Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance
Abstract
1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Structural Characterization
2.3. Polishing Test
3. Results and Discussion
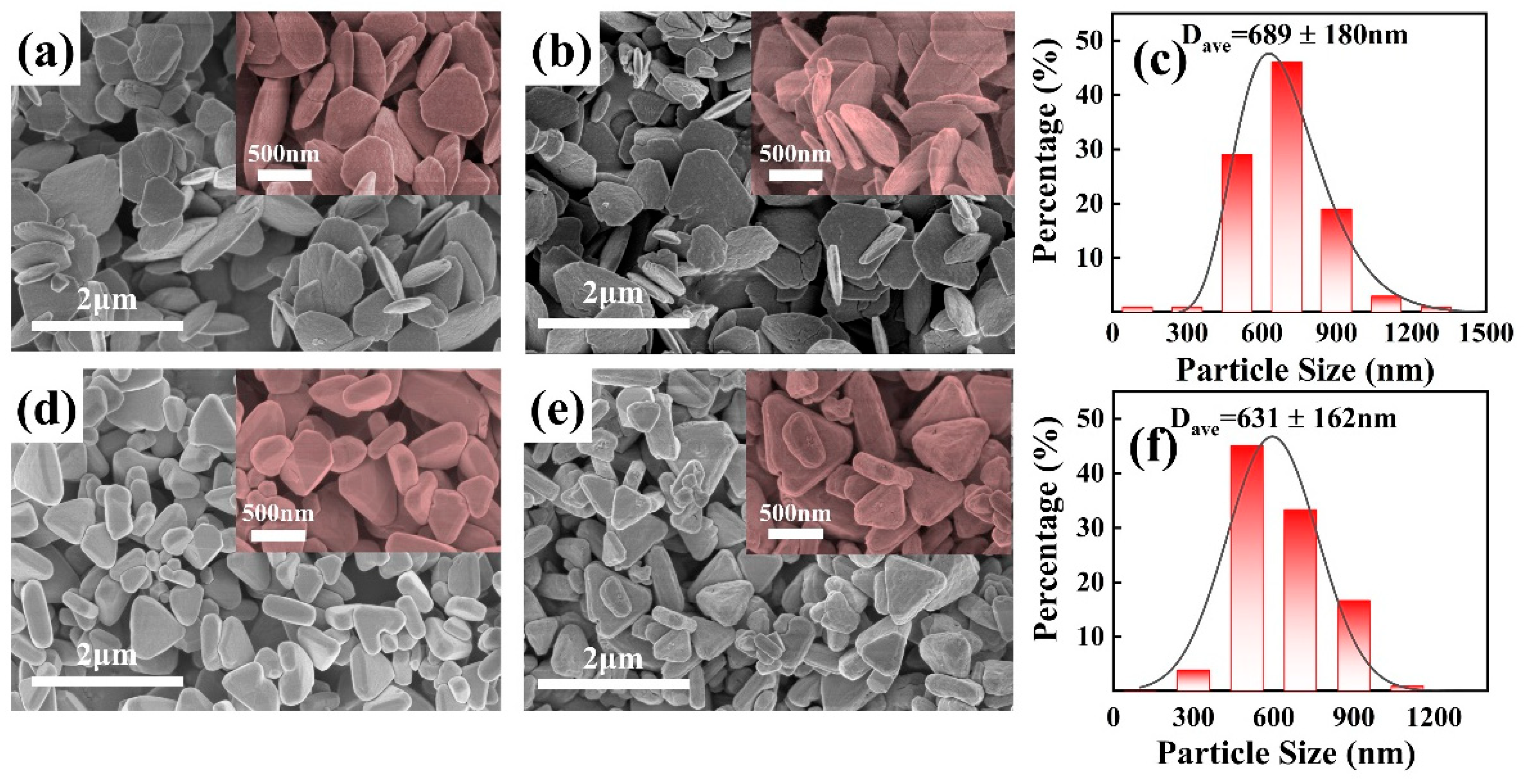
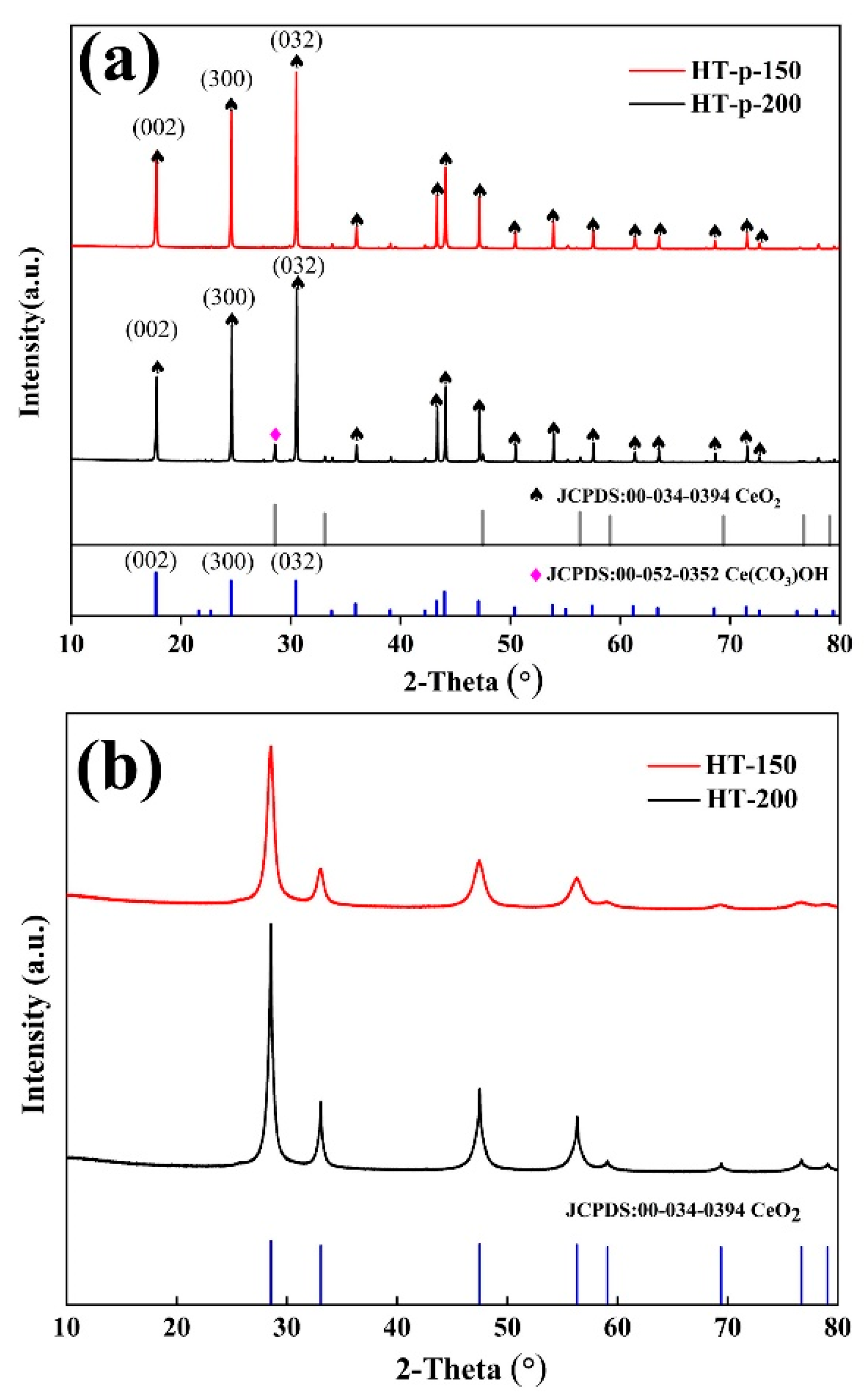
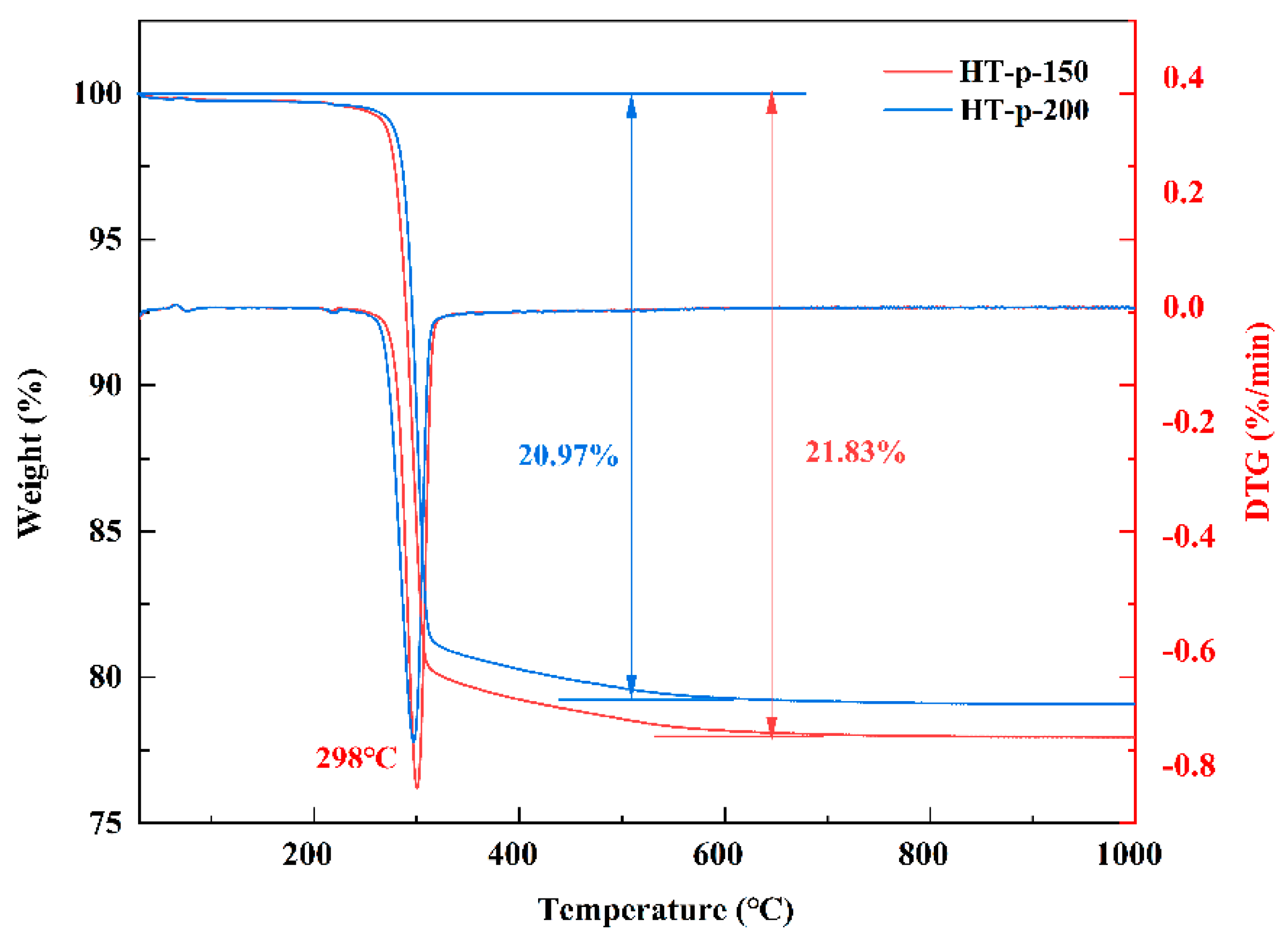
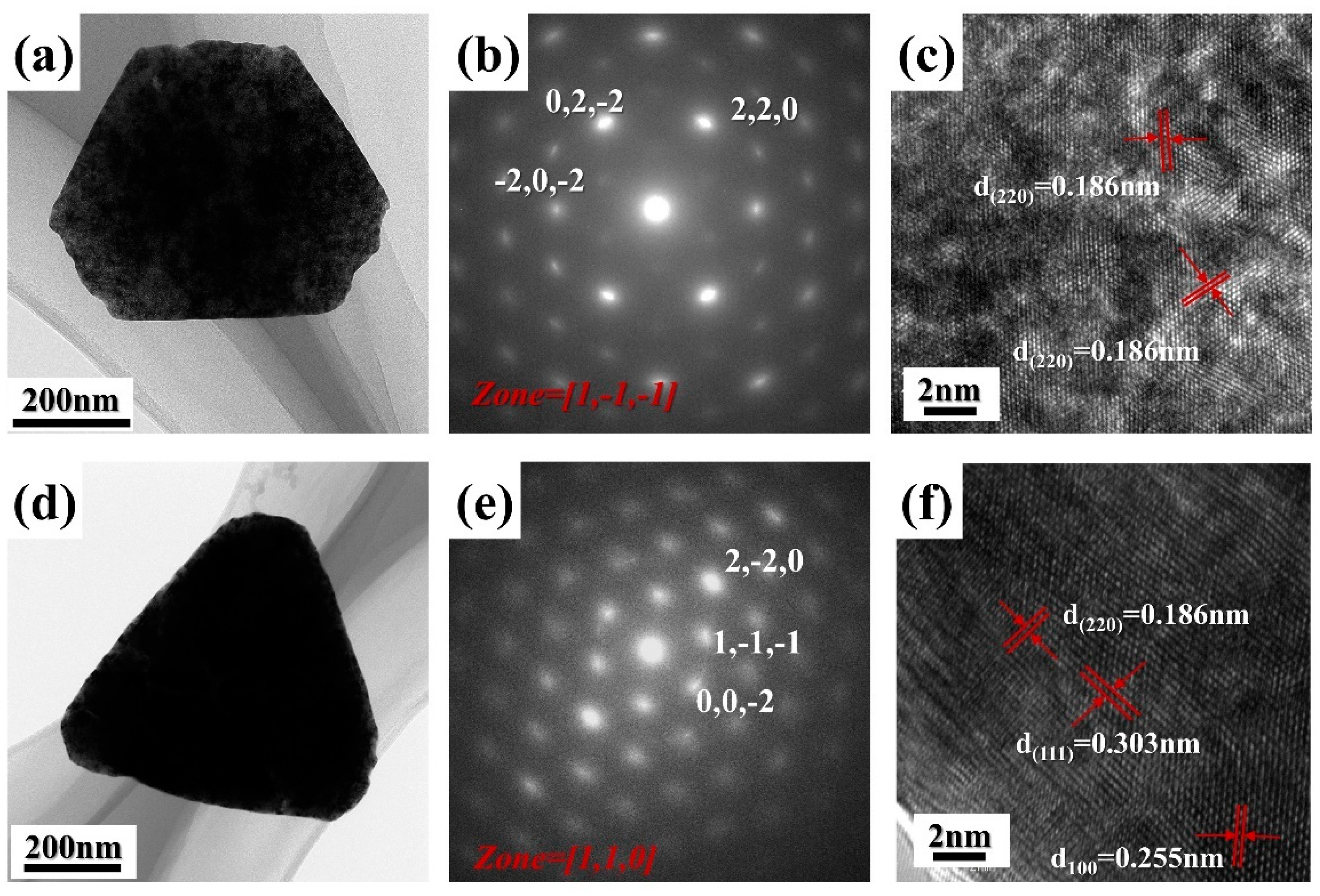
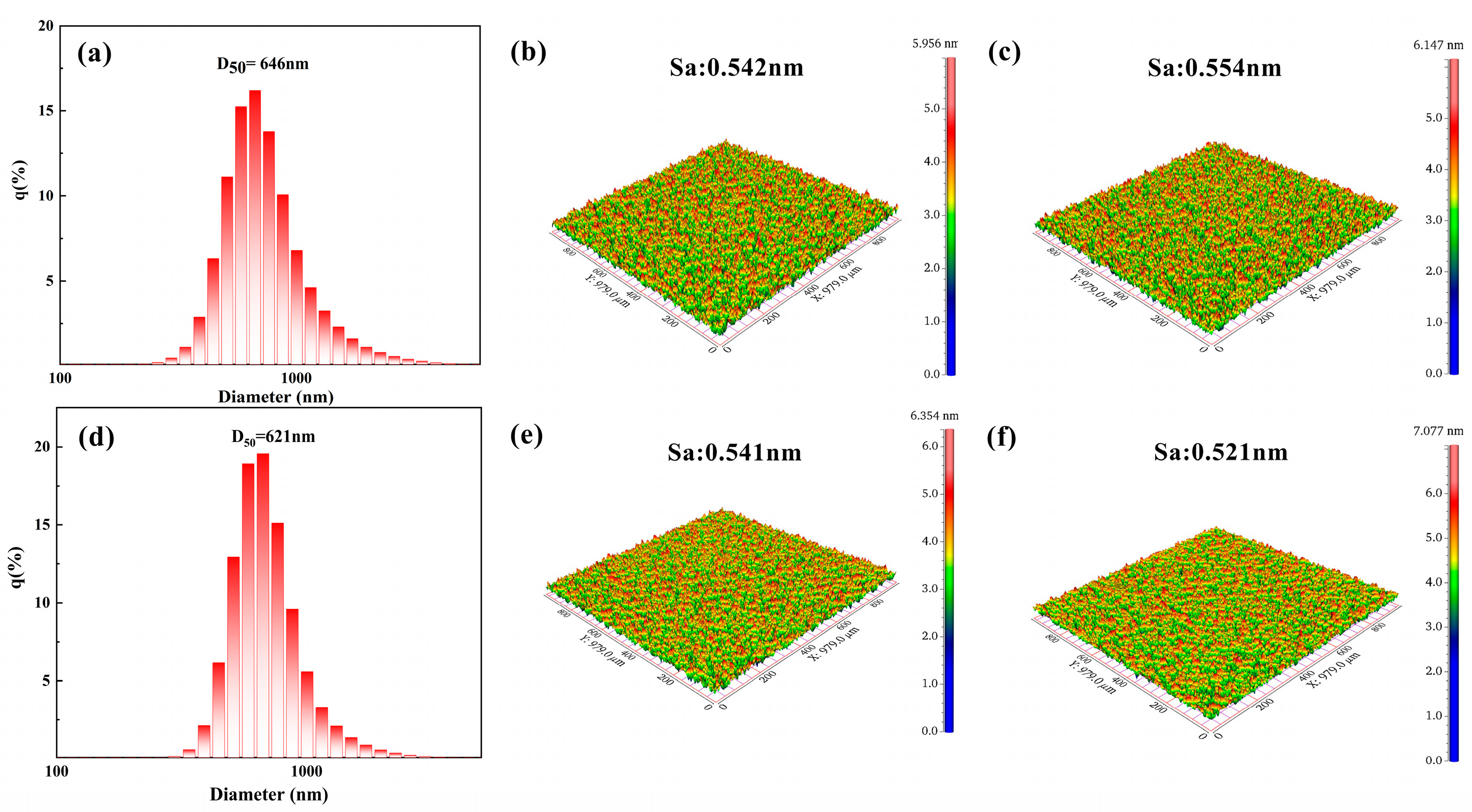
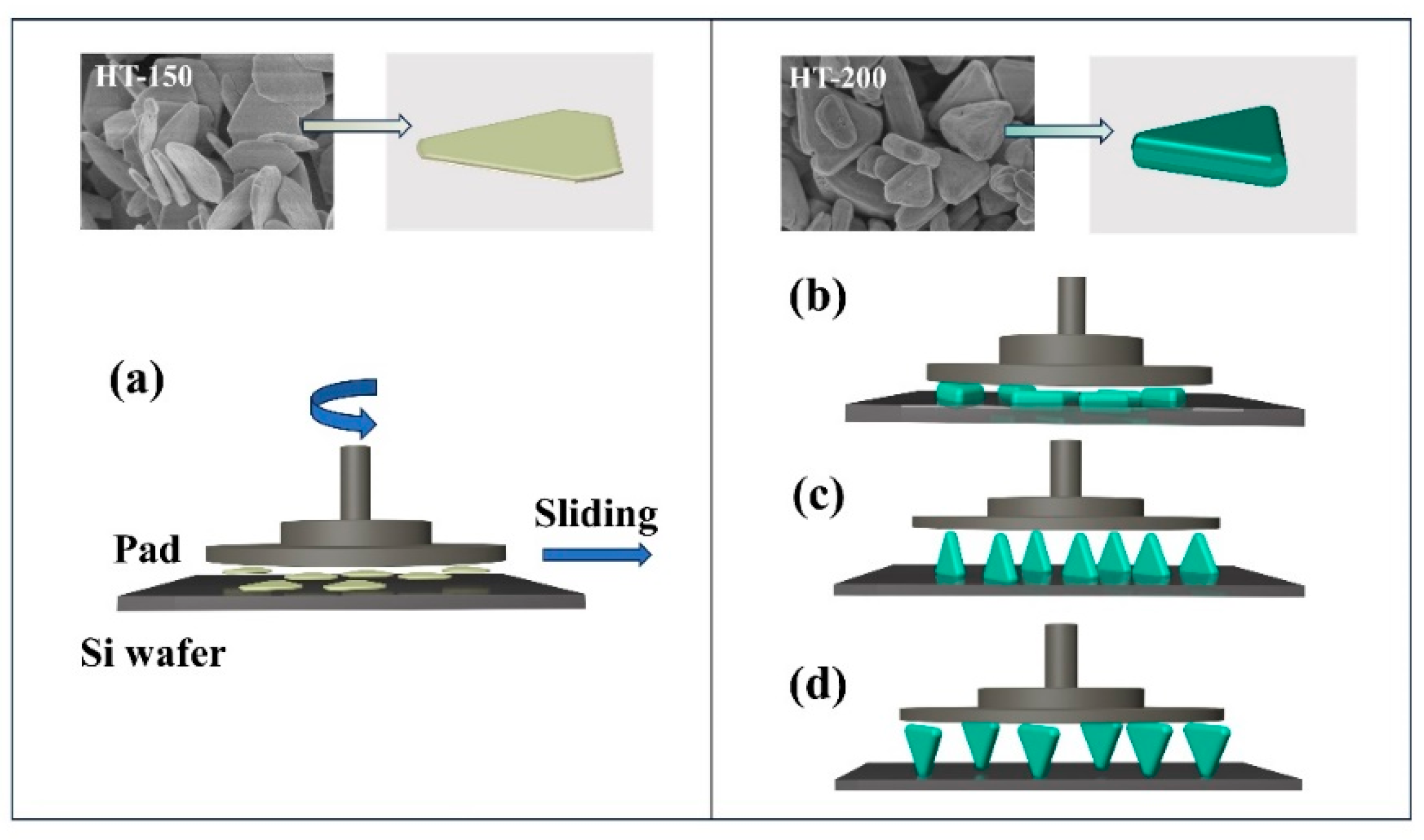
3.1. Morphology and Structure Characterization of CeO2 Abrasives
3.2. The Polishing Performance of CeO2 Abrasives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Lu, X. Chemical mechanical polishing: Theory and experiment. Friction 2013, 1, 306–326. [Google Scholar] [CrossRef]
- Moon, Y. Advances in Chemical Mechanical Planarization (CMP), 2nd ed.; Woodhead Publishing: New York, NY, USA, 2022; pp. 3–28. [Google Scholar]
- Lee, H.; Kim, H.; Jeong, H. Approaches to Sustainability in Chemical Mechanical Polishing (CMP): A Review. Int. J. Precis. Eng. Manuf.-Green Technol. 2022, 9, 349–367. [Google Scholar] [CrossRef]
- Srinivasan, R.; Dandu, P.V.; Babu, S.V. Shallow Trench Isolation Chemical Mechanical Planarization: A Review. ECS J. Solid State Sci. Technol. 2015, 4, P5029–P5039. [Google Scholar] [CrossRef]
- Cook, L.M. Chemical processes in glass polishing. J. Non-Cryst. Solids 1990, 120, 152–171. [Google Scholar] [CrossRef]
- Yang, J.C.; Oh, D.W.; Lee, G.W.; Song, C.L.; Kim, T. Step height removal mechanism of chemical mechanical planarization (CMP) for sub-nano-surface finish. Wear 2010, 268, 505–510. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ashika, S.A.; Jainshaa, J. Influence of synthesis methods (combustion and precipitation) on the formation of nanocrystalline CeO2, MgO, and NiO phase materials. Results Chem. 2023, 5, 100941. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of annealing temperature on the microstructural, optical and electrical properties of CeO2 nanoparticles by chemical precipitation method. Appl. Surf. Sci. 2013, 273, 457–464. [Google Scholar] [CrossRef]
- Ramachandran, M.; Subadevi, R.; Sivakumar, M. Role of pH on synthesis and characterization of cerium oxide (CeO2) nano particles by modified co-precipitation method. Vacuum 2019, 161, 220–224. [Google Scholar] [CrossRef]
- Hernández-Arteaga, J.; Ojeda-Galván, H.J.; Alanis, J.; Rodríguez-Aranda, M.; Villabona-Leal, E.; Mendoza-Mendoza, E.; Ulloa-Castillo, N.A.; Quintana, M.; Navarro-Contreras, H.R.; Rodríguez, A. Thermal tuning of the morphology of hydrothermally synthesized CeO2 nanotubes for photocatalytic applications. Ceram. Int. 2022, 48, 17802–17815. [Google Scholar] [CrossRef]
- Divya, T.; Anjali, C.; Sunajadevi, K.R.; Anas, K.; Renuka, N.K. Influence of hydrothermal synthesis conditions on lattice defects in cerium oxide. J. Solid State Chem. 2021, 300, 122253. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Zhang, X. The gas sensor utilizing CeO2 nanorods for the low temperature detection of hydrogen. Inorg. Chem. Commun. 2021, 130, 108692. [Google Scholar] [CrossRef]
- Sonawane, L.D.; Mandawade, A.S.; Ahemad, H.I.; Aher, Y.B.; Gite, A.B.; Nikam, L.K.; Shinde, S.D.; Jain, G.H.; Patil, G.E.; Shinde, M.S. Sol-gel and hydrothermal synthesis of CeO2 NPs: Their physiochemical properties and applications for gas sensor with photocatalytic activities. Inorg. Chem. Commun. 2024, 2024, 112313. [Google Scholar] [CrossRef]
- Hadi, A.; Yaacob, I.I. Novel synthesis of nanocrystalline CeO2 by mechanochemical and water-in-oil microemulsion methods. Mater. Lett. 2007, 61, 93–96. [Google Scholar] [CrossRef]
- Mao, X.; Xia, X.; Li, J.; Chen, C.; Gu, X.; Li, S.; Lan, Y.-P. Self-assembly of structured CeCO3OH and its decomposition in H2 for a novel tactic to obtain CeO2-with excellent photocatalytic property. J. Alloys Compd. 2021, 870, 159424. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wu, S.-H.; Hsiao, C.-H.; Lee, A.-T.; Huang, M.H. Mild Synthesis of Size-Tunable CeO2 Octahedra for Band Gap Variation. Chem. Mater. 2020, 32, 2631–2638. [Google Scholar] [CrossRef]
- Sonsupap, S.; Waehayee, A.; Siritanon, T.; Saenrang, W.; Chanlek, N.; Nakajima, H.; Rattanachata, A.; Maensiri, S. Structural, optical, and photocatalytic properties of La3+ doped CeO2 nanospheres for enhanced photodegradation of tetracycline. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130650. [Google Scholar] [CrossRef]
- Ozawa, N.; Ishikawa, M.; Nakamura, M.; Kubo, M. Polishing Process Simulation of SiO2 by CeO2 Abrasive Grain under Wet Environment. Hyomen Kagaku 2012, 33, 351–356. [Google Scholar] [CrossRef]
- Kim, E.; Hong, J.; Hong, S.; Kanade, C.; Seok, H.; Kim, H.-U.; Kim, T. Improvement of oxide removal rate in chemical mechanical polishing by forming oxygen vacancy in ceria abrasives via ultraviolet irradiation. Mater. Chem. Phys. 2021, 273, 124967. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E.; Bae, C.; Seok, H.; Cho, J.; Aydin, K.; Kim, T. Improvement of oxide chemical mechanical polishing performance by increasing Ce3+/Ce4+ ratio in ceria slurry via hydrogen reduction. Mater. Sci. Semicond. Process. 2023, 159, 107349. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, S.; Li, Y.; Wang, T.; Xie, L.; Lu, X. RE (La, Nd and Yb) doped CeO2 abrasive particles for chemical mechanical polishing of dielectric materials: Experimental and computational analysis. Appl. Surf. Sci. 2020, 506, 144668. [Google Scholar] [CrossRef]
- Hu, P.; Chen, Y.; Sun, R.; Chen, Y.; Yin, Y.; Wang, Z. Synthesis, characterization and frictional wear behavior of ceria hybrid architectures with {111} exposure planes. Appl. Surf. Sci. 2017, 401, 100–105. [Google Scholar] [CrossRef]
- Xu, N.; Ma, J.; Liu, Q.; Luo, Y.; Pu, Y. Preparation of CeO2 abrasives by reducing atmosphere-assisted molten salt method for enhancing their chemical mechanical polishing performance on SiO2 substrates. J. Rare Earths 2023, 41, 1627–1635. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Van Hecke, K.; Van Deun, R. Nano- and micro-sized rare-earth carbonates and their use as precursors and sacrificial templates for the synthesis of new innovative materials. Chem. Soc. Rev. 2015, 44, 2032–2059. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Raghavan, C.; Ghosh, C.; Divakar, R.; Mohandas, E.; Song, J.I.; Bae, S.; Kim, T.G. X-ray diffraction, Raman and photoluminescence studies of nanocrystalline cerium oxide thin films. Ceram. Int. 2013, 39, 8327–8333. [Google Scholar] [CrossRef]
- Tsuda, Y.; Uda, K.; Chiba, M.; Sun, H.; Sun, L.; White, M.S.; Masuhara, A.; Yoshida, T. Selective hybridization of organic dyes with CuSCN during its electrochemical growth. Microsyst. Technol. 2018, 24, 715–723. [Google Scholar] [CrossRef]
- Uchiyama, H.; Sakaue, R.; Kozuka, H. Preparation of nanostructured CeCO3OH particles from aqueous solutions and gels containing biological polymers and their thermal conversion to CeO2. RSC Adv. 2013, 3, 20106. [Google Scholar] [CrossRef]
- Sims, C.M.; Maier, R.A.; Johnston-Peck, A.C.; Gorham, J.M.; Hackley, V.A.; Nelson, B.C. Approaches for the quantitative analysis of oxidation state in cerium oxide nanomaterials. Nanotechnology 2019, 30, 085703. [Google Scholar] [CrossRef] [PubMed]
- Han, K.M.; Han, S.Y.; Sahir, S.; Yerriboina, N.P.; Kim, T.G.; Mahadev, N.; Park, J.G. Contamination Mechanism of Ceria Particles on the Oxide Surface after the CMP Process. ECS J. Solid State Sci. Technol. 2020, 9, 124004. [Google Scholar] [CrossRef]
- Li, L.; Hu, G.S.; Lu, J.Q.; Luo, M.F. Review of Oxygen Vacancies in CeO2-doped Solid Solutions as Characterized by Raman Spectroscopy. Acta Phys.-Chim. Sin. 2012, 28, 1012–1020. [Google Scholar]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing Defect Sites on CeO2 Nanocrystals with Well-Defined Surface Planes by Raman Spectroscopy and O 2 Adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Spanier, J.E.; Robinson, R.D.; Zhang, F.; Chan, S.W.; Herman, I.P. Size-dependent properties of CeO2−y nanoparticles as studied by Raman scattering. Phys. Rev. B 2001, 64, 245407. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Popović, Z.; Dohčević-Mitrović, Z.; Šćepanović, M.; Grujić-Brojčin, M.; Aškrabić, S. Raman scattering on nanomaterials and nanostructures. Ann. Phys. 2011, 523, 62–74. [Google Scholar] [CrossRef]
- Pu, Z.Y.; Liu, X.S.; Jia, A.P.; Xie, Y.L.; Lu, J.Q.; Luo, M.F. Enhanced Activity for CO Oxidation over Pr- and Cu-Doped CeO2 Catalysts: Effect of Oxygen Vacancies. J. Phys. Chem. C 2008, 112, 15045–15051. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Zhu, X.; Wu, T.; Tang, B.; Li, Y. Synthesis of hierarchical CeO2 octahedrons with tunable size and the catalytic properties. Mater. Lett. 2019, 240, 73–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Bals, S.; Van Tendeloo, G. Understanding CeO2-Based Nanostructures through Advanced Electron Microscopy in 2D and 3D. Part. Part. Syst. Charact. 2019, 36, 1800287. [Google Scholar] [CrossRef]
- Marciniak, A.A.; Henrique, F.J.; de Lima, A.F.; Alves, O.C.; Moreira, C.R.; Appel, L.G.; Mota, C.J. What are the preferred CeO2 exposed planes for the synthesis of dimethyl carbonate? Answers from theory and experiments. Mol. Catal. 2020, 493, 111053. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? ACS Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.; Jeong, H. Mechanical aspects of the chemical mechanical polishing process: A review. Int. J. Precis. Eng. Manuf. 2016, 17, 525–536. [Google Scholar] [CrossRef]
- Han, X.; Liu, R.; Tan, B.; Wang, F.; Yan, M.; Zhao, X.; Zhao, J. Research progress on the application of ceria nanoparticles as abrasives in dielectric layer CMP and post cleaning: Structure, morphology, doping, and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132551. [Google Scholar] [CrossRef]


| Parameter | Specifications |
|---|---|
| Head rotating speed | 45 rpm |
| Pad rotating speed | 15 rpm |
| Slurry flow rate | 50 mL/min |
| Polishing pressure | 5.98 Psi |
| Polishing time | 2 min |
| HT-p-150 | HT-p-200 | Standard (JCPDS No. 52-0352) | |
|---|---|---|---|
| Intensity ratio of (300)/(002) | 1.50 | 1.79 | 0.79 |
| Intensity ratio of (032)/(002) | 1.85 | 2.35 | 0.79 |
| HT-150 | HT-200 | CC1 | CC2 | |
|---|---|---|---|---|
| MRR (nm/min) | 216 | 324 | 136 | 154 |
| Sa (nm) | 0.554 | 0.521 | 0.592 | 0.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, N.; Zhang, Z.; Tan, X.; Zheng, Y.; Yang, J. Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance. Materials 2024, 17, 2001. https://doi.org/10.3390/ma17092001
Wang X, Wang N, Zhang Z, Tan X, Zheng Y, Yang J. Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance. Materials. 2024; 17(9):2001. https://doi.org/10.3390/ma17092001
Chicago/Turabian StyleWang, Xingzi, Ning Wang, Zhenyu Zhang, Xianmin Tan, Yuanyuan Zheng, and Juanyu Yang. 2024. "Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance" Materials 17, no. 9: 2001. https://doi.org/10.3390/ma17092001
APA StyleWang, X., Wang, N., Zhang, Z., Tan, X., Zheng, Y., & Yang, J. (2024). Controlled Synthesis of Triangular Submicron-Sized CeO2 and Its Polishing Performance. Materials, 17(9), 2001. https://doi.org/10.3390/ma17092001




