Optimization of Bilayer Resistive Random Access Memory Based on Ti/HfO2/ZrO2/Pt
Abstract
:1. Introduction
2. Simulation Methods
2.1. Electrical–Thermal Coupling Physical Model
2.1.1. Electrical Conduction Model
2.1.2. Joule Heat Model
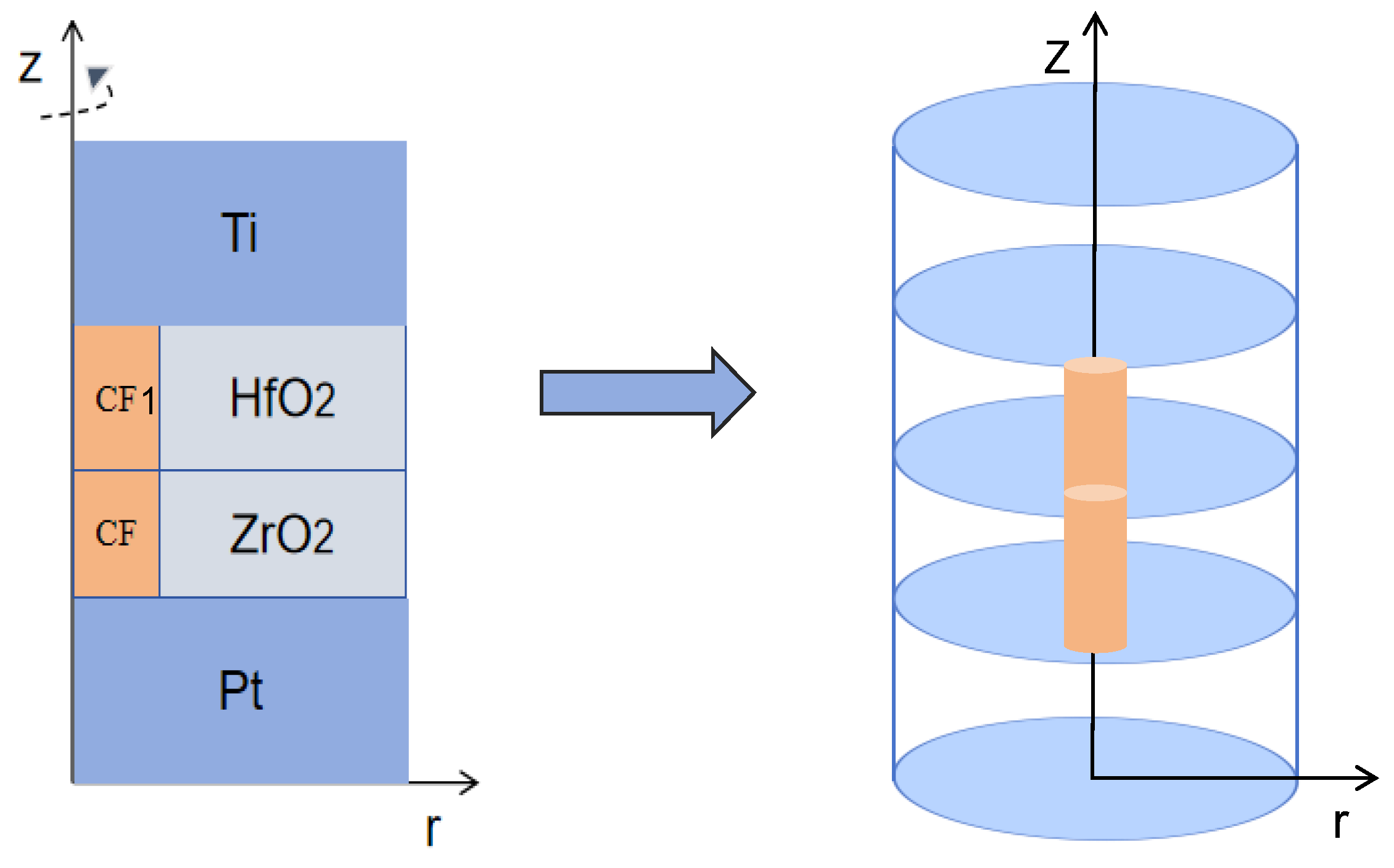
2.2. Model Establishment in COMSOL
Geometric Model
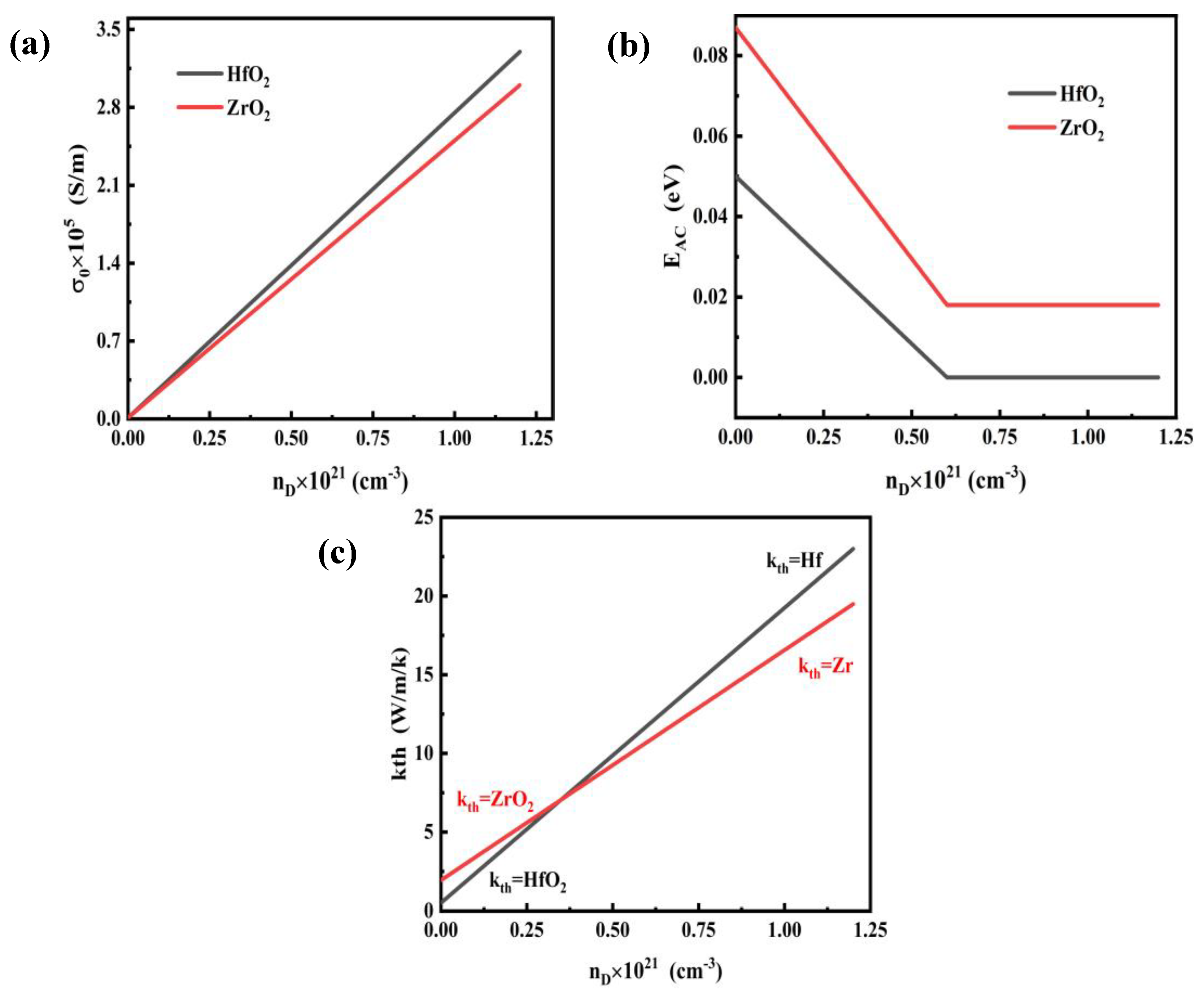
2.3. Definition of Material Properties
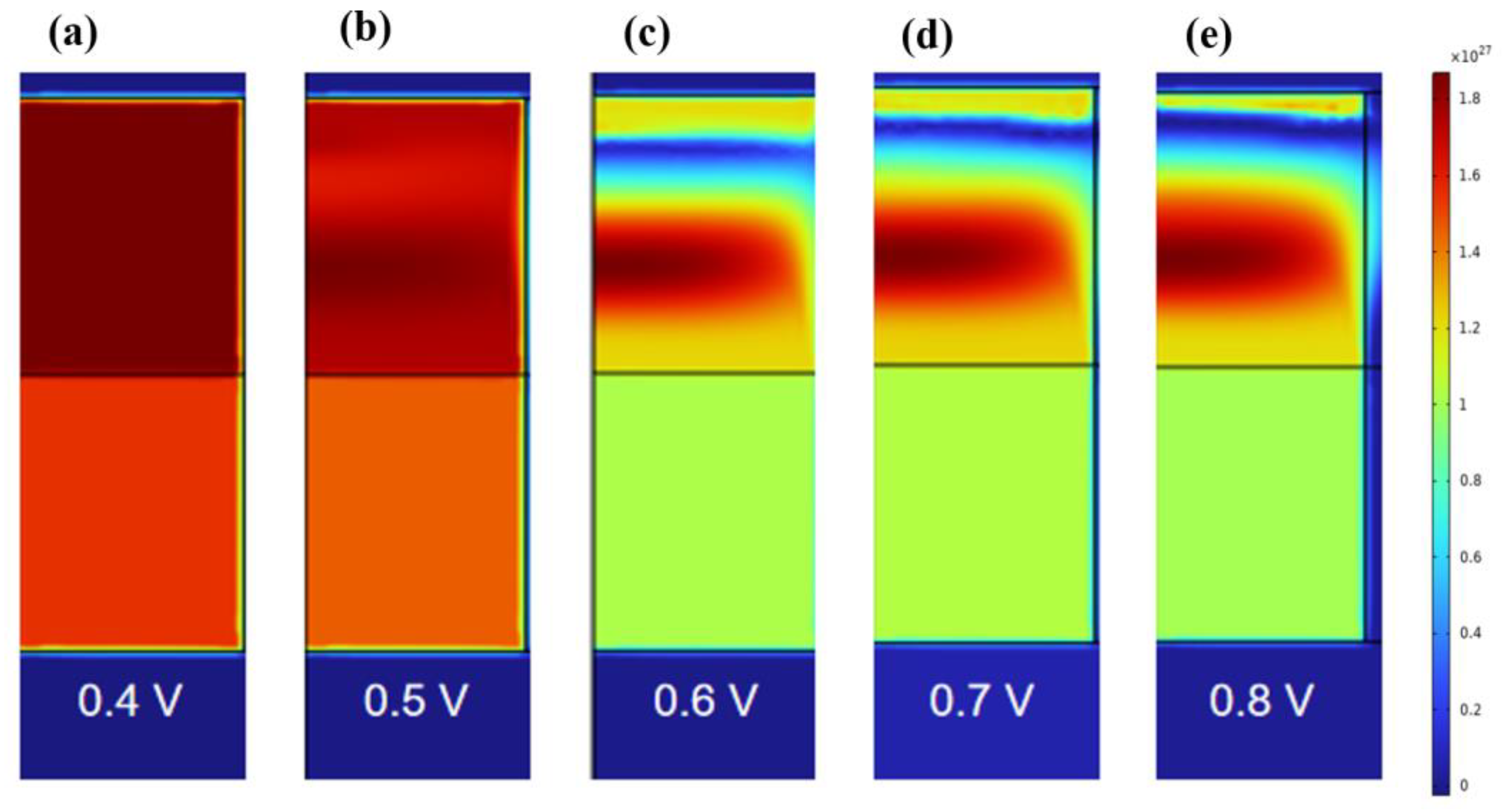
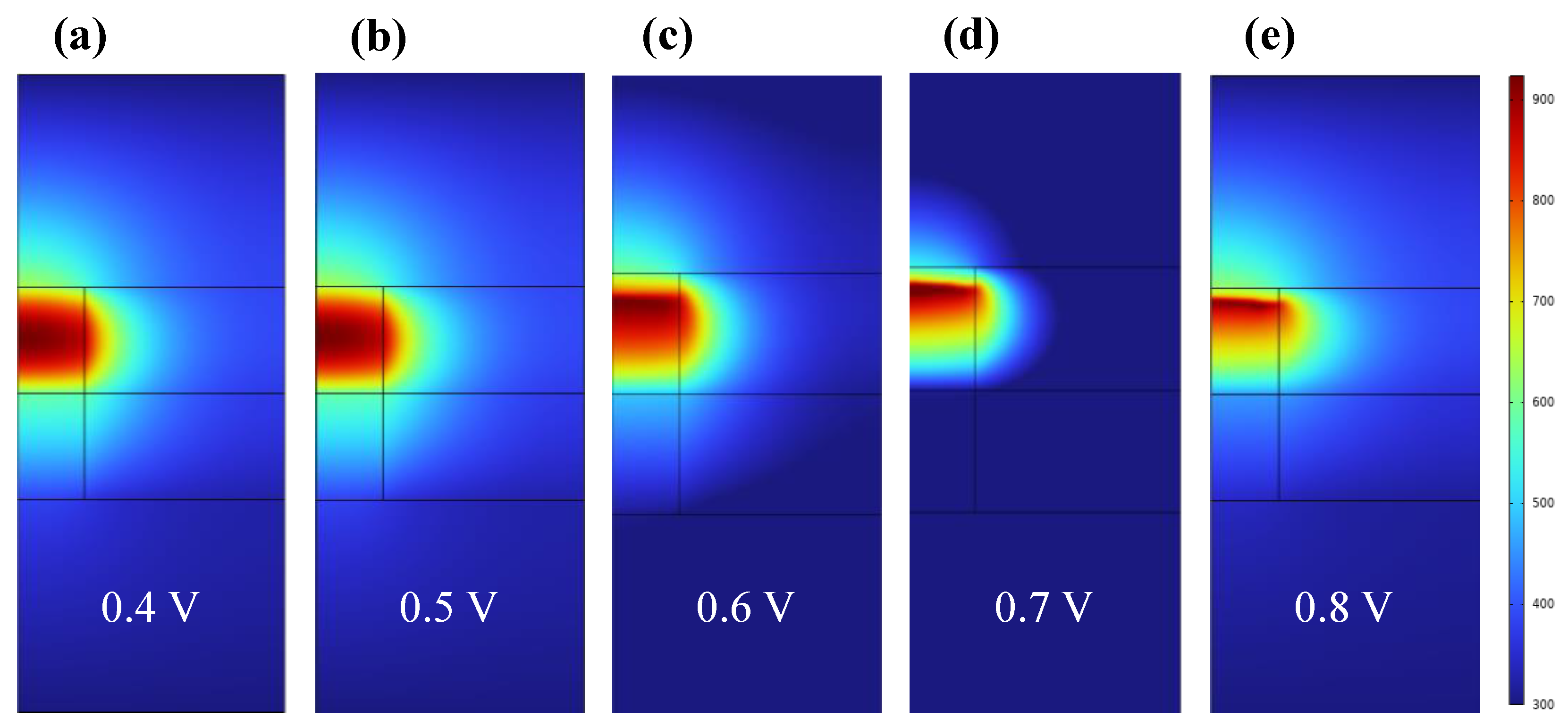
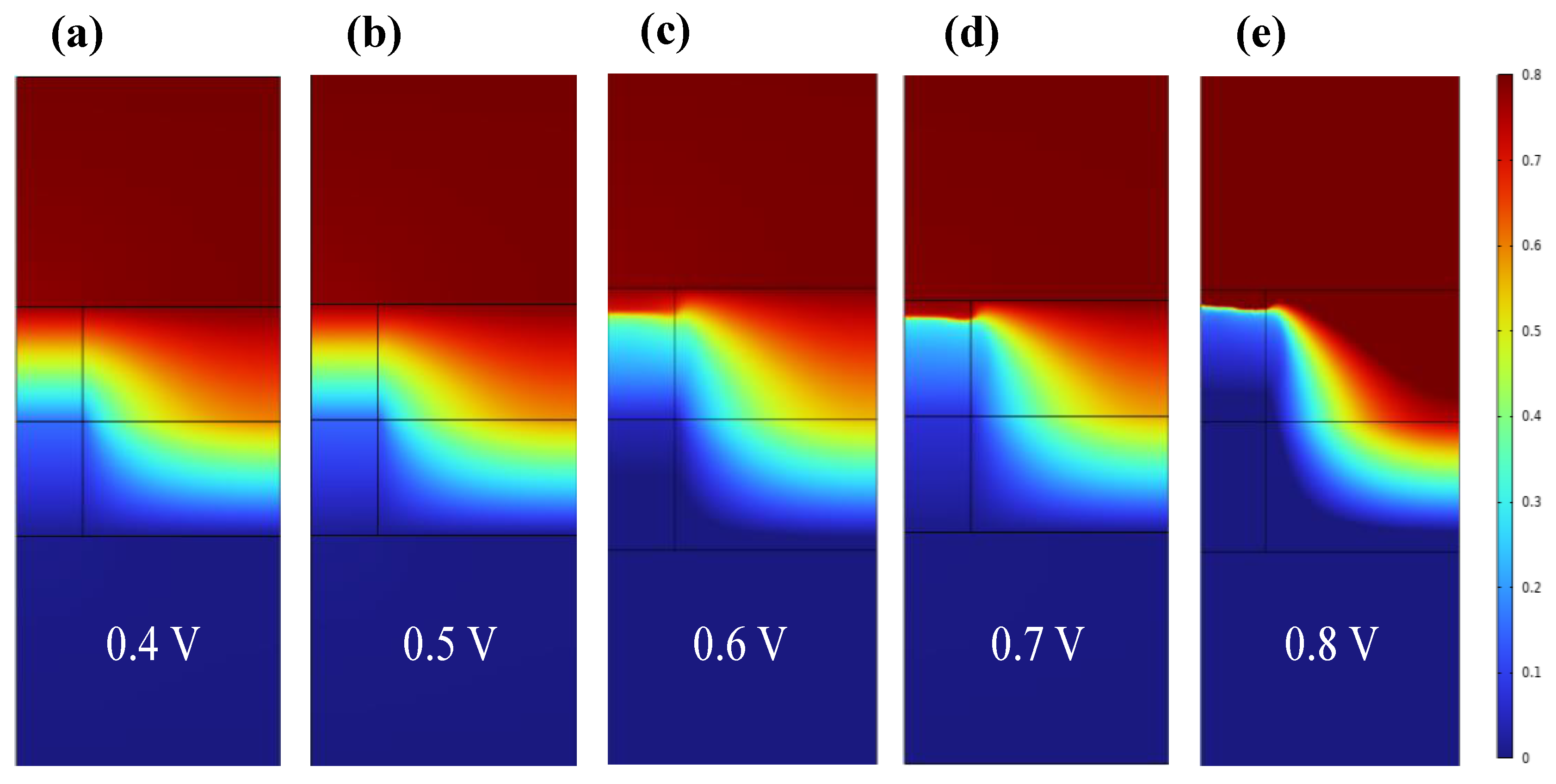
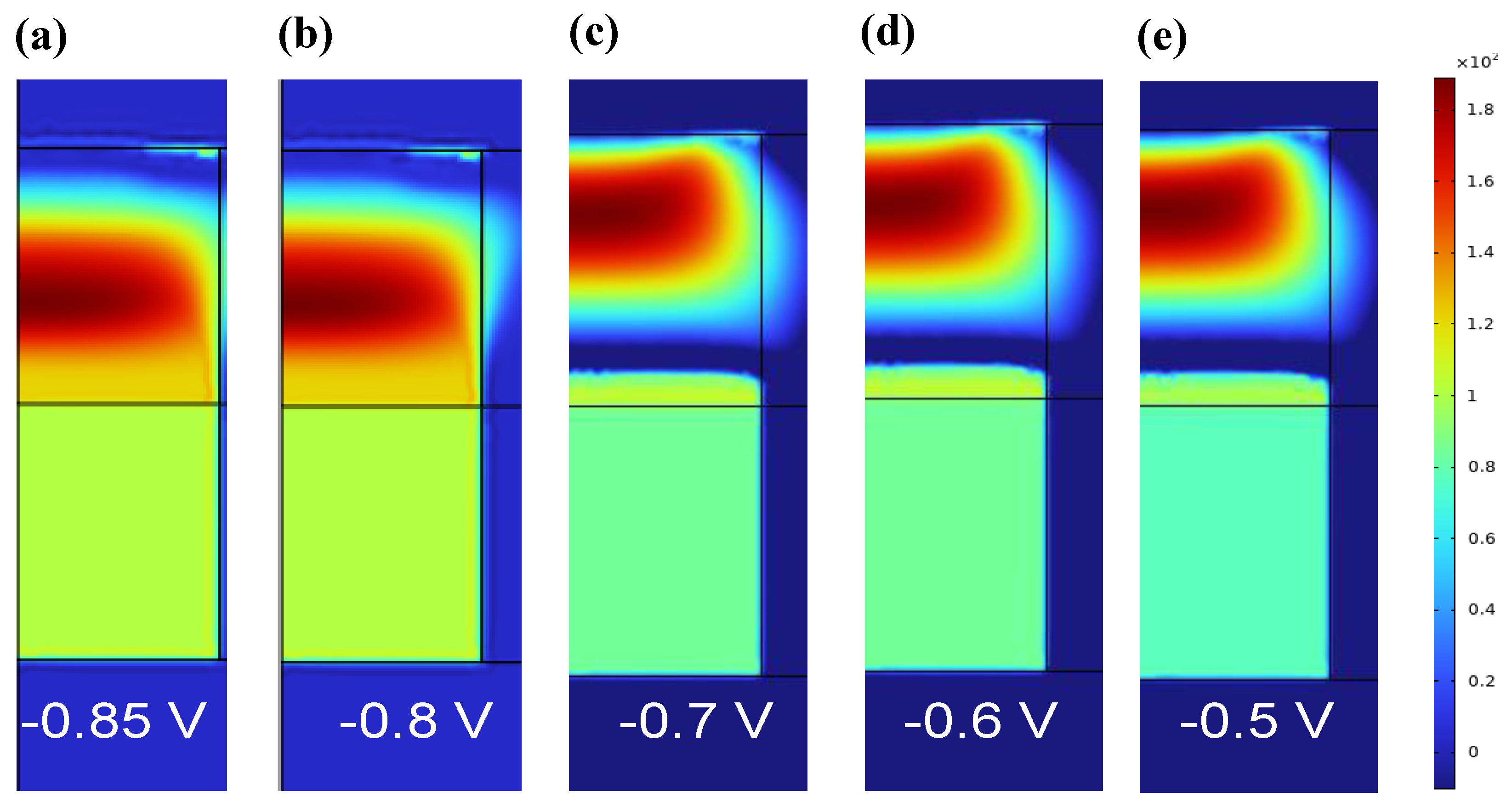
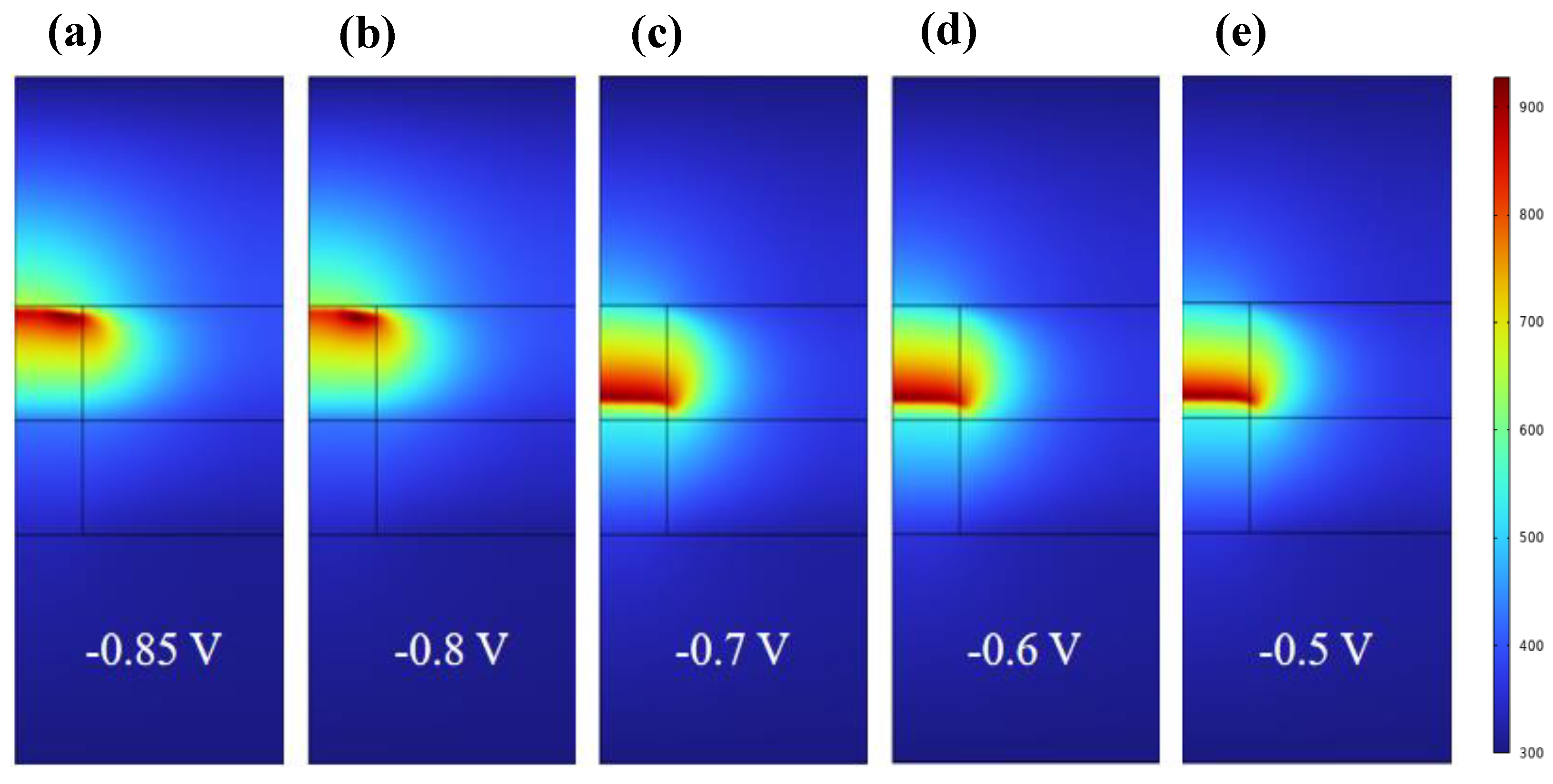
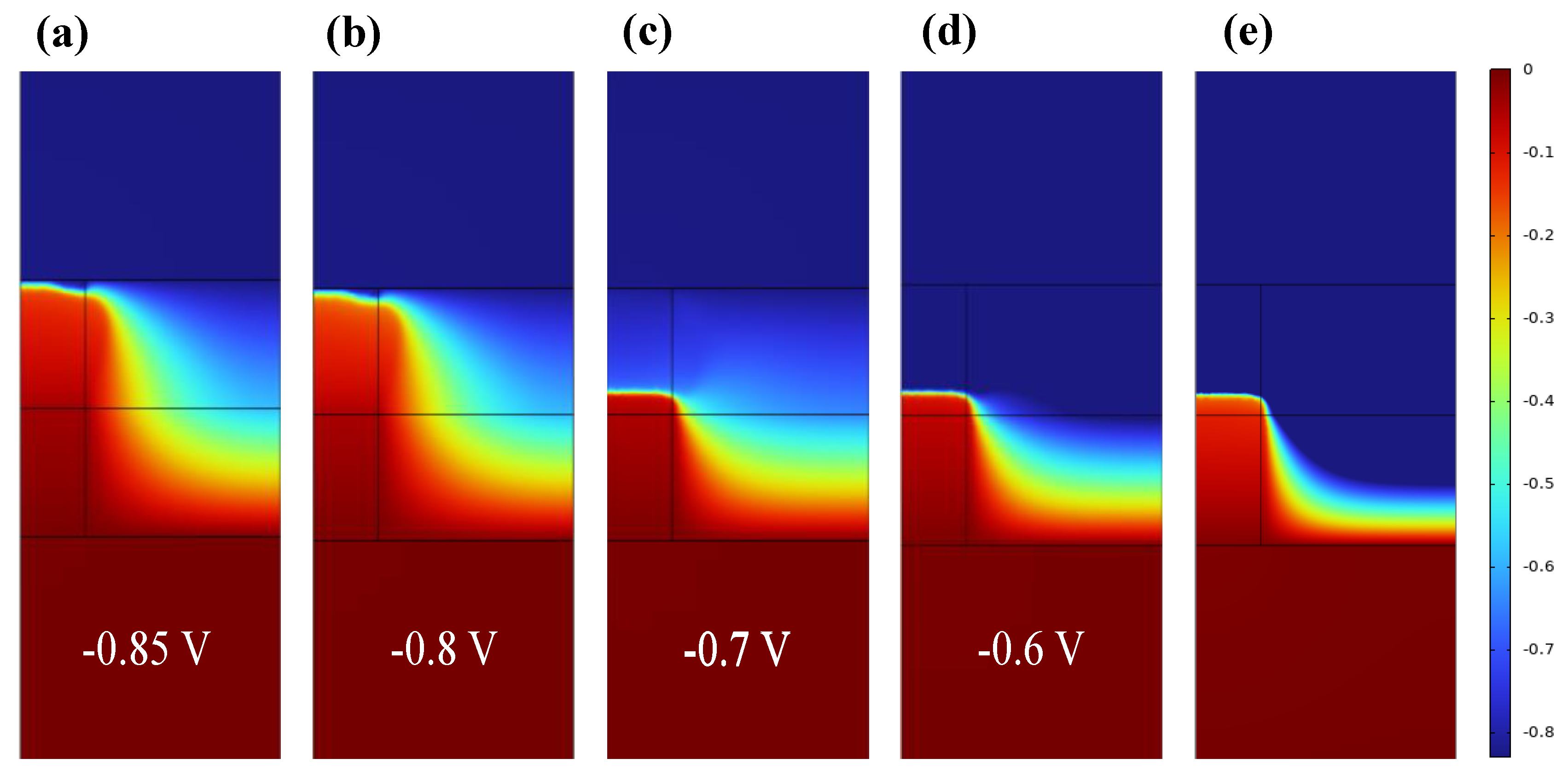
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, Z.; Zhao, C.; Qi, Y.; Xu, W.; Liu, Y.; Mitrovic, I.Z.; Yang, L.; Zhao, C. Advances of RRAM Devices: Resistive Switching Mechanisms, Materials and Bionic Synaptic Application. Nanomaterials 2020, 10, 1437. [Google Scholar] [CrossRef]
- Yin, S.; Sun, X.; Yu, S. High-Throughput In-Memory Computing for Binary Deep Neural Networks with Monolithically Integrated RRAM and 90-nm CMOS. IEEE Trans. Electron Devices 2020, 67, 4185–4192. [Google Scholar] [CrossRef]
- Kim, D.; Shin, J.; Kim, S. Resistive switching characteristics of ZnO-based RRAM on silicon substrate. Metals 2021, 11, 1572. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Song, G.; Zhang, C.W. Study on Multilevel Resistive Switching Behavior with Tunable ON/OFF Ratio Capability in Forming-Free ZnO QDs-Based RRAM. IEEE Trans. Electron Devices 2020, 67, 4884–4890. [Google Scholar] [CrossRef]
- Yi, Y.; Weng, Y.; Ding, X.; Feng, Y.; Liu, L. Pulsed Operation on HfO2/Al2O3 RRAM Device as Electronic Dendrite. In Proceedings of the 2021 IEEE International Conference on Integrated Circuits, Technologies and Applications (ICTA), Zhuhai, China, 24–26 November 2021; pp. 96–98. [Google Scholar]
- Pebay-Peyroula, F.; Dalgaty, T.; Vianello, E. Entropy source characterization in HfO2 RRAM for TRNG applications. In Proceedings of the 2020 15th Design & Technology of Integrated Systems in Nanoscale Era (DTIS), Marrakech, Morocco, 1–3 April 2020; pp. 1–2. [Google Scholar]
- Tang, L.; Maruyama, H.; Han, T.; Nino, J.C.; Chen, Y.; Zhang, D. Resistive switching in atomic layer deposited HfO2/ZrO2 nanolayer stacks. Appl. Surf Sci. 2020, 515, 146015. [Google Scholar] [CrossRef]
- Nauman, M.M.; Zulfikre Esa, M.; Zaini, J.H.; Iqbal, A.; Bakar, S.A. Zirconium Oxide based memristors fabrication via Electrohydrodynamic Printing. In Proceedings of the 2020 IEEE 11th International Conference on Mechanical and Intelligent Manufacturing Technologies (ICMIMT), Cape town, South Africa, 20–22 January 2020; pp. 167–171. [Google Scholar]
- Dwivedi, A. Effect of Temperature Induced Phase Variation in ALD TiO2 Dielectric on the Switching Behaviour of RRAM Devices. In Proceedings of the 2022 IEEE International Conference on Emerging Electronics (ICEE), Bangalore, India, 11–14 December 2022; pp. 1–4. [Google Scholar]
- Youssef, A.N.; Jagath, A.L.; Thulasiraman, N.K.; Almurib, H.A.F. Effect of Sneak Path Current in TiOx/HfOx Based 1S1R RRAM Crossbar Memory Array. In Proceedings of the 2021 IEEE 19th Student Conference on Research and Development (SCOReD), Kota Kinabalu, Malaysia, 23–25 November 2021; pp. 267–272. [Google Scholar]
- Soumi, S.; Subhradeep, P.; Sounak, R.; Parikshit, S.; Surya, S. Experimental Demonstration of CeO2-Based Tunable Gated Memristor for RRAM Applications. ACS Appl. Electron. Mater. 2023, 5, 6392–6400. [Google Scholar]
- Lan, T.T.B.; Li, Y.-T.; Sun, A.-C.A.; Lu, H.-C.; Wang, S.-F. Chemical defect-dependent resistive switching characterization in CeO2 thin films. Mater. Sci. Semicond. Process. 2022, 137, 106177. [Google Scholar] [CrossRef]
- Pligovka, A. Reflectant photonic crystals produced via porous-alumina-assisted-anodizing of Al/Nb and Al/Ta systems. Surf. Rev. Lett. 2021, 28, 2150055. [Google Scholar] [CrossRef]
- Tsai, M.H.; Shih, C.J.; Chang, C.W.; Chu, Y.T.; Wu, Y.S.; Huang, C.L. Resistive switching properties of Mn-doped amorphous Nb2O5 thin films for resistive RAM application. Mater. Sci. Semicond. Process. 2022, 152, 107059. [Google Scholar] [CrossRef]
- Lata, L.K.; Jain, P.K. Bipolar resistive switching behavior and endurance in RF-sputtered bilayer HfO2/ZrO2 resistive random access memory. Emergent Mater. 2023, 6, 1979–1989. [Google Scholar] [CrossRef]
- Arun, N.; Nageswara Rao, S.V.S.; Pathak, A.P. Effects of Bottom Electrode Materials on the Resistive Switching Characteristics of HfO2-Based RRAM Devices. J. Electron. Mater. 2023, 52, 1541–1551. [Google Scholar] [CrossRef]
- Lee, J. Effect of ZrOx/HfOx bilayer structure on switching uniformity and reliability in nonvolatile memory applications. Appl. Phys. Lett. 2010, 97, 172105. [Google Scholar] [CrossRef]
- Ismail, M.; Batool, Z.; Mahmood, K.; Rana, A.M.; Yang, B.D.; Kim, S. Resistive switching characteristics and mechanism of bilayer HfO2/ZrO2 structure deposited by radio-frequency sputtering for nonvolatile memory. Results Phys. 2020, 18, 103275. [Google Scholar] [CrossRef]
- Szot, K. Switching the electrical resistance of individual dislocations in single-crystalline SrTiO3. Nat. Mater. 2009, 5, 312–320. [Google Scholar] [CrossRef]
- Nian, Y.B. Evidence for an oxygen diffusion model for the electric pulse induced resistance change effect in transition-metal oxides. Phys. Rev. Lett. 2008, 98, 146403. [Google Scholar] [CrossRef]
- Yang, J.J.; Pickett, M.D.; Xuema, L. Memristive switching mechanism for metal/oxide/metal nanodevices. Nat. Nanotechnol. 2008, 3, 429–433. [Google Scholar] [CrossRef]
- Ielmini, D. Modeling the Universal SET/RESET Characteristics of Bipolar RRAM by Field-and Temperature-Driven Filament Growth. IEEE Trans. Electron. Device 2011, 58, 4309–4317. [Google Scholar] [CrossRef]
- Larentis, S.; Nardi, F. Resistive Switching by Voltage-Driven Ion Migration in Bipolar RRAM-Part II: Modeling. IEEE Trans. Electron Device 2012, 59, 2468–2475. [Google Scholar] [CrossRef]
- Yu, S.; Wu, Y.; Wong, H.S.P. Investigating the switching dynamics and multilevel capability of bipolar metal oxide resistive switching memory. Appl. Phys. Lett. 2011, 98, 103514. [Google Scholar] [CrossRef]
- Menzel, S.; Waters, M.; Marchewka, A. Origin of the ultra-nonlinar switching kinetics in oxide-based resistive switches. Adv. Funct. Mater. 2011, 21, 4487–4492. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.J.; Kim, K.M. Physical electro-thermal model of resistive switching in bi-layered resistance-change memory. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Huang, P.; Liu, X.Y.; Li, W.H. A Physical Based Analytic Model of RRAM Operation for Circuit Simulation. In Proceedings of the International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 10–13 December 2012; pp. 26.6.1–26.6.4. [Google Scholar]
- Kim, S.; Choi, S.H.; Lu, W. Comprehensive physical model of dynamic resistive switching in an oxide memristor. ACS Nano 2014, 8, 2369–2376. [Google Scholar] [CrossRef]
- Basnet, P.; Pahinkar, D.G.; West, M.P.; Perini, C.J.; Graham, S.; Vogel, E.M. Substrate dependent resistive switching in amorphous-HfOx memristors: An experimental and computational investigation. J. Mater. Chem. C 2020, 8, 5092–5101. [Google Scholar] [CrossRef]
- Li, H.T.; Huang, P.; Gao, B. A Spice model of resistive random access memory for large-scale memory array simulation. IEEE Electron. Device Lett. 2014, 35, 211–213. [Google Scholar] [CrossRef]
- Lin, J.; Liu, H.; Wang, S.; Zhang, S. Modeling and Simulation of Hafnium Oxide RRAM Based on Oxygen Vacancy Conduction. Crystals 2021, 11, 1462. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, M.K.; Gill, G.S.; Mukherjee, S. 3-D Physical Electro-Thermal Modeling of Nanoscale Y2O3 Memristors for Synaptic Application. IEEE Trans. Electron. Devices 2022, 69, 3124–3129. [Google Scholar] [CrossRef]
- Bature, U.I.; Nawi, I.M.; Khir, M.H.M. Analysis of thermodynamic resistive switching in ZnO-based RRAM. Phys. Scr. 2020, 98, 035020. [Google Scholar] [CrossRef]
- Nandi, S.K. Resistive Switching in Transition Metal Oxides for Integrated Non-volatile Memory. Nanotechnology 2017, 11, 28–36. [Google Scholar]
- Bonanos, N.; Butler, E.P. Ionic conductivity of monoclinic and tetragonal yttria-zirconia single crystals. J. Mater. Sci. Lett. 1985, 4, 561–564. [Google Scholar] [CrossRef]
- Niraula, D.; Karpov, V. Numerical modeling of resistive switching in RRAM device. In Proceedings of the 2017 COMSOL Conference in Boston, Boston, MA, USA, 8–10 October 2017. [Google Scholar]
- Crema, A.P.S.; Istrate, M.C.; Silva, A. Ferroelectric Orthorhombic ZrO2 Thin Films Achieved Through Nanosecond Laser Annealing. Adv. Sci. 2023, 10, 2207390. [Google Scholar] [CrossRef]
- Ismail, M.; Talib, I.; Rana, A.M.; Ahmed, E.; Nadeem, M.Y. Performance stability and functional reliability in bipolar resistive switching of bilayer ceria based resistive random access memory devices. J. Appl. Phys. 2015, 117, 8. [Google Scholar] [CrossRef]
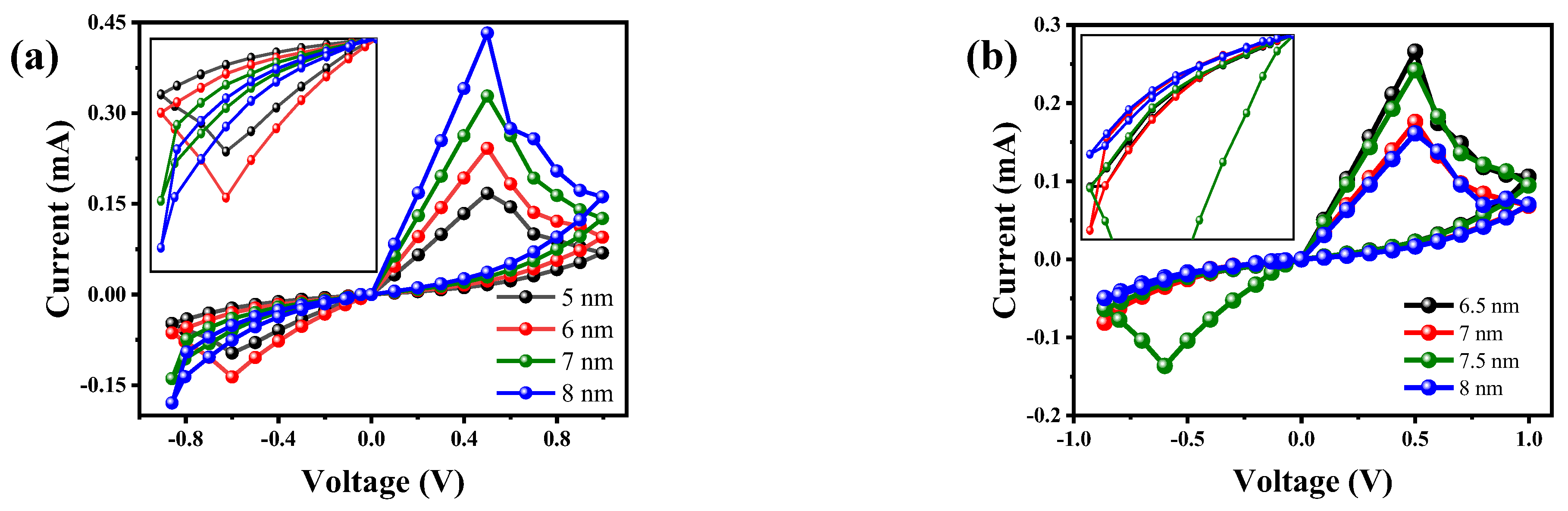
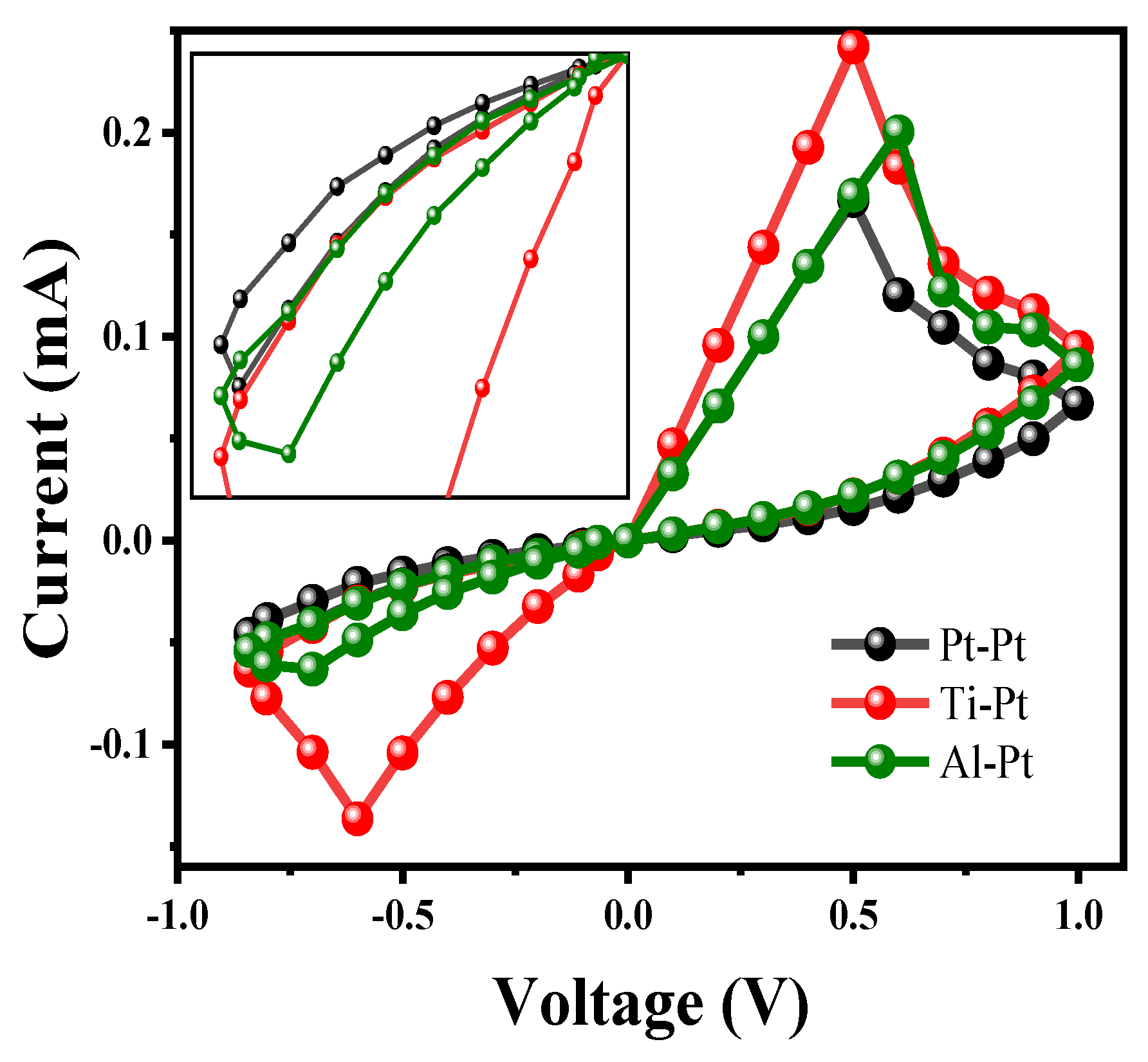
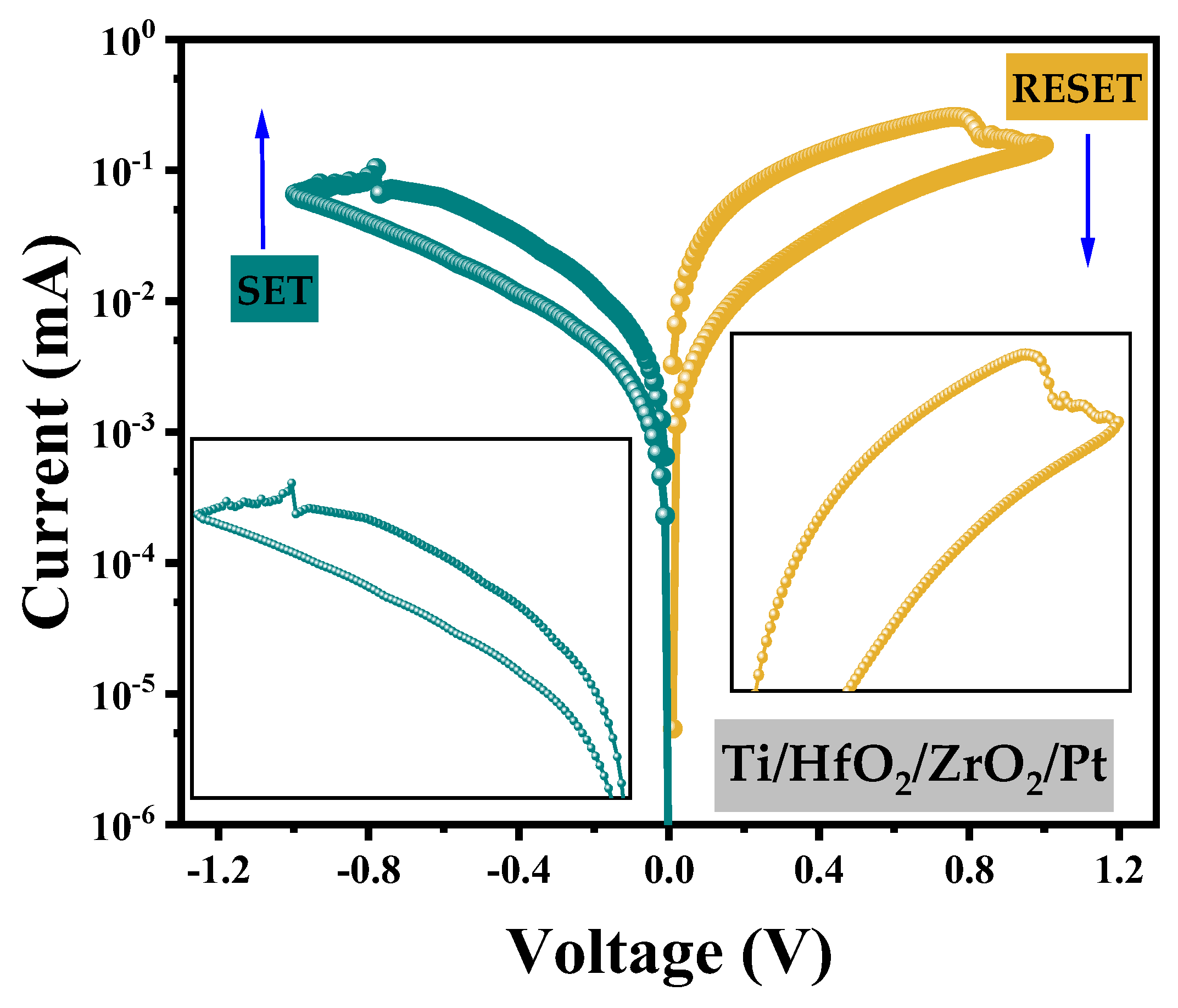
| Parameters | Values | Description |
|---|---|---|
| Ea 1 | 1.5 [eV] [29] | Oxygen vacancy diffusion activation energy |
| k | 1.380 × 10−23 [J/K] | Boltzmann constant |
| a 1 | 0.1 [nm] [30] | Ionic transition distance |
| f 2 | 1 × 1013 [ Hz] [30] | Escape frequency |
| q | 1.602 × 10−19 [C] | The unit charge |
| A 3 | 1 × 1024 [cm−3 s−1] [31] | A constant associated with the frequency of vibration |
| b 3 | 0.75 [nm] [31] | Cell mesh size |
| Ec 3 | 0.8 [eV] [31] | Relaxation energy during recombination |
| Size Modules | Width (nm) | Thickness (nm) |
|---|---|---|
| Top Electrode (TE) | 20 | 15 |
| Conductive Filament 1 (CF1) | 5 | 7.5 |
| HfO2 switching layer (SL) | 15 | 7.5 |
| Conductive Filament 2 (CF2) | 5 | 7.5 |
| ZrO2 switching layer (SL) | 15 | 7.5 |
| Bottom Electrode (BE) | 20 | 15 |
| Materials Parameters | Ti | Pt | HfO2 | ZrO2 |
|---|---|---|---|---|
| σ (S/m) | 2.6 × 106 [33] | 8.9 × 106 [34] | 1 × 10−6 [31] | 7.6 × 10−6 [35] |
| kth (W/m/K) | 21.9 [33] | 71.6 [34] | 0.5 [36] | 1.94 [37] |
| Cp (J/kg/K) | 522 | 133 | 120 [31] | 453 [37] |
| ρ (g/cm3) | 4.506 | 21.450 | 9.680 [31] | 6.100 [37] |
| εr | 1 | 1 | 25 [35] | 20 |
| Device Structure | Deposition Technique | SET- Voltage (V) | RESET- Voltage (V) | Roff/Ron |
|---|---|---|---|---|
| TiN/ZrO2/HfO2/Pt [7] | ALD 1 | −1.0 to −0.8 | 1.5 to 2.0 | 5 |
| TaN/ZrO2/HO2/TiN [38] | Sputtering | 1.0 to 0.8 | −1. 0 to −0.8 | 10 |
| TaN/HfO2/ZO2/Pt [18] | Sputtering | 1.6 to 1.1 | −1.4 to −0.9 | 14 |
| Ti/HfO2/ZrO2/Pt This work | ~ | −0.7 | 0.6 | 10.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wang, P.; Li, X.; Chen, L.; Yang, Y.; Wang, C. Optimization of Bilayer Resistive Random Access Memory Based on Ti/HfO2/ZrO2/Pt. Materials 2024, 17, 1852. https://doi.org/10.3390/ma17081852
Sun Z, Wang P, Li X, Chen L, Yang Y, Wang C. Optimization of Bilayer Resistive Random Access Memory Based on Ti/HfO2/ZrO2/Pt. Materials. 2024; 17(8):1852. https://doi.org/10.3390/ma17081852
Chicago/Turabian StyleSun, Zhendong, Pengfei Wang, Xuemei Li, Lijia Chen, Ying Yang, and Chunxia Wang. 2024. "Optimization of Bilayer Resistive Random Access Memory Based on Ti/HfO2/ZrO2/Pt" Materials 17, no. 8: 1852. https://doi.org/10.3390/ma17081852






