. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; El-Fattah, A.A.; Kandil, S. Microwave-assisted preparation of Nano-hydroxyapatite for bone substitutes. Ceram. Int. 2016, 42, 3725–3744. [Google Scholar] [CrossRef]
- Hassan, M.N.; Mahmoud, M.M.; Link, G.; El-Fattah, A.A.; Kandil, S. Sintering of naturally derived hydroxyapatite using high frequency microwave processing. J. Alloys Compd. 2016, 682, 107–114. [Google Scholar] [CrossRef]
- Targonska, S.; Szyszka, K.; Watras, A.; Wiglusz, R.J. Influence of the fluorine ion content on luminescence properties of the EuII+/III+-doped silicate-substituted apatite. J. Alloys Compd. 2022, 911, 164985. [Google Scholar] [CrossRef]
- Muthu, D.; Kumar, G.S.; Kattimani, V.S.; Viswabaskaran, V.; Girija, E.K. Optimization of a lab scale and pilot scale conversion of eggshell biowaste into hydroxyapatite using microwave reactor. Ceram. Int. 2020, 46, 25024–25034. [Google Scholar] [CrossRef]
- Ardanova, L.I.; Get’man, E.I.; Loboda, S.N.; Prisedsky, V.V.; Tkachenko, T.V.; Marchenko, V.I.; Antonovich, V.P.; Chivireva, N.A.; Chebishev, K.A.; Lyashenko, A.S. Isomorphous Substitutions of Rare Earth Elements for Calcium in Synthetic Hydroxyapatites. Inorg. Chem. 2010, 49, 10687–10693. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Folz, D.C.; Folgar, C.; Mahmoud, M.M. Microwave Solutions for Ceramic Engineers; Wiley-American Ceramic Society: Hoboken, NJ, USA, 2006. [Google Scholar]
- Singh, C.; Khanna, V.; Singh, S. Sustainability of microwave heating in materials processing technologies. Mater. Today Proc. 2023, 73, 241–248. [Google Scholar] [CrossRef]
- Alias, N.; Ahmad Zaini, M.A.; Kamaruddin, M.J. Microwave heating rate and dielectric properties of some agricultural wastes. Nord. Pulp Pap. Res. J. 2023, 38, 1–7. [Google Scholar] [CrossRef]
- Siddique, I.J.; Salema, A.A.; Antunes, E.; Vinu, R. Technical challenges in scaling up the microwave technology for biomass processing. Renew. Sustain. Energy Rev. 2022, 153, 111767. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Nyambura, S.M.; Wang, J.; Li, C.; Zhu, X.; Feng, X.; Wang, Y. Food waste pyrolysis by traditional heating and microwave heating: A review. Fuel 2022, 324, 124574. [Google Scholar] [CrossRef]
- Khalid, M.; Jikan, S.S.B.; Adzila, S.; Murni, Z.; Badarulzaman, N.A.; Rosley, R.; Hameed, M.U. Synthesis and characterizations of hydroxyapatite using precursor extracted from chicken egg shell waste. Biointerface Res. Appl. Chem. 2022, 12, 5663–5671. [Google Scholar]
- Nuñez, N.; Raineri, M.; Troiani, H.; Tobia, D.; Zysler, R.; Lima, E.; Winkler, E. Zinc ferrite nanoparticles embedded in hydroxyapatite for magnetic hyperthermia and sensitive to ionizing radiation. J. Alloys Compd. 2022, 920, 165887. [Google Scholar] [CrossRef]
- André, R.S.; Paris, E.; Gurgel, M.; Rosa, I.; Paiva-Santos, C.; Li, M.; Varela, J.; Longo, E. Structural evolution of Eu-doped hydroxyapatite nanorods monitored by photoluminescence emission. J. Alloys Compd. 2012, 531, 50–54. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Locs, J. Novel preparation route of stable amorphous calcium phosphate nanoparticles with high specific surface area. J. Alloys Compd. 2017, 700, 215–222. [Google Scholar] [CrossRef]
- Mignardi, S.; Archilletti, L.; Medeghini, L.; De Vito, C. Valorization of Eggshell Biowaste for Sustainable Environmental Remediation. Sci. Rep. 2020, 10, 2436. [Google Scholar] [CrossRef]
- Hanein, T.; Simoni, M.; Woo, C.L.; Provis, J.L.; Kinoshita, H. Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate. Energy Environ. Sci. 2021, 14, 6595–6604. [Google Scholar] [CrossRef]
- Kumar, G.S.; Thamizhavel, A.; Girija, E.K. Microwave conversion of eggshells into flower-like hydroxyapatite nanostructure for biomedical applications. Mater. Lett. 2012, 76, 198–200. [Google Scholar] [CrossRef]
- Harabor, A.; Rotaru, P.; Harabor, N.A. Two phases in a commercial anhydrous sodium carbonate by air contact. Ann. Univ. Craiova Phys. 2013, 23, 79–88. [Google Scholar]
- Brouns, E.; Visser, J.; De Wolff, P. An anomaly in the crystal structure of Na2CO3. Acta Crystallogr. 1964, 17, 614. [Google Scholar] [CrossRef]
- Withers, R.; Wilson, J. An examination of the formation and characteristics of charge-density waves in inorganic materials with special reference to the two-and one-dimensional transition-metal chalcogenides. J. Phys. C Solid. State Phys. 1986, 19, 4809. [Google Scholar] [CrossRef]
- Khachani, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci. 2014, 5, 615–624. [Google Scholar]
- Sari, Y.W.; Saputra, A.; Bahtiar, A.; Nuzulia, N. Effects of microwave processing parameters on the properties of nanohydroxyapatite: Structural, spectroscopic, hardness, and toxicity studies. Ceram. Int. 2021, 47, 30061–30070. [Google Scholar] [CrossRef]
- Kalbarczyk, M.; Szcześ, A. Potential biomedical application of calcium phosphates obtained using eggshells as a biosource of calcium at different initial pH values. Ceram. Int. 2021, 47, 33687–33696. [Google Scholar] [CrossRef]
- Kalita, S.J.; Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C-Mater. Biol. Appl. 2010, 30, 295–303. [Google Scholar] [CrossRef]
- Agalya, P.; Kumar, G.S.; Prabu, K.; Cholan, S.; Karunakaran, G.; Hakami, J.; Shkir, M.; Ramalingam, S. One-pot ultrasonic-assisted synthesis of magnetic hydroxyapatite nanoparticles using mussel shell biowaste with the aid of trisodium citrate. Ceram. Int. 2022, 48, 28299–28307. [Google Scholar] [CrossRef]
- Ashokan, A.; Rajendran, V.; Kumar, T.S.; Jayaraman, G. Eggshell derived hydroxyapatite microspheres for chromatographic applications by a novel dissolution-precipitation method. Ceram. Int. 2021, 47, 18575–18583. [Google Scholar] [CrossRef]
- Cuisinier, F.; Bres, E.F.; Hemmerle, J.; Voegel, J.C.; Frank, R.M. Transmission electron microscopy of lattice planes in human alveolar bone apatite crystals. Calcif. Tissue Int. 1987, 40, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.I.; Lee, K.; Outslay, M.; Kohn, D. Ultrastructural analyses of nanoscale apatite biomimetically grown on organic template. J. Mater. Res. 2008, 23, 478–485. [Google Scholar] [CrossRef]
- Ibrahim, A.R.; Wei, W.; Zhang, D.; Wang, H.; Li, J. Conversion of waste eggshells to mesoporous hydroxyapatite nanoparticles with high surface area. Mater. Lett. 2013, 110, 195–197. [Google Scholar] [CrossRef]
- EPA. Greenhouse Gas Equivalencies Calculator. 2024. Available online: https://www.epa.gov/energy/greenhouse-gas-equivalencies-calculator (accessed on 30 March 2024).
- U.S. Environmental Protection Agency, Inventory of U.S. Greenhouse Gas Emissions and Sinks. 1990–2021. Annex 2 (Methodology for Estimating CO2 Emissions from Fossil Fuel Combustion), Table A-29. In Carbon Content Coefficients and Underlying Data for Petroleum Products; EPA: Washington, DC, USA, 2023; p. 760. [Google Scholar]
- IPCC. Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change—IPCC: Geneva, Switzerland, 2006. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).