A Study on the Microstructure Regulation Effect of Niobium Doping on LiNi0.88Co0.05Mn0.07O2 and the Electrochemical Performance of the Composite Material under High Voltage
Abstract
:1. Introduction
2. Experimental Component
2.1. Material Preparation
2.2. Material Characterization
2.3. Electrochemical Testing
3. Results and Discussion
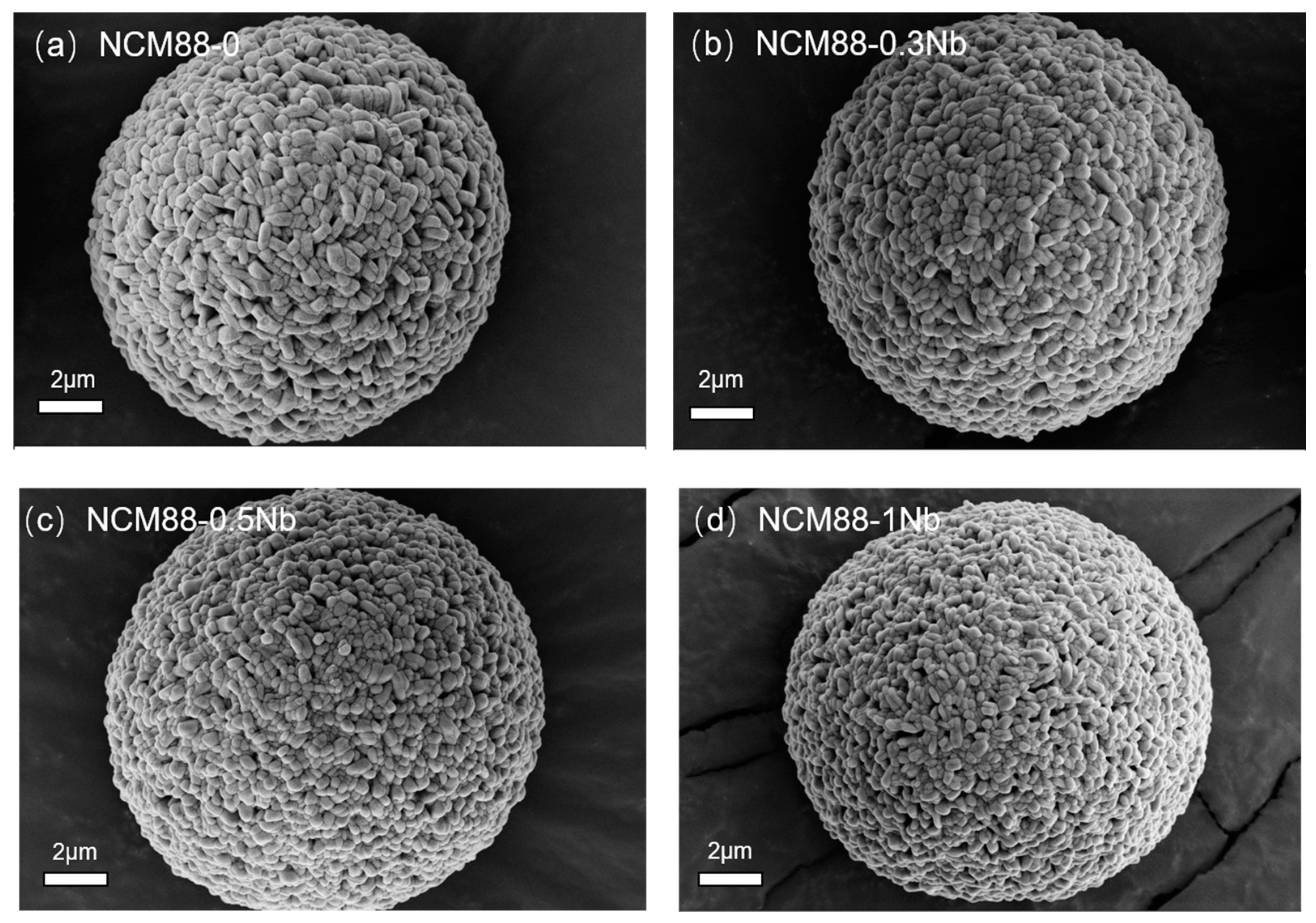
3.1. LiNi0.88Co0.05Mn0.07O2@Nb2O5 Physical Characterization
3.2. LiNi0.88Co0.05Mn0.07O2@Nb2O5 High-Voltage Electrochemical Performance Test
3.3. Surface and Cross-Sectional Morphological Characterization of LiNi0.88Co0.05Mn0.07O2@Nb2O5 after Cycling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, Y.; Huang, S.; Zhao, Y.; Roy, S.; Lu, X.; Hou, Y.; Zhang, J. A review of nickel-rich layered oxide cathodes: Synthetic strategies, structural characteristics, failure mechanism, improvement approaches and prospects. Appl. Energy 2022, 305, 117849. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, D.; Zhang, C. An overview of electricity powered vehicles: Lithium-ion battery energy storage density and energy conversion efficiency. Renew. Energy 2020, 162, 1629–1648. [Google Scholar] [CrossRef]
- Ding, Y.; Mu, D.; Wu, B.; Wang, R.; Zhao, Z.; Wu, F. Recent progresses on nickel-rich layered oxide positive electrode materials used in lithium-ion batteries for electric vehicles. Appl. Energy 2017, 195, 586–599. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2016, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Zuo, Q.; Liu, W.; Su, Y.; Cao, Y.; Ren, K.; Wang, Y. Synthesis of LiCoO2 cathode materials for Li-ion batteries at low temperatures. Scr. Mater. 2023, 233, 115511. [Google Scholar] [CrossRef]
- Meng, D.; Duan, H.; Wu, S.; Ren, X.; Yuan, S. Lithium iron phosphate with high-rate capability synthesized through hydrothermal reaction in low Li concentration solution. J. Alloys Compd. 2023, 967, 171570. [Google Scholar] [CrossRef]
- Tang, H.; Chang, Z.; Zhao, H.; Yuan, X.-Z.; Wang, H.; Gao, S. Effects of precursor treatment on the structure and electrochemical properties of spinel LiMn2O4 cathode. J. Alloys Compd. 2013, 566, 16–21. [Google Scholar] [CrossRef]
- Shan, R.; Lu, X.; Xu, Y.; Shen, K.; Xia, Y.; Cai, Y.; Yao, J.; Mao, Q.; Wang, Y.; Ji, T. A ternary MOF derived single crystalline LiNi1/3Mn1/3Co1/3O2 as high-voltage cathodes for lithium-ion batteries. Chem. Eng. Sci. 2023, 268, 118416. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhu, J.H.; Wang, J.M.; Gan, Z.X.; Li, G.X.; Meng, C.Z. Experimental Study on Long Cycling Performance of NCM523 Lithium-Ion Batteries and Optimization of Charge-Discharge Strategy. J. Therm. Sci. 2020, 29, 1180–1192. [Google Scholar] [CrossRef]
- Yang, F.M.; Zhou, X.Y.; Li, X.D.; Yi, Z.C.; Feng, R.; He, G.W. Hollow urchin-shaped NCM811 ternary-structure for high rate charge/discharge capability and efficient CO2 adsorption. J. Environ. Chem. Eng. 2023, 11, 109445. [Google Scholar] [CrossRef]
- Azambou, C.I.; Djioko, F.H.K.; Obiukwu, O.O.; Tsobnang, P.K.; Kalu, E.E.; Kenfack, I.T.; Oguzie, E.E. Structural, electronic, mechanical and thermodynamic properties of lithium-rich layered oxides cathode materials for lithium-ion battery: Computational study. Mater. Today Commun. 2023, 35, 105738. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.-L.; Deng, Y.-P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Yuan, H.; Lu, L.; Xu, Z.; Lu, Z. Critical review on the degradation mechanisms and recent progress of Ni-rich layered oxide cathodes for lithium-ion batteries. Energy Chem. 2023, 5, 100103. [Google Scholar] [CrossRef]
- Xiao, P.; Li, W.; Chen, S.; Li, G.; Dai, Z.; Feng, M.; Chen, X.; Yang, W. Effects of Oxygen Pressurization on Li+/Ni2+ Cation Mixing and the Oxygen Vacancies of LiNi0.8Co0.15Al0.05O2 Cathode Materials. ACS Appl. Mater. Interfaces. 2022, 14, 31851–31861. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, X.; Li, F.; Zhang, J.; Liu, B.; Wang, L.; Yang, Y.; Hu, Z.; Ma, J.; Li, C.; et al. Bulk oxygen release inducing cyclic strain domains in Ni-rich ternary cathode materials. Energy Storage Mater. 2023, 55, 691–697. [Google Scholar] [CrossRef]
- Sun, H.-H.; Manthiram, A. Impact of Microcrack Generation and Surface Degradation on a Nickel-Rich Layered Li[Ni0.9Co0.05Mn0.05]O2 Cathode for Lithium-Ion Batteries. Chem. Mater. 2017, 29, 8486–8493. [Google Scholar] [CrossRef]
- Yu, R.; Zeng, W.; Zhou, L.; Van Tendeloo, G.; Mai, L.; Yao, Z.; Wu, J. Layer-by-layer delithiation during lattice collapse as the origin of planar gliding and microcracking in Ni-rich cathodes. Cell Rep. Phys. Sci. 2023, 4, 101480. [Google Scholar] [CrossRef]
- Yan, W.; Yang, S.; Huang, Y.; Yang, Y.; Guohui, Y. A review on doping/coating of nickel-rich cathode materials for lithium-ion batteries. J. Alloys Compd. 2020, 819, 153048. [Google Scholar] [CrossRef]
- Lv, H.; Li, C.; Zhao, Z.; Wu, B.; Mu, D. A review: Modification strategies of nickel-rich layer structure cathode (Ni ≥ 0.8) materials for lithium ion power batteries. J. Energy Chem. 2021, 60, 435–450. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, P.-b.; He, Z.-j.; Yang, Z.; Tang, L.-b.; An, C.-s.; Zheng, J.-c. Effect of MgO and TiO2 Coating on the Electrochemical Performance of Li-Rich Cathode Materials for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800829. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Q. Improving the electrochemical performance of LiNi0.7Mn0.3O2 cathode material by CeO2 coating via Ce doping. Solid State Ion. 2023, 399, 116296. [Google Scholar] [CrossRef]
- Herzog, M.J.; Esken, D.; Janek, J. Improved Cycling Performance of High-Nickel NMC by Dry Powder Coating with Nanostructured Fumed Al2O3, TiO2, and ZrO2: A Comparison. Batteries & Supercaps 2021, 4, 1003–1017. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Wang, Q.; Xu, G.; Miao, C.; Xu, M.; Wang, C.; Xiao, W. Investigation of W6+-doped in high-nickel LiNi0.83Co0.11Mn0.06O2 cathode materials for high-performance lithium-ion batteries. J. Colloid Interface Sci. 2022, 628, 338–349. [Google Scholar] [CrossRef]
- Yuan, S.; Yu, H.; Zhou, T.; Liang, R.; Jiang, G.; Xiong, J.; Zheng, A.; Li, k.; Wang, X. Preparation and electrochemical performance of La2O3 coated LiNi0.8Co0.1Mn0.1O2 material. Int. J. Electrochem. Sci. 2023, 18, 100141. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, L.; Wang, K.; Wu, X.; Zhou, P.; Miao, Z.; Zhou, J.; Zhao, Y.; Zhuo, S. Stabilizing the high-voltage cycle performance of LiNi0.8Co0.1Mn0.1O2 cathode material by Mg doping. J. Power Sources 2019, 438, 227017. [Google Scholar] [CrossRef]
- Chen, G.; Qian, G.; Zan, G.; Lun, M.; Su, F.; Stripe, B.; Chu, Y.S.; Pianetta, P.; Huang, X.; Li, J. Stabilizing Ni-rich layered cathode for high-voltage operation through hierarchically heterogeneous doping with concentration gradient. Mater. Today Chem. 2024, 35, 101845. [Google Scholar] [CrossRef]
- Che, W.; Wan, X.; Zhang, D.; Chang, C. Stabilized Performance of LiNi0.90Co0.07Al0.03O2 Cathodes via Zr4+ Doping upon 4.5 V Application due to the Suppression of H2–H3 Phase Transitions. ACS Sustain. Chem. Eng. 2021, 9, 5536–5545. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, R.; Xiao, F.; Zeng, L.; Wang, Y.; Huang, H. Elevated rate and cycling performance of nickel-rich single-crystal at high voltage enabled by Al/Ce co-doping. J. Power Sources 2024, 597, 234133. [Google Scholar] [CrossRef]
- Bizzotto, F.; Dachraoui, W.; Grissa, R.; Zhao, W.; Pagani, F.; Querel, E.; Kühnel, R.-S.; Battaglia, C. Modification of NMC811 with titanium for enhanced cycling and high-voltage stability. Electrochim. Acta 2023, 462, 142758. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, S.; Niu, H.; Wang, Z. Understanding the role of TiO2 coating for stabilizing 4.6V high-voltage LiCoO2 cathode materials. Electrochim. Acta 2024, 478, 143862. [Google Scholar] [CrossRef]
- Teng, T.; Xiao, L.; Shen, L.; Ran, J.; Xiang, G.; Zhu, Y.; Chen, H. Simultaneous Li2MoO4 coating and Mo6+ doping improves the structural stability and electrochemical properties of nickel-rich LiNi0.83Co0.11Mn0.06O2. Appl. Surf. Sci. 2022, 601, 154101. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Yoo, Y.-W.; Hwang, D.-Y.; Shim, T.-Y.; Kang, C.-Y.; Park, H.-J.; Kim, H.-S.; Lee, S.-H. Effect of niobium doping to enhance electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode material. Solid State Ion. 2023, 389, 116108. [Google Scholar] [CrossRef]
- Huang, W.; Li, W.; Wang, L.; Zhu, H.; Gao, M.; Zhao, H.; Zhao, J.; Shen, X.; Wang, X.; Wang, Z.; et al. Structure and Charge Regulation Strategy Enabling Superior Cyclability for Ni-Rich Layered Cathode Materials. Small 2021, 17, 2104282. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.-d.; Li, J.-y.; Lei, C.-l.; He, Z.-j.; Cheng, Y.; Wu, F.-x.; Li, Y.-j. B-doped nickel-rich ternary cathode material for lithium-ion batteries with excellent rate performance. Ionics 2023, 29, 4559–4567. [Google Scholar] [CrossRef]
- Teng, T.; Xiao, L.; Zheng, J.; Wen, D.; Chen, H.; Zhu, Y. High-Ni layered LiNi0.83Co0.11Mn0.06O2 modified by Nb for Li-ion batteries. Ceram. Int. 2022, 48, 8680–8688. [Google Scholar] [CrossRef]
- Liu, J.; Chu, C.; Qin, X.; Meng, W.; Xu, X.; Wang, B.; Cai, F. The influence of surface lithium residue to the performance of LiNi0.9Co0.05Mn0.05O2 cathode materials. J. Korean Ceram. Soc. 2023, 60, 462–473. [Google Scholar] [CrossRef]
- Chu, M.; Huang, Z.; Zhang, T.; Wang, R.; Shao, T.; Wang, C.; Zhu, W.; He, L.; Chen, J.; Zhao, W.; et al. Enhancing the Electrochemical Performance and Structural Stability of Ni-Rich Layered Cathode Materials via Dual-Site Doping. ACS Appl. Mater. Interfaces 2021, 13, 19950–19958. [Google Scholar] [CrossRef]
- Qu, X.; Huang, H.; Wan, T.; Hu, L.; Yu, Z.; Liu, Y.; Dou, A.; Zhou, Y.; Su, M.; Peng, X.; et al. An integrated surface coating strategy to enhance the electrochemical performance of nickel-rich layered cathodes. Nano Energy 2022, 91, 106665. [Google Scholar] [CrossRef]
- Xin, F.; Goel, A.; Chen, X.; Zhou, H.; Bai, J.; Liu, S.; Wang, F.; Zhou, G.; Whittingham, M.S. Electrochemical Characterization and Microstructure Evolution of Ni-Rich Layered Cathode Materials by Niobium Coating/Substitution. Chem. Mater. 2022, 34, 7858–7866. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, H.; Cai, F.; Liu, Z.; Yang, G.; Qin, X.; Świerczek, K. Surface engineering with ammonium niobium oxalate: A multifunctional strategy to enhance electrochemical performance and thermal stability of Ni-rich cathode materials at 4.5V cutoff potential. Electrochim. Acta 2022, 403, 139636. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, P.; Zhou, B.; Zhao, H.; Wang, Z.; Ma, J.; Ren, Y. Multifunctional Electrolyte Additive for High-Nickel LiNi0.8Co0.1Mn0.1O2 Cathodes of Lithium-Metal Batteries. Energy & Fuels 2023, 37, 11388–11396. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, H.; Lu, S.; He, H. Y-Element Doping Improves Electrochemical Performance and Single-Crystal Structural Stability of Cathode LiNi0.8Co0.1Mn0.1O2. ACS Appl. Energy Mater. 2023, 6, 9487–9498. [Google Scholar] [CrossRef]
- Peng, F.; Chu, Y.; Li, Y.; Pan, Q.; Yang, G.; Zhang, L.; Hu, S.; Zheng, F.; Wang, H.; Li, Q. Mg,Ti-base surface integrated layer and bulk doping to suppress lattice oxygen evolution of Ni-rich cathode material at a high cut-off voltage. J. Energy Chem. 2022, 71, 434–444. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, B.; Chen, M.; Li, C.; Zhao, D.; Zhang, X.; Guo, S.; Li, S. Revisiting the impact of Co at high voltage for advanced nickel-rich cathode materials. Energy Storage Mater. 2024, 67, 103311. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, N.-Y.; Park, G.-T.; Kim, H.; Yoon, C.S.; Sun, Y.-K. High-Energy W-Doped Li[Ni0.95Co0.04Al0.01]O2 Cathodes for Next-Generation Electric Vehicles. Energy Storage Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Park, G.-T.; Ryu, H.-H.; Park, N.-Y.; Yoon, C.S.; Sun, Y.-K. Tungsten doping for stabilization of Li[Ni0.90Co0.05Mn0.05]O2 cathode for Li-ion battery at high voltage. J. Power Sources 2019, 442, 227242. [Google Scholar] [CrossRef]














| Sample | a (A) | c (A) | c/a | V (A3) | R (I003/I104) | Rwp (%) |
|---|---|---|---|---|---|---|
| NCM88-0 | 2.88372 | 14.2388 | 4.937651 | 102.444 | 1.41 | 3.1 |
| NCM88-0.3Nb | 2.88411 | 14.2395 | 4.937218 | 102.474 | 1.52 | 2.14 |
| NCM88-0.5Nb | 2.88423 | 14.2428 | 4.938179 | 102.539 | 1.68 | 2.28 |
| NCM88-1Nb | 2.88517 | 14.2493 | 4.938808 | 102.704 | 1.48 | 2.76 |
| Samples | Voltage Range | Discharge Capacity | Rate/Capacity Retentions/ Cycle Number | Reference |
|---|---|---|---|---|
| LiNi0.88Co0.05Mn0.07O2@Nb2O5 | 2.7 V–4.5 V | 200.3/1C | 1C/92.9%/100 | This work |
| NCM811-PFPN | 3.0 V–4.5 V | 187.6/1C | 1C/89.5%/200 | [46] |
| SC-NCM811 (Ce/Al doping) | 2.8 V–4.5 V | 188.5/1C | 1C/85.38%/100 | [33] |
| SC-NCM811 (Y doping) | 2.7 V–4.5 V | 189.5/1C | 1C/94.53%/100 | [47] |
| NCM811@MTP | 2.7 V–4.5 V | 201.5/1C | 1C/89.3%/200 | [48] |
| NCM8155 | 2.7 V–4.5 V | 202.1/1C | 1C/88.02%/100 | [49] |
| Sample | NCM88-0 | NCM88-0.5Nb | ||||
|---|---|---|---|---|---|---|
| Ro (Ω) | RSEI (Ω) | Rct (Ω) | Ro (Ω) | RSEI (Ω) | Rct (Ω) | |
| Cycling | ||||||
| 1st | 2.206 | 28.915 | 80.03 | 2.2996 | 52.769 | 37.646 |
| 50th | 2.468 | 27.803 | 154.22 | 2.4016 | 28.147 | 109.11 |
| 100th | 2.588 | 23.363 | 292.9 | 2.752 | 20.909 | 170.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, J.; Wang, B.; Wang, J.; Wang, Y.; Meng, W.; Cai, F. A Study on the Microstructure Regulation Effect of Niobium Doping on LiNi0.88Co0.05Mn0.07O2 and the Electrochemical Performance of the Composite Material under High Voltage. Materials 2024, 17, 2127. https://doi.org/10.3390/ma17092127
Xu X, Liu J, Wang B, Wang J, Wang Y, Meng W, Cai F. A Study on the Microstructure Regulation Effect of Niobium Doping on LiNi0.88Co0.05Mn0.07O2 and the Electrochemical Performance of the Composite Material under High Voltage. Materials. 2024; 17(9):2127. https://doi.org/10.3390/ma17092127
Chicago/Turabian StyleXu, Xinrui, Junjie Liu, Bo Wang, Jiaqi Wang, Yunchang Wang, Weisong Meng, and Feipeng Cai. 2024. "A Study on the Microstructure Regulation Effect of Niobium Doping on LiNi0.88Co0.05Mn0.07O2 and the Electrochemical Performance of the Composite Material under High Voltage" Materials 17, no. 9: 2127. https://doi.org/10.3390/ma17092127
APA StyleXu, X., Liu, J., Wang, B., Wang, J., Wang, Y., Meng, W., & Cai, F. (2024). A Study on the Microstructure Regulation Effect of Niobium Doping on LiNi0.88Co0.05Mn0.07O2 and the Electrochemical Performance of the Composite Material under High Voltage. Materials, 17(9), 2127. https://doi.org/10.3390/ma17092127





