The Influence of Al and Nb on the Low Oxygen Pressure Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl-yNb Alloys at 1000 °C
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Pre-Oxidation Experiment
2.3. Sample Analysis
3. Results and Discussion
3.1. Effect of Al on the Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl Alloy
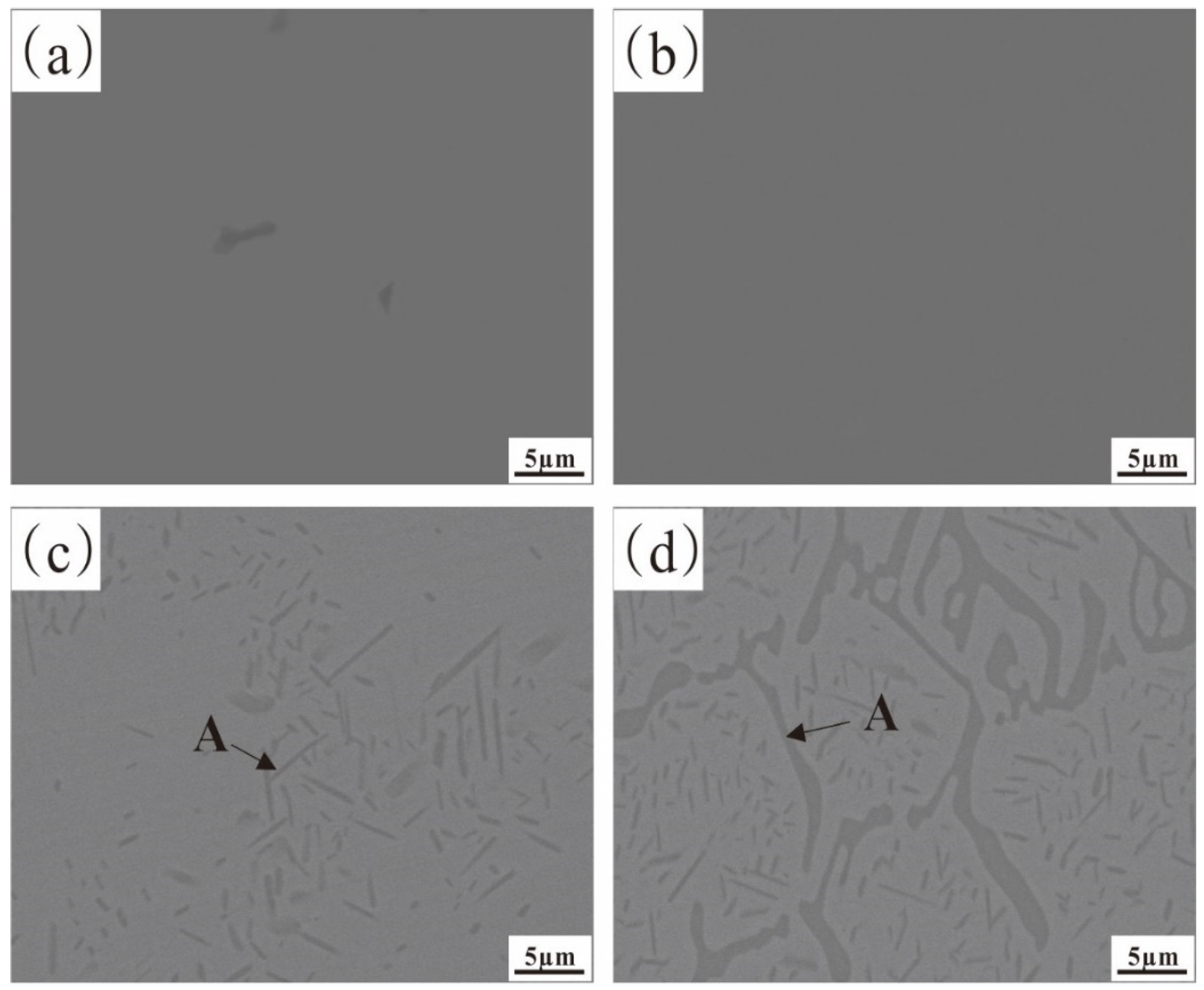
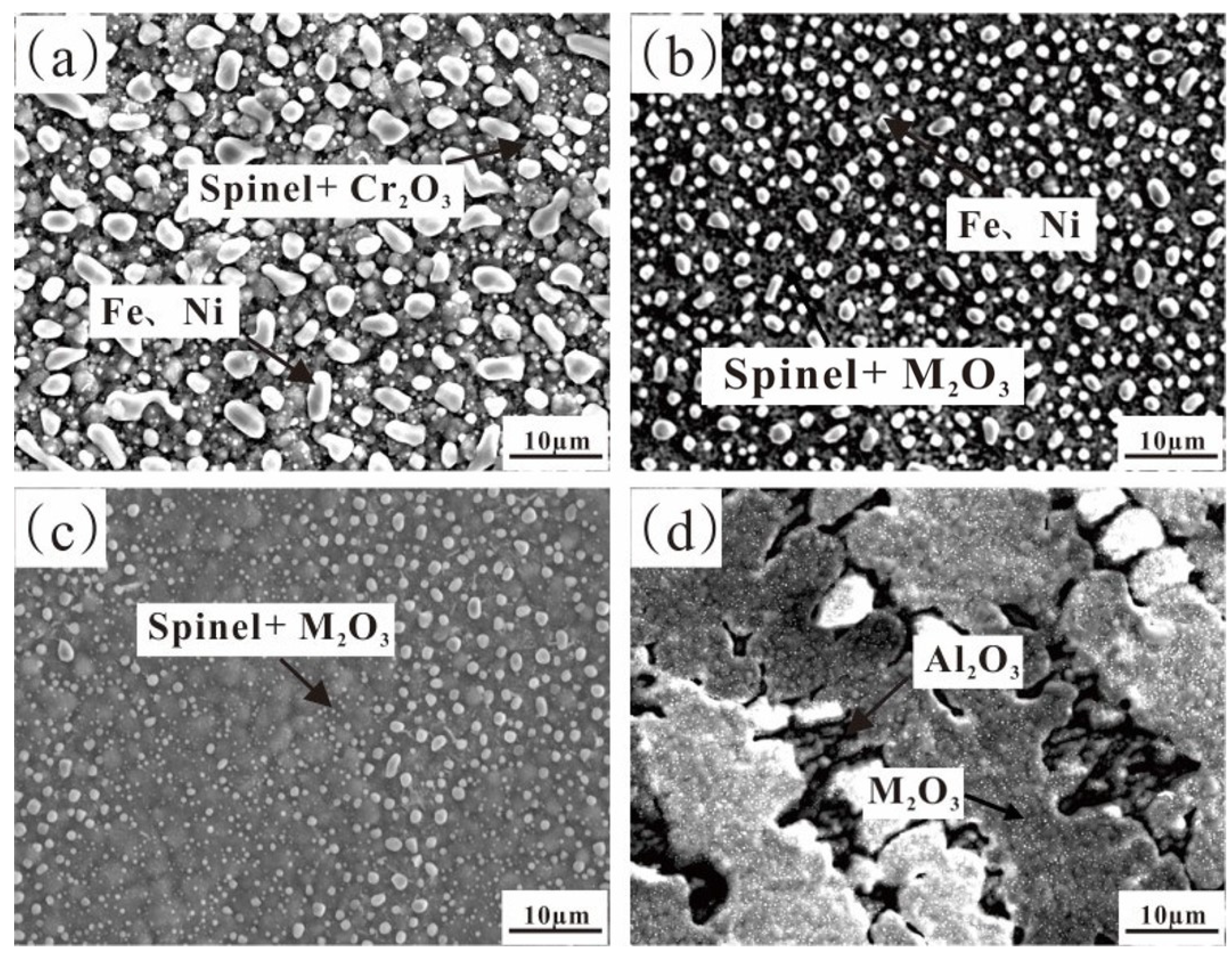
3.1.1. The Microstructure of the Fe-35Ni-20Cr-xAl Alloy
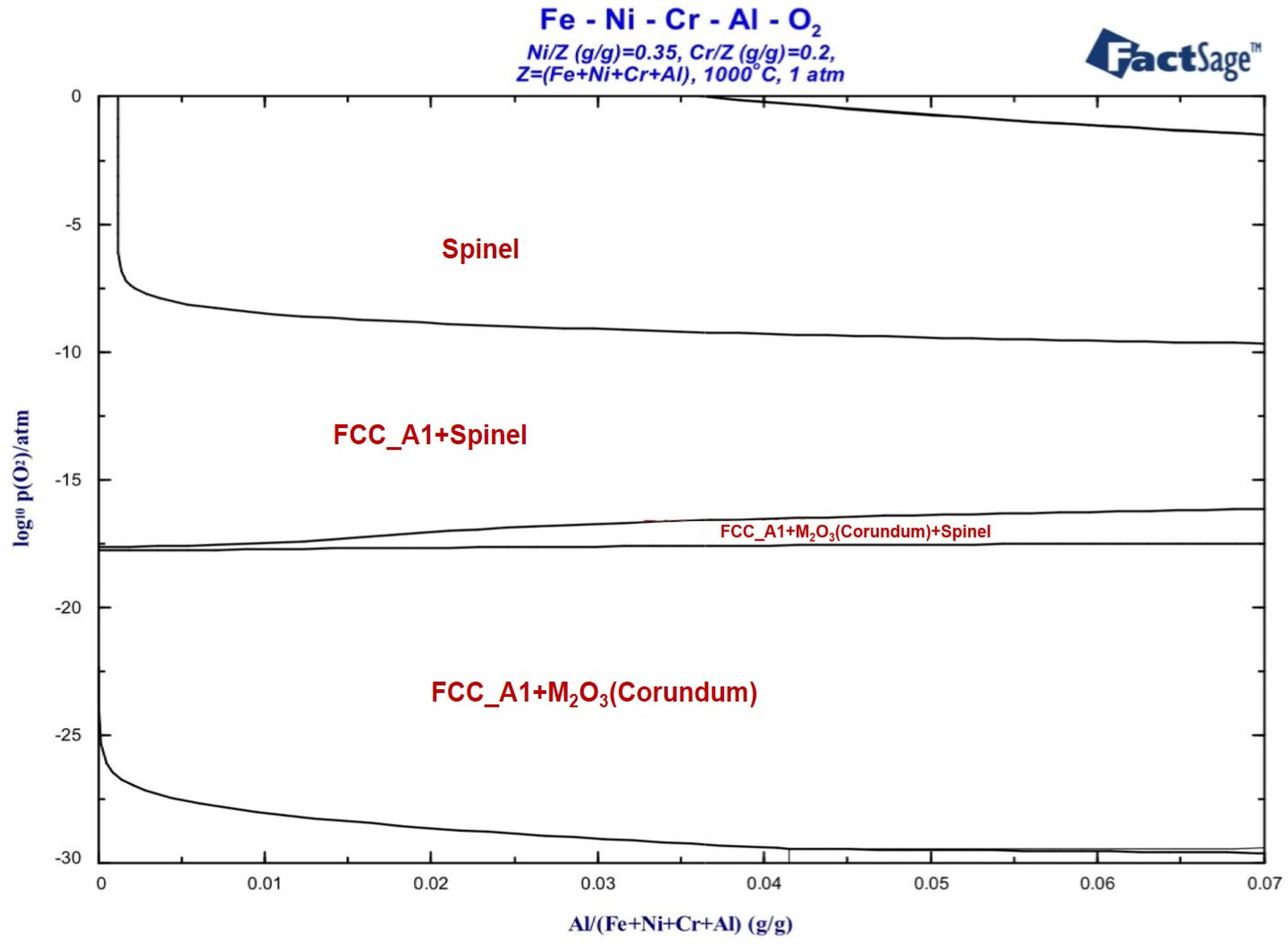
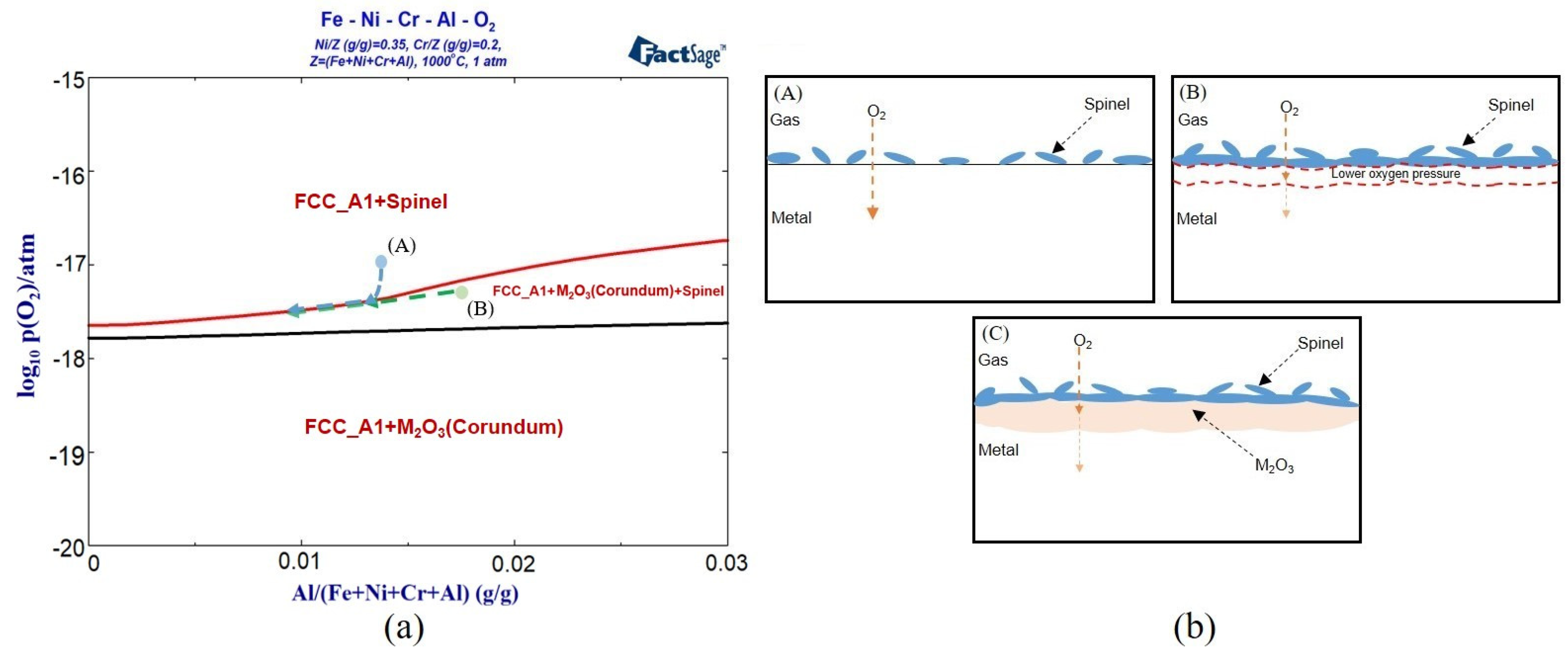
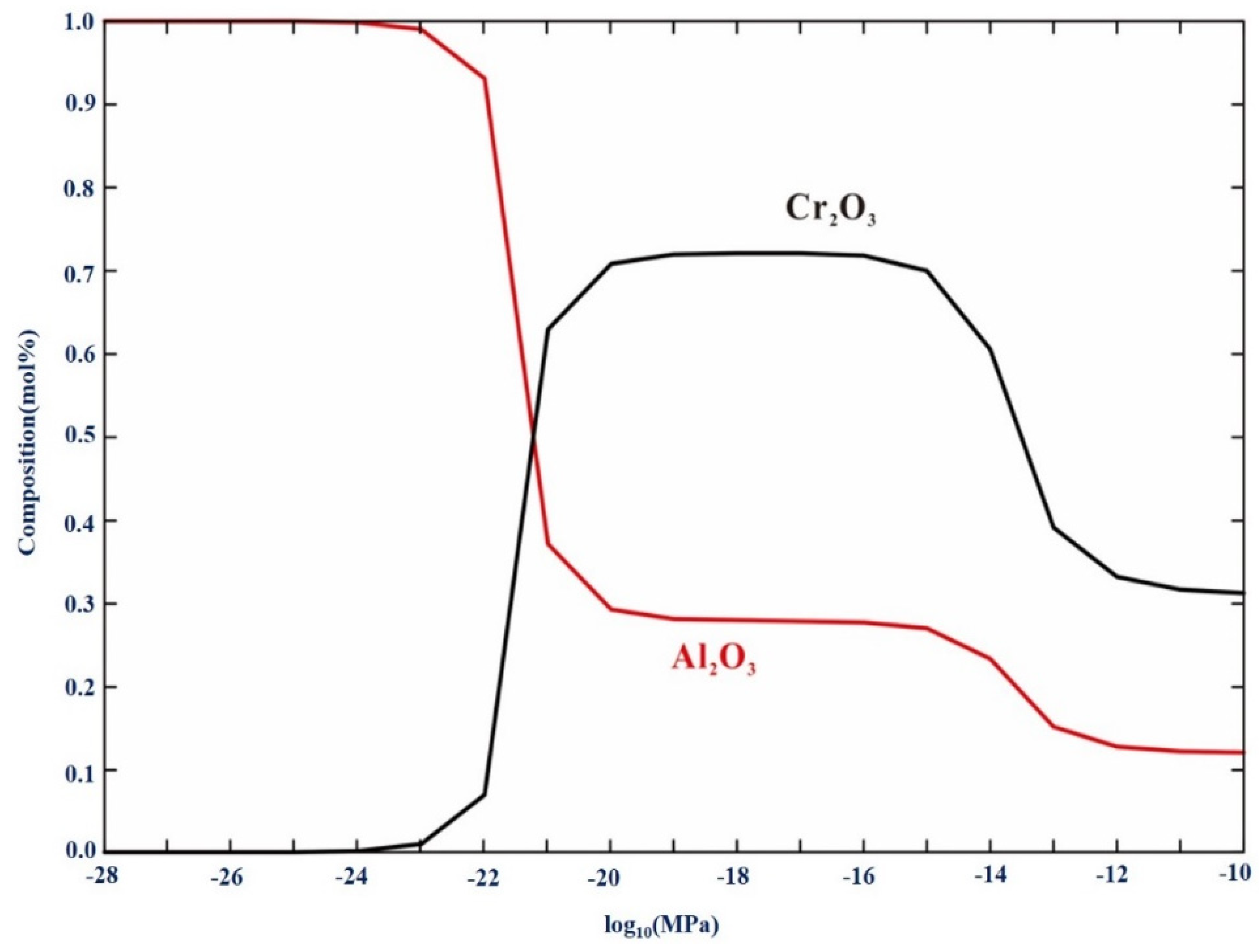
3.1.2. Calculation of Oxidation Phase Diagram
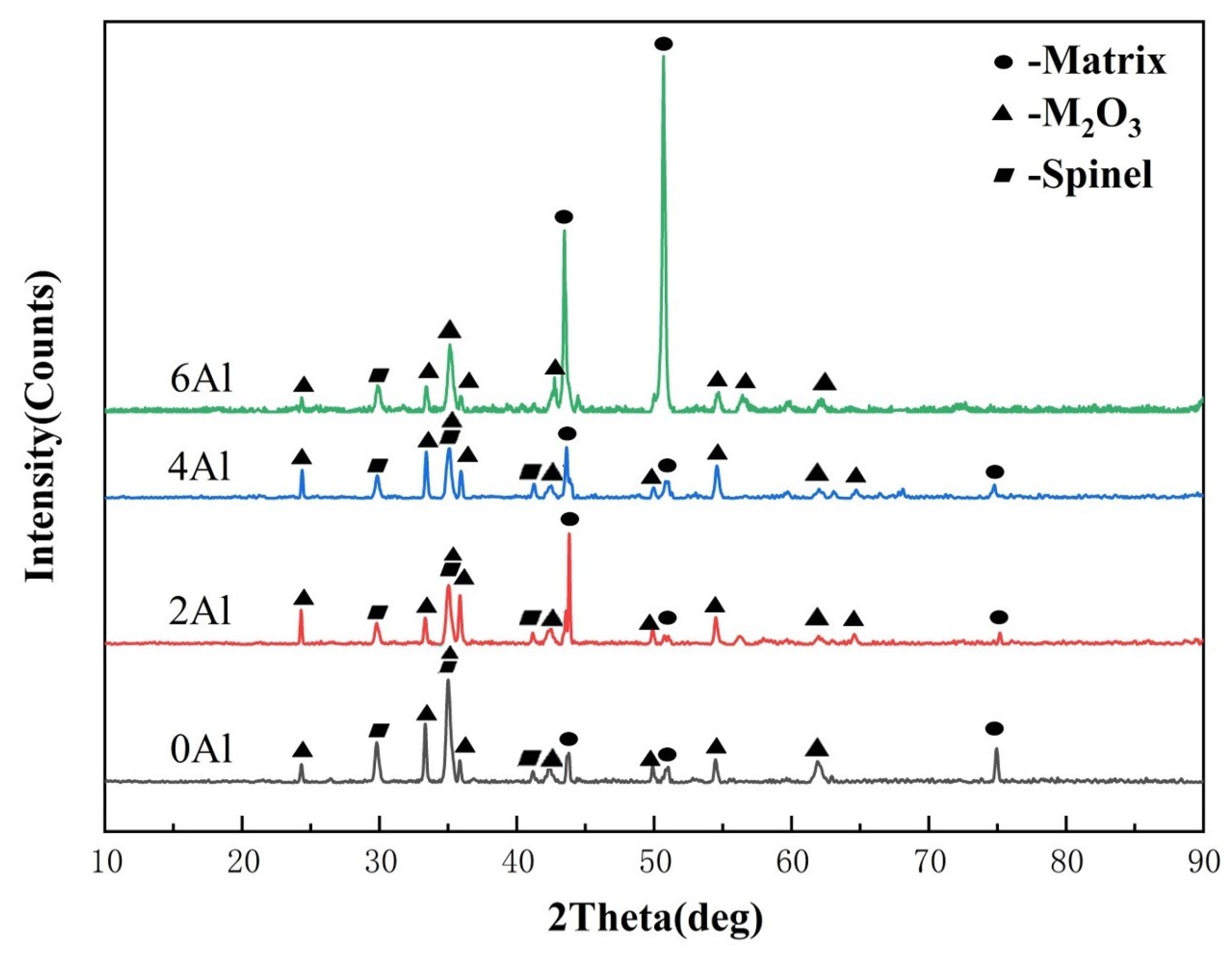
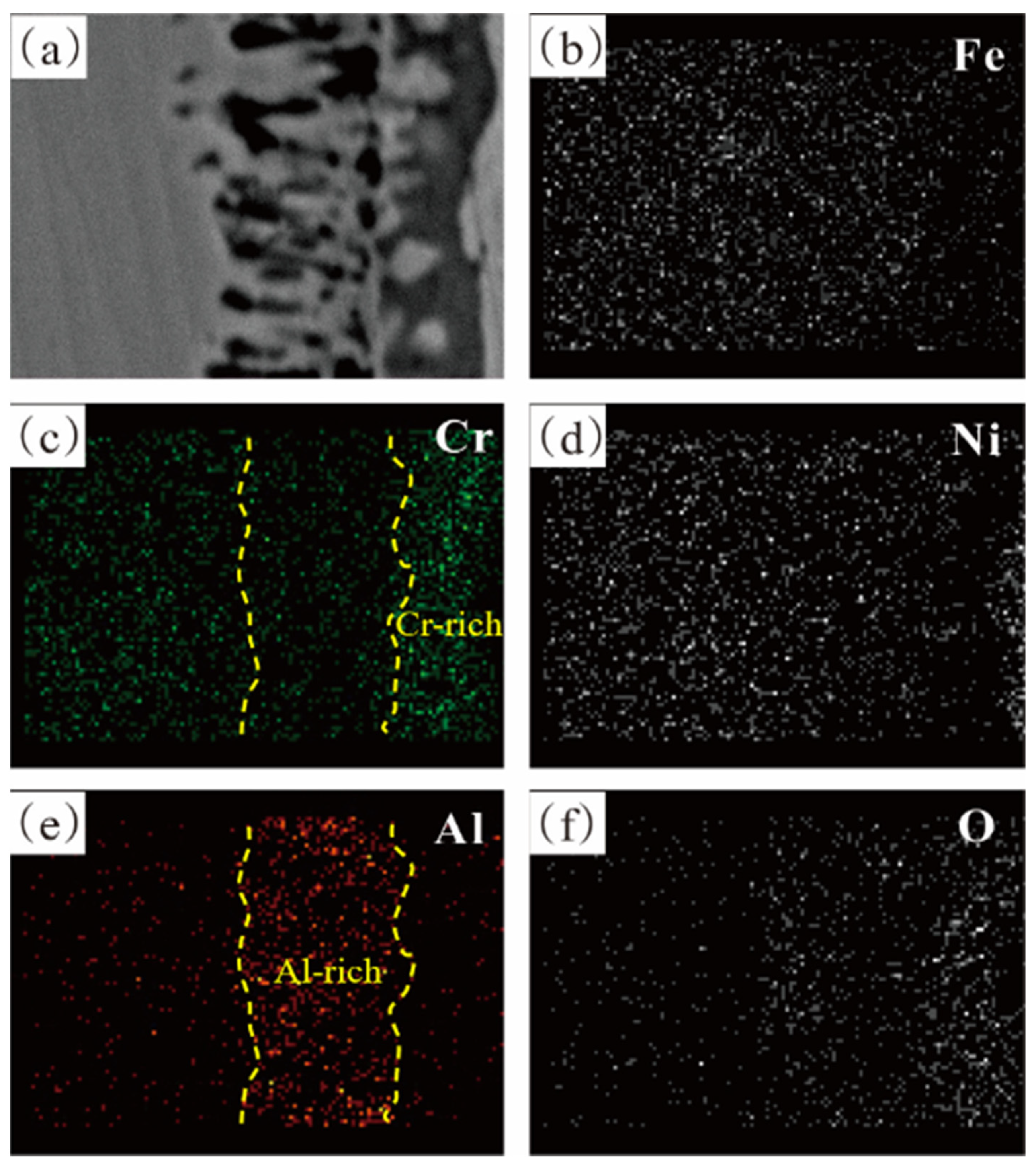
3.1.3. Analysis of Pre-Oxidation Products
3.2. The Influence of Nb on the Pre-Oxidation Behavior of Fe-35Ni-20Cr-4Al-yNb Alloy
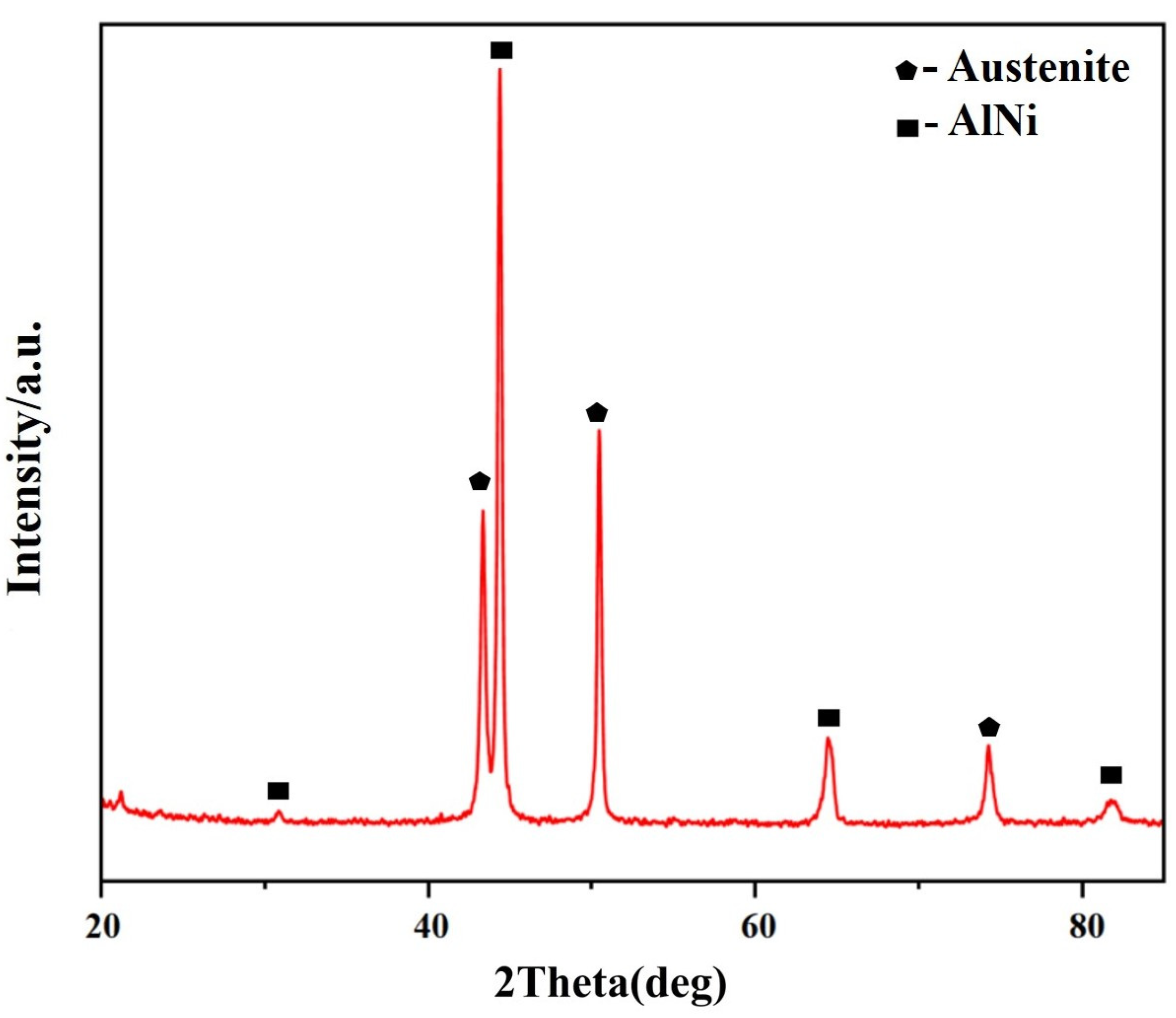
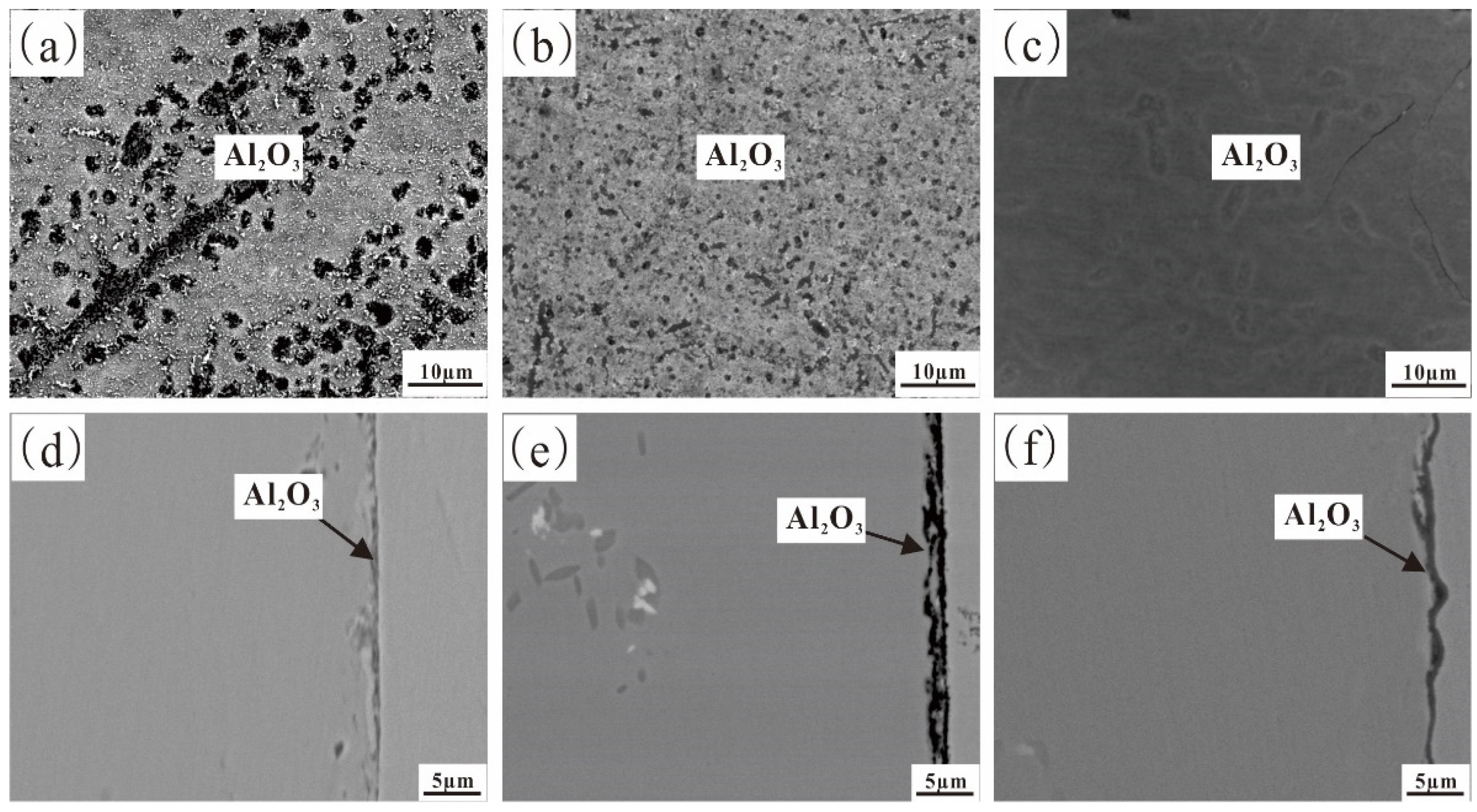
3.2.1. The Microstructure of the Fe-35Ni-20Cr-4Al-yNb Alloy
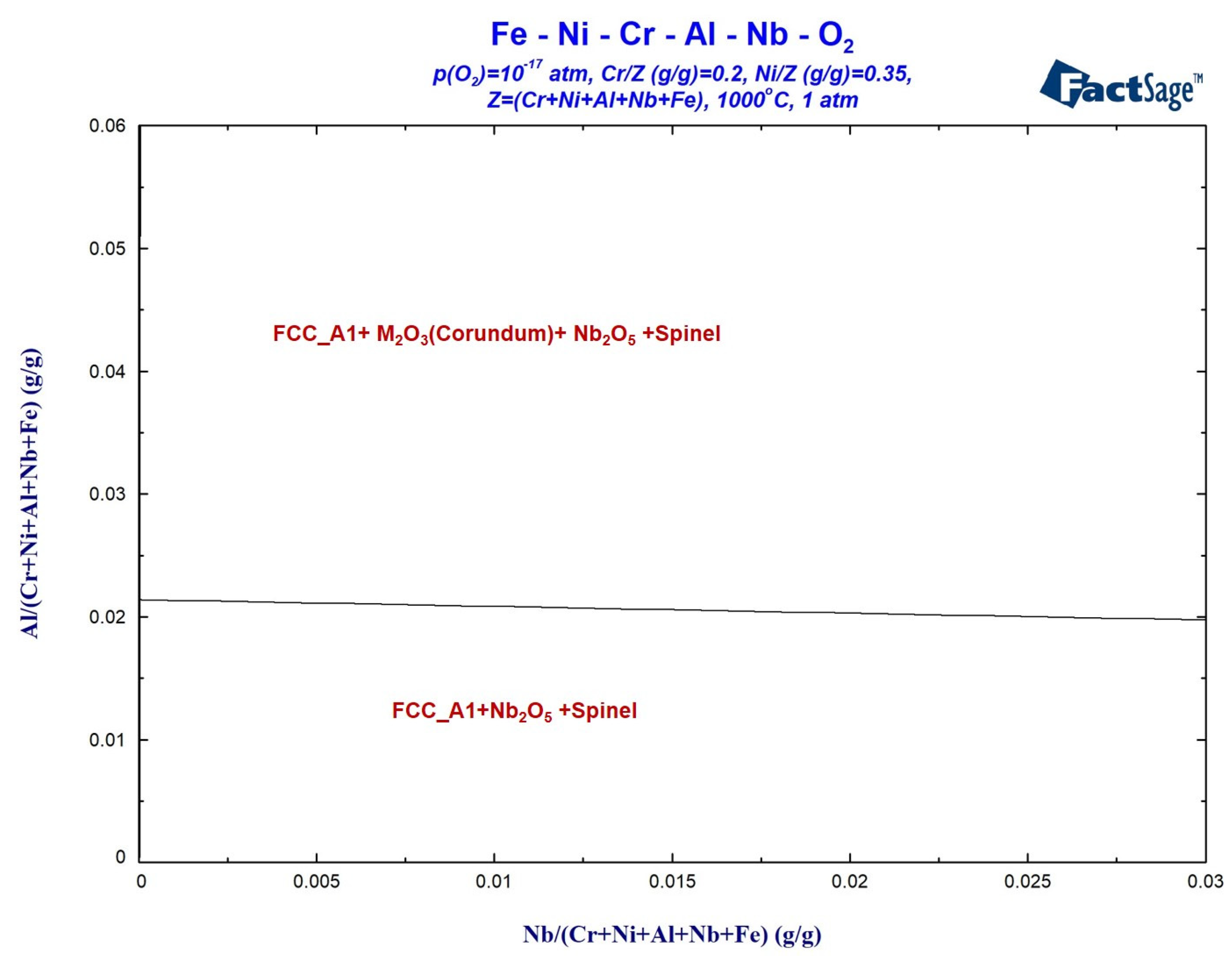
3.2.2. Calculation of Oxidation Phase Diagram
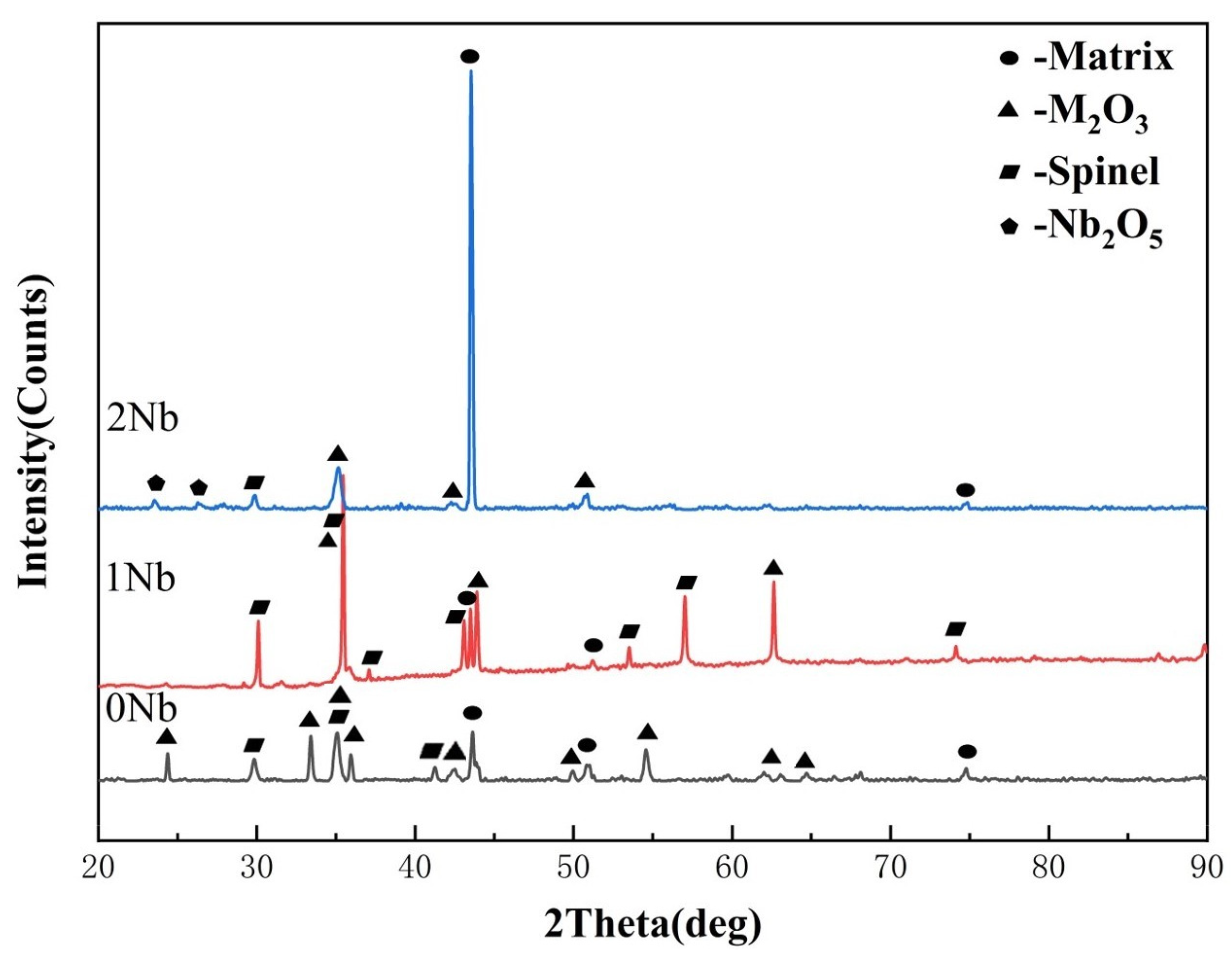
3.2.3. Analysis of Pre-Oxidation Products
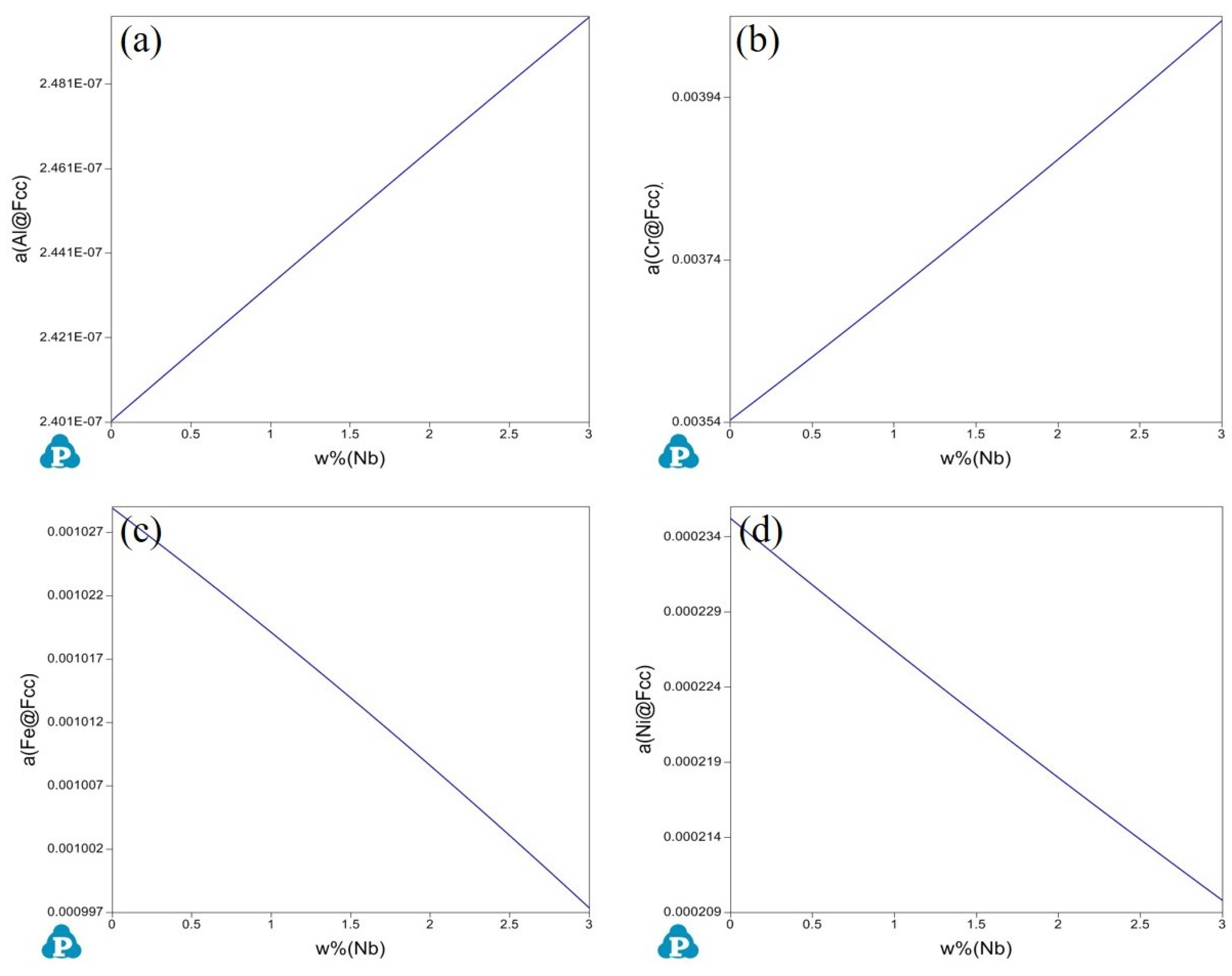
3.3. Thermodynamic Calculation and Analysis
4. Conclusions
- (1)
- For Fe-35Ni-20Cr-xAl (x = 0, 2, 4, 6) alloys, at 1000 °C under oxygen pressures of 10−17 atm., the pre-oxidation protective film consisted of a continuous outer layer of FeCr2O4, M2O3 (M: Al and Cr), and an inner layer composite oxide film of Al2O3. Increasing the content of Al resulted in an increase in Al2O3 content in the oxidation products. Al promoted the formation of M2O3 and prevented the growth of FeCr2O4, leading to the formation of a continuous inner Al2O3 oxide film. When the Al content reached 6%, partial delamination of the oxide film occurred on the alloy surface.
- (2)
- The addition of Nb enhanced the activity of Cr and Al elements while reducing the activity of Ni and Fe elements. Increase in element activity enhanced the driving force for the formation of their oxides. At an oxidation temperature of 1000 °C under oxygen pressure of 10−17 atm., the amount of M2O3 formed on the alloy surface increased with the addition of Nb, resulting in a gradually denser oxidation layer. More continuous internal oxidation of Al2O3 occurred, which inhibited the formation of spinel, and a Nb-rich oxide, Nb2O5, appeared internally.
- (3)
- As the oxygen pressure decreased, the driving force for oxide formation also decreased. The experiment and calculations show that the amount of Cr2O3 in the oxide layer decreased, while the amount of Al2O3 increased. When the oxygen pressure was as low as 10−25 atm., only Al2O3 was formed.
- (4)
- By adding Nb and reducing the oxygen pressure, a continuous and dense Al2O3 oxide film on Fe-35Ni-20Cr-4Al-2Nb alloy pre-oxidized for 5 h at 1000 °C under oxygen pressure of 10−25 atm. was obtained.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lai, G.Y. High-Temperature Corrosion of Engineering Alloys; ASM International: Almere, The Netherlands, 1990. [Google Scholar]
- Lu, B.Y.; Gao, Q.Z.; Zhang, H.L.; Ma, Q.S.; Li, H.J.; Liu, Z.Y.; Sun, L.L. Strengthening and fracture mechanisms of Fe-20Ni-14Cr-2Cu alumina-forming austenitic steel during creeping. J. Mater. Sci. 2022, 57, 20472–20484. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Santella, M.L.; Bei, H.; Maziasz, P.J.; Pint, B.A. Overview of strategies for high-temperature creep and oxidation resistance of alumina-forming austenitic stainless steels. Metall. Mater. Trans. A 2011, 42, 922–931. [Google Scholar] [CrossRef]
- Stott, F.H. Protective oxide scales and their breakdown. Corros. Sci. 1997, 39, 1497–1498. [Google Scholar] [CrossRef]
- Parimin, N.; Hamzah, E. Oxidation kinetics of Fe-Ni-Cr alloy at 900 °C. Mater. Sci. Forum. 2020, 1010, 58–64. [Google Scholar] [CrossRef]
- Wanderka, N.; Mousa, M.S.; Henke, P.; Korchuganova, O.; Mukherji, D.; Rösler, J.; Banhart, J. Carbides in Co-Re-Cr-based high-temperature alloys. J. Mater. Sci. 2016, 51, 7145–7155. [Google Scholar] [CrossRef]
- Young, D.J.; Pint, B.A. Chromium volatilization rates from Cr2O3 scales into flowing gases containing water vapor. Oxid. Met. 2006, 66, 137–153. [Google Scholar] [CrossRef]
- Asteman, H.; Svensson, J.-E.; Johansson, L.-G.; Norell, M. Indication of chromium oxide hydroxide evaporation during oxidation of 304 L at 873 K in the presence of 10% water vapor. Oxid. Met. 1999, 52, 95–111. [Google Scholar] [CrossRef]
- Asteman, H.; Svensson, J.-E.; Norell, M.; Johansson, L.-G. Influence of water vapor and flow rate on the high-temperature oxidation of 304 L; effect of chromium oxide hydroxide evaporation. Oxid. Met. 2000, 54, 11–26. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, S.P.; Bai, X.; Zhou, X.H.; Han, X.X. Oxidation behavior of Fe-Al-Cr alloy at high temperature: Experiment and a first principle study. Vacuum 2020, 173, 109144. [Google Scholar] [CrossRef]
- Fujikawa, H.; Newcomb, S.B. High temperature oxidation behaviour of high Al content ferritic and austenitic stainless steels with and without rare-earth element addition. Oxid. Met. 2012, 77, 85–92. [Google Scholar] [CrossRef]
- Meier, G.H. Research on oxidation and embrittlement of Intermetallic Compounds in the U.S. Mater. Corros. 1996, 47, 595–618. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Chen, G.; Lu, Z. Improvement of high-temperature oxidation resistance and strength in alumina-forming austenitic stainless steels. Mater. Lett. 2011, 65, 3285–3288. [Google Scholar] [CrossRef]
- Brady, M.P.; Magee, J.; Yamamoto, Y.; Helmick, D.; Wang, L. Co-optimization of wrought alumina-forming austenitic stainless steel composition ranges for high-temperature creep and oxidation/corrosion resistance. Mater. Sci. Eng. A 2014, 590, 101–115. [Google Scholar] [CrossRef]
- Asteman, H.; Hartnagel, W.; Jakobi, D. The influence of Al content on the high temperature oxidation properties of state-of-the-art cast Ni-base alloys. Oxid. Met. 2013, 80, 3–12. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433–436. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al alloys between 1000 ℃ and 1200 ℃. J. Electrochem. Soc. 1971, 118, 1782. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C.; Stringer, J. The influence of alloying elements on the development and maintenance of protective scales. Oxid. Met. 1995, 44, 113–145. [Google Scholar] [CrossRef]
- Hall, J.; Hellström, K.; Svensson, J.-E.; Norell, M.; Lundberg, M.; Helander, T.; Johansson, L.-G. The initial oxide scale development on a model FeNiCrAl alloy at 900 ℃ in dry and humid atmosphere: A detailed investigation. Oxid. Met. 2014, 82, 225–247. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Maziasz, P.J.; Pint, B.A.; Liu, C.T.; Lu, Z.P.; Bei, H. The development of alumina-forming austenitic stainless steels for high-temperature structural use. J. Miner. Met. Mater. Soc. 2008, 60, 12–18. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Santella, M.L.; Pint, B.A. Effects of minor alloy additions and oxidation temperature on protective alumina scale formation in creep-resistant austenitic stainless steels. Scr. Mater. 2007, 57, 1117–1120. [Google Scholar] [CrossRef]
- Brady, M.P.; Yamamoto, Y.; Pint, B.A.; Santella, M.L.; Maziasz, P.J.; Walker, L.R. On the loss of protective scale formation in creep-resistant, alumina-forming austenitic stainless steels at 900 ℃ in air. Mater. Sci. Forum. 2008, 595, 725–732. [Google Scholar] [CrossRef]
- Chen, G.; Sun, Z.; Zhou, X. Oxidation of intermetallic alloys in Ti-Al-Nb ternary system. Corrosion 1992, 48, 939–946. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Shen, L.; Wu, B.J.; Zhao, K.; Peng, H.B.; Wen, Y.H. Reason for negative effect of Nb addition on oxidation resistance of alumina-forming austenitic stainless steel at 1323 K. Corros. Sci. 2021, 191, 109754. [Google Scholar] [CrossRef]
- Ranganathan, S.; Hajra, J.P. Alloy oxide equilibria in the Cr-Mn-O system. Bull. Mater. Sci. 1987, 9, 149–158. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melançon, J.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Chen, S.L.; Daniel, S.; Zhang, F.; Chang, Y.A.; Yan, X.Y.; Xie, F.Y.; Schmid-Fetzer, R.; Oates, W.A. The PANDAT software package and its applications. Calphad 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Xu, C.; Gao, W. Pilling-Bedworth ratio for oxidation of alloys. Mater. Res. Innov. 2000, 3, 231–235. [Google Scholar] [CrossRef]
- Li, S.M.; Qian, H.Q.; Qin, L. The analysis of the volume ratio of oxide on the alloy. Corros. Sci. Prot. Technol. 1999, 11, 284–289. [Google Scholar]
- Jung, I.H.; Paliwal, M.; Kim, Y.M.; Lee, S.K.; Kim, J.S. Thermodynamic Analysis of the Oxidation of High-Strength Steels. In Proceedings of the Asia-Pacific Galvanizing Conference, Jeju, Republic of Korea, 8–12 November 2009. [Google Scholar]
- Zhang, M.; Xu, B.J.; Ling, G.P. Preparation and characterization of α-Al2O3 film by low temperature thermal oxidation of Al8Cr5 coating. Appl. Surf. Sci. 2015, 331, 1–7. [Google Scholar] [CrossRef]
- Hancock, P.; Hurst, R.C. The mechanical properties and breakdown of surface oxide films at elevated temperatures. Adv. Corros. Sci. Technol. 1974, 4, 1–84. [Google Scholar]
- Yuan, M.M.; Zhao, J.Y.; Liu, Y.; Li, D.H.; Kolawole, S.K.; Su, X.P. Effect of silicon on the formation of protective scale on Fe-10Ni-10Cr-4Al alloy pre-oxidized at low oxygen pressure. Mater. Today Commun. 2023, 34, 105167. [Google Scholar] [CrossRef]







| Powder | Temperature | P(O2) |
|---|---|---|
| Fe/FeO | 860 °C | 10−17 atm. |
| Cr/Cr2O3 | 900 °C | 10−25 atm. |
| Alloys | Phase | O | Al | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Fe-35Ni-20Cr | Fe + Ni particles | 8.7 | -- | 4.6 | 32.6 | 54.0 |
| Spinel + Cr2O3 | 58.3 | -- | 26.9 | 13.8 | 1.0 | |
| 0Fe-35Ni-20Cr-2Al | Spinel + M2O3 | 56.2 | 0.8 | 30.0 | 11.9 | 1.1 |
| Fe-35Ni-20Cr-4Al | Spinel + M2O3 | 60.7 | 1.1 | 30.5 | 6.4 | 1.3 |
| Fe-35Ni-20Cr-6Al | M2O3 | 59.8 | 1.4 | 28.2 | 8.9 | 1.7 |
| Al2O3 | 59.2 | 36.5 | 2.2 | 1.6 | 0.5 |
| Alloys | Phase | O | Al | Cr | Fe | Ni |
|---|---|---|---|---|---|---|
| Fe-35Ni-20Cr | Spinel + Cr2O3 | 56.5 | -- | 27.0 | 13.2 | 3.3 |
| Fe-35Ni-20Cr-2Al | Spinel + M2O3 | 57.0 | 0.8 | 27.2 | 9.3 | 5.7 |
| Al2O3 | 42.1 | 21.7 | 5.6 | 17.1 | 13.5 | |
| Fe-35Ni-20Cr-4Al | Spinel + M2O3 | 57.4 | 0.5 | 28.0 | 8.7 | 5.4 |
| Al2O3 | 50.6 | 29.1 | 3.2 | 10.3 | 6.8 | |
| Fe-35Ni-20Cr-6Al | M2O3 | 60.6 | 1.6 | 24.0 | 11.4 | 2.4 |
| Al2O3 | 48.7 | 29.5 | 4.8 | 9.1 | 7.9 |
| Alloys | Phase | O | Al | Cr | Fe | Ni | Nb |
|---|---|---|---|---|---|---|---|
| Fe-35Ni-20Cr-4Al | Spinel + M2O3 | 57.4 | 0.5 | 28.0 | 8.7 | 5.4 | -- |
| Al2O3 | 50.6 | 29.1 | 3.2 | 10.3 | 6.8 | -- | |
| Fe-35Ni-20Cr-4Al-1Nb | Spinel + M2O3 | 61.1 | 0.3 | 30.8 | 5.1 | 2.6 | 0.1 |
| Al2O3 | 60.4 | 34.7 | 1.2 | 2.0 | 1.6 | 0.1 | |
| Fe-35Ni-20Cr-4Al-2Nb | Spinel + M2O3 | 64.6 | 0.6 | 30.8 | 2.3 | 1.4 | 0.3 |
| Al2O3 | 60.0 | 36.0 | 1.8 | 1.1 | 1.0 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Yuan, M.; Liu, Y.; Chen, J.; Wu, C.; Su, X. The Influence of Al and Nb on the Low Oxygen Pressure Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl-yNb Alloys at 1000 °C. Materials 2024, 17, 2086. https://doi.org/10.3390/ma17092086
Chen L, Yuan M, Liu Y, Chen J, Wu C, Su X. The Influence of Al and Nb on the Low Oxygen Pressure Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl-yNb Alloys at 1000 °C. Materials. 2024; 17(9):2086. https://doi.org/10.3390/ma17092086
Chicago/Turabian StyleChen, Lang, Manman Yuan, Ya Liu, Junxiu Chen, Changjun Wu, and Xuping Su. 2024. "The Influence of Al and Nb on the Low Oxygen Pressure Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl-yNb Alloys at 1000 °C" Materials 17, no. 9: 2086. https://doi.org/10.3390/ma17092086
APA StyleChen, L., Yuan, M., Liu, Y., Chen, J., Wu, C., & Su, X. (2024). The Influence of Al and Nb on the Low Oxygen Pressure Pre-Oxidation Behavior of Fe-35Ni-20Cr-xAl-yNb Alloys at 1000 °C. Materials, 17(9), 2086. https://doi.org/10.3390/ma17092086








