A Study on the Materials Used in Ancient Wooden Architectural Paintings at DaZhong Gate in Confucius Temple, Qufu, Shandong, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Pigment Sample Information
2.2. Experimental Methods and Instrumentation
2.2.1. Polarized Light Microscopy (PLM)
2.2.2. Energy-Dispersive X-ray Spectroscopy (EDX)
2.2.3. Micro-Raman Spectroscopy (m-RS)
2.2.4. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.2.5. Pyrolysis–Gas Chromatography/Mass Spectrometry (Py-GC/MS)
3. Results and Discussion
3.1. Pigments
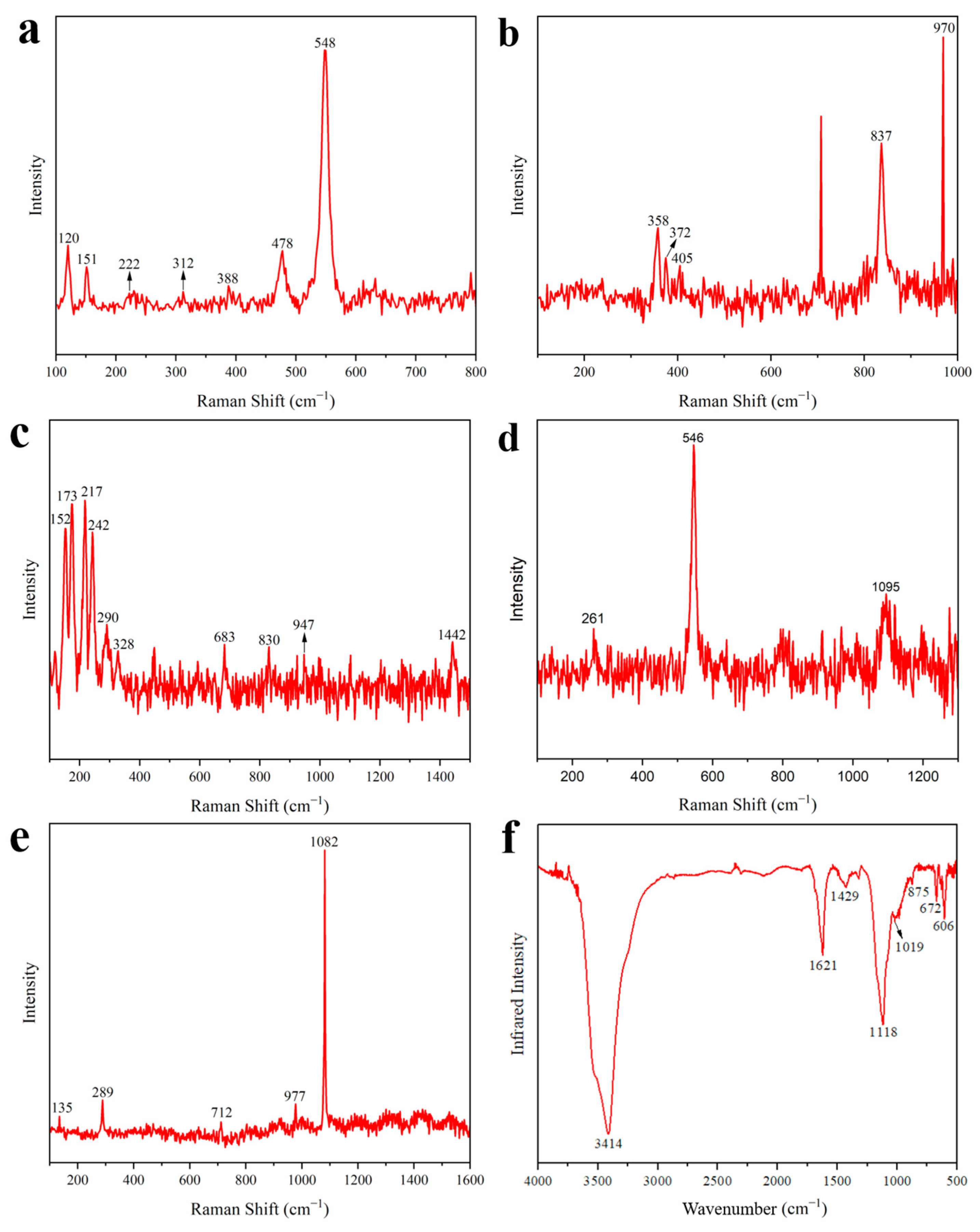
3.1.1. Red
3.1.2. Yellow
3.1.3. Green
3.1.4. Blue
3.1.5. White
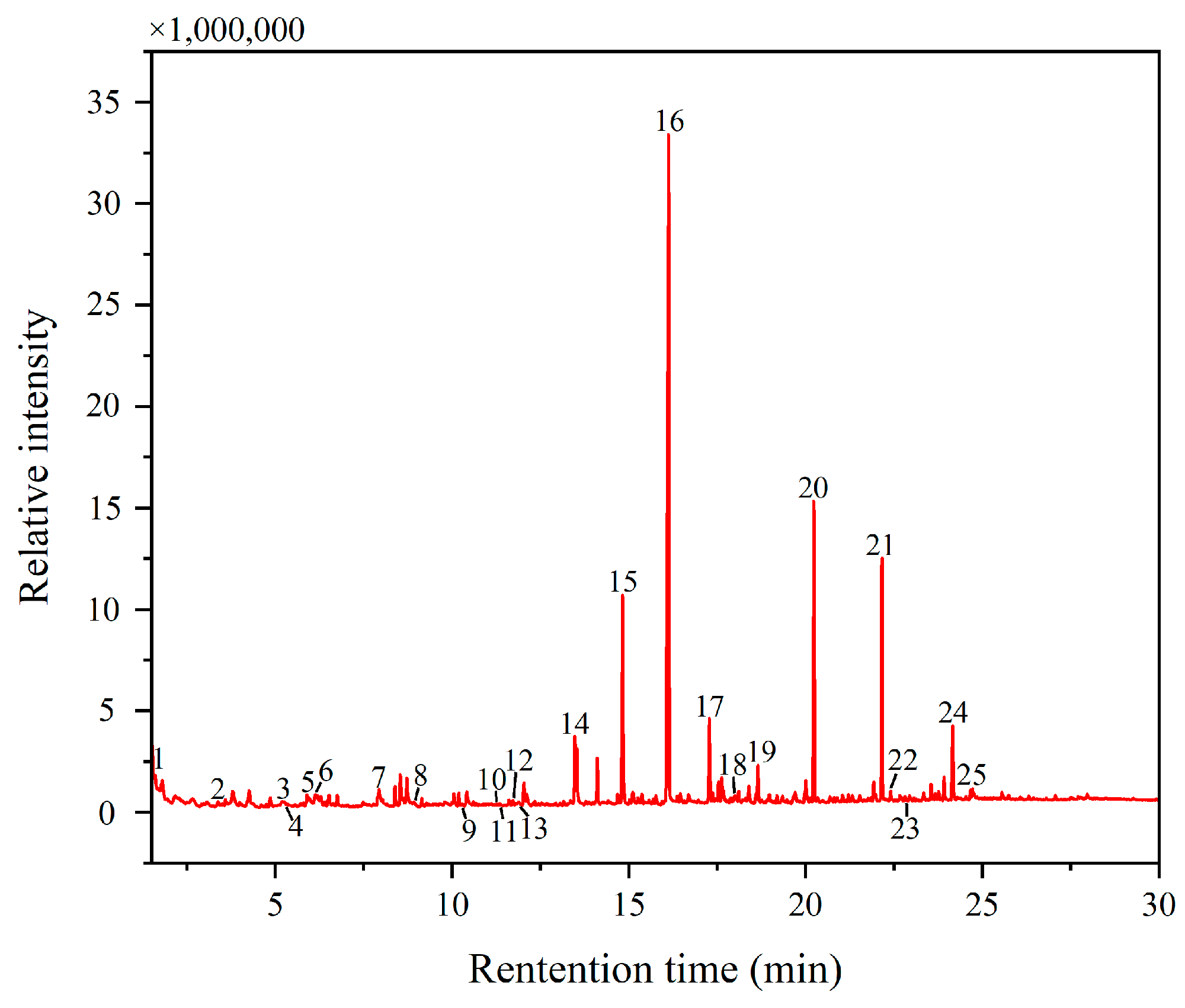
3.2. Binder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Z.; Wu, W.; Shang, G.; Wu, Y.; Wang, J. Study on colored pattern pigments of a royal Taoist temple beside the Forbidden City (Beijing, China). Vib. Spectrosc. 2017, 92, 234–244. [Google Scholar] [CrossRef]
- Zou, W.; Zou, W.; Yeo, S.-Y. Investigation on the painting materials and profile structures used in ancient chinese folk architectural paintings by multiple analytical methods. Coatings 2022, 12, 320. [Google Scholar] [CrossRef]
- Han, K.; Yang, H.; Teri, G.; Hu, S.; Li, J.; Li, Y.; Ma, E.; Tian, Y.; Fu, P.; Luo, Y. Spectroscopic investigation of a color painting on an ancient wooden architecture from the taiping heavenly kingdom prince dai’s mansion in Jiangsu, China. Minerals 2023, 13, 224. [Google Scholar] [CrossRef]
- Chen, E.; Zhang, B.; Zhao, F.; Wang, C. Pigments and binding media of polychrome relics from the central hall of Longju temple in Sichuan, China. Herit. Sci. 2019, 7, 45. [Google Scholar] [CrossRef]
- Teri, G.; Han, K.; Huang, D.; Li, Y.; Tian, Y.; Chao, X.; Jia, Z.; Fu, P.; Li, Y. A study on the materials used in the ancient architectural paintings from the qing dynasty tibetan buddhist monastery of Puren, China. Materials 2023, 16, 6404. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhen, G.; Hao, X.; Zhou, P.; Wang, Z.; Jia, J.; Gao, Y.; Dong, S.; Tong, H. Micro-raman, XRD and THM-Py-GC/MS analysis to characterize the materials used in the eleven-faced guanyin of the Du Le Temple of the Liao Dynasty, China. Microchem. J. 2021, 171, 106828. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B. Study of identification results of proteinous binding agents in Chinese painted cultural relics. J. Cult. Herit. 2020, 43, 73–79. [Google Scholar] [CrossRef]
- Bonaduce, I.; Blaensdorf, C.; Dietemann, P.; Colombini, M.P. The binding media of the polychromy of Qin Shihuang’s Terracotta Army. J. Cult. Herit. 2008, 9, 103–108. [Google Scholar]
- Yan, H.; An, J.; Zhou, T.; Xia, Y.; Rong, B. Identification of proteinaceous binding media for the polychrome terracotta army of Emperor Qin Shihuang by MALDI-TOF-MS. Chin. Sci. Bull. 2014, 59, 2574–2581. [Google Scholar] [CrossRef]
- Wei, S.; Ma, Q.; Schreiner, M. Scientific investigation of the paint and adhesive materials used in the Western Han dynasty polychromy terracotta army, Qingzhou, China. J. Archaeol. Sci. 2012, 39, 1628–1633. [Google Scholar] [CrossRef]
- Peifan, Q.; Deqi, Y.; Qi, M.; Aijun, S.; Jingqi, S.; Zengjun, Z.; Jianwei, H. Study and restoration of the Yi Ma Wu Hui Layer of the ancient coating on the Putuo Zongcheng Temple. Int. J. Archit. Herit. 2020, 15, 1707–1721. [Google Scholar] [CrossRef]
- Zhu, T.; Li, T.; Liu, N.; Chen, J.; Huang, H.; Fu, Q.; Zhang, S. Hexi painting on Xitian Fanjing, a Qing imperial Buddhist temple in Beijing, China: Technology revealed by analytical approaches (an initial report). Herit. Sci. 2016, 4, 42. [Google Scholar] [CrossRef]
- Fu, P.; Teri, G.; Li, J.; Huo, Y.; Yang, H.; Li, Y. Analysis of an ancient architectural painting from the Jiangxue Palace in the Imperial Museum, Beijing, China. Anal. Lett. 2021, 54, 684–697. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Xiao, K.; Liu, P.; Chao, X.; Fu, P.; Xing, H.; Li, Y. A Study of pigment, adhesive, and firing temperature in pottery figurines excavated from the tomb of Qibi Ming, China. Molecules 2023, 28, 7739. [Google Scholar] [CrossRef]
- Go, I.; Mun, S.; Lee, J.; Jeong, H. A case study on Hoeamsa Temple, Korea: Technical examination and identification of pigments and paper unearthed from the temple site. Herit. Sci. 2022, 10, 20. [Google Scholar] [CrossRef]
- Klisińska-Kopacz, A. Non-destructive characterization of 17th century painted silk banner by the combined use of Raman and XRF portable systems. J. Raman Spectrosc. 2015, 46, 317–321. [Google Scholar] [CrossRef]
- Cerrato, E.J.; Cosano, D.; Esquivel, D.; Otero, R.; Jimémez-Sanchidrián, C.; Ruiz, J.R. A multi-analytical study of a wall painting in the satyr domus in Córdoba, Spain. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 232, 118148. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P. The vibrational spectrum of α-mercuric sulphide. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 2305–2310. [Google Scholar] [CrossRef]
- Hao, X.; Schilling, M.R.; Wang, X.; Khanjian, H.; Heginbotham, A.; Han, J.; Auffret, S.; Wu, X.; Fang, B.; Tong, H. Use of THM-PY-GC/MS technique to characterize complex, multilayered Chinese lacquer. J. Anal. Appl. Pyrolysis 2019, 140, 339–348. [Google Scholar] [CrossRef]
- Xia, Y. Chinese Historical Pigments in Polarized Light Microscope; Science Press: Beijing, China, 2017. [Google Scholar]
- Arjonilla, P.; Domínguez-Vidal, A.; Correa-Gómez, E.; Domene-Ruiz, M.J.; Ayora-Cañada, M.J. Raman and fourier transform infrared microspectroscopies reveal medieval Hispano–Muslim wood painting techniques and provide new insights into red lead production technology. J. Raman Spectrosc. 2019, 50, 1537–1545. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J. Raman spectroscopic library of natural and synthetic pigments (pre- ≈ 1850 AD). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Doménech-Carbó, M.T.; Mas-Barberá, X. Identification of lead pigments in nanosamples from ancient paintings and polychromed sculptures using voltammetry of nanoparticles/atomic force microscopy. Talanta 2007, 71, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, F.; Ma, J.; He, K.; Zhang, M. Study on the pigments of Chinese architectural colored drawings in the Altar of Agriculture (Beijing, China) by portable Raman spectroscopy and ED-XRF spectrometers. Vib. Spectrosc. 2021, 116, 103291. [Google Scholar] [CrossRef]
- Mazzocchin, G.A.; Agnoli, F.; Mazzocchin, S.; Colpo, I. Analysis of pigments from Roman wall paintings found in Vicenza. Talanta 2003, 61, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhen, G.; Hao, X.; Tong, T.; Ni, F.; Wang, Z.; Jia, J.; Li, L.; Tong, H. Spectroscopic investigation and comprehensive analysis of the polychrome clay sculpture of Hua Yan Temple of the Liao Dynasty. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Y.; Tong, T.; Sun, Z.; Yu, Z.; Zhu, Y.; Tong, H. Red lead degradation: Monitoring of color change over time. New J. Chem. 2016, 40, 3686–3692. [Google Scholar] [CrossRef]
- Daniilia, S.; Sotiropoulou, S.; Bikiaris, D.; Salpistis, C.; Karagiannis, G.; Chryssoulakis, Y.; Price, B.A.; Carlson, J.H. Panselinos’ Byzantine wall paintings in the Protaton Church, Mount Athos, Greece: A technical examination. J. Cult. Herit. 2000, 1, 91–110. [Google Scholar] [CrossRef]
- Jinyu, W. Challenge of lithargyrum’s extraterritorial introduction. Chin. Med. Cult. 2022, 17, 216–225. [Google Scholar] [CrossRef]
- Weidon, K.; Shangxin, Z.; Qianli, F. Pigment study of the stone coffin from Yang Hui’s tomb of the Tang Dynasty. Sci. Conserv. Archaeol. 2023, 35, 114–122. [Google Scholar] [CrossRef]
- Yanying, M.; Jianhua, Z.; Hu, D. Scientific analysis of a Ming Dynasty polychrome star sculpture from Shanxi Art Museum, Taiyuan, China. Sci. Conserv. Archaeol. 2015, 27, 50–60. [Google Scholar] [CrossRef]
- Petrova, O.; Pankin, D.; Povolotckaia, A.; Borisov, E.; Beznosova, M.; Krivul’ko, T.; Kurochkin, A. Identification of pigments in colored layers of a painting by raman spectroscopy. Opt. Spectrosc. 2017, 123, 965–969. [Google Scholar] [CrossRef]
- Rosi, F.; Miliani, C.; Borgia, I.; Brunetti, B.; Sgamellotti, A. Identification of nineteenth century blue and green pigments by in situ X-ray fluorescence and micro-Raman spectroscopy. J. Raman Spectrosc. 2004, 35, 610–615. [Google Scholar] [CrossRef]
- Castillejo, M.; Martín, M.; Silva, D.; Stratoudaki, T.; Anglos, D.; Burgio, L.; Clark, R.J. Laser-induced breakdown spectroscopy and Raman microscopy for analysis of pigments in polychromes. J. Cult. Herit. 2000, 1, S297–S302. [Google Scholar] [CrossRef]
- Shen, A.G.; Wang, X.H.; Xie, W.; Shen, J.; Li, H.Y.; Liu, Z.A.; Hu, J.M. Pigment identification of colored drawings from Wuying Hall of the Imperial Palace by micro-Raman spectroscopy and energy dispersive X-ray spectroscopy. J. Raman Spectrosc. 2006, 37, 230–234. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, L.; Yan, J.; Zhou, W.; Pitthard, V.; Bayerova, T.; Krist, G. Chromatographic, microscopic, and spectroscopic characterization of a wooden architectural painting from the Summer Palace, Beijing, China. Anal. Lett. 2019, 52, 1670–1680. [Google Scholar] [CrossRef]
- Favaro, M.; Guastoni, A.; Marini, F.; Bianchin, S.; Gambirasi, A. Characterization of lapis lazuli and corresponding purified pigments for a provenance study of ultramarine pigments used in works of art. Anal. Bioanal. Chem. 2012, 402, 2195–2208. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Zhang, B.; Yang, H.; Zhang, Q. The analysis of the colored paintings from the Yanxi Hall in the Forbidden City. Guangpuxue Yu Guangpu Fenxi Spectrosc. Spectr. Anal. 2018, 38, 2054–2063. [Google Scholar]
- Zhang, C.; Huang, J.; Zhu, T.; Zhang, R. Guangzhou Tongcao painting in late China Qing Dynasty (1840–1912 AD): Technology revealed by analytical approaches. Herit. Sci. 2021, 9, 9. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S.; Scattering, R. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Petrova, O.; Pankin, D.; Povolotckaia, A.; Borisov, E.; Krivul’ko, T.; Kurganov, N.; Kurochkin, A. Pigment palette study of the XIX century plafond painting by Raman spectroscopy. J. Cult. Herit. 2019, 37, 233–237. [Google Scholar] [CrossRef]
- Vagnini, M.; Vivani, R.; Viscuso, E.; Favazza, M.; Brunetti, B.; Sgamellotti, A.; Miliani, C. Investigation on the process of lead white blackening by Raman spectroscopy, XRD and other methods: Study of Cimabue’s paintings in Assisi. Vib. Spectrosc. 2018, 98, 41–49. [Google Scholar] [CrossRef]
- Bruni, S.; Cariati, F.; Casadio, F.; Toniolo, L. Spectrochemical characterization by micro-FTIR spectroscopy of blue pigments in different polychrome works of art. Vib. Spectrosc. 1999, 20, 15–25. [Google Scholar] [CrossRef]
- Zaffino, C.; Guglielmi, V.; Faraone, S.; Vinaccia, A.; Bruni, S. Exploiting external reflection FTIR spectroscopy for the in-situ identification of pigments and binders in illuminated manuscripts. Brochantite and posnjakite as a case study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Hou, X. Deposit in bleached pulp production system and its infrared spectrum identification. China Pulp Pap. Ind. 2022, 43, 20–24. [Google Scholar]
- Xin, H.; Anxin, L.; Jin, Z. Simultaneous quantitative analysis of SO42− and NO3− in soil by diffuse reflectance fourier transform infrared spectroscopy. Chin. J. Anal. Lab. 2015, 34, 1428–1431. [Google Scholar] [CrossRef]
- Kurouski, D.; Zaleski, S.; Casadio, F.; Van Duyne, R.P.; Shah, N.C. Tip-enhanced Raman spectroscopy (TERS) for in situ identification of indigo and iron gall ink on paper. J. Am. Chem. Soc. 2014, 136, 8677–8684. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Maiqian, N.; Chiavari, G.; Mazzeo, R. Analytical characterization of binding medium used in ancient Chinese artworks by pyrolysis–gas chromatography/mass spectrometry. Microchem. J. 2007, 85, 347–353. [Google Scholar] [CrossRef]
- Wang, N.; He, L.; Zhao, X.; Simon, S. Comparative analysis of eastern and western drying-oil binding media used in polychromic artworks by pyrolysis–gas chromatography/mass spectrometry under the influence of pigments. Microchem. J. 2015, 123, 201–210. [Google Scholar] [CrossRef]



| Color | Pigment Name | Chemical Formula | Location of Use |
|---|---|---|---|
| Red | Cinnabar | HgS | Du Le Temple (10th century AD), etc. |
| Red lead | Pb3O4 | Royal Residence (19th century AD) | |
| Litharge | α-PbO | Du Le Temple (10th century AD) | |
| Yellow | Orpiment | As2S3 | Assembly Hall (19th century) |
| Realgar | As4S4 | Royal Taoist Temple (16th century) | |
| Massicot | β-PbO | Du Le Temple (10th century AD) | |
| green | Emerald Green | Cu(CH3COO)2·3Cu(AsO2)2 | Jiangxue Palace (15th century AD) |
| Atacamite | Cu2(OH)3Cl | Longju Temple (15th century AD), etc. | |
| Phthalocyanine | CuC32N8Cl16 | Prince Dai’s Mansion (19th century AD) | |
| Blue | Lapis lazuli | (Na,Ca)8(AlSiO4)6(S,Cl)2 | Du Le Temple (10th century AD) |
| Azurite | 2CuCO3·Cu(OH)2 | Longju Temple (15th century AD) | |
| Indigo blue | C16H8N2Na2O8S2 | Prince Dai’s Mansion (19th century AD) | |
| White | Lead white | 2PbCO3·Pb(OH)2 | Puren Temple (18th century AD) |
| Black | Carbon black | C | Longju Temple (15th century AD), etc. |
| Sample | Color | Experimental Methods | Elements (Wt %) |
|---|---|---|---|
| DZ-1 | Red | PLM, EDX, m-RS | Pb (57.8), Ba (20.6), Ca (6.6), Si (6.0), Ce (3.7) |
| DZ-2 | Yellow | PLM, EDX, m-RS | Ca (32.5), Ba (18.9), Zn (16.9), Pb (16.7), Si (7.2), Cr (4.3) |
| DZ-3 | Green | PLM, EDX, m-RS | As (35.9), Cu (33.1), Ca (11.5), Si (11.3), S (6.2) |
| DZ-4 | Blue | PLM, EDX, m-RS | Ca (33.1), S (27.2), Si (25.2), Pb (7.6), K (3.0) |
| DZ-5 | White | PLM, EDX, m-RS, FT-IR | Ca (33.4), S (22.1), Ba (19.9), Zn (8.5), Si (7.3), Pb (3.3) |
| Peak Number | Retention Time (min) | Area (%) | Compound |
|---|---|---|---|
| 1 | 1.6057 | 0.26 | Methylamine, N,N-dimethyl- |
| 2 | 3.381 | 0.27 | 1H-Pyrrole, 1-methyl- |
| 3 | 5.2443 | 0.04 | glycine |
| 4 | 5.2507 | 0.02 | TMAH reagent |
| 5 | 5.9213 | 0.44 | 1,3,5-Triazine, hexahydro-1,3,5-trimethyl- |
| 6 | 6.1153 | 0.08 | Alanine |
| 7 | 7.873 | 0.01 | valine |
| 8 | 8.9157 | 0.06 | L-Proline, 1-methyl-, methyl ester |
| 9 | 10.237 | 0.05 | Methyl 1-methylpyrrole-2-carboxylate |
| 10 | 11.2533 | 0.04 | Naphthalene |
| 11 | 11.2567 | 0.06 | 1H-Indene, 1-methylene- |
| 12 | 11.7583 | 0.06 | Protein |
| 13 | 11.8607 | 0.03 | hydroxyproline |
| 14 | 13.4727 | 2.99 | Heptanedioic acid, dimethyl ester |
| 15 | 14.8307 | 8.81 | Octanedioic acid, dimethyl ester |
| 16 | 16.127 | 38.56 | Nonanedioic acid, dimethyl ester |
| 17 | 17.279 | 3.09 | Decanedioic acid, dimethyl ester |
| 18 | 17.992 | 0.11 | Protein—blood and glue |
| 19 | 18.6543 | 1.44 | Drying oil |
| 20 | 20.2363 | 10.70 | Hexadecanoic acid, methyl ester |
| 21 | 22.164 | 8.95 | Octadecanoic acid, methyl ester |
| 22 | 22.409 | 0.27 | Heat bodied oil |
| 23 | 22.844 | 0.01 | Benzene, isocyanato |
| 24 | 24.1617 | 3.62 | Drying oil |
| 25 | 24.6707 | 0.29 | Drying oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Han, K.; Teri, G.; Tian, Y.; Cui, M.; Qi, Y.; Li, Y. A Study on the Materials Used in Ancient Wooden Architectural Paintings at DaZhong Gate in Confucius Temple, Qufu, Shandong, China. Materials 2024, 17, 2170. https://doi.org/10.3390/ma17092170
Li K, Han K, Teri G, Tian Y, Cui M, Qi Y, Li Y. A Study on the Materials Used in Ancient Wooden Architectural Paintings at DaZhong Gate in Confucius Temple, Qufu, Shandong, China. Materials. 2024; 17(9):2170. https://doi.org/10.3390/ma17092170
Chicago/Turabian StyleLi, Kuiju, Kezhu Han, Gele Teri, Yuxiao Tian, Menglei Cui, Yunpeng Qi, and Yuhu Li. 2024. "A Study on the Materials Used in Ancient Wooden Architectural Paintings at DaZhong Gate in Confucius Temple, Qufu, Shandong, China" Materials 17, no. 9: 2170. https://doi.org/10.3390/ma17092170
APA StyleLi, K., Han, K., Teri, G., Tian, Y., Cui, M., Qi, Y., & Li, Y. (2024). A Study on the Materials Used in Ancient Wooden Architectural Paintings at DaZhong Gate in Confucius Temple, Qufu, Shandong, China. Materials, 17(9), 2170. https://doi.org/10.3390/ma17092170






