The Potential of Using Shungite Mineral from Eastern Kazakhstan in Formulations for Rubber Technical Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Testing
2.2.1. Determination of the Plastoelastic Properties of the Rubber Compounds
2.2.2. Determination of Vulcanization Kinetics of Rubber Compounds
2.2.3. Methodology for Determination of the Physical–Mechanical Indicators of Vulcanizates
2.2.4. Determination of Resistance of Rubber to Aggressive Factors
2.2.5. Determination of Relative Residual Compressive Deformation (RRCD)
3. Results and Discussion
3.1. Technological Properties
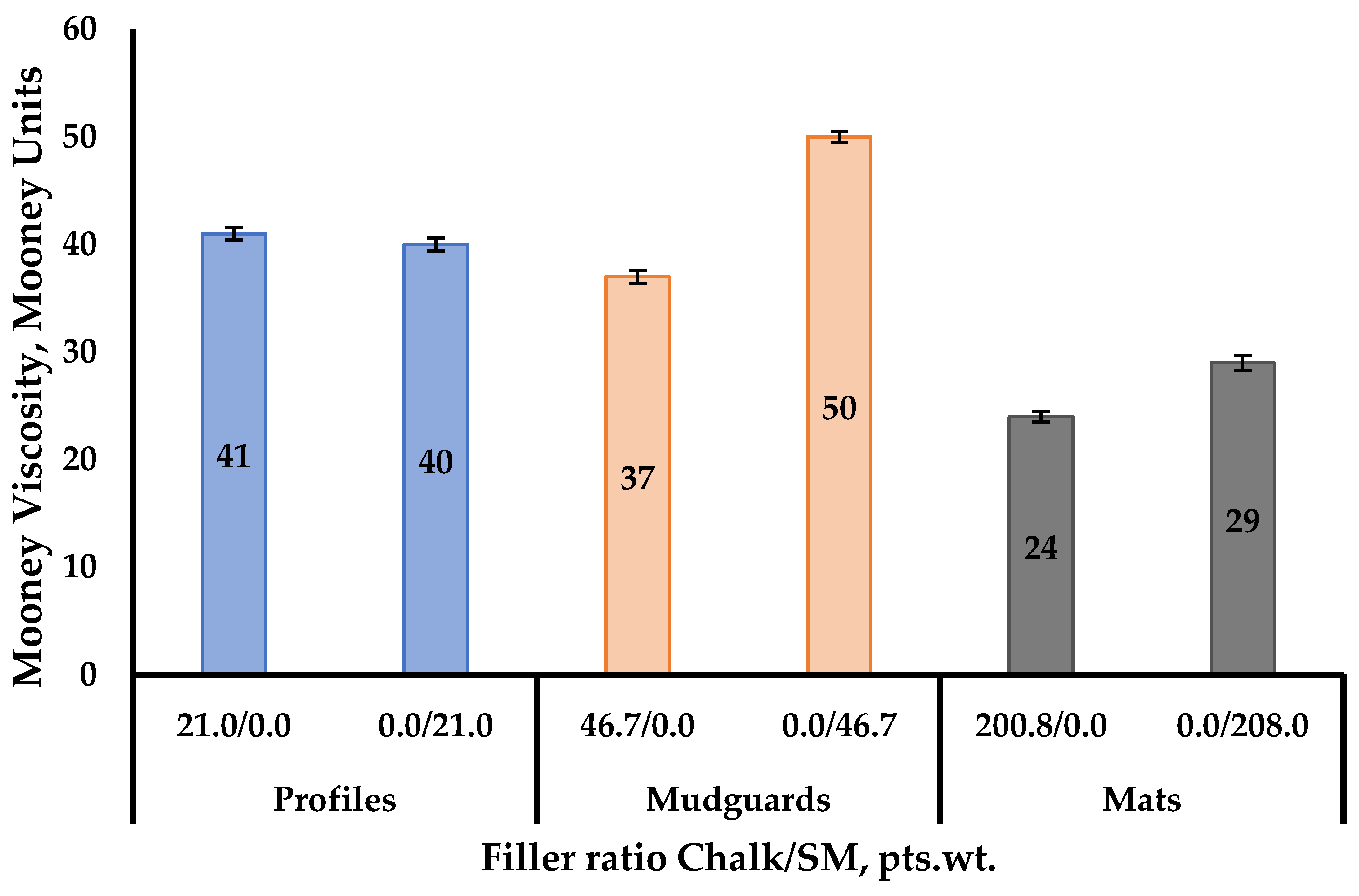
3.1.1. Investigation of Mooney Viscosity of Rubber Compounds
3.1.2. Determination of the Kinetic Parameters of the Vulcanization Process
3.2. Physical–Mechanical and Performance Properties of the Studied Elastomer Compositions
3.2.1. Investigation of Elastic and Strength Properties of Rubbers
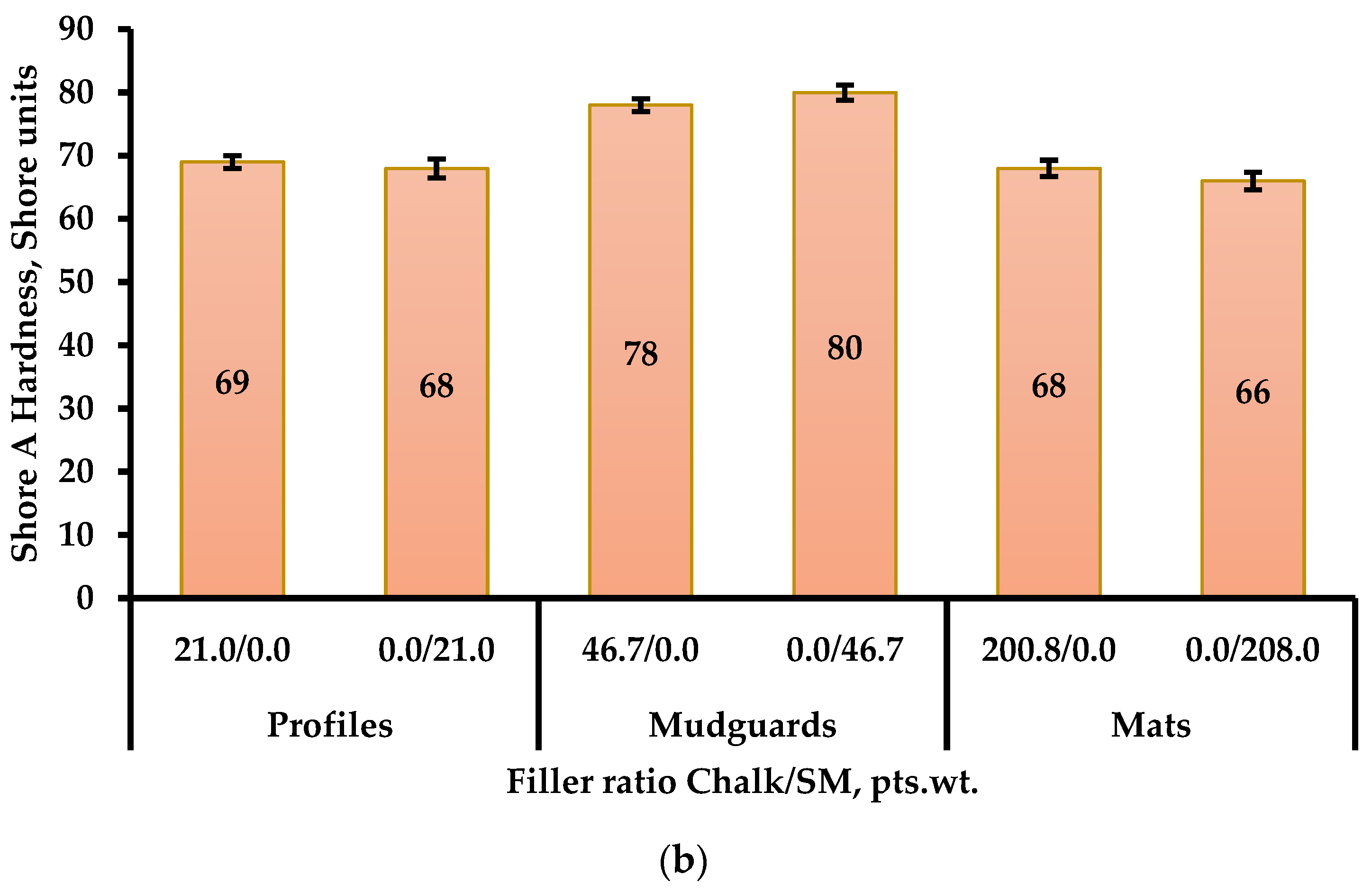
3.2.2. Investigation of Shore A Hardness of Elastomer Compositions
3.2.3. Determination of Relative Residual Compressive Deformation (RRCD)
3.2.4. Study of Rubbers with Shungite Mineral for Resistance to Liquid Aggressive Media
- Absorption of the solvent on the polymer surface;
- Penetration of the solvent into the polymer, initially by filling pores and free volume, followed by diffusion of solvent molecules into the polymer;
- The polymer structure expands as the solvent absorbed into the pores penetrates the network of polymer chains, causing them to swell [6].
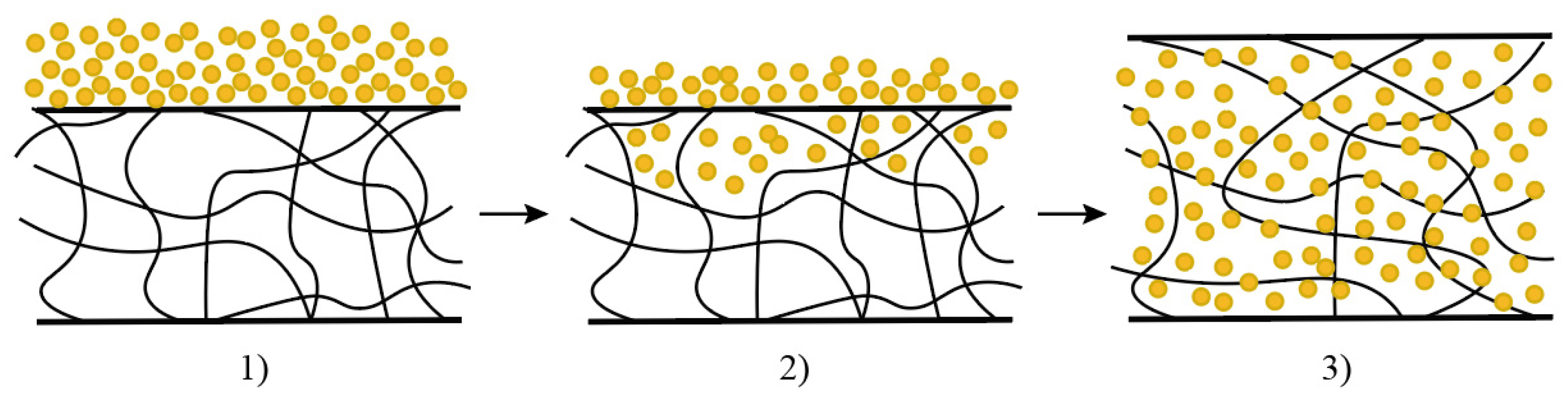
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sattayanurak, S.; Sahakaro, K.; Kaewsakul, W.; Dierkes, W.K.; Reuvekamp, L.A.E.M.; Blume, A.; Noordermeer, J.W.M. Synergistic Effect by High Specific Surface Area Carbon Black as Secondary Filler in Silica Reinforced Natural Rubber Tire Tread Compounds. Polym. Test. 2020, 81, 106173. [Google Scholar] [CrossRef]
- Garishin, O.K.; Shadrin, V.; Beliaev, A.; Kornev, Y.V. Micro and Nanoshungites—Perspective Mineral Fillers for Rubber Composites Used in the Tires. Mater. Phys. Mech. 2018, 40, 56–62. [Google Scholar] [CrossRef]
- Basak, G.K.; Goswami, L.; Chattopadhyay, B.; Bandyopadhyay, A. Plasticizing Polystyrene with Waste Leather Buffing Dust: A Drive Towards Waste-Polymer Composite Synthesis. Polym. Polym. Compos. 2012, 20, 279–288. [Google Scholar] [CrossRef]
- Fan, Y.; Fowler, G.D.; Zhao, M. The Past. Present and Future of Carbon Black as a Rubber Reinforcing Filler—A Review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectroscopy as a Tool for the Analysis of Carbon-Based Materials (Highly Oriented Pyrolytic Graphite. Multilayer Graphene and Multiwall Carbon Nanotubes) and of Some of Their Elastomeric Composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Wang, X.; Rodrigues, A.M.; Zhang, R. Reinforcement of the Mechanical Properties in Nitrile Rubber by Adding Graphene Oxide/Silicon Dioxide Hybrid Nanoparticles. J. Appl. Polym. Sci. 2017, 135, 46091. [Google Scholar] [CrossRef]
- Devi, U.K.S.; Maria, H.J.; Thomas, S.; Ponnamma, D.; Causin, V. Enhanced Morphology and Mechanical Characteristics of Clay/Styrene Butadiene Rubber Nanocomposites. Appl. Clay Sci. 2015, 114, 568–572. [Google Scholar] [CrossRef]
- Yadav, R.; Tirumali, M.; Wang, X.; Naebe, M.; Kandasubramanian, B. Polymer Composite for Antistatic Application in Aerospace. Def. Technol. 2019, 5, 107–118. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Wang, W.; Zhang, L.; Fakhrullin, R.F. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Das, A.; Jurk, R.; Stöckelhuber, K.W.; Engelhardt, T.; Fritzsche, J.; Klüppel, M.; Heinrich, G. Processing and Properties of Nanocomposites Based on Layered Silicate and Carboxylated Nitrile Rubber. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 45, 144. [Google Scholar] [CrossRef]
- Das, A.; Jurk, R.; Stöckelhuber, K.W.; Heinrich, G. Jamming in Filled Polymer Systems. Macromol. Symp. 2010, 292, 193–201. [Google Scholar] [CrossRef]
- Mujkanović, A.; Vasiljević, L.; Ostojić, G. Non-Black Fillers for Elastomers. In Proceedings of the 13th International Research/Expert Conference Trends in the Development of Machinery and Associated Technology (TMT), Hammamet, Tunisia, 16–21 October 2009; pp. 865–868. [Google Scholar]
- Masłowski, M.; Miedzianowska, J.; Strzelec, K. Natural Rubber Composites Filled with Crop Residues as an Alternative to Vulcanizates with Common Fillers. Polymers 2019, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A Review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Bhat, A.H.; Yusra, A.F.I. Green Composites from Sustainable Cellulose Nanofibrils: A Review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agricultural Residues: Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Murtaja, Y.; Lapčík, L.; Sepetcioglu, H.; Vlček, J.; Lapčíková, B.; Ovsík, M.; Staněk, M. Enhancement of the Mechanical Properties of HDPE Mineral Nanocomposites by Filler Particles Modulation of the Matrix Plastic/Elastic Behavior. Nanotechnol. Rev. 2022, 11, 312–320. [Google Scholar] [CrossRef]
- Post, W.; Kuijpers, L.J.; Zijlstra, M.; van der Zee, M.; Molenveld, K. Effect of Mineral Fillers on the Mechanical Properties of Commercially Available Biodegradable Polymers. Polymers 2021, 13, 394. [Google Scholar] [CrossRef]
- Kwaśniewska, A.; Chocyk, D.; Gładyszewski, G.; Borc, J.; Świetlicki, M.; Gładyszewska, B. The Influence of Kaolin Clay on the Mechanical Properties and Structure of Thermoplastic Starch Films. Polymers 2020, 12, 73. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, S.; Li, H.; Sun, J.; Tang, W.; Zhao, L.; Gu, X. Preparation of Intumescent Flame Retardant Poly(Butylene Succinate) Using Urea Intercalated Kaolinite as Synergistic Agent. Fibers Polym. 2019, 20, 1631–1640. [Google Scholar] [CrossRef]
- Valera-Zaragoza, M.; Yescas-Yescas, A.; Juarez-Arrelano, E.A.; Aguirre-Cruz, A.; Aparicio-Saguilan, A.; Ramirez-Vargas, E.; Sanchez-Valdes, S.; Sepulveda-Guzman, S. Immobilization of TiO2 Nanoparticles on Montmorillonite Clay and Its Effect on the Morphology of Natural Rubber Nanocomposites. Polym. Bull. 2014, 71, 1295–1313. [Google Scholar] [CrossRef]
- Shakun, A.; Vuorinen, J.; Hoikanen, N.M.; Poikelispaa, M.; Das, A. Hard Nanodiamonds in Soft Rubbers: Past, Present and Future—A Review. Compos. Part A Appl. Sci. Manuf. 2014, 64, 49–69. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Bassyouni, M.; Abdel-Hamid, S.M.-S. Effect of Modified Kaolinite by Using Fatty Acids on NR- and SBR-Reinforced Composites. Rubber Chem. Technol. 2015, 88, 449–462. [Google Scholar] [CrossRef]
- Lua, Y.; Liua, J.; Houa, G.; Mac, J.; Wang, W.; Weid, F.; Zhang, L. Thermal Degradation and Flame Retarding Characteristics of Polypropylene Composites Incorporated with Boron Mud. Compos. Sci. Technol. 2016, 137, 94–102. [Google Scholar] [CrossRef]
- DeArmitt, C.; Rothon, R. Particulate Fillers. Selection. and Use in Polymer Composites. In Encyclopedia of Polymers and Composites; Sanjay, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–19. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.; Ascione, L.; Mantia, F. Mechanical, Thermomechanical and Reprocessing Behavior of Green Composites from Biodegradable Polymer and Wood Flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef] [PubMed]
- Fengge, G. Clay/Polymer Composites: The Story. Mater. Today 2004, 11, 50–55. [Google Scholar] [CrossRef]
- Theng, B.K.G. Formation and Properties of Clay-Polymer Complexes. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 153–162. [Google Scholar]
- Saba, N.; Tahir, P.; Jawaid, M. A Review on Potentiality of Nano Filler/Natural Fiber Filled Polymer Hybrid Composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Post, W.; Bose, R.K.; Garcia, S.J.; Van Der Zwaag, S. Healing of Early Stage Fatigue Damage in Ionomer/Fe3O4 Nanoparticle Composites. Polymers 2016, 8, 436. [Google Scholar] [CrossRef]
- Zhong, N.; Post, W. Self-Repair of Structural and Functional Composites with Intrinsically Self-Healing Polymer Matrices: A Review. Compos. Part A Appl. Sci. Manuf. 2015, 69, 226–239. [Google Scholar] [CrossRef]
- Armentano, I.; Puglia, D.; Luzi, F.; Arciola, C.R.; Morena, F.; Martino, S.; Torre, L. Nanocomposites Based on Biodegradable Polymers. Materials 2018, 11, 795. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Reuter, U.; Heinrich, G. Macromolecular Materials and Engineering. Macromol. Mater. Eng. 2012, 297, 369–375. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Stöckelhuber, K.W.; Mukhopadhyay, N.; Bhattacharyya, A.R.; Jehnichen, D. Pre-Intercalation of Long Chain Fatty Acid in the Interlayer Space of Layered Silicates and Preparation of Montmorillonite/Natural Rubber Nanocomposites. Appl. Clay Sci. 2012, 68, 50–56. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Morozov, I.A.; Stöckelhuber, K.W.; Stocek, R.; Heinrich, G. Reinforcement of Solution Styrene Butadiene Rubber by Silane Functionalized Halloysite Nanotubes. J. Macromol. Sci. Part A Pure Appl. Chem. 2013, 50, 1091–1106. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Q.; Zhang, Y.; Cheng, H.; Lu, Y. Formation and Properties of Clay-Polymer Complexes. Appl. Clay Sci. 2012, 65, 134–142. [Google Scholar] [CrossRef]
- Saidi, M.A.; Mazlan, F.S.; Hassan, A.; Abd Rashid, R.; Rahmat, A.R. Flammability. Thermal and Mechanical Properties of Polybutylene Terephthalate/Dolomite Composites. J. Phys. Sci. 2019, 30, 175–189. [Google Scholar] [CrossRef]
- Osman, A.F.; Siah, L.; Alrashdi, A.A.; Ul-Hamid, A.; Ibrahim, I. Improving the Tensile and Tear Properties of Thermoplastic Starch/Dolomite Biocomposite Film through Sonication Process. Polymers 2021, 13, 274. [Google Scholar] [CrossRef]
- Lim, K.C.; Osman, A.F.; Ahmad Fauzi, A.A.; Alrashdi, A.A.; Abdul Halim, K.A. The Mechanical and Thermal Properties of Poly(Ethylene-Co-Vinyl Acetate) (PECoVA) Composites with Pristine Dolomite and Organophilic Microcrystalline Dolomite (OMCD). Polymers 2021, 13, 3034. [Google Scholar] [CrossRef]
- ASTM D1765-03; Standard Classification System for Carbon Blacks Used in Rubber Products. ASTM International: West Conshohocken, PA, USA, 2003.
- GOST R 54552–2011; Rubbers and Rubber Mixtures. Determination of Viscosity. Stress Relaxation. and Pre-Vulcanization Characteristics Using a Mooney Viscometer. Standardinform: Moscow, Russia, 2013; 9p. (In Russian)
- GOST 12535-84; Rubber Mixtures. Method for Determining Vulcanization Characteristics Using a Vulcameter. Standards Publishing: Moscow, Russia, 1985; 33p. (In Russian)
- GOST 270-75; Rubber. Method for Determining Tensile Elastic Properties. Standards Publishing: Moscow, Russia, 1975; 29p. (In Russian)
- GOST 263-75; Method for Determining Hardness According to Shore A. Standards Publishing: Moscow, Russia, 1989; 7p. (In Russian)
- GOST 9.024-74; Unified System of Protection Against Corrosion and Aging. Rubbers. Methods for Testing Thermal Aging Resistance. Standards Publishing: Moscow, Russia, 1974; 12p. (In Russian)
- GOST 9.030-74; Rubbers. Methods for Testing Resistance in a Non-Stressed State to Exposure to Liquid Aggressive Media. Standards Publishing: Moscow, Russia, 2006; 10p. (In Russian)
- GOST 9.029-74; Rubbers. Methods for Testing Resistance to Aging under Static Compression Deformation. Standards Publishing: Moscow, Russia, 1974; 8p. (In Russian)
- Ovcharov, V.B. Properties of Rubber Mixtures and Rubbers: Evaluation. Regulation. Stabilization. SANT-TM: Moscow, Russia, 2001; 400p. (In Russian)
- Reznychenko, S.V.; Morozova, Y.L. Comprehensive Handbook for Rubber Technologists; Tekhinform: Moscow, Russia, 2012; Volume 2, 735p. (In Russian) [Google Scholar]
- Zhovner, N.A.; Chirkova, N.V.; Khlebov, G.A. Structure and Properties of Materials Based on Elastomers: A Textbook; Filial RosZITLP: Omsk, Russia, 2003; 276p. (In Russian) [Google Scholar]
- Chaikun, A.M.; Naumov, I.S.; Alifanova, I.S. Rubber Sealing Materials (Review). Tr. VIAM 2017, 1, 99–106. (In Russian) [Google Scholar]
- Hu, H.; Zhao, L.; Liu, J.; Liu, Y.; Cheng, J.; Luo, J.; Liang, Y.; Tao, Y.; Wang, X.; Zhao, J. Enhanced Dispersion of Carbon Nanotube in Silicone Rubber Assisted by Graphene. Polymer 2012, 53, 3378–3385. [Google Scholar] [CrossRef]
- Pradhan, B.; Srivastava, S.K. Synergistic Effect of Three-Dimensional Multi-Walled Carbon Nanotube-Graphene Nanofiller in Enhancing the Mechanical and Thermal Properties of High-Performance Silicone Rubber. Polym. Int. 2014, 63, 1219–1228. [Google Scholar] [CrossRef]




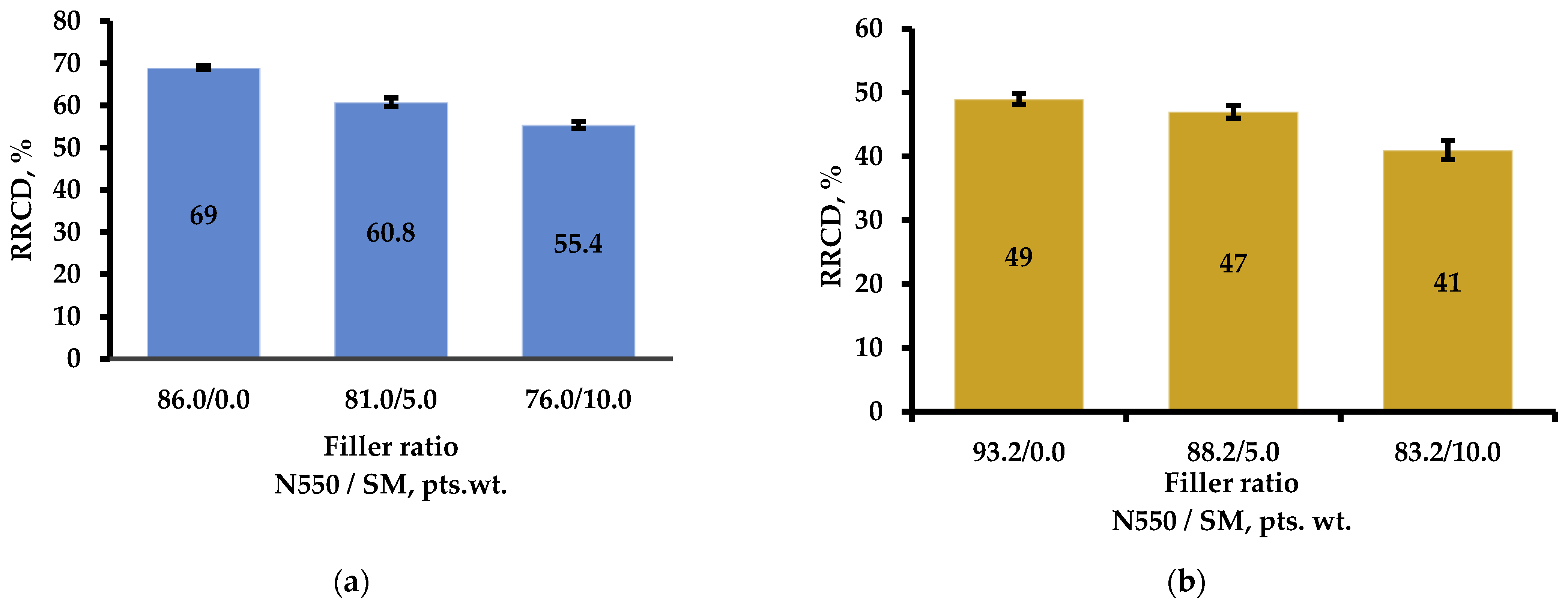
| Ingredient Name | The Content of Ingredients, pts. wt. per 100 pts. wt. of Rubber | |||
|---|---|---|---|---|
| Profiles | Mudguards | Mats | Sealing Rings | |
| SKEPT-50 | 20.0 | – | – | – |
| SBR-30 ARKM-15 | 80.0 | 23.0 | 42.0 | – |
| IR (SKI-3) | – | 55.0 | 58.0 | – |
| BNR-28AN | – | – | – | 93.0 |
| Zinc white | 6.0 | 1.8 | 2.3 | 6.8 |
| Acetoanil N | 2.1 | 1.5 | – | 0.9 |
| Diafen IPPD | 2.1 | 1.5 | – | 0.9 |
| Stearic acid | 2.1 | – | 2.3 | 0.5 |
| Carbon black N550 | 86.0 | 90.0 | 95.4 | 93.2 |
| Chalk | 21.0 | 46.7 | 200.8 | – |
| Calcium naphthenate M1 | 7.1 | – | – | – |
| Santogard PVI | 1.0 | 0.3 | – | – |
| Oil I-20A | 25.5 | 11.0 | – | – |
| Protective wax | 3.4 | 2.1 | 2.3 | 2.3 |
| PEG-4000 | 2.9 | – | – | – |
| Sulfur | 2.6 | 0.6 | 1.5 | – |
| Diphenylguanidine (DFG) | 0.8 | – | – | – |
| Sulfenamide C | 2.1 | – | – | 0.5 |
| Regenerate | – | 55.0 | 143.5 | – |
| Thiuram D | – | 0.6 | 0.8 | 0.5 |
| Altax | – | 1.2 | 0.8 | – |
| Petroleum bitumen | – | 9.8 | 16.0 | – |
| Benzoic acid | – | 0.3 | 0.8 | – |
| Softener | – | – | 61.8 | – |
| Rubber crumb SP | – | – | 137.4 | – |
| DBP (dibutyl phthalate) | – | – | – | 0.9 |
| DTDM (dithiodimorpholine) | – | – | – | 0.5 |
| Classification (ASTM) | Iodine Adsorption Number, g/kg | DBP Absorption, cm³/100 g | pH of Aqueous Suspension | Surface Area for Multi Point Nitrogen Adsorption, m²/g | External Surface Area for Nitrogen Adsorption, m²/g | Bulk Density, kg/m³ |
|---|---|---|---|---|---|---|
| N550 | 43 ± 4 | 101 ± 4 | 6.0–8.0 | 41 | 39 | 360 |
| Characteristic | Numerical Value, Standardized According to Technical Specifications (TS) |
|---|---|
| Total mass fraction of calcium carbonate and magnesium carbonate (calculated as calcium carbonate), minimum | 98.5% |
| Mass fraction of substances that are insoluble in hydrochloric acid, maximum | 1.3% |
| Mass fraction of iron and aluminum oxides, maximum | 0.4% |
| Mass fraction of moisture, maximum | 0.2% |
| Reflectance coefficient, minimum | 86.0% |
| Characteristic | RTP Type | |||
|---|---|---|---|---|
| Profile | Mudguards | Mats | Sealing Rings | |
| Tensile strength, MPa, minimum | 5.9 | 9.8 | 4.0 | 10.8 |
| Elongation at break, %, minimum | 180 | 250 | 110 | 150 |
| Hardness, Shore A units | 60–80 | 70–75 | 60–75 | 75–85 |
| Compression set at 20–30% compression, %, maximum | 70.0 | – | – | 60.0 |
| Change in mass after exposure to a 70:30 by volume mixture of isooctane and toluene for 24 h at (23 ± 3) °C, %, maximum | – | – | – | 25.0 |
| Characteristics | Value |
|---|---|
| Appearance | Highly dispersed gray powder |
| Bulk density, kg/m³ | 500–600 |
| Grinding fraction | 15.0 ÷ 35.0 µm |
| Moisture content, % | 0.5–1.5 |
| Loss on ignition, % | 8–14 |
| pH of aqueous extract | 7–9 |
| Dibutyl phthalate absorption, cm³/100 g | 28–35 |
| Mass fraction of residue after sieving using sieve No. 0045 | Less than 0.08 |
| Specific external surface area by nitrogen adsorption, m²/g | 23–25 |
| Elements | Content, % w/w |
|---|---|
| C | 17.16 |
| O | 50.28 |
| Si | 16.52 |
| Al | 9.68 |
| K | 1.98 |
| Fe | 2.11 |
| Na | 0.88 |
| Mg | 0.57 |
| Ca | 0.43 |
| Ti | 0.39 |
| Product Name | |||
|---|---|---|---|
| Profiles | Mudguards | Mats | Sealing Rings |
| Partial Replacement of Carbon Black N550 with Shungite Mineral | |||
| Ratio of N550/SM, pts. wt. per 100 pts. wt. of rubber | |||
| 86.0/0.0 (industrial mix) | – | – | 93.2/0.0 (industrial mix) |
| 81.0/5.0 (experimental mixture) | – | – | 88.0/5.0 (experimental mixture) |
| 76.0/10.0 (experimental mixture) | – | – | 83.2/10.0 (experimental mixture) |
| Complete Replacement of Chalk with SM | |||
| Ratio of Chalk/SM, pts. wt. per 100 pts. wt. of rubber | |||
| 21.0/0.0 (industrial mix) | 46.7/0.0 (industrial mix) | 200.8/0.0 (industrial mix) | – |
| 0.0/21.0 (experimental mixture) | 0.0/46.7 (experimental mixture) | 0.0/200.8 (experimental mixture) | – |
| Type of Product | Filler Ratio N550/SM, pts. wt. | Kinetic Parameters | ||
|---|---|---|---|---|
| ts2, min | t90, min | ΔM, dN∙m | ||
| Rubber profiles | 86.0/0.0 (industrial mixture) | 1.46 ± 0.07 | 3.29 ± 0.16 | 22.4 ± 1.1 |
| 81.0/5.0 (experimental mixture) | 1.15 ± 0.06 | 2.93 ± 0.15 | 18.5 ± 0.9 | |
| 76.0/10.0 (experimental mixture) | 1.02 ± 0.05 | 2.51 ± 0.13 | 16.4 ± 0.8 | |
| Sealing rings | 93.2/0.0 (industrial mixture) | 1.27 ± 0.06 | 2.51 ± 0.13 | 35.6 ± 1.8 |
| 88.2/5.0 (experimental mixture) | 1.28 ± 0.06 | 2.51 ± 0.13 | 33.4 ± 1.7 | |
| 83.2/10.0 (experimental mixture) | 1.3 ± 0.6 | 2.49 ± 0.12 | 31 ± 2 | |
| Type of Product | Filler Ratio N550/SM, pts. wt. | Kinetic Parameters | ||
|---|---|---|---|---|
| ts2, min | t90, min | ΔM, dN∙m | ||
| Rubber profiles | 86.0/0.0 (industrial mixture) | 1.46 ± 0.07 | 3.29 ± 0.16 | 22.4 ± 1.1 |
| 0.0/86.0 (experimental mixture) | 1.44 ± 0.07 | 3.22 ± 1.16 | 22.3 ± 1.1 | |
| Mudguards | 46.7/0.0 (industrial mixture) | 0.77 ± 0.04 | 1.32 ± 0.07 | 21.2 ± 1.1 |
| 0.0/46.7 (experimental mixture) | 0.72 ± 0.04 | 1.24 ± 0.06 | 20.8 ± 1.0 | |
| Mats | 200.8/0.0 (industrial mixture) | 0.63 ± 0.03 | 0.93 ± 0.05 | 13.4 ± 0.7 |
| 0.0/200.8 (experimental mixture) | 0.57 ± 0.03 | 0.87 ± 0.04 | 13.5 ± 0.7 | |
| Type of Product | Filler Ratio N550/SM, pts. wt. | fp, MPa | εp, % | ||
|---|---|---|---|---|---|
| Before Aging | After Aging | Before Aging | After Aging | ||
| Rubber profiles | 86.0/0.0 (industrial mixture) | 8.1 ± 0.8 | 8.4 ± 0.8 | 290 ± 29 | 190 ± 19 |
| 81.0/5.0 (experimental mixture) | 7.6 ± 0.8 | 7.7 ± 0.8 | 340 ± 34 | 230 ± 23 | |
| 76.0/10.0 (experimental mixture) | 7.3 ± 0.7 | 7.5 ± 0.7 | 380 ± 38 | 250 ± 25 | |
| Sealing rings | 93.2/0.0 (industrial mixture) | 12 ± 1 | 12.8 ± 1.3 | 160 ± 16 | 190 ± 19 |
| 88.2/5.0 (experimental mixture) | 11.3 ± 1.1 | 13.7 ± 1.4 | 140 ± 14 | 230 ± 23 | |
| 83.2/10.0 (experimental mixture) | 10.9 ± 1.1 | 14.1 ± 1.4 | 130 ± 13 | 260 ± 26 | |
| Type of Product | Filler Ratio Chalk/SM, pts. wt. | fp, MPa | εp, % | ||
|---|---|---|---|---|---|
| Before Aging | After Aging | Before Aging | After Aging | ||
| Rubber profiles | 86.0/0.0 (industrial mixture) | 8.1 ± 0.8 | 8.4 ± 0.8 | 290 ± 29 | 190 ± 19 |
| 0.0/86.0 (experimental mixture) | 8 ± 1 | 7.9 ± 0.8 | 300 ± 30 | 210 ± 21 | |
| Mudguards | 46.7/0.0 (industrial mixture) | 10.3 ± 1.0 | 10.6 ± 1.1 | 340 ± 34 | 150 ± 15 |
| 0.0/46.7 (experimental mixture) | 12.2 ± 1.2 | 11.6 ± 1.2 | 330 ± 33 | 190 ± 19 | |
| Mats | 200.8/0.0 (industrial mixture) | 4.5 ± 0.4 | 3.7 ± 0.4 | 240 ± 24 | 150 ± 15 |
| 0.0/200.8 (experimental mixture) | 8.1 ± 0.8 | 8.4 ± 0.8 | 290 ± 29 | 190 ± 19 | |
| Product Type | Ratio of Fillers N550/SM. pts. wt. | Change in Mass of Vulcanizate, % |
|---|---|---|
| Sealing rings | 93.2/0.0 (industrial mix) | 18.0 |
| 88.2/5.0 (experimental mixture) | 16.4 | |
| 83.2/10.0 (experimental mixture) | 15.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nechipurenko, S.V.; Bobrova, V.V.; Kasperovich, A.V.; Yermaganbetov, M.Y.; Yefremov, S.A.; Kaiaidarova, A.K.; Makhayeva, D.N.; Yermukhambetova, B.B.; Mun, G.A.; Irmukhametova, G.S. The Potential of Using Shungite Mineral from Eastern Kazakhstan in Formulations for Rubber Technical Products. Materials 2025, 18, 114. https://doi.org/10.3390/ma18010114
Nechipurenko SV, Bobrova VV, Kasperovich AV, Yermaganbetov MY, Yefremov SA, Kaiaidarova AK, Makhayeva DN, Yermukhambetova BB, Mun GA, Irmukhametova GS. The Potential of Using Shungite Mineral from Eastern Kazakhstan in Formulations for Rubber Technical Products. Materials. 2025; 18(1):114. https://doi.org/10.3390/ma18010114
Chicago/Turabian StyleNechipurenko, Sergey V., Valeriya V. Bobrova, Andrey V. Kasperovich, Mubarak Ye. Yermaganbetov, Sergey A. Yefremov, Aigerim K. Kaiaidarova, Danelya N. Makhayeva, Bayana B. Yermukhambetova, Grigoriy A. Mun, and Galiya S. Irmukhametova. 2025. "The Potential of Using Shungite Mineral from Eastern Kazakhstan in Formulations for Rubber Technical Products" Materials 18, no. 1: 114. https://doi.org/10.3390/ma18010114
APA StyleNechipurenko, S. V., Bobrova, V. V., Kasperovich, A. V., Yermaganbetov, M. Y., Yefremov, S. A., Kaiaidarova, A. K., Makhayeva, D. N., Yermukhambetova, B. B., Mun, G. A., & Irmukhametova, G. S. (2025). The Potential of Using Shungite Mineral from Eastern Kazakhstan in Formulations for Rubber Technical Products. Materials, 18(1), 114. https://doi.org/10.3390/ma18010114






