Effects of Temperature, Ionic Strength and Humic Acid on the Transport of Graphene Oxide Nanoparticles in Geosynthetic Clay Liner
Abstract
:1. Introduction
2. Materials and Methods
2.1. GONPs
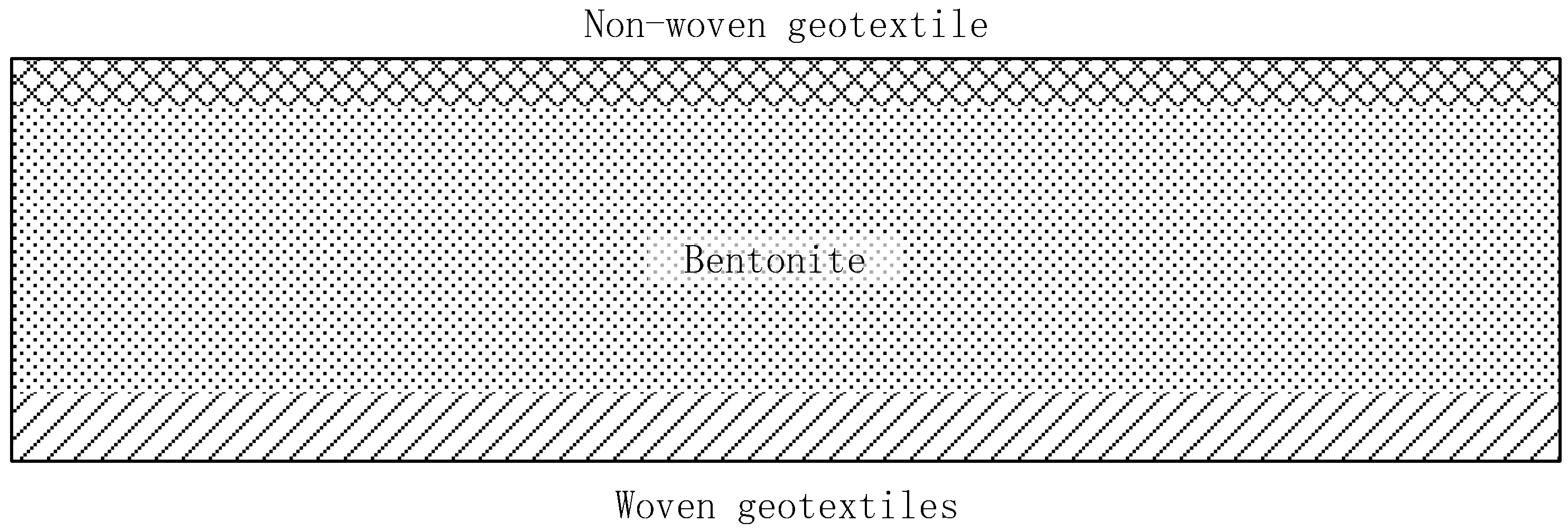
2.2. Geosynthetic Clay Liner
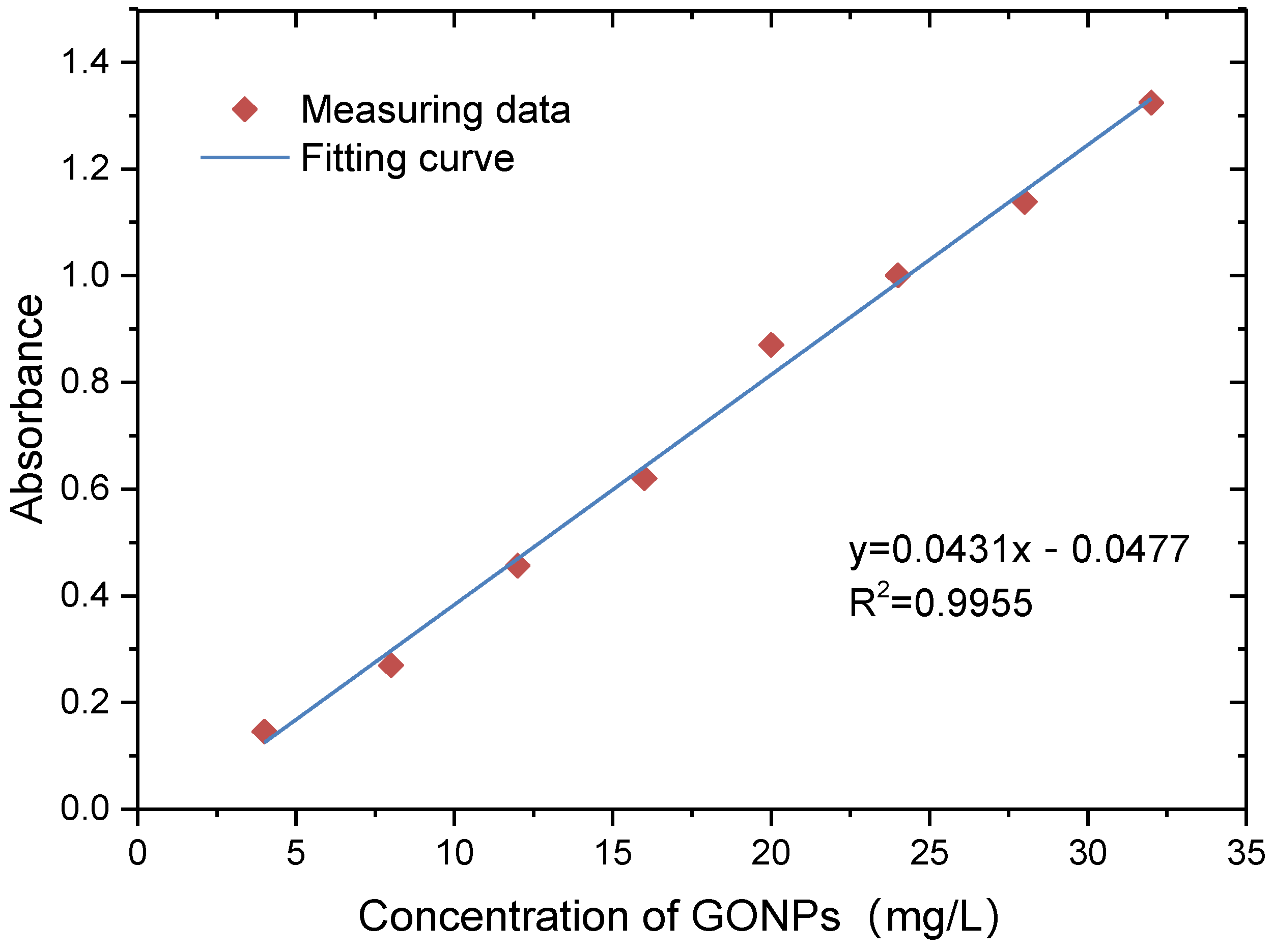
2.3. Concentration Determination of GONPs
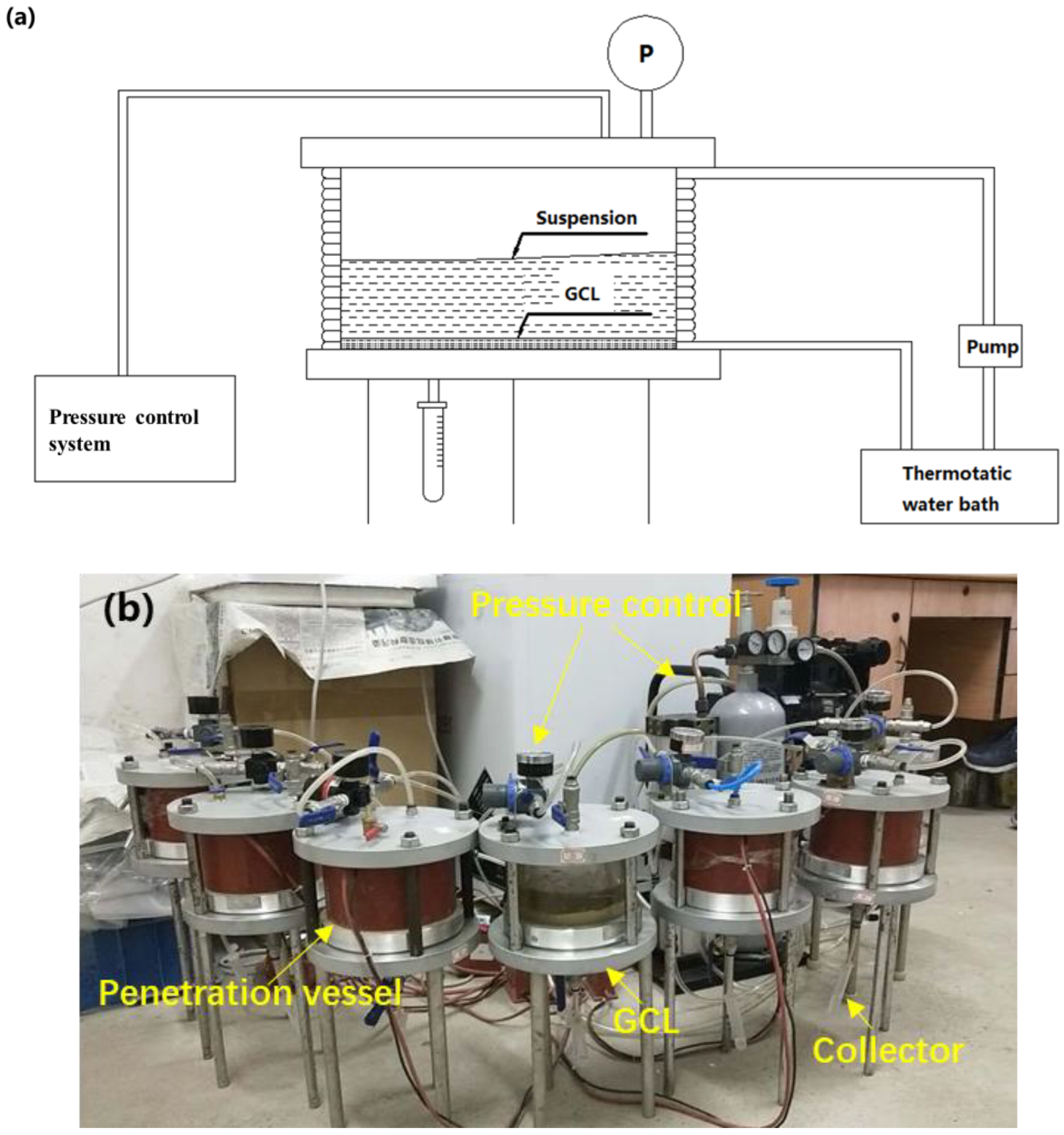
2.4. Experimental Setup
2.5. Column Experiment
- (1)
- GCL was placed in the bottom of column. Deionized water (20 PVs) was injected into the column. The value of pore volumes (PVs) was determined by dividing effluent volume by GCL pore volume [33]. The deionized water was transported through the GCL and cleared the mini-impurity in GCL.
- (2)
- NaCl crystal was dissolved in deionized water, and specific NaCl solution was mixed with GONP suspension to acquire GONP suspension with specific IS [33]. NaCl was chosen to control the IS because Na+ was the most-widespread and common positive ion in leachate. IS could be acquired through NaCl concentration and IS formula. IS value in each experiment is shown in Table 2.
- (3)
- Specific content of HA was added into the deionized water, and the impurity of HA was removed using 0.45 μm filter membrane. Then, the HA solution was added into the GONP suspension to obtain the suspension with different HA content (Table 2).
- (4)
- The GONP suspension with different IS and HA was injected into the column. The temperature was controlled by a temperature control system. To highlight the effect of single factor, the experimental scheme was designed based on simple variable method, as shown in Table 2.
- (5)
- The initial concentration was 50 mg/L, and the pressure was 0.1 MPa in each column experiment. The values for temperature, IS and HA were determined based on previous studies, measured data and hydrogeological reports in many landfills [33].
- (6)
- Effluent samples (1/3 PV each sample) were collected through automated collector until the volume of effluent exceeded 10 PV. The GONPs remaining in the GCL were washed by 6 PV deionized water.
- (7)
- The concentration of GONPs in the effluents was measured through UV-vis.
3. Results and Discussion
3.1. Results of GONP Concentration Determination
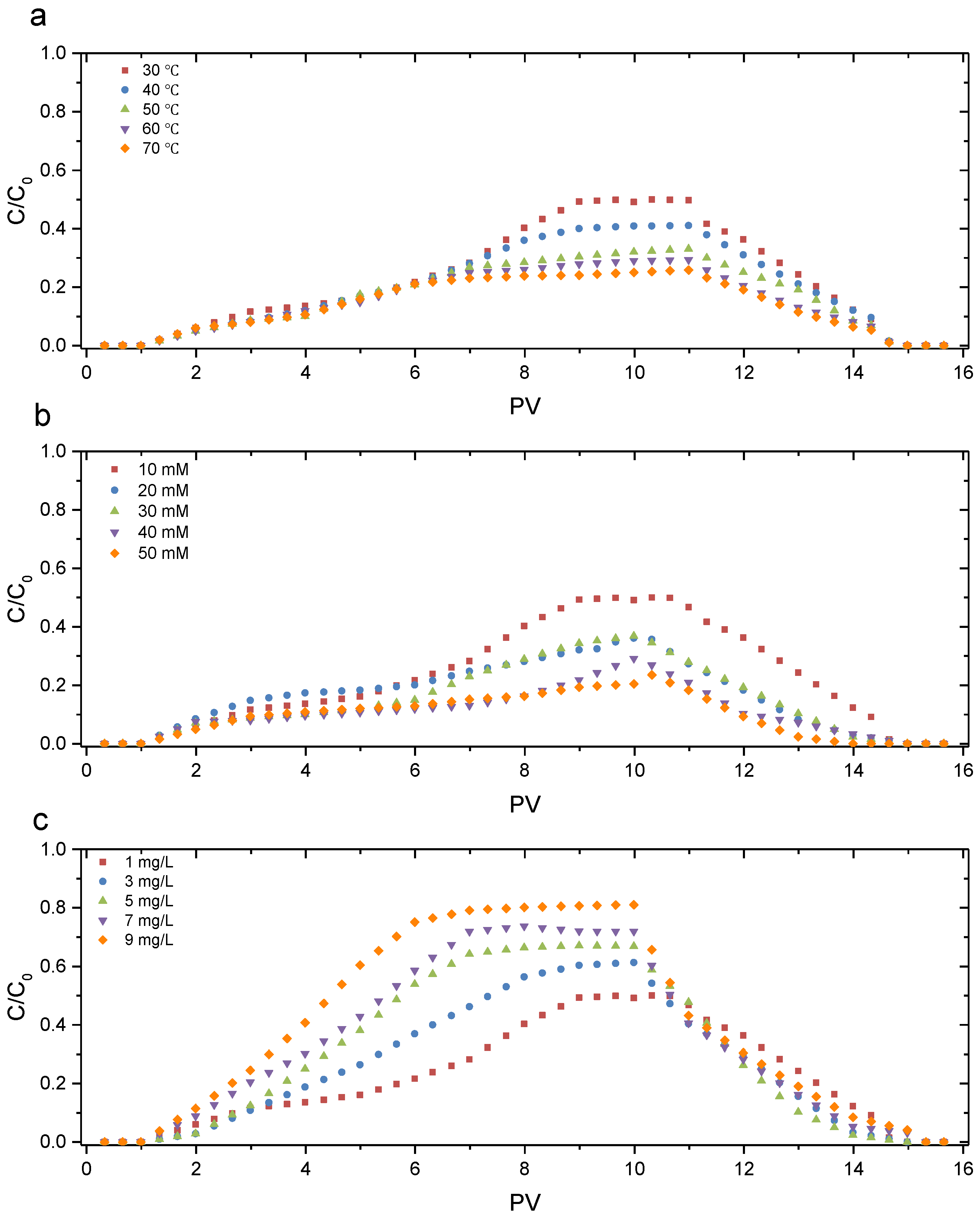
3.2. Transport of GONPs in GCL
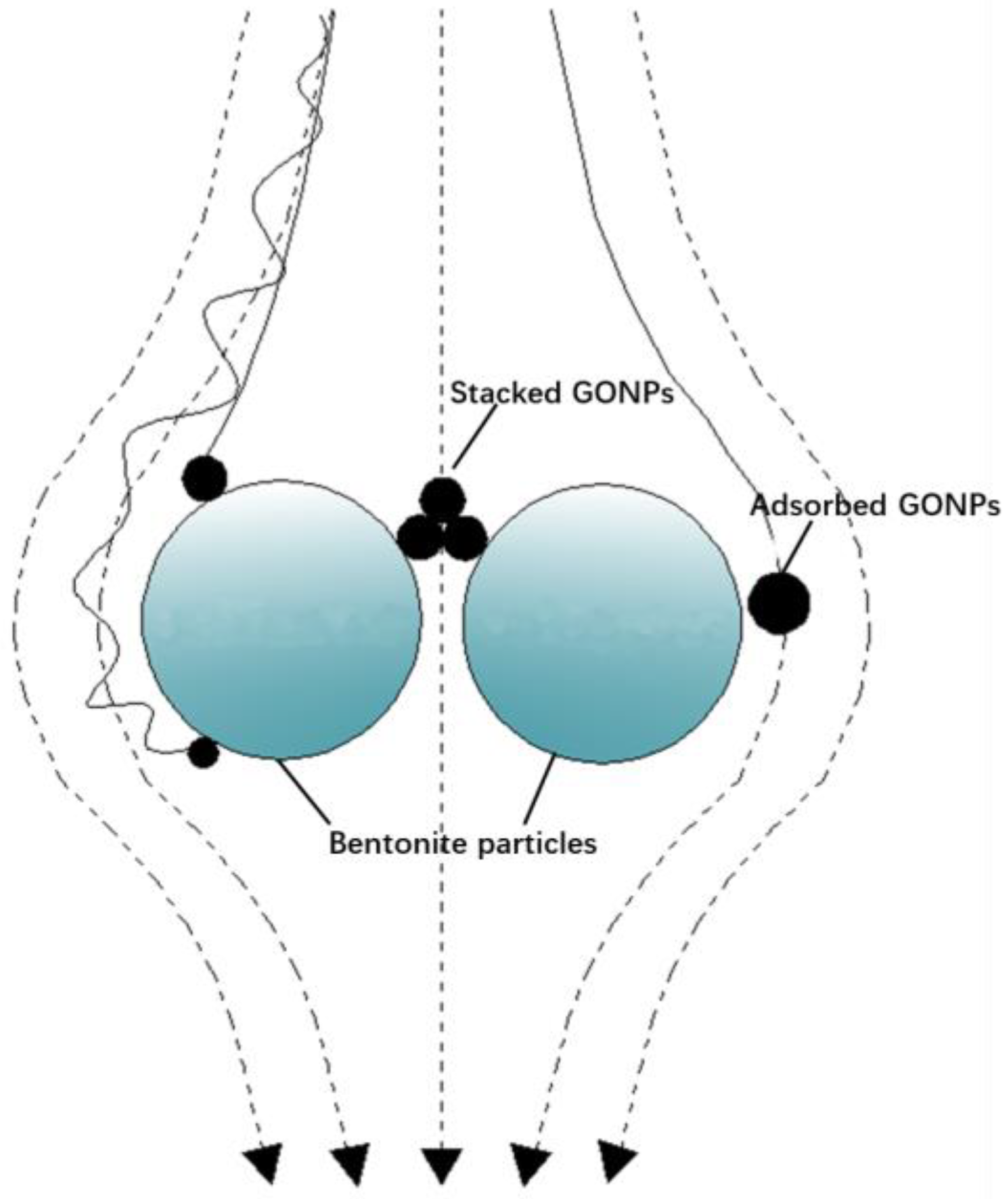
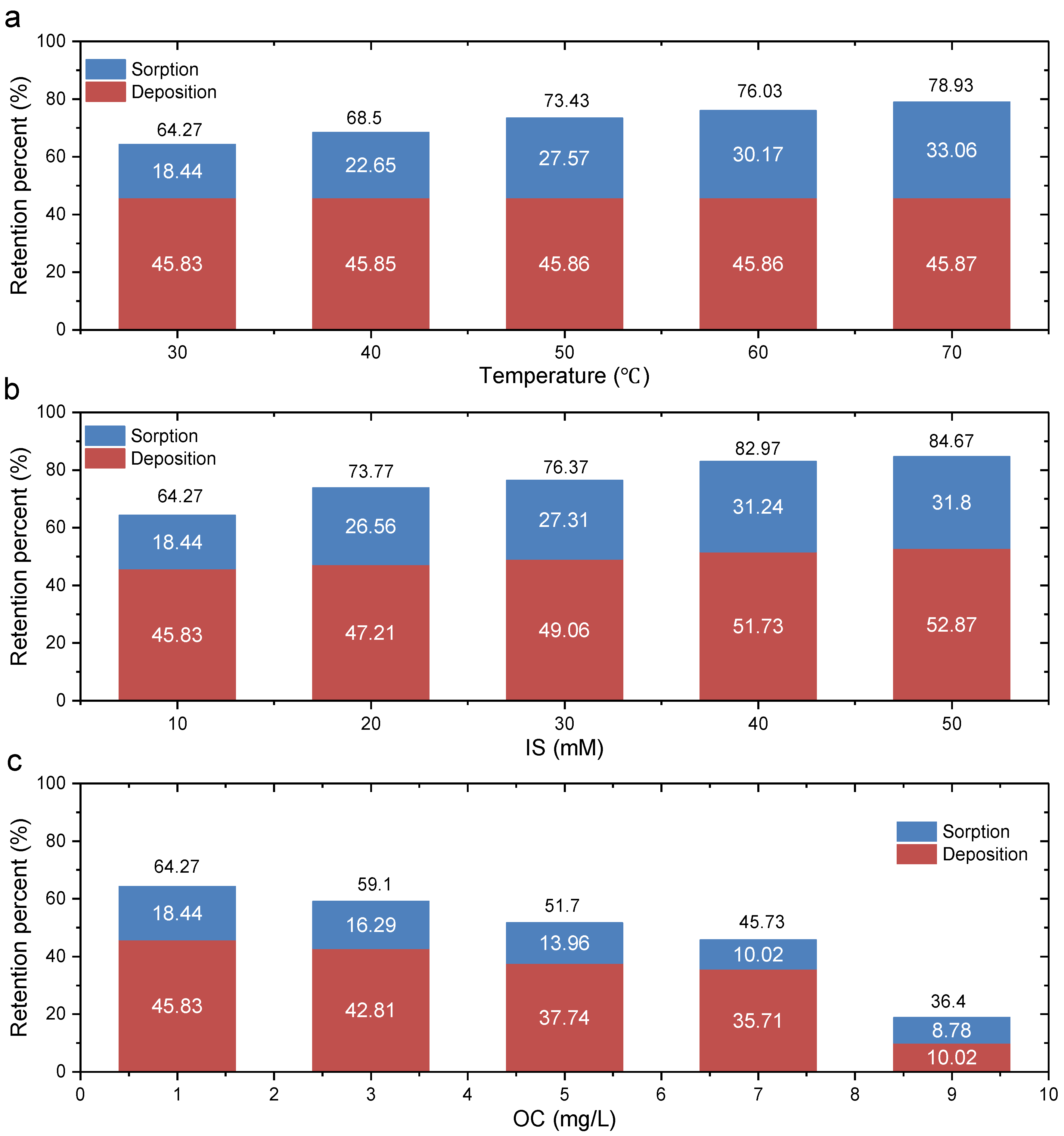
3.3. Retention of GONPs in GCL
3.4. Comparison between Transport of GONPs in GCL and in Other Porous Materials
4. Limitations and Discussion
5. Conclusions
- (1)
- GONPs could be transported through the GCL, and the transport behaviors under various conditions were different: Cmax decreased (0.49–0.25) with an increase in temperature (30–70 °C) because a higher temperature will aggravate the Brownian movement of GONPs; Cmax decreased (0.49–0.20) with an increase in IS (10–50 MM) because a greater IS could reduce the electrostatic repulsion between nanoparticles and bentonite particles; Cmax increased (0.49–0.8) with an increase in HA (1–9 mg/L) because HA covered the surface of GONPs and affected the characteristics of the GONP surface.
- (2)
- Higher temperature, greater IS and lower HA will increase the adsorption ratio of GONPs. The deposition ratio increased with an increase in IS, and temperature had little effect on deposition ratio.
- (3)
- The comparison between the transport of GONPs in the GCL and in other porous materials showed that the BTCs in the GCL were much smoother, and the retention ability of the GCL (0.36 Cmax) was much better than glass beads (0.71 Cmax) and quartz sand (0.69 Cmax).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTC | breakthrough curve |
| GCL | geosynthetic clay liner |
| GONPs | graphene oxide nanoparticles |
| IS | ionic strength |
| HA | humic acid |
References
- Ozcoban, M.S.; Acarer, S. Investigation of the effect of leachate on permeability and heavy metal removal in soils improved with nano additives. Appl. Sci. 2022, 12, 6104. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Huang, W.; Liu, Z.L.; Wang, D.B. Application of supramolecular nano-material adsorbent in the treatment of heavy metal pollution in acid selenium-rich soils in south China. Integr. Ferroelectr. 2021, 217, 69–81. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, J.R.; Yang, R.; Zhou, D.; Fang, C.Q.; He, X.Y.; Wang, D.; Lei, W.Q.; Hu, J.B.; Li, Y. Facile preparation and characterization of fibrous carbon nanomaterial from waste polyethylene terephthalate. Waste Manag. 2020, 107, 172–181. [Google Scholar] [CrossRef]
- Gambardella, C.; Pinsino, A. Nanomaterial Ecotoxicology in the Terrestrial and Aquatic Environment: A Systematic Review. Toxics 2022, 10, 393. [Google Scholar] [CrossRef]
- Barlaz, M.A.; Mike, M.W.; Ham, R.K. Gas production parameters in sanitary landfill simulations. Waste Manag. Res. 1987, 5, 27–39. [Google Scholar] [CrossRef]
- Khan, I.A.; Berge, N.D.; Sabo-Attwood, T.; Ferguson, P.L.; Saleh, N.B. Single-walled carbon nanotube transport in representative municipal solid waste landfill conditions. Environ. Sci. Technol. 2013, 47, 8425–8433. [Google Scholar] [CrossRef]
- Kamenska, T.; Abrashev, M.; Georgiven, M.; Krasteva, N. Impact of Polyethylene Glycol Functionalization of Graphene Oxide on Anticoagulation and Haemolytic Properties of Human Blood. Materials 2021, 14, 4853. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Ming, J.; Ling, M.; Wu, K.L.; Ye, Y.; Wei, X.W. Solvothermal Synthesis of Bi Nanoparticles/Reduced Graphene Oxide Composites and Their Catalytic Applications for Dye Degradation and Fast Aromatic Nitro Compounds Hydrogenation. Chem. Lett. 2020, 49, 318–322. [Google Scholar] [CrossRef]
- Yang, P.; Liu, Y.H.; Yang, K.; Ouyang, Z.B. Effects of pressure on graphene oxide nanoparticle deposition and transport in GCLs. Geosynth. Int. 2020, 27, 573–580. [Google Scholar] [CrossRef]
- Cui, J.L.; Qi, M.; Gao, S.B.; Xu, N.; Wang, X.H.; Li, N.; Chen, G.Y. Disposal and resource utilization of waste masks: A review. Environ. Sci. Pollut. Res. 2023, 30, 19683–19704. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Jaworski, S.; Sawosz, E.; Jung, A.; Gielerak, G.; Jaremek, H.; Lojkowski, W.; Wozniak, B.; Stobinski, L.; Malolepszy, A.; et al. Graphene Oxide in a Composite with Silver Nanoparticles Reduces the Fibroblast and Endothelial Cell Cytotoxicity of an Antibacterial Nanoplatform. Nanoscale Res. Lett. 2019, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- An, W.Z.; Zhang, Y.; Zhang, X.; Li, K.; Kang, Y.J.; Akhtar, S.; Sha, X.L.; Gao, L. Ocular toxicity of reduced graphene oxide or graphene oxide exposure in mouse eyes. Exp. Eye Res. 2018, 174, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, S.; Santiago, R.; Lopez-Diaz, D.; Merchan, M.D.; Velazquez, M.M.; Fierro, J.L.G.; Palomar, J. Role of the Structure of Graphene Oxide Sheets on the CO2 Adsorption Properties of Nanocomposites Based on Graphene Oxide and Polyaniline or Fe3O4-Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 12464–12473. [Google Scholar] [CrossRef]
- Dibyanshu, K.; Chhaya, T.; Raychoudhury, T. A review on the fate and transport behavior of engineered nanoparticles: Possibility of becoming an emerging contaminant in the groundwater. Int. J. Environ. Sci. Technol. 2022, 20, 4649–4672. [Google Scholar] [CrossRef]
- Lin, J.D.; Yin, X.B.; Pang, H.; Zhang, L.; Liao, B.; Xin, M.L.; Su, Y.; Liu, Y.; Wu, L.; Jia, K.L.; et al. Tuning the Wrinkles in 3D Graphene Architectures for Mass and Electron Transport. Adv. Mater. Interfaces 2020, 7, 1902190. [Google Scholar] [CrossRef]
- Seetha, N.; Hassanizadeh, S.M. A two-way coupled model for the co-transport of two different colloids in porous media. J. Contam. Hydrol. 2022, 244, 103922. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, M.P.; Syngouna, V.I.; Chrysikopoulos, C.V. Influence of graphene oxide nanoparticles on the transport and cotransport of biocolloids in saturated porous media. Colloids Surf. B Biointerfaces 2020, 189, 110841. [Google Scholar] [CrossRef]
- Wang, M.; Gao, B.; Tang, D.S.; Sun, H.; Yin, X.; Yu, C. Effect of temperature on graphene oxide deposition and transport in saturated porous media. J. Hazard Mater. 2017, 331, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhang, L.; Wang, F.; Hou, L.; Chen, W. Factors controlling transport of graphene oxide nanoparticles in saturated sand columns. Environ. Toxicol. Chem. 2014, 33, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, J.F.; Cao, R.Y.; Zhang, Y.Z.; Li, J.X. The detection and characterization techniques for the interaction between graphene oxide and natural colloids: A review. Sci. Total Environ. 2022, 808, 151906. [Google Scholar] [CrossRef]
- Esfahani, A.R.; Batelaan, O.; Hutson, J.L.; Fallowfield, H.J. Transport and retention of graphene oxide nanoparticles in sandy and carbonaceous aquifer sediments: Effect of physicochemical factors and natural biofilm. J. Environ. Manag. 2021, 278, 111419. [Google Scholar] [CrossRef]
- Dong, L.L.; Shi, C.; Guo, L.L.; Yang, T.; Sun, Y.X.; Cui, X.J. Fabrication of redox and pH dual-responsive magnetic graphene oxide microcapsules via sonochemical method. Ultrason. Sonochemistry 2017, 36, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.J.; Lin, Y.X.; Guo, X.T.; Li, S.L.; Cui, J.S.; Ping, H.X.; Zhang, J.; Zhong, R.W.; Du, L.S.; Han, C.X.; et al. Co-transport of graphene oxide and titanium dioxide nanoparticles in saturated quartz sand: Influences of solution pH and metal ions. Environ. Pollut. 2019, 251, 723–730. [Google Scholar] [CrossRef]
- Cao, J.J.; Bai, X.; Ye, Z.F.; Chen, W.; Ge, H.Y.; Ding, Y.Y.; Hua, Z.L. Enhanced Transport of TiO2-Reduced Graphene Oxide Nanocomposites in Saturated Porous Media: The Impact of Loaded TiO2 Shape and Solution Conditions. Water Air Soil Pollut. 2020, 231, 124. [Google Scholar] [CrossRef]
- Wang, M.J.; Zhang, H.J.; Chen, W.F.; Lu, T.T.; Yang, H.Y.; Wang, X.H.; Lu, M.H.; Qi, Z.C.; Li, D.L. Graphene oxide nanoparticles and hematite colloids behave oppositely in their co-transport in saturated porous media. Chemosphere 2021, 265, 129081. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, B.; Tang, D.; Yu, C. Concurrent aggregation and transport of graphene oxide in saturated porous media: Roles of temperature, cation type, and electrolyte concentration. Environ. Pollut. 2018, 235, 350–357. [Google Scholar] [CrossRef]
- Bai, B.; Nie, Q.K.; Wu, H.Y.; Hou, J.P. The attachment-detachment mechanism of ionic/nanoscale/microscale substances on quartz sand in water. Powder Technol. 2021, 394, 1158–1168. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Sotirelis, N.P.; Kallithrakas-kontos, N.G. Cotransport of Graphene Oxide Nanoparticles and Kaolinite Colloids in Porous Media. Transp. Porous Media 2017, 119, 181–204. [Google Scholar] [CrossRef]
- Tan, S.; Zhou, B.; Wang, Q. Effects of nanocarbon on the hydraulic parameters and the solute transport process for disturbed loessial soil. Arab. J. Geosci. 2016, 9, 4. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lu, T.T.; Wang, Y.; Li, J.Q.; Fu, X.W.; Qi, Z.C.; Zhang, Q. Transport of graphene oxide nanoparticles in saturated kaolinite- and goethite-coated sand columns: Effects of low-molecular-weight organic acids. Environ. Sci. Pollut. Res. 2019, 26, 24922–24932. [Google Scholar] [CrossRef]
- Esfahani, A.R.; Batelaan, O.; Hutson, J.L.; Fallowfield, H.J. Effect of bacteria and virus on transport and retention of graphene oxide nanoparticles in natural limestone sediments. Chemosphere 2020, 248, 125929. [Google Scholar] [CrossRef] [PubMed]
- Brachman, R.W.I.; Rowe, R.K.; Take, W.A. Reductions in GCL Overlap Beneath an Exposed Geomembrane. J. Geotech. Geoenvironmental Eng. 2018, 144, 12. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, T.; Zhang, Y.L.; Li, Z.C. Effect of Osmotic Pressure on Migration Behavior of nZnO in GCL. Adv. Civ. Engineering 2018, 2018, 6703485. [Google Scholar] [CrossRef]
- Ozcoban, M.S.; Acarer, S.; Tufekci, N. Effect of solid waste landfill leachate contaminants on hydraulic conductivity of landfill liners. Water Sci. Technol. 2022, 85, 1581–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, C.H.; Zhang, J.W.; Zhang, Z.G. Review of the Anti-Pollution Performance of Triple-Layer GM/GCL/AL Composite Liners. Membranes 2022, 12, 922. [Google Scholar] [CrossRef]
- Zeng, Z.M.; Lu, Y.; Wan, T.; Lin, S.; Nong, X.Z.; Sun, J.J. Modeling the Salinity Effect on the Water Retention Curve of Geosynthetic Clay Liner (GCL) on the Drying Path. Materials 2023, 16, 5468. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Peng, C.J.; Liu, H.; Meng, L.F. Breakthrough times for barrier systems at typical municipal solid waste landfills in China. Environ. Sci. Pollut. Res. 2023, 30, 58773–58782. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jiang, X.H.; Yang, W.; Geng, Z.; Huo, M.X.; Liu, Z.M.; Zhou, H. Transport of graphene oxide in saturated porous media: Effect of cation composition in mixed Na–Ca electrolyte systems. Sci. Total Environ. 2015, 511, 509–515. [Google Scholar] [CrossRef]
- Sun, C.; Sun, B.; Zhong, Y.; Jiang, J. Numerical Simulation of the Intensified Heat Exchange of CUO-H2O Nano-fluid. J. Eng. Therm. Energy Power 2015, 30, 200–204. [Google Scholar]
- Lu, T.T.; Xia, T.J.; Zhang, C.D.; Chen, W. Effects of clay minerals on transport of graphene oxide in saturated porous media. Environ. Toxicol. Chem. 2017, 36, 655–660. [Google Scholar] [CrossRef]
- Jones, E.H.; Su, C. Transport and retention of zinc oxide nanoparticles in porous media: Effects of natural organic matter versus natural organic ligands at circumneutral PH. J. Hazard. Mater. 2014, 275, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shang, J.Y.; Zheng, X.; Zhao, K.; Yan, C.R.; Sharma, P.; Liu, K. Effect of physicochemical factors on transport and retention of graphene oxide in saturated media. Environ. Pollut. 2018, 236, 168–176. [Google Scholar] [CrossRef] [PubMed]
| Specific Surface Area (cm2/g) | Rate of Single Layer (%) | Thickness of Single Layer (nm) | Solubility (%) |
|---|---|---|---|
| 1217 | 98% | 1.0 nm | 25% |
| Test Number | Temperature (°C) | IS (mmol/L) | OC (mg/L) | |
|---|---|---|---|---|
| Temperature group | 1 (reference test) | 30 | 10 | 1 |
| 2 | 40 | 10 | 1 | |
| 3 | 50 | 10 | 1 | |
| 4 | 60 | 10 | 1 | |
| 5 | 70 | 10 | 1 | |
| IS group | 1 | 30 | 10 | 1 |
| 6 | 30 | 20 | 1 | |
| 7 | 30 | 30 | 1 | |
| 8 | 30 | 40 | 1 | |
| 9 | 30 | 50 | 1 | |
| OC group | 1 | 30 | 10 | 1 |
| 10 | 30 | 10 | 3 | |
| 11 | 30 | 10 | 5 | |
| 12 | 30 | 10 | 7 | |
| 13 | 30 | 10 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, T. Effects of Temperature, Ionic Strength and Humic Acid on the Transport of Graphene Oxide Nanoparticles in Geosynthetic Clay Liner. Materials 2024, 17, 2082. https://doi.org/10.3390/ma17092082
Liu Y, Jiang T. Effects of Temperature, Ionic Strength and Humic Acid on the Transport of Graphene Oxide Nanoparticles in Geosynthetic Clay Liner. Materials. 2024; 17(9):2082. https://doi.org/10.3390/ma17092082
Chicago/Turabian StyleLiu, Yaohui, and Tao Jiang. 2024. "Effects of Temperature, Ionic Strength and Humic Acid on the Transport of Graphene Oxide Nanoparticles in Geosynthetic Clay Liner" Materials 17, no. 9: 2082. https://doi.org/10.3390/ma17092082
APA StyleLiu, Y., & Jiang, T. (2024). Effects of Temperature, Ionic Strength and Humic Acid on the Transport of Graphene Oxide Nanoparticles in Geosynthetic Clay Liner. Materials, 17(9), 2082. https://doi.org/10.3390/ma17092082







