Advancing in Cesium Retention: Application of Magnesium Phosphate Cement Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analysis Method
2.3. Parameter Optimization
2.3.1. Base
2.3.2. Acid
2.3.3. Mg/P Ratio
2.3.4. Setting Retarders
2.3.5. Quantity of Water
2.3.6. Preparation
3. Results
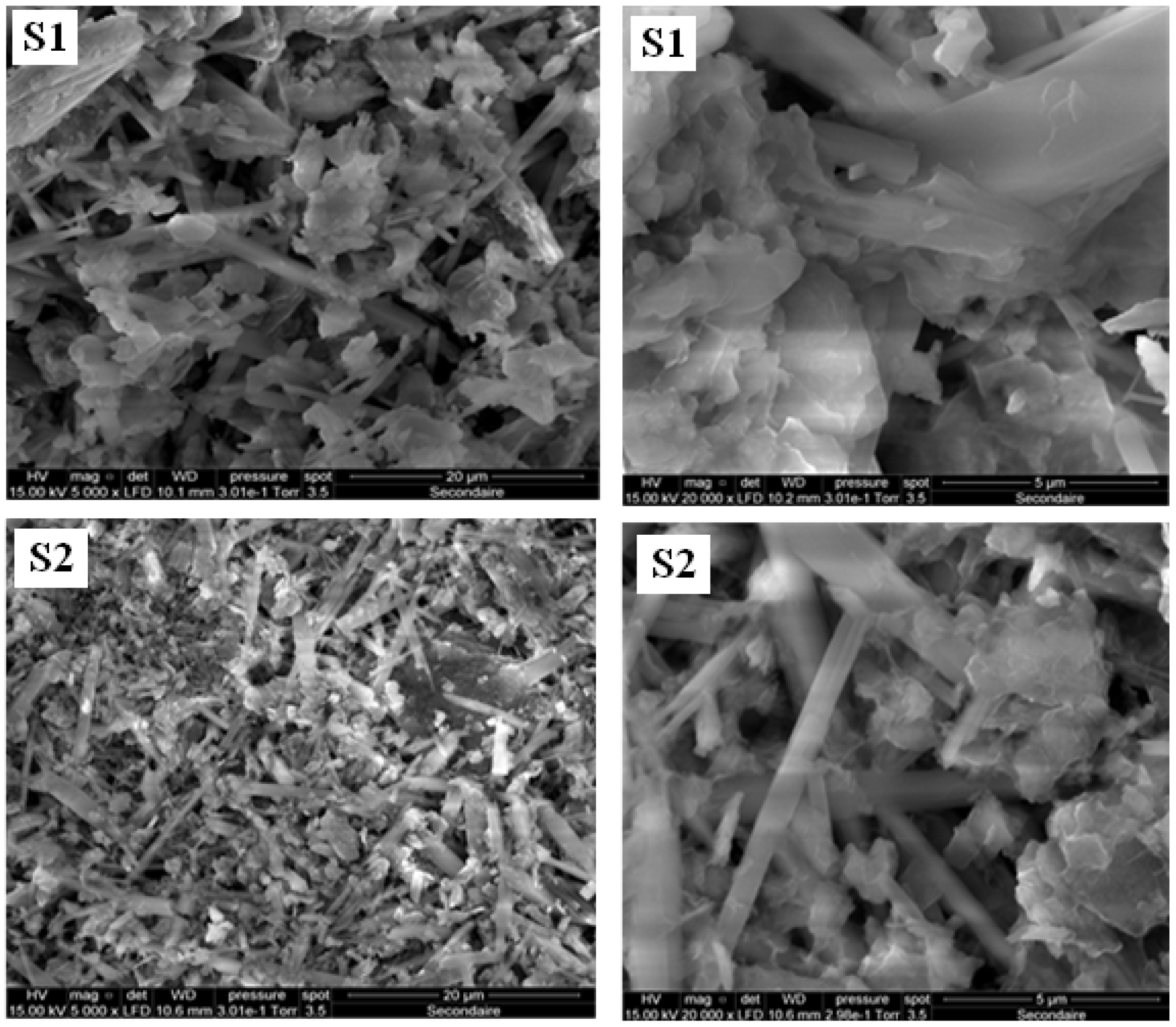
3.1. SEM Analysis
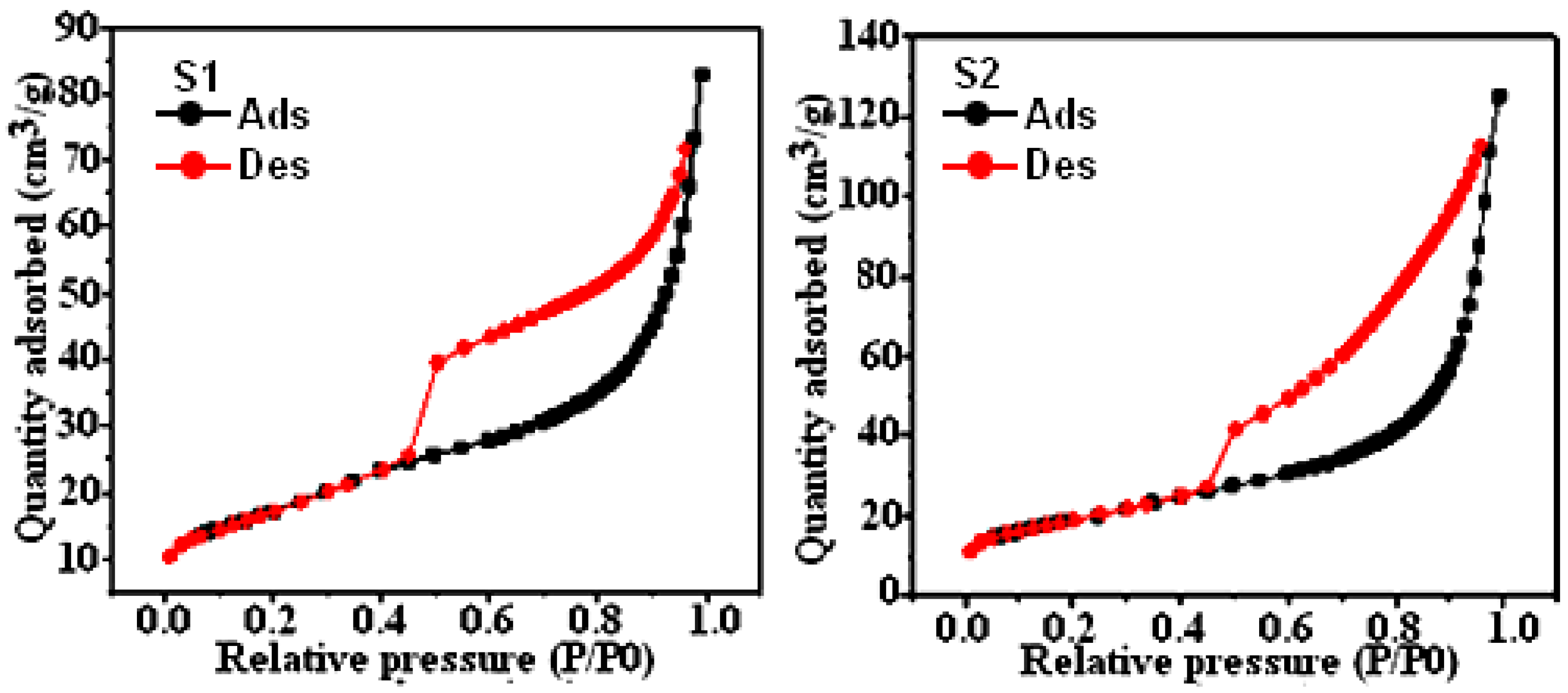
3.2. BET-Analysis
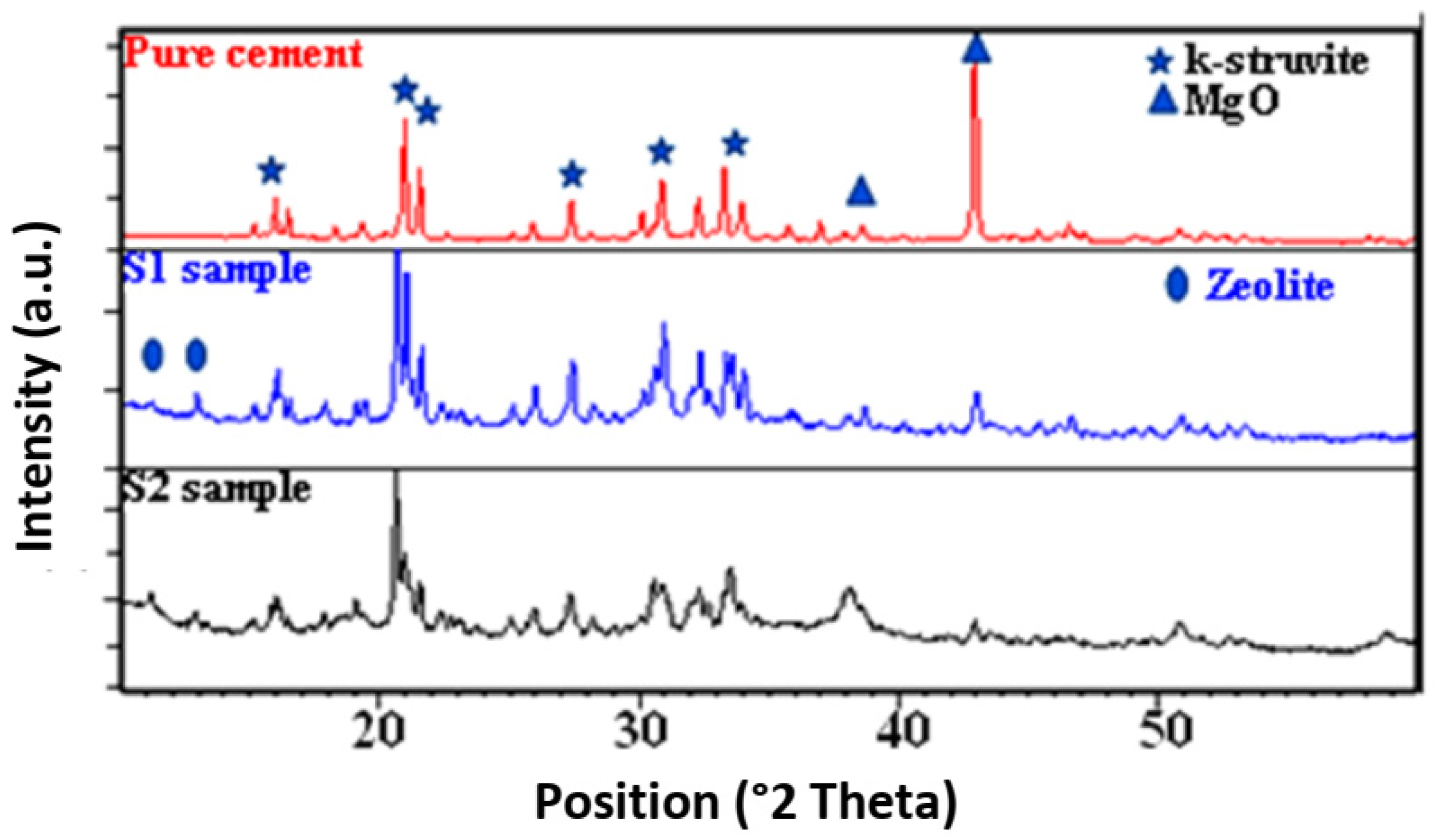
3.3. XRD Analysis
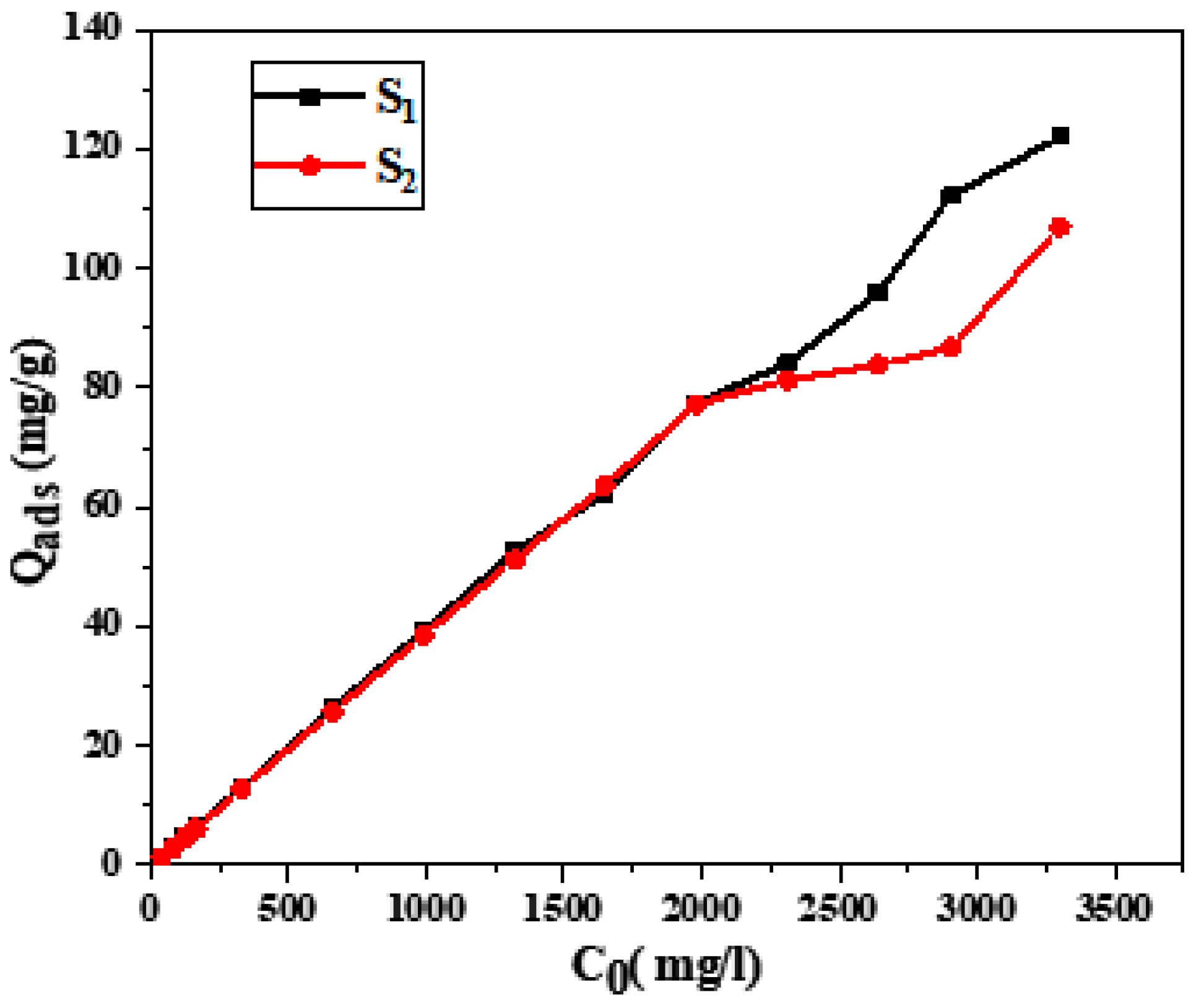
3.4. Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chauhan, C.K.; Vyas, P.M.; Joshi, M.J. Growth and characterization of Struvite-K crystals. Cryst. Res. Technol. 2011, 46, 187–194. [Google Scholar] [CrossRef]
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of magnesium potassium phosphate cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- Gharsallah, S.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Synthesis and Characterization of New Composite Materials Based on Magnesium Phosphate Cement for Fluoride Retention. Materials 2023, 16, 718. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of k-struvite formation in magnesium phosphate cements. Cem. Concr. Res. 2007, 91, 117–122. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, H.S.S. Dehydration characteristics of struvite-K pertaining to magnesium potassium phosphate cement system in non-isothermal condition. J. Therm. Anal. Calorim. 2013, 111, 35–40. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing mechanism of potassium phosphate-based magnesium phosphate cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Aguilera, G.R.; Yang, S.; Junaid, A.S.M.; Kuznicki, S.M.; Mccaffrey, W.C. Ni and V Removal from Oil and Model Compounds without Hydrogenation: Natural Chabazite as Solid Acid. Can. J. Chem. Eng. 2016, 94, 938–946. [Google Scholar] [CrossRef]
- Ribeiro, D.; Rocha de Paula, G.; Morelli, M. Effect of MgO/NH4H2PO4 Ratio on the Properties of Magnesium Phosphate Cements. Mater. Res. 2020, 23, e20200034. [Google Scholar] [CrossRef]
- Peng, L.; Chen, B. Study on the basic properties and mechanism of waste sludge solidified by magnesium phosphate cement containing different active magnesium. Constr. Build. Mater. 2021, 281, 122609. [Google Scholar] [CrossRef]
- Lahalle, H.; Patapy, C.; Glid, M.; Renaudin, G.; Cyr, M. Microstructural evolution/durability of magnesium phosphate cement paste over time in neutral and basic environments. In Cement and Concrete Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 122, pp. 42–58. [Google Scholar]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Effects of K-struvite on hydration behavior of magnesium potassium phosphate cement. Constr. Build. Mater. 2021, 275, 121741. [Google Scholar] [CrossRef]
- Aysan, H.; Edebali, S.; Ozdemir, C.; Celi, M. Microporous and Mesoporous Materials Use of chabazite, a naturally abundant zeolite, for the investigation of the adsorption kinetics and mechanism of methylene blue dye. Microporous Mesoporous Mater. 2016, 235, 78–86. [Google Scholar] [CrossRef]
- Atun, G.; Bodur, N. Retention of Cs on zeolite, bentonite and their mixtures. J. Radioanal. Nucl. Chem. 2002, 253, 275–279. [Google Scholar] [CrossRef]
- Abusafa, A.; Yucel, H. Removal of 137 Cs from aqueous solutions using different cationic forms of a natural zeolite: Clinoptilolite. Sep. Purif. Technol. 2002, 28, 103–116. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Luo, Q.; Long, W. Effect of silt modification on the properties of magnesium phosphate cement. Front. Mater. 2023, 10, 1274489. [Google Scholar] [CrossRef]
- Lee, K.; Kim, K.; Park, M.; Kim, J.; Oh, M.; Lee, E.; Chung, D.; Moon, J. Novel application of nanozeolite for radioactive cesium removal from high-salt wastewater. Water Res. 2016, 95, 134–141. [Google Scholar] [CrossRef]
- Lestaevel, P.; Racine, R.; Bensoussan, H.; Rouas, C.; Gueguen, Y.; Dublineau, I.; Bertho, J.; Gourmelon, P.; Jourdain, J.; Souidi, M. Caesium 137: Properties and biological effects resulting of an internal contamination. Med. Nucleaire. Imag. Fonct. Metab. 2010, 34, 108–118. [Google Scholar]
- He, R.; Lu, N. Unveiling the dielectric property change of concrete during hardening process by ground penetrating radar with the antenna frequency of 1.6 GHz and 2.6 GHz. Cem. Concr. Compos. 2023, 144, 105279. [Google Scholar] [CrossRef]
- He, R.; Nantung, T.; Olek, J.; Lu, N. Field study of the dielectric constant of concrete: A parameter less sensitive to environmental variations than electrical resistivity. J. Build. Eng. 2023, 74, 106938. [Google Scholar] [CrossRef]
- Gharsallah, S.; Mallah, A.; Alsawi, A.; Hammami, B.; Khitouni, M.; Charnay, C.; Chemingui, M. Study of Modified Magnesium Phosphate Cement for Fluoride Removal. Materials 2023, 16, 5749. [Google Scholar] [CrossRef]
- Leng, D.; Li, X.; Lv, Y.; Tan, H.; Li, N.; Liu, Z.; Jiang, W. Cesium immobilization by K-struvite crystal in aqueous solution: Ab initio calculations and experiments. J. Hazard. Mater. 2020, 387, 121872. [Google Scholar] [CrossRef]
- Murthy, G.S.; Sivaiah, M.V.; Kumar, S.S.; Reddy, V.N.; Krishna, R.M.; Lakshminarayana, S. Adsorption of cesium on a composite inorganic exchanger zirconium phosphate—Ammonium molybdophosphate. J. Radioanal. Nucl. Chem. 2004, 260, 109–114. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Moradi, M.; Azizpour, H.; Zarghami, R. Evaluation of AMP—PAN composite for adsorption of Cs+ ions from aqueous solution using batch and fixed bed operations. J. Radioanal. Nucl. Chem. 2012, 292, 609–617. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Microporous and Mesoporous Materials Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater. 2017, 242, 238–244. [Google Scholar] [CrossRef]
- Sponge, P.; Wang, H.; Ma, G.; Zhang, K.; Jia, Z.; Wang, Y.; Gao, L.; Liu, B. Adsorption Behavior and Mechanism of Cesium Ions in Low-Concentration Brine Using Ammonium Molybdophosphate—Zirconium Phosphate on polyurethane sponge. Materials 2023, 16, 4583. [Google Scholar] [CrossRef]
- Gharsallah, S.; Alsawi, A.; Alsulami, A.H.; Charnay, C.; Chemingui, M. Enhancing Magnesium Phosphate Cement Paste for Efficient Fluoride Adsorption. Coatings 2024, 14, 9. [Google Scholar] [CrossRef]
- Nilchi, A.; Saberi, R.; Moradi, M.; Azizpour, H.; Zarghami, R. Adsorption of cesium on copper hexacyanoferrate-PAN composite ion exchanger from aqueous solution. Chem. Eng. J. 2011, 172, 572–580. [Google Scholar] [CrossRef]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef]
- Awual, M.R.; Suzuki, S.; Taguchi, T.; Shiwaku, H.; Okamoto, Y.; Yaita, T. Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 2014, 242, 127–135. [Google Scholar] [CrossRef]
- Park, Y.; Lee, Y.C.; Shin, W.S.; Choi, S.J. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate polyacrylonitrile (AMP-PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar] [CrossRef]
- Ma, B.; Oh, S.; Shin, W.S.; Choi, S.J. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM). Desalination 2011, 276, 336–346. [Google Scholar] [CrossRef]
- Park, Y.; Shin, W.S.; Choi, S.J. Ammonium salt of heteropoly acid immobilized on mesoporous silica (SBA-15): An efficient ion exchange for cesium ion. Chem. Eng. J. 2013, 220, 204–213. [Google Scholar] [CrossRef]
| MgO (g) | KH2PO4 (g) | Borax (g) | Water (mL) | |
|---|---|---|---|---|
| MgO not calcined | 1.097 | 0.830 | 0.105 | 2 |
| MgO (550 °C/3 h) | 1.064 | 0.864 | 0.099 | 1.5 |
| MgO (1005 °C/2 h) | 1.0054 | 0.8456 | 0.0965 | 3.5 |
| MgO (1200 °C/2 h) | 1.074 | 0.844 | 0.098 | 1.5 |
| MgO (1500 °C/6 h) | 1.0664 | 0.8128 | 0.0923 | 1 |
| MgO (g) | Borax (g) | Water (mL) | Observation | |
|---|---|---|---|---|
| KH2PO4 (powder) = 0.816 g | 1.132 | 0.094 | 1.5 | Very resistant |
| KH2PO4 crist = 0.837 g | 1.049 | 0.096 | 1.5 | Very resistant |
| K2HPO4 = 0.265 g | 1.133 | 0.067 | 1.4 | Very resistant |
| H3PO4 = 1 mL | 1.032 | 0.096 | 2 | Very exothermic with gas release and very resistant structure |
| MgHPO4·3H2O = 1.073 g | 1.042 | 0.126 | 2 | Very resistant |
| NH4H2PO4 = 2.8720 ((Mg/P) = 1) | 1.078 | 0.319 | 2 | Very durable with gas release |
| Mg/P ratio | MgO (g) | KH2PO4 (g) | Borax (g) | Water (mL) | Observation |
|---|---|---|---|---|---|
| Mg/P = 1 | 1.054 | 3.036 | 0.270 | 1.3 | Very resistant |
| Mg/P = 2 | 1.062 | 1.045 | 0.137 | 1.2 | Very resistant |
| Mg/P = 3 | 0.547 | 0.523 | 0.059 | 1 | Very resistant |
| Mg/P = 4 | 1.066 | 0.812 | 0.092 | 1 | Very resistant |
| Mg/P = 5 | 0.570 | 0.370 | 0.043 | 1 | Resistant |
| Mg/P = 8 | 1.023 | 0.467 | 0.072 | 1 | Less resistant |
| Mg/P = 12 | 1.001 | 0.270 | 0.064 | 1.3 | Fragile |
| MgO (g) | KH2PO4 (g) | Water (mL) | Observation | |
|---|---|---|---|---|
| Without retarders | 1.001 | 0.823 | 2 | Quick take |
| Borax = 0.092 g. | 1.066 | 0.812 | 1 | Moderate hold |
| Boric acid = 0.096 g. | 1.037 | 0.820 | 1 | Moderate hold |
| NaCl = 0.093 g. | 1.001 | 0.866 | 1.3 | Quick take |
| Water | e/c | MgO (g) | KH2PO4 (g) | Observation |
|---|---|---|---|---|
| 2 mL | 1.089 | 1.002 | 0.833 | Formation of a homogeneous paste. quick to set and resistant. |
| 4 mL | 2.168 | 1.006 | 0.839 | Weak setting. no dough formation. totally crumbly. |
| 6 mL | 3.260 | 1.006 | 0.836 | Very weak setting. no dough formation. totally crumbly. |
| 8 mL | 4.347 | 1.007 | 0.837 | Very weak setting. no dough formation. totally crumbly. |
| M/P | MgO | KH2PO4 | Borax | Zeolite | Water | pH | |
|---|---|---|---|---|---|---|---|
| S1 | 2 | 1.0016 | 1.6892 | 0.1340 | 1.3470 | 4 | 9–10 |
| S2 | 4 | 1.0054 | 0.8456 | 0.0965 | 0.9232 | 3.5 | 7–8 |
| Model | Sample 1 | Sample 2 |
|---|---|---|
| Langmuir | b = 0.025 L/g q0 = 1.636 mg/g RL = 0.187 Kap = 0.144 R2 = 0.946 | b = −0.035 L/g q0 = 4.65 mg/g RL = 0.042 Kap = −0.744 R2 = 0.906 |
| Freundlich | 1/n = 0.588 Kf = 4.636 R2 = 0.925 | 1/n = 0.724 Kf = 1.23 R2 = 0.6513 |
| Temkin | A = 1.288 B = 16.49 R2 = 0.903 | A = 1.31 B = 13.8 R2 = 0.814 |
| Dubinin–Radushkevich | qd = 39.067 B = 0.253 R2 = 0.591 | qd = 85.66 B = 97.89 R2 = 0.873 |
| Adsorbent | Capacity (mg/g) | Reference |
|---|---|---|
| CHCF-PAN composite | 12.5 | [27] |
| Prussian Blue | 25.52 | [28] |
| Inorganic adsorbent | 27.40 | [29] |
| Conjugate adsorbent | 50.23 | [29] |
| AMP-PAN | 81.31 | [30] |
| Phosphate-modified Montmorillonite | 93.87 | [31] |
| NH4PMA | 97.23 | [32] |
| S1 | 122.108 | This study |
| S2 | 106.997 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharsallah, S.; Khitouni, N.; Mallah, A.; Alsawi, A.; Alluhayb, A.H.; Khitouni, M.; Charnay, C.; Chemingui, M. Advancing in Cesium Retention: Application of Magnesium Phosphate Cement Composites. Materials 2024, 17, 2132. https://doi.org/10.3390/ma17092132
Gharsallah S, Khitouni N, Mallah A, Alsawi A, Alluhayb AH, Khitouni M, Charnay C, Chemingui M. Advancing in Cesium Retention: Application of Magnesium Phosphate Cement Composites. Materials. 2024; 17(9):2132. https://doi.org/10.3390/ma17092132
Chicago/Turabian StyleGharsallah, Sana, Nawel Khitouni, Abdulrahman Mallah, Abdulrahman Alsawi, Abdullah H. Alluhayb, Mohamed Khitouni, Clarence Charnay, and Mahmoud Chemingui. 2024. "Advancing in Cesium Retention: Application of Magnesium Phosphate Cement Composites" Materials 17, no. 9: 2132. https://doi.org/10.3390/ma17092132
APA StyleGharsallah, S., Khitouni, N., Mallah, A., Alsawi, A., Alluhayb, A. H., Khitouni, M., Charnay, C., & Chemingui, M. (2024). Advancing in Cesium Retention: Application of Magnesium Phosphate Cement Composites. Materials, 17(9), 2132. https://doi.org/10.3390/ma17092132







