Abstract
Co-doping at Ba and Ti sites with double rare-earth elements has proven an effective strategy for enhancing the dielectric properties of BaTiO3 ceramics. Among intermediate-sized rare-earth ions, Tb and Ho exhibit amphoteric behavior, occupying both Ba and Ti sites. Investigating the site occupation, defect chemistry, and dielectric effects of Tb and Ho in BaTiO3 is therefore valuable. In this work, Tb/Ho-co-doped BaTiO3 ceramics with the composition (Ba1−xTbx)(Ti1−xHox)O3 (x = 0.01~0.10) were fabricated at 1400 °C via solid-state reaction, and their solid solubility and crystal structures are confirmed. Microstructure, dielectric properties, photoluminescence, and valence states of samples with a single phase were systematically studied. Both the lattice parameter a and unit cell volume increase with doping level. The ceramic with x = 0.02 meets the X5S dielectric specification. Ho and Tb ions both demonstrate amphoteric site occupancy: Ho exists solely as Ho3+ at both Ba and Ti sites, while Tb exhibits mixed valence states as Ba-site Tb3+ and Ti-site Tb4+. As the doping content increases, the concentration of Tb4+ at Ti sites decreases, and the quantity of Ba-site Ho3+ ions initially increases to a maximum before decreasing. Defect compensation mechanisms within the samples are also discussed.
1. Introduction
High-performance dielectric ceramics with perovskite structure (ABO3) have attracted huge attention for their unique properties. For example, BaTiO3 [1,2], SrTiO3 [3], and (K, Na)NbO3 [4] have been widely used in high-k dielectrics, piezoelectric devices and so on. Among these compounds, BaTiO3-based ceramics are widely used in multilayer ceramic capacitors (MLCCs) [5,6], positive temperature coefficient (PTC) thermistors [7,8], and other applications. Rare-earth elements are found to be crucial to the electrical properties of BaTiO3, and various doping strategies have been adopted to improve the performance of the above devices. Hu et al. prepared Bi- and Ho-co-doped BaTiO3 ceramics and studied the synergistic effects of Bi and Ho on the dielectric performance. By adjusting the doping concentration, the samples satisfied the X8R specification and exhibited good temperature coefficient of capacitance stability [9]. Lu et al. designed rare-earth element and Ca co-doped BaTiO3 with the formula of (Ba1−xREx)(Ti1−x/2Cax/2)O3 (RE = La, Tb, Dy) and studied their dielectric properties in detail, and the samples showed high dielectric performance and satisfied the X5R, X7R, or X8R specifications [10,11,12]. These studies demonstrate convincingly that rare-earth elements play an important role in tailoring performance for BaTiO3 ceramics. Apart from the above doping strategy, simultaneous doping at Ba and Ti sites with double rare-earth elements is also proved to be effective for improving the dielectric properties of BaTiO3 [13].
The site occupation of rare-earth ions in the BaTiO3 lattice depends on the ionic radii [14,15]. Large rare-earth ions, for example, La3+ and Sm3+, occupy exclusively the Ba site, whereas the small ions such as Yb3+ enter the Ti site. Using a double rare-earth doping strategy, the researchers could prepare dielectric ceramics with fine grains and low loss [16,17]. Ions with intermediate size, for example, Ho3+, Er3+, and Dy3+, exhibit an amphoteric behavior of occupying Ba sites as donors and Ti sites as acceptors [18,19].
Among the intermediate-size rare-earth ions, Ho is important for the densification and dielectric properties of BaTiO3 ceramics. Lu et al. studied Ho-doped BaTiO3 ceramics with a self-compensation mechanism, and they found that Ho-doped BaTiO3 ceramics have a high relative density and a low dielectric loss [20]. Tb also exhibits amphoteric behavior, i.e., Tb ions can occupy both Ba and Ti sites. However, unlike Ho, which keeps a stable trivalent state, Tb ions may exist in the BaTiO3 lattice as Tb3+ or Tb4+. Some authors proposed the possibility that Tb ions exist at Ti sites as Tb3+ [19,21,22], whereas Tsur et al. pointed out that Tb could be incorporated into Ti sites as Tb4+ during the sintering process [23]. Lu et al. proved that the self-compensation mode of Tb () does not exist in the case of Ba/Ti = 1 because Tb occupies Ba and Ti sites as Tb3+ and Tb4+, respectively [24]. The results of Lu et al. demonstrated that the self-adjustable amphoteric behavior of valence states of Tb ions is important for the lattice electroneutrality of the samples. Based on the above studies and discussion, it is meaningful to study the interaction of Tb and Ho in BaTiO3. In this work, Tb- and Ho-co-doped BaTiO3 samples are designed to explore the crystal structure, site occupancy, and dielectric properties.
2. Materials and Methods
(Ba1−xTbx)(Ti1−xHox)O3 (x = 0.01~0.10) (abbreviated as BTTH) ceramics were prepared by the conventional solid-state reaction method. Starting reagents BaCO3 (99.4%, Sinopharm Chemical Regent Co., Ltd., Shanghai, China), TiO2 (99.5%, Yuejiang Chemical Co., Ltd., Shanghai, China), Tb4O7 (99.99%, Diyang Co., Ltd., Shanghai, China), and Ho2O3 (99.99%, Diyang Co., Ltd., Shanghai, China) were weighed according to their stoichiometric ratios. These raw material oxides were thoroughly ground in an agate mortar and calcined at 1100 °C for 5 h. The calcined powders were mixed with 12 wt% PVA and pressed into cylindrical pellets (12 mm in diameter) under 200 MPa. Finally, these pellets were sintered at 1400 °C for 12 h in an air conditioner.
The room-temperature crystal structures were determined by powder X-ray diffraction (XRD) using a diffractometer (DX-2700, Dandong, China) with Cu Kα radiation (1.540562 Å), and the measurement step and counting time were 0.02° and 3 s, respectively. The microstructures of the pellets were characterized by scanning electron microscopy (SEM, EVO MA10, Zeiss, Oberkochen, Germany). The Nano Measurer software (Version 1.02) was used to estimate the grain size distribution of SEM images. X-ray photoelectron spectroscopy (XPS) measurements were carried out on a Thermo ESCALAB 250Xi spectrometer (Waltham, MA, USA) with Al Kα radiation to study the valence states. The XPS curves were fitted by a mixed Gaussian-Lorentzian function, and a Shirley-type background subtraction was used. Photoluminescence (PL) spectroscopy was adopted to investigate the site occupancy of Ho3+ ions on a LabRAM XploRA Raman spectrometer (HORIBA France SAS, Longjumeau, France) with a 532 nm laser (Horiba Jobin Yvon). The pellets were polished on both sides, and then silver paste was painted as an electrode before dielectric measurement. The dielectric properties were performed on a broadband dielectric spectrometer (Concept 41, Novocontrol Technologies, Montabaur, Germany) in the temperature range of −75–200 °C with a heating rate of 2 °C/min. Room-temperature electron paramagnetic resonance (EPR) measurement was carried out with an X-band (≈9.85 GHz) spectrometer (A300, Bruker, Berlin, Germany).
3. Results and Discussion
3.1. Crystal Structure and Microstructure Characterization
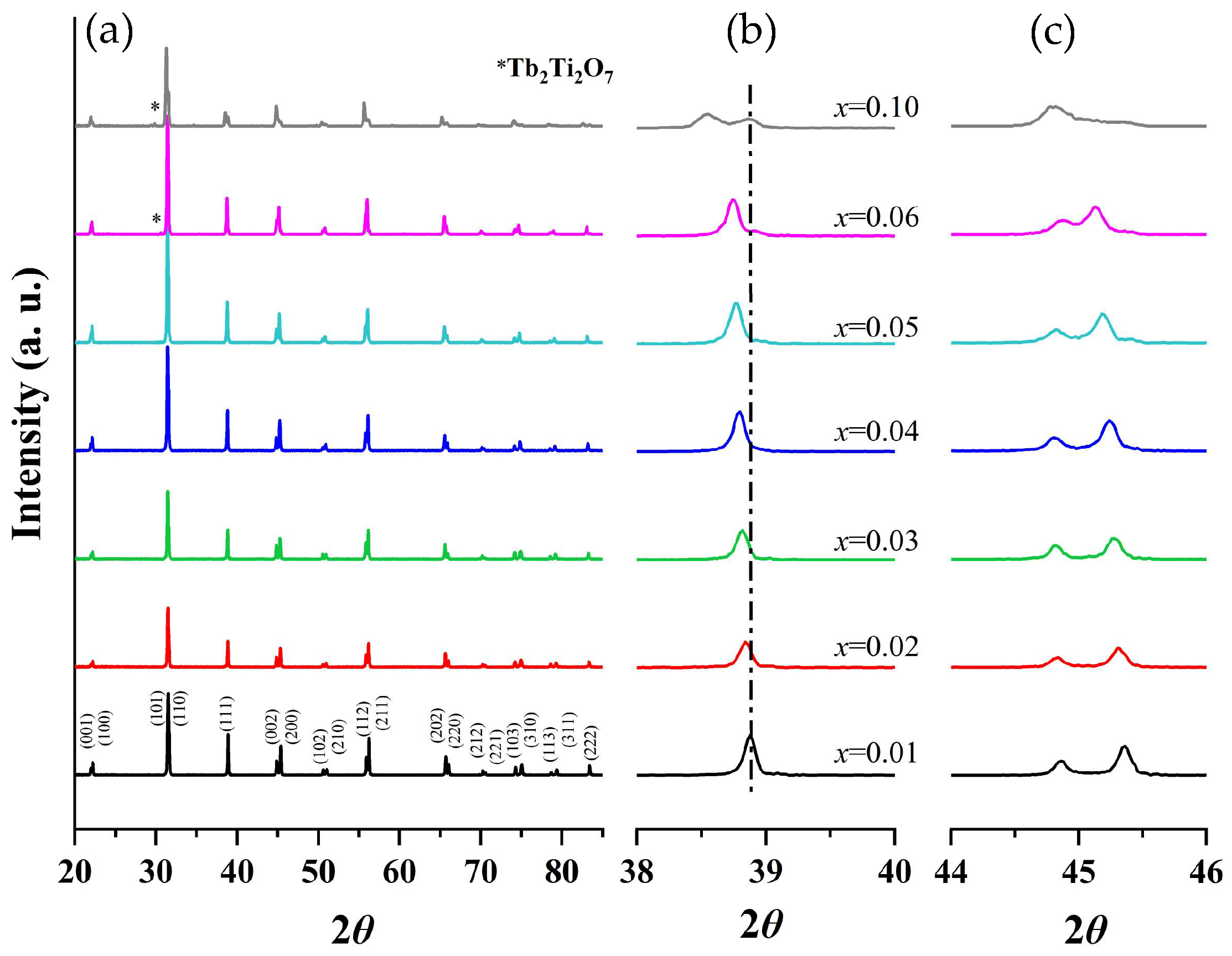
Powder XRD patterns of BTTH ceramics are shown in Figure 1a. When x ≤ 0.05, the samples can be indexed by a single perovskite structure. However, a secondary phase of Tb2Ti2O7 (JCPDS Card No. 15-9785) emerges when x ≥ 0.06, indicating that the solid solubility of Tb/Ho is 0.05. The secondary phase of Tb2Ti2O7 was also reported in Tb-doped barium titanate ceramics with self-compensation of Tb ions [25]. Secondary phases such as Ho2O3, Ho2Ti2O7, or Ba6Ho2Ti4O17 are not observed in all samples within the resolution limit of the XRD equipment, suggesting that Ho ions can completely enter the BaTiO3 lattice.
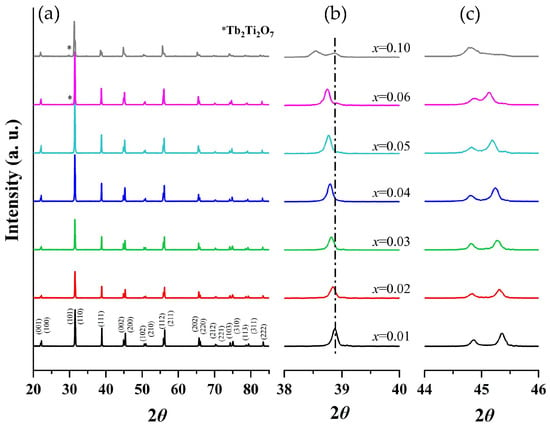
Figure 1.
(a) Room-temperature XRD patterns of (Ba1−xTbx)(Ti1−xHox)O3 (BTTH) ceramics, and magnified diffraction peaks at (b) 39° and (c) 45°.
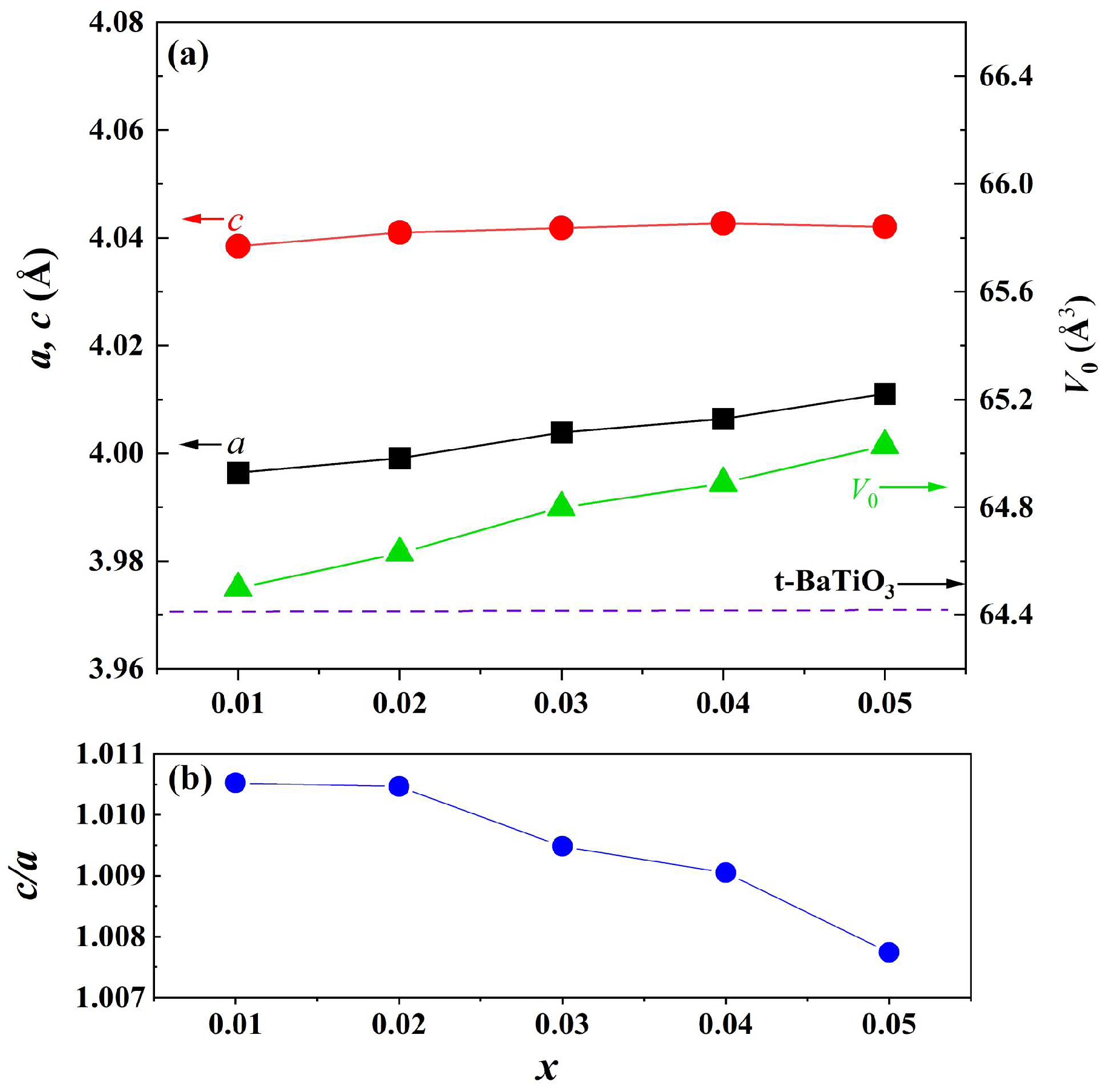
The magnified diffraction peaks in the vicinity of 39° and 45° are presented in Figure 1b,c. The peak at 39° shifts towards lower diffraction angles as the doping content increases, indicating an expansion of the lattice parameter. Two separate peaks are observed in the region of 44–46 degrees and are assigned to (002) and (200) crystal planes, indicating that the samples possess a tetragonal structure when x ≤ 0.06. These two peaks are close together and merge into one peak when x = 0.1. To analyze the structural evolution quantitatively, Rietveld refinements for XRD data of samples with pure phase (x ≤ 0.05) were performed, and the lattice parameters are shown in Figure 2a. With increasing the doping content, lattice parameter a increases from 3.99 to 4.01 Å, whereas c remains nearly constant. The unit cell volume (V0) also increases almost linearly with doping concentration, obeying Vegard’s law very well [26]. Figure 2b shows the lattice parameter ratio of c/a, which decreases slightly from 1.011 to 1.008, indicating tetragonality (c/a) of the crystal structure decreases with doping content, which is consistent with the result of Figure 1.
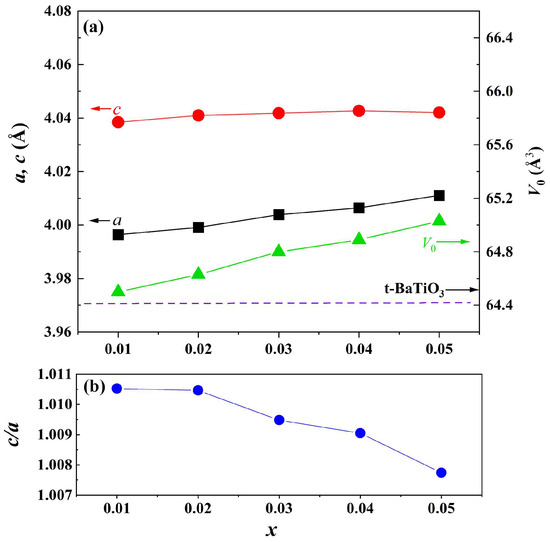
Figure 2.
(a) Lattice parameters (a, c) and unit cell volume (V0) (the dashed line shows the cell volume of tetragonal BaTiO3 ceramic) and (b) lattice parameter ratio c/a as a function of doping content.
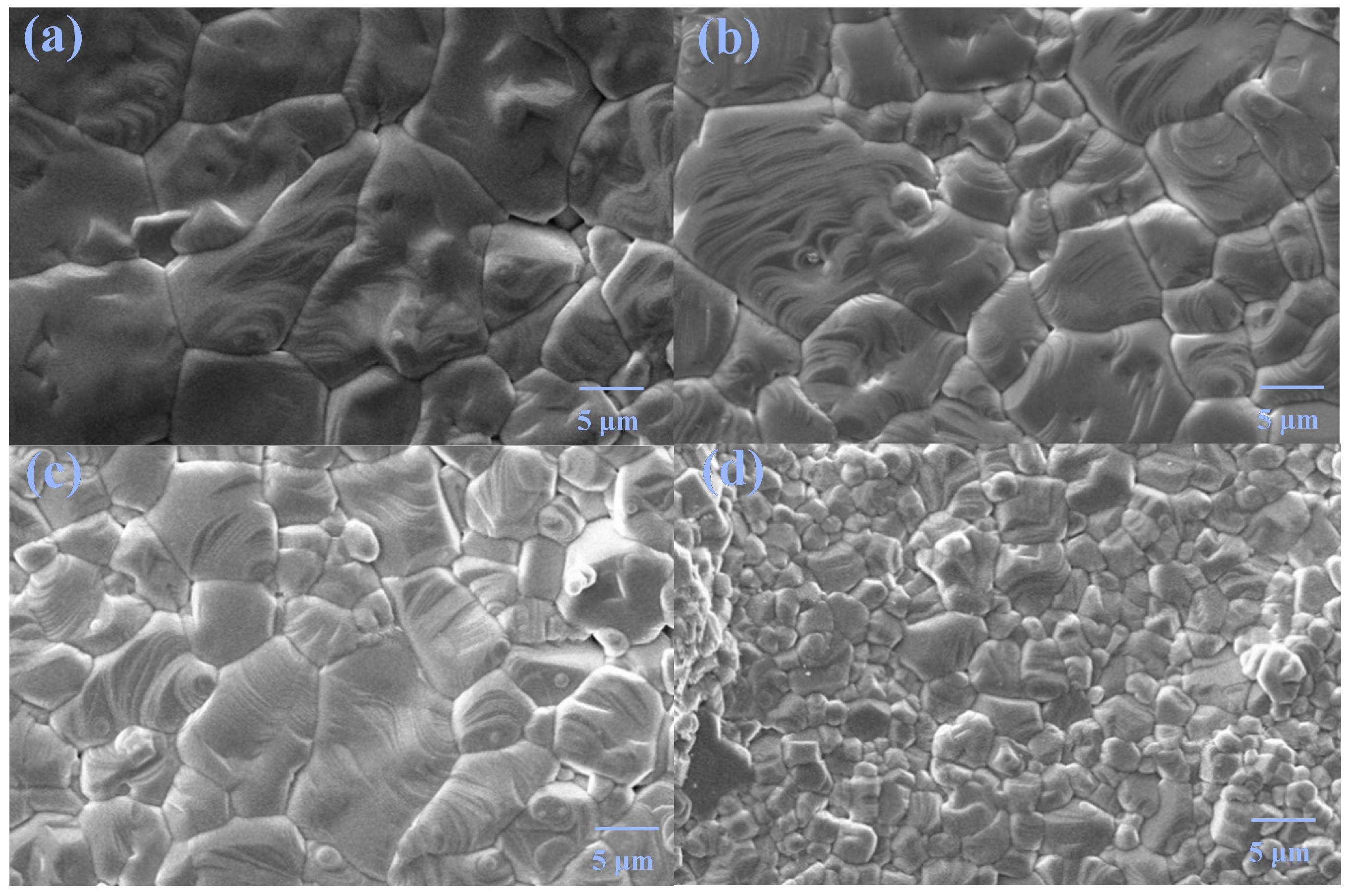
Figure 3 shows the micrographs of pure phase samples. All samples possess good crystallinity with very few pores. The grain size decreases with increasing doping content, suggesting that Tb/Ho co-doping can inhibit the grain growth. Using the Nano Measure software, the grain sizes of the samples are calculated to be 9.17, 6.48, 5.74, and 2.80 μm for x = 0.02, 0.03, 0.04, and 0.05, respectively.
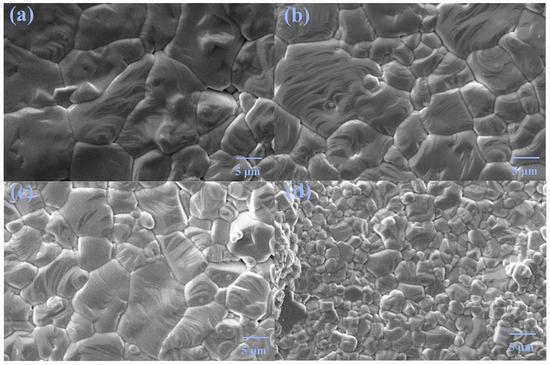
Figure 3.
SEM images of x = (a) 0.02, (b) 0.03, (c) 0.04, and (d) 0.05.
3.2. Dielectric Properties
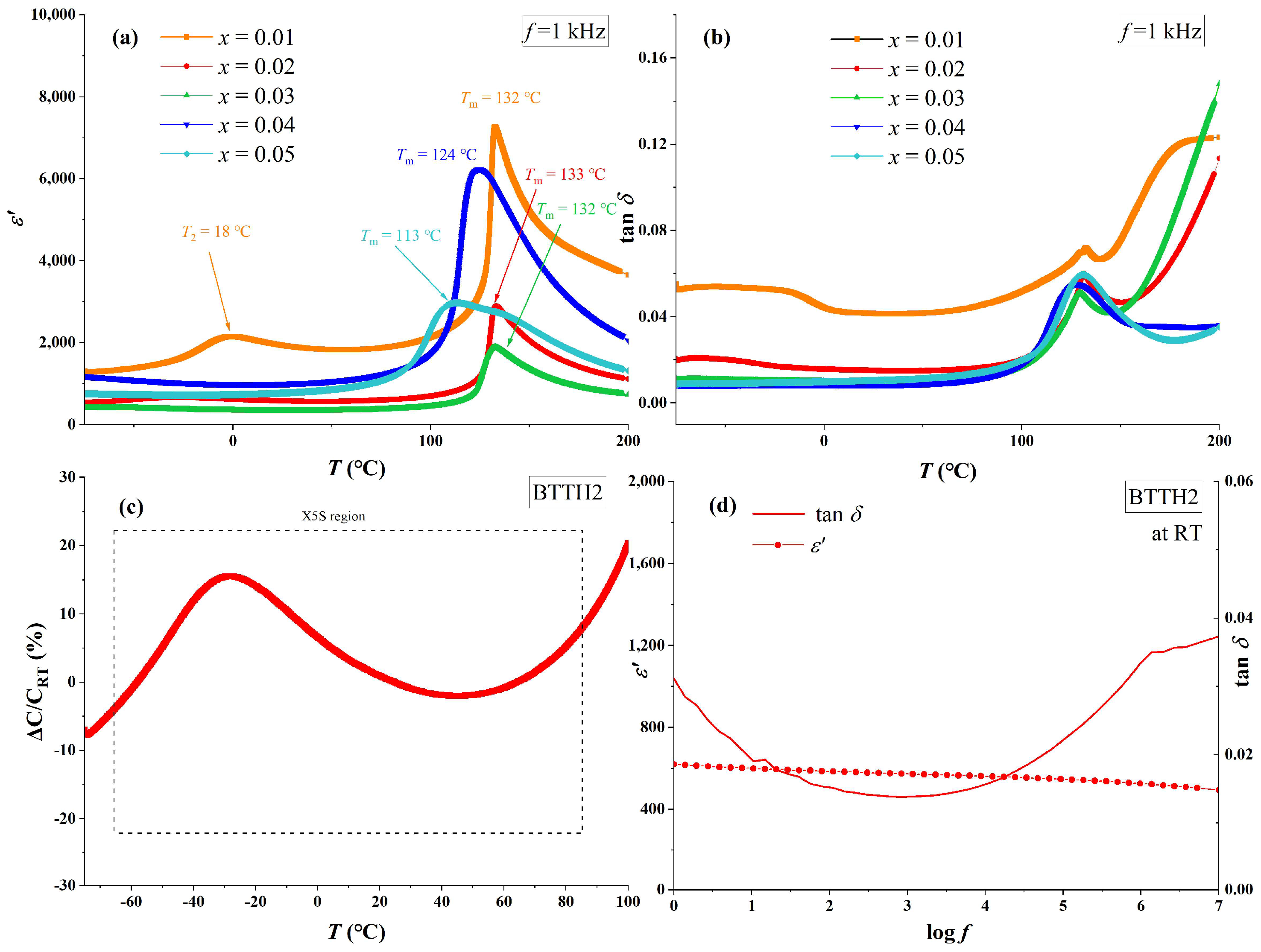
The temperature-dependent real-part permittivity (ε′) and loss (tan δ) of BTTH ceramics at 1 kHz are shown in Figure 4a,b. For sample x = 0.01, the phase transitions of tetragonal to cubic (Tm) and orthorhombic to tetragonal (T2) are clearly observed at 132 and 18 °C, which are higher than those of pure BaTiO3. This result indicates that a lower doping concentration of Tb/Ho can raise the phase transition temperature, which is also reported by Lu et al. in their study of Tb-doped barium titanate (Ba1−xTbx)(Ti1−xTbx)O3 with self-compensation mode [24]. The values of Tm for x = 0.01, 0.02, and 0.03 are almost the same and decrease to 124 and 113 °C for x = 0.04 and 0.05, respectively. Sample x = 0.01 possesses the highest permittivity among all samples and the sharpest permittivity peak at Tm, which broadens as doping content increases, and the phase transition of orthorhombic to tetragonal (T2) disappears when x ≥ 0.02. Compared with other samples, the loss values for x ≥ 0.02 are much lower than that of x = 0.01, which is similar to Nd/Yd co-doped BaTiO3 ceramics [17]. This result suggests that charges in sample x = 0.01 are not compensated and thus lead to higher electrical conductivity.
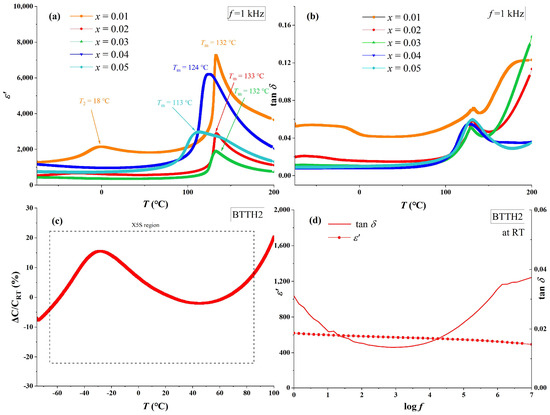
Figure 4.
(a) Real part of permittivity (ε′), (b) loss (tan δ) of BTTH ceramics, and (c,d) dielectric performance of x = 0.02.
For sample x = 0.02, the dielectric performance satisfies the X5S dielectric specification (−55~85 °C, ǀε′ − ε′RTǀ/ε′RT ≤ 22%). The real part of permittivity and loss at room temperature and 1 kHz for x = 0.02 are 617 and 0.015, respectively. In the study of Lu et al., the sample (Ba1−xTbx)(Ti1−xTbx)O3 (x = 0.05) also satisfies the X5S specification [24]; however, the room temperature permittivity of (Ba1−xTbx)(Ti1−xTbx)O3 (x = 0.05) is much higher than that of BTTH (x = 0.05) samples. This result indicates that the permittivity is significantly influenced when Tb is replaced by Ho. Figure 4d also presents the frequency dependence of permittivity at room temperature for x = 0.02. The tanδ values are lower than 0.02 in the frequency range between 10 and 8.8 × 104 Hz, and the values of ε′ decrease slowly from 621 to 500 when frequency increases from 1 to 107 Hz, suggesting that non-intrinsic polarization can be neglected in the sample [27].
3.3. Site Occupation
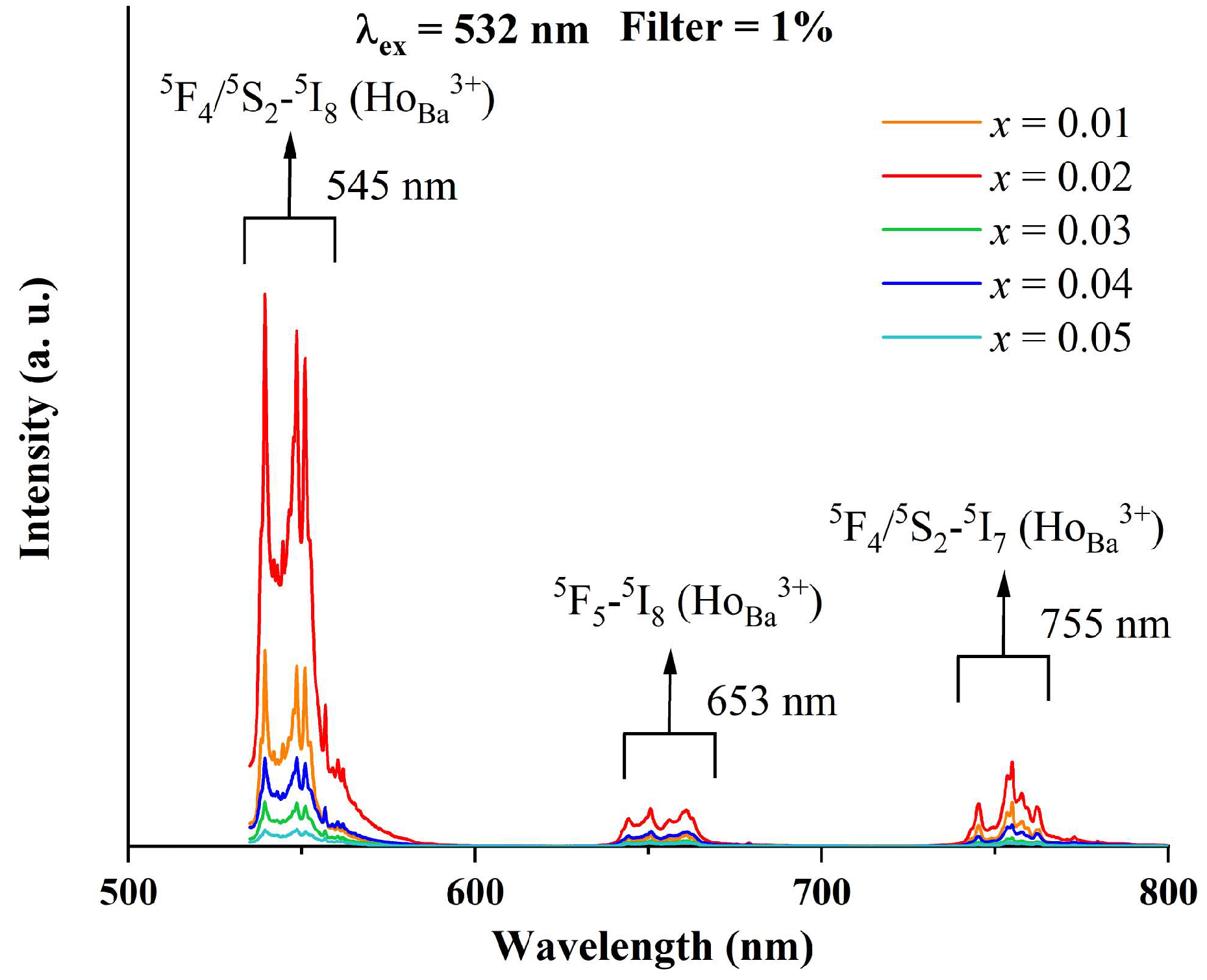
Figure 5 exhibits the photoluminescence spectra of BTTH samples upon the excitation of the 532 nm laser line. There are three PL bands located around 545, 653, and 755 nm with different intensities in the wavelength range of 500~800 nm. Kumar et al. examined the PL spectra of Ho3+ in alkali bismuth gallate glasses using excitation wavelengths of 488 and 450 nm [28]. They reported three PL bands: the strong green emission located at 545 nm is assigned to the 5F4/5S2 → 5I8 transition, and the weak red emissions located at 662 and 756 nm are attributed to 5F5 → 5I8 and 5S2 → 5I7 transitions [28]. Babu et al. reported a similar result of Ho3+ as a luminescence center in multicomponent fluorophosphate-based glasses [29]. Battisha and Secu et al. studied Ho-doped BaTiO3 ceramics and declared that Ho3+ is mainly substituted for the Ba sites under the low sintering temperature [30,31]. They observed four luminescence bands at 435, 545, 660, and 760 nm, respectively, and the last three bands were associated with the 5F4/5S2 → 5I8, 5F5 → 5I8 and 5F4/5S2 → 5I7 transitions, respectively. Based on the above discussion, the observed PL spectra of BTTH ceramics in this paper are associated with Ho3+ ions at Ba sites. Lu et al. reported three PL bands at 545, 653, and 755 nm and also assigned them to the 5F4/5S2 → 5I8, 5F5 → 5I8, and 5F4/5S2 → 5I7 transitions of Ho3+ ions on the Ba sites [32]. Makovec et al. investigated the solubility and site occupancy of Ho ions in BaTiO3 and drew the conclusion that the solubility limit of Ho3+ ions on Ba sites is less than 1.4% at the sintering temperature of 1400 °C in the TiO2-rich samples, and Ho3+ ions entered the Ti sites in BaO-rich samples [33]. The results of Makovec et al. convincingly proved that Ho3+ ions possess an amphoteric behavior of site occupancy, which is influenced by the ratio of Ba/Ti. In Figure 5, the PL intensity of x = 0.02 is much higher than that of x = 0.01, suggesting a higher concentration of Ho3+ at Ba sites with increasing doping content. However, the PL intensity decreases dramatically when x > 0.02, suggesting that Ho3+ ions at Ba sites are strongly suppressed. Since there is no impurity phase that contains the Ho element in XRD spectra, it is therefore reasonable to suppose that Ho3+ ions prefer to occupy Ti sites at higher doping levels.
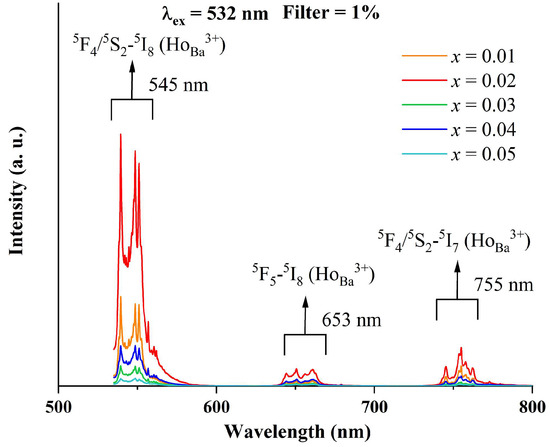
Figure 5.
Photoluminescence spectra of BTTH ceramics.
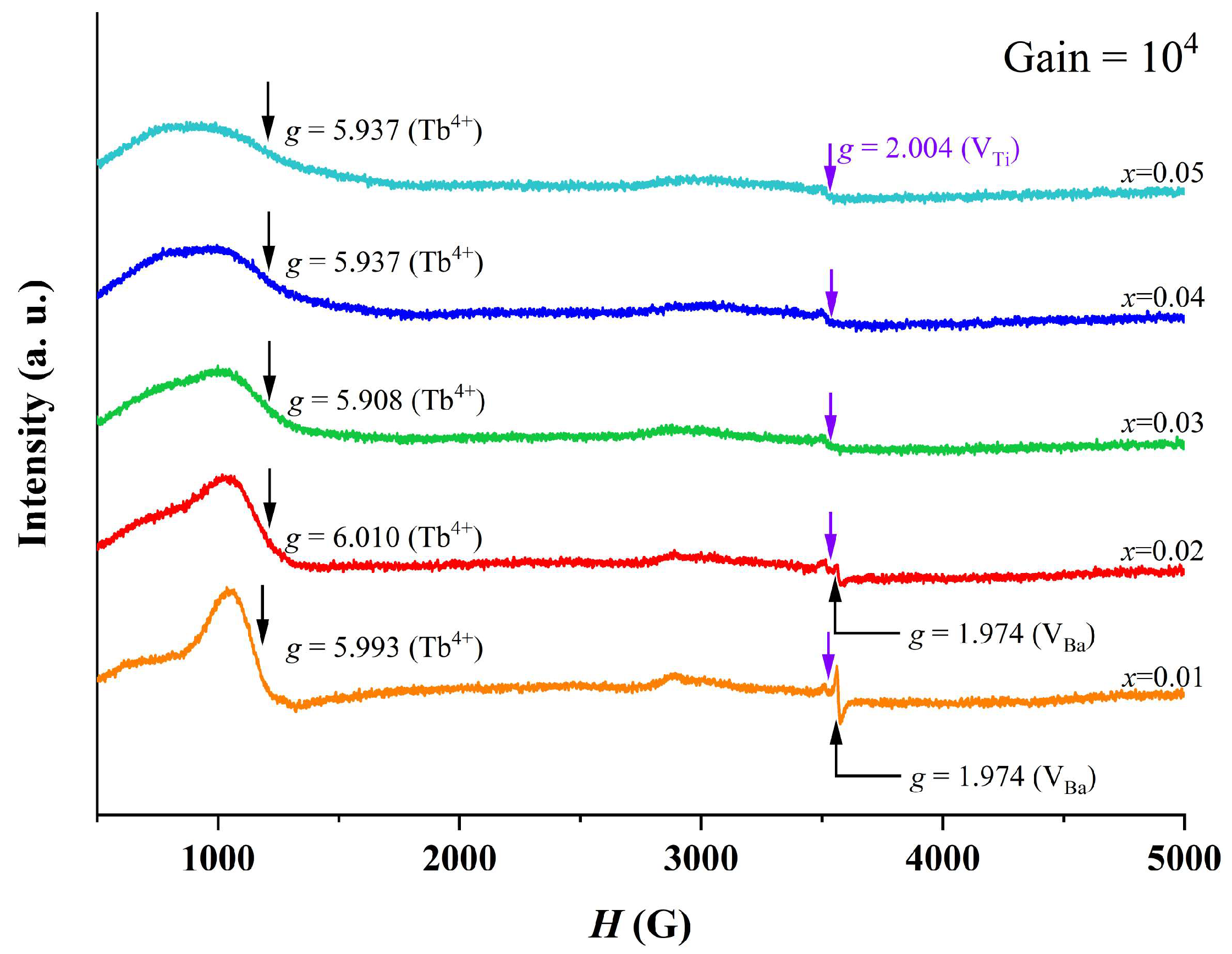
Figure 6 shows room temperature EPR spectra of BTTH ceramics, and three signals at g = 1.974, 2.004, and 6.010 are observed. The g = 1.974 signal is assigned to ionized Ba vacancy defect () [34,35], which only exists in x = 0.01 and 0.02. For the weak signal, g = 2.004, which is associated with Ti vacancies () [35], can be observed in all samples. A broad signal is located at g = 5.908~6.010 and is attributed to the electron paramagnetic resonance caused by Tb4+ (4f7, 8S7/2) Kramers ions at Ti sites () [25]. The intensity of the Tb4+ signal decreases with increasing doping content, suggesting that the concentration of Tb4+ ions incorporated into Ti sites decreases with increasing doping level. Because no impurity phase is observed in XRD results for x ≤ 0.05, which means that Tb ions completely enter the BaTiO3 lattice, the decrease in the Tb4+ signal in Figure 6 suggests that more and more Tb ions occupy the Ba sites as Tb3+ [12]. In this study, Tb ions are designed to occupy the Ba sites to compensate for the Ti-site Ho ions, and this compensation mechanism will be partly broken when Tb occupies the Ti sites as Tb4+. Ba or Ti vacancies will be produced to compensate the Ba-site rare-earth ions. For x = 0.01, the quantity of is larger than other samples; therefore, the largest quantity of Ba vacancies in x = 0.01 is reasonable. With the decrease in the Tb4+ EPR peak and more Tb3+ ions occupying the Ba sites, it is easy to understand the decrease in the Ba vacancy signal. The above results provide solid evidence of multivalent states and amphoteric behavior of site occupancy of Tb ions in BTTH ceramics.
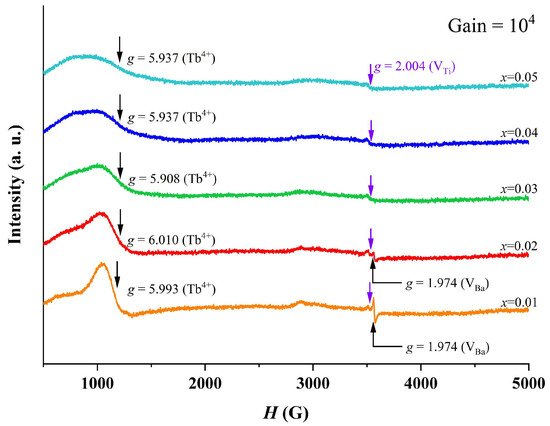
Figure 6.
Room temperature EPR spectra of BTTH ceramics.
3.4. Valence States
The mixed valence states of Tb ions have been widely confirmed in Tb-doped BaTiO3 ceramics. Lu et al. studied the self-compensation of Tb ions in BaTiO3 and found that the complete self-compensation mode of Tb3+ in BaTiO3, i.e., , could not be formed like Eu3+, Dy3+, or Ho3+ because Tb ions exist in the mixed-valence states of Ba-site Tb3+ and Ti-site Tb4+ [25]. In their study, Tb ions exist in the forms of Ba-site Tb3+ and Ti-site Tb4+, and the concentrations of Tb3+ and Tb4+ are adjustable and deviate from the designed values to maintain the lattice electroneutrality. These phenomena are also observed in other systems with Tb doping [36,37].
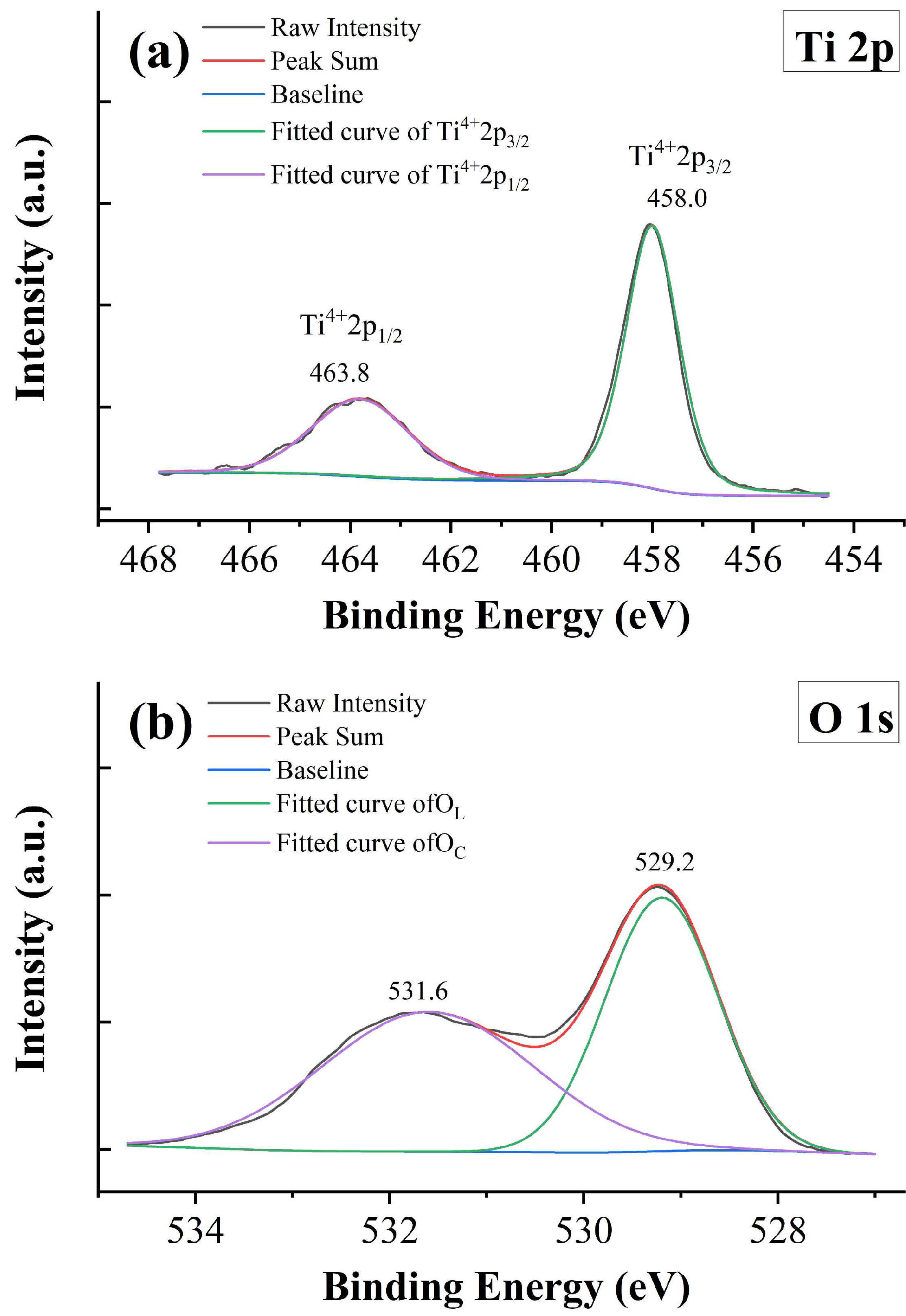
XPS measurement is employed to gain a better understanding of the valences of Tb ions. Figure 7 shows the raw data and fitting results of XPS spectra for sample x = 0.05. Figure 7a presents the data for the Ti-2p core level, which contains two main peaks originating from the spin-orbit splitting of Ti 2p1/2 and Ti 2p3/2. The data are fitted with a combination of Gaussian–Lorentzian functions, and only two peaks located at 463.8 and 458.0 eV are obtained. These peaks are identified as a complete Ti4+ state, suggesting that the Ti3+ state is absent and all Ti ions are tetravalent in the samples. The energy separation of these two peaks is 5.8 eV, which is consistent with the previous studies [38]. It is well known that oxygen vacancies are unavoidable in transition metal oxides under high-temperature sintering conditions and low partial oxygen pressure. For example, Sinclair and West pointed out that La-doped BaTiO3 ceramics sintered in an air atmosphere may be oxygen deficient inside the grains [39]. Wang et al. also declared that partial oxidation occurred only in the surface layer of the sample, while the inner part is oxygen deficient [40]. The conducting electrons created by ionization of the oxygen vacancies can be captured by transition metal cations and thus result in the reduction in transition metal cations; for example, Ti4+ is reduced to Ti3+ [41,42]. However, the mixed valence states of Ti ions do not exist in BTTH. To probe the state of the oxygen anion, the 1s core level spectrum of oxygen is also analyzed, and the result is exhibited in Figure 7b. Two peaks are observed at the binding energies of 531.6 and 529.2 eV with an energy separation of 2.4 eV. The XPS spectra of the O 1s core level in BaTiO3 or similar systems have been studied a lot [43,44]. According to the literature, the O 1s spectra are usually asymmetric and can be deconvoluted into three peaks, which are associated with lattice oxygen ions in BaTiO3 (OL), defect oxygen within the BaTiO3 matrix (OD), and loosely bound oxygen from chemisorbed or dissociated surface oxygen species (OC), respectively [43,45]. The energy separation in Figure 7b coincides well with the value of OC with respect to OL [43]; therefore, the XPS spectra of the O 1s core level in this study are assigned to OL and OC, and the defect oxygen species is absent, which is consistent with the tetravalent state of Ti ions. According to the literature, defect oxygen is usually associated with the Ti3+ state [43,44].
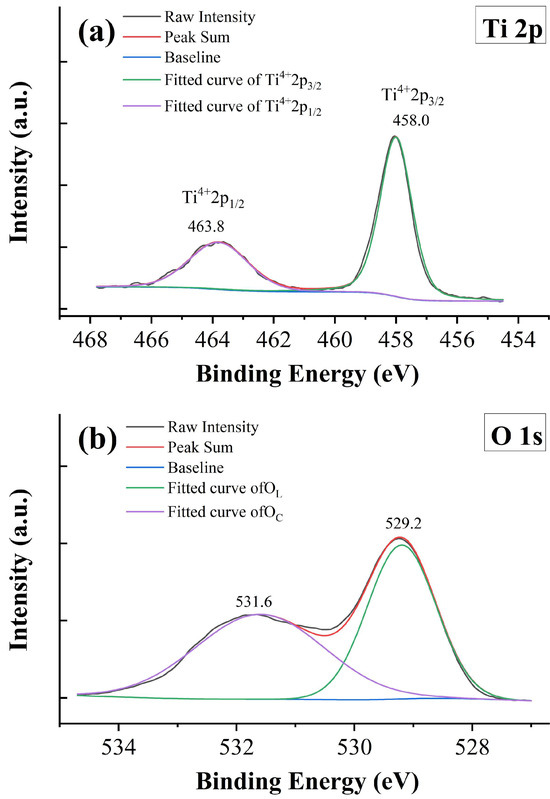
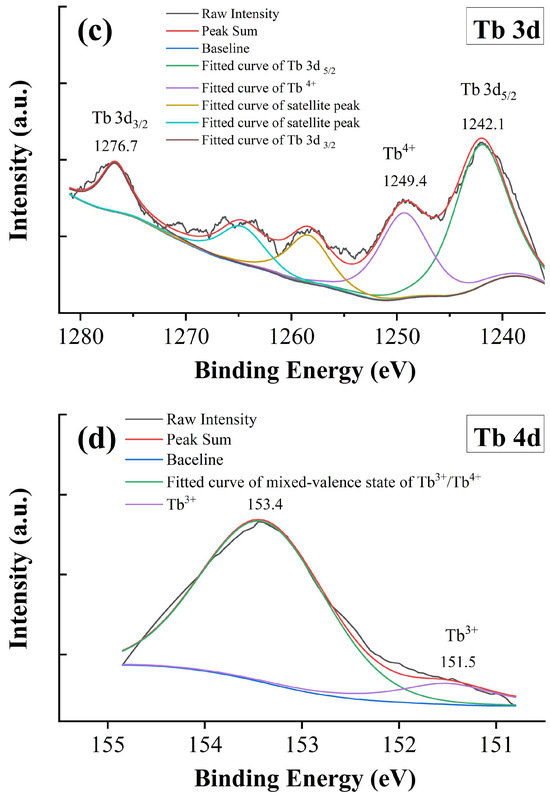
Figure 7.
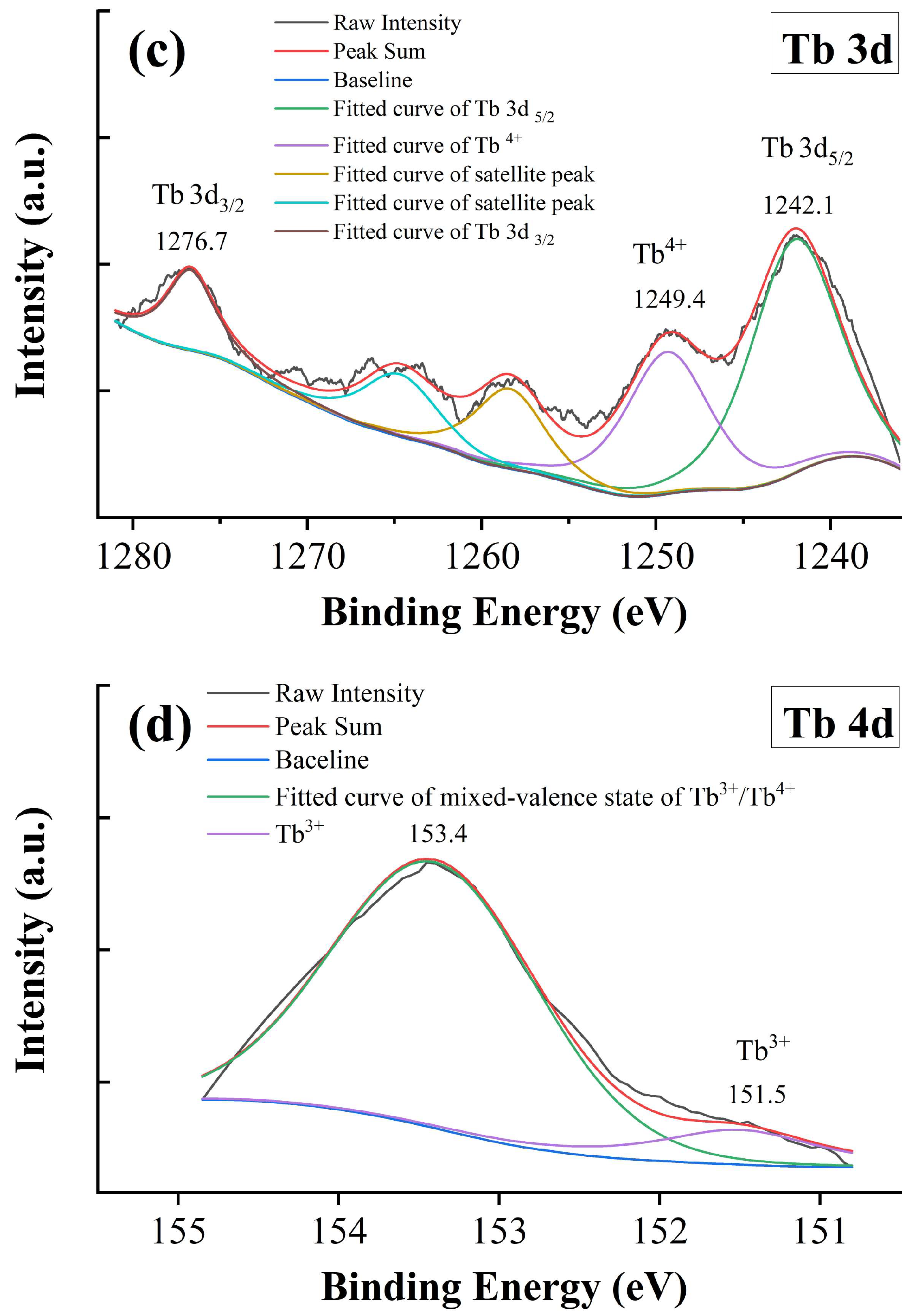
XPS spectra of x = 0.05. (a) Ti 2p, (b) O 1s, (c) Tb 3d, and (d) Tb 4d.
The Tb 3d and 4d XPS spectra of x = 0.05 are shown in Figure 7c,d. Two main peaks are located at 1242.1 and 1276.7 eV, which are associated with the Tb 3d5/2 and 3d3/2 signals, respectively. Ramesh and Liang et al. attributed the above signals observed in Tb-doped Zn2(BO3)(OH)0.75F0.25 and NaSrB5O9 phosphor to the +3 state of Tb [46,47]. Colomer et al. reported a mixed-valence state of Tb3+/Tb4+ in Tb-doped TiO2 using the XPS spectrum of the Tb 3d region [48]. However, it is still difficult to identify the Tb3+ and Tb4+ states within the Tb 3d region because of their overlapping [12]. Generally, there are satellite peaks with binding energy 10 eV higher than those of the main peaks, whereas no 3d satellite of Tb3+ exists [49,50]. In this study, the satellite at 1249.4 eV is an indicator of the Tb4+ state; however, the Tb3+ should not be excluded, especially when the satellite peaks are not significant [49]. Figure 7d shows the XPS spectrum of Tb 4d, and the signal within the measurement range can be deconvoluted and fitted with two peaks at 151.5 and 153.4 eV, respectively. The peak at 153.4 eV is related to a mixed-valence state of Tb3+/Tb4+ [12], whereas the signal at 151.5 eV can be attributed to the Tb3+ state [51]. These results provide convincing evidence of the mixed-valence state of Tb ions in BTTH ceramics.
3.5. Defect Chemistry
Based on the above results and discussion, the dominant defects in BTTH ceramics are , , and . In addition, a small quantity of Ti and Ba vacancies exists in all samples, and x ≤ 0.02, respectively. Ho3+ prefers to occupy the Ba sites () and Tb4+ prefers to occupy Ti sites () when x ≤ 0.02, whereas Tb3+ ions at Ba sites () and Ho3+ at Ti sites () will be significant when x ≥ 0.03. Therefore, the majority of defects are and when x ≤ 0.02. In this case, Ti and Ba vacancies are produced to preserve the lattice electroneutrality. Thus, the dominant defect complexes in x = 0.01 and 0.02 are and , which are in agreement with the EPR result. Because the signal of in EPR is stronger than that of in x = 0.01, the number of should be much larger than that of , which means that Ba vacancy is the predominant compensation mechanism for . However, due to the largest quantity of in x = 0.01, the quantity of cannot completely compensate , and the extra act as donors, resulting in a high loss value of tanδ. For sample x ≥ 0.03, the dominant defects are and , and they can form the defect complex of without the necessary formation of or . The vanishing of signal in the EPR measurement also proves this result. The small amount of is related to , which cannot be compensated by or .
4. Conclusions
Single-phase Tb- and Ho-co-doped BaTiO3 ceramics with a solubility limit of 0.05 were successfully prepared by the solid-state-reaction method at 1400 °C. The tetragonality and grain sizes of the samples decrease with increasing doping content. The dielectric constant peaks broaden with increasing Ho and Tb content, and the dielectric performance of x = 0.02 satisfies X5S dielectric specification. Both Ho and Tb ions exhibit amphoteric behavior of site occupancy. Tb ions exhibit mixed-valence states of Ba-site Tb3+ and Ti-site Tb4+, whereas Ho ions are trivalent at both Ba and Ti sites. At low doping levels, Ho and Tb ions prefer to occupy Ba and Ti sites, respectively. For samples with high doping concentration, Ho ions have a tendency to occupy Ti sites, and more Tb ions enter Ba sites. The defect compensation mechanism is also influenced by site occupancy.
Author Contributions
Conceptualization, J.L. and Q.L. (Qiaoli Liu); funding acquisition, J.L. and Q.L. (Qiaoli Liu); investigation, X.W., Y.R., G.X. and Q.L. (Qi Liu); data curation, Q.L. (Qi Liu); project administration, J.L. and Q.L. (Qiaoli Liu); supervision, Q.L. (Qiaoli Liu); visualization, J.L. and Q.L. (Qiaoli Liu); writing—original draft, J.L. and X.W.; writing—review and editing, J.L. and Q.L. (Qiaoli Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Science and Technology Research Project of Henan Province (No. 232102231056 and No. 242102230150) and the Doctoral Project of Pingdingshan University (PXY-BSQD-2024004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the experimental support of Jilin Institute of Chemical Technology.
Conflicts of Interest
Author Xin Wei was employed by the company Huangmei Longyuan Gypsum Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Acosta, M.; Novak, N.; Rojas, V.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, G.A., Jr.; Rödel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Adediji, Y.B.; Adeyinka, A.M.; Yahya, D.I.; Mbelu, O.V. A review of energy storage applications of lead-free BaTiO3-based dielectric ceramic capacitors. Energy Ecol. Environ. 2023, 8, 401–419. [Google Scholar] [CrossRef]
- Palani, P.; Fasquelle, D.; Tachafine, A. A review on (Sr,Ca)TiO3-based dielectric materials: Crystallography, recent progress and outlook in energy-storage aspects. J. Mater. Sci. 2022, 57, 12279–12317. [Google Scholar] [CrossRef]
- Li, J.-F.; Wang, K.; Zhu, F.-Y.; Cheng, L.-Q.; Yao, F.-Z. (K, Na)NbO3-Based Lead-Free Piezoceramics: Fundamental Aspects, Processing Technologies, and Remaining Challenges. J. Am. Ceram. Soc. 2013, 96, 3677–3696. [Google Scholar] [CrossRef]
- Li, J.-H.; Wang, S.-F.; Chung, T.-F.; Yang, J.-R. Effects of addition of Sc2O3 on microstructure and dielectric properties of BaTiO3-based X8R MLCCs. J. Phys. Chem. Solids 2019, 127, 194–201. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Y.; Yang, S.; Li, J.; Liu, D.; Wu, J.; Wang, M.; Li, C.; Li, F. Simultaneous enhancement of dielectric properties and temperature stability in BaTiO3-based ceramics enabling X8R multilayer ceramic capacitor. Int. J. Appl. Ceram. Technol. 2023, 20, 3735–3742. [Google Scholar] [CrossRef]
- Bell, J.G.; Graule, T.; Stuer, M. Barium titanate-based thermistors: Past achievements, state of the art, and future perspectives. Appl. Phys. Rev. 2021, 8, 031318. [Google Scholar] [CrossRef]
- Fang, T.-T. New insights into the positive temperature coefficient of resistance model of BaTiO3-based ceramics. J. Am. Ceram. Soc. 2024, 107, 2048–2051. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.; Lu, Z.; Wang, X.; Fu, X. Effect of Bi2O3 and Ho2O3 co-doping on the dielectric properties and temperature reliability of X8R BaTiO3-based ceramics. Ceram. Int. 2021, 47, 24982–24987. [Google Scholar] [CrossRef]
- Lu, D.Y.; Yue, Y.; Sun, X.Y. Novel X7R BaTiO3 ceramics co-doped with La3+ and Ca2+ ions. J. Alloys Compd. 2014, 586, 136–141. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Yin, S.; Cui, S.-Z. A fine-grained and low-loss X8R (Ba1–xDyx) (Ti1–x/2Cax/2)O3 ceramic. J. Alloys Compd. 2018, 762, 282–288. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Gao, X.-L.; Liu, Q.-L. Synergistic effect of terbium and calcium ions on the temperature stability and dielectric loss of BaTiO3-based ceramics. J. Alloys Compd. 2019, 808, 151713. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Toda, M.; Sugano, M. High-Permittivity Double Rare-Earth-Doped Barium Titanate Ceramics with Diffuse Phase Transition. J. Am. Ceram. Soc. 2006, 89, 3112–3123. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zhang, J.; Jin, L.; Wang, D.; Wei, J.; Ye, Z.-G.; Jia, C.-L. Charge effects in donor-doped perovskite ferroelectrics. J. Am. Ceram. Soc. 2020, 103, 5392–5399. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, E.J.; Han, Y.H. Effects of Ho2O3 addition on defects of BaTiO3. Mater. Chem. Phys. 2006, 100, 434–437. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Cui, S.-Z.; Liu, Q.-L.; Sun, X.-Y. Dielectric properties and defect chemistry of barium titanate ceramics co-doped R and Dy ions (R = Eu, Gd, Tb). Ceram. Int. 2016, 42, 14364–14373. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Wei, X.; Cai, Q. Mixed valence states of Yb3+/Yb2+ in low-loss (Ba1−xNdx)(Ti1−xYbx)O3 dielectric ceramics. J. Alloys Compd. 2021, 884, 161049. [Google Scholar] [CrossRef]
- Hwang, J.H.; Han, Y.H. Defect chemistry of Er-doped BaTiO3. Solid State Ionics 2001, 140, 181–186. [Google Scholar] [CrossRef]
- Park, K.-J.; Kim, C.-H.; Yoon, Y.-J.; Song, S.-M.; Kim, Y.-T.; Hur, K.-H. Doping behaviors of dysprosium, yttrium and holmium in BaTiO3 ceramics. J. Eur. Ceram. Soc. 2009, 29, 1735–1741. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Gao, X.-L.; Wang, S. Abnormal Curie-temperature shift in Ho-doped BaTiO3 ceramics with the self-compensation mode. Results Phys. 2019, 12, 585–591. [Google Scholar] [CrossRef]
- Xue, L.A.; Chen, Y.; Brook, R.J. The influence of ionic radii on the incorporation of trivalent dopants into BaTiO3. Mater. Sci. Eng. B 1988, 1, 193–201. [Google Scholar] [CrossRef]
- Li, Y.-X.; Yao, X.; Wang, X.-S.; Hao, Y.-B. Studies of dielectric properties of rare earth (Dy, Tb, Eu) doped barium titanate sintered in pure nitrogen. Ceram. Int. 2012, 38, S29–S32. [Google Scholar] [CrossRef]
- Tsur, Y.; Dunbar, T.D.; Randall, C.A. Crystal and Defect Chemistry of Rare Earth Cations in BaTiO3. J. Electroceram. 2001, 7, 25–34. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Peng, Y.-Y. Dielectric properties and exploration of self-compensation mode of Tb in BaTiO3 ceramics. J. Ceram. Soc. Jpn. 2016, 124, 455–459. [Google Scholar] [CrossRef]
- Lu, D.-Y. Self-adjustable site occupations between Ba-site Tb3+ and Ti-site Tb4+ ions in terbium-doped barium titanate ceramics. Solid State Ionics 2015, 276, 98–106. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Zhu, P. Dielectric relaxations without colossal permittivity in Mg and Nb co-doped BaTiO3 ceramics. Ceram. Int. 2023, 49 Pt A, 40623–40629. [Google Scholar] [CrossRef]
- Kumar, G.A.; Martinez, A.; Mejia, E.; Eden, J.G. Fluorescence and upconversion spectral studies of Ho3+ in alkali bismuth gallate glasses. J. Alloys Compd. 2004, 365, 117–120. [Google Scholar] [CrossRef]
- Babu, S.; Seshadri, M.; Balakrishna, A.; Reddy Prasad, V.; Ratnakaram, Y.C. Study of multicomponent fluoro-phosphate based glasses: Ho3+ as a luminescence center. Phys. B Condens. Matter 2015, 479, 26–34. [Google Scholar] [CrossRef]
- Battisha, I.K. Visible Up-Conversion Luminescence in Ho3+: BaTiO3 Nano-Crystals Prepared by Sol Gel Technique. J. Sol-Gel Sci. Technol. 2004, 30, 163–172. [Google Scholar] [CrossRef]
- Secu, M.; Cernea, M.; Secu, C.E.; Vasile, B.S. Structural characterization and photoluminescence of nanocrystalline Ho-doped BaTiO3 derived from sol–gel method. J. Nanopart. Res. 2011, 13, 3123–3128. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Guan, D.-X. Photoluminescence associated with the site occupations of Ho3+ ions in BaTiO3. Sci. Rep. 2017, 7, 6125. [Google Scholar] [CrossRef] [PubMed]
- Makovec, D.; Samardžija, Z.; Drofenik, M. Solid Solubility of Holmium, Yttrium, and Dysprosium in BaTiO3. J. Am. Ceram. Soc. 2004, 87, 1324–1329. [Google Scholar] [CrossRef]
- Kolodiazhnyi, T.; Petric, A. Analysis of point defects in polycrystalline BaTiO3 by electron paramagnetic resonance. J. Phys. Chem. Solids 2003, 64, 953–960. [Google Scholar] [CrossRef]
- Dunbar, T.D.; Warren, W.L.; Tuttle, B.A.; Randall, C.A.; Tsur, Y. Electron Paramagnetic Resonance Investigations of Lanthanide-Doped Barium Titanate: Dopant Site Occupancy. J. Phys. Chem. B 2004, 108, 908–917. [Google Scholar] [CrossRef]
- Josse, M.; Dubois, M.; El-Ghozzi, M.; Avignant, D. Synthesis and crystal structures of new mixed-valence terbium (III/IV) fluorides with a random distribution between Tb3+ and Tb4+. J. Alloys Compd. 2004, 374, 213–218. [Google Scholar] [CrossRef]
- Ueda, K.; Shimizu, Y.; Nagamizu, K.; Matsuo, M.; Honma, T. Luminescence and Valence of Tb Ions in Alkaline Earth Stannates and Zirconates Examined by X-ray Absorption Fine Structures. Inorg. Chem. 2017, 56, 12625–12630. [Google Scholar] [CrossRef]
- Herrera-Pérez, G.; Solis-Canto, O.; Silva-Vidaurri, G.; Pérez-García, S.; Borja-Urby, R.; Paraguay-Delgado, F.; Rojas-George, G.; Reyes-Rojas, A.; Fuentes-Cobas, L. Multiplet structure for perovskite-type Ba0.9Ca0.1Ti0.9Zr0.1O3 by core–hole spectroscopies. J. Appl. Phys. 2020, 128, 064106. [Google Scholar] [CrossRef]
- Morrison, F.D.; Sinclair, D.C.; West, A.R. Characterization of lanthanum-doped barium titanate ceramics using impedance spectroscopy. J. Am. Ceram. Soc. 2001, 84, 531–538. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, L.W. Surface-layer effect in CaCu3Ti4O12. Appl. Phys. Lett. 2006, 88, 042906. [Google Scholar] [CrossRef]
- Badapanda, T.; Senthil, V.; Rout, S.K.; Panigrahi, S.; Sinha, T.P. Dielectric relaxation on Ba1−xBi2x/3Zr0.25Ti0.75O3 ceramic. Mater. Chem. Phys. 2012, 133, 863–870. [Google Scholar] [CrossRef]
- Raengthon, N.; DeRose, V.J.; Brennecka, G.L.; Cann, D.P. Defect mechanisms in high resistivity BaTiO3–Bi(Zn1/2Ti1/2)O3 ceramics. Appl. Phys. Lett. 2012, 101, 112904. [Google Scholar] [CrossRef]
- Chun, H.-J.; Lee, Y.; Kim, S.; Yoon, Y.; Kim, Y.; Park, S.-C. Surface Termination of BaTiO3(111) Single Crystal: A Combined DFT and XPS Study. Appl. Surf. Sci. 2022, 578, 152018. [Google Scholar] [CrossRef]
- Panchal, G.; Shukla, D.K.; Choudhary, R.J.; Reddy, V.R.; Phase, D.M. The effect of oxygen stoichiometry at the interface of epitaxial BaTiO3/La0.7Sr0.3MnO3 bilayers on its electronic and magnetic properties. J. Appl. Phys. 2017, 122, 085310. [Google Scholar] [CrossRef]
- Clabel H., J.L.; Awan, I.T.; Lozano, G.; Pereira-da-Silva, M.A.; Romano, R.A.; Rivera, V.A.G.; Ferreira, S.O.; Marega, E. Understanding the electronic properties of BaTiO3 and Er3+ doped BaTiO3 films through confocal scanning microscopy and XPS: The role of oxygen vacancies. Phys. Chem. Chem. Phys. 2020, 22, 15022–15034. [Google Scholar] [CrossRef]
- Ramesh, B.; Dillip, G.R.; Raju, B.D.P.; Somasundaram, K.; Peddi, S.P.; de Carvalho dos Anjos, V.; Joo, S.W. Facile one-pot synthesis of hexagons of NaSrB5O9:Tb3+ phosphor for solid-state lighting. Mater. Res. Express 2017, 4, 046201. [Google Scholar] [CrossRef]
- Liang, P. Co-existence phenomenon of Ce3+/Ce4+ and Tb3+ in Ce/Tb co-doped Zn2(BO3)(OH)0.75F0.25 phosphor: Luminescence and energy transfer. Adv. Powder Technol. 2019, 30, 974–982. [Google Scholar] [CrossRef]
- Colomer, M.T.; Rodríguez, E.; Morán-Pedroso, M.; Vattier, F.; de Andrés, A. Impact of Tb4+ and morphology on the thermal evolution of Tb-doped TiO2 nanostructured hollow spheres and nanoparticles. J. Alloys Compd. 2021, 853, 156973. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Hungría, A.B.; Fernández-García, M.; Iglesias-Juez, A.; Conesa, J.C.; Mather, G.C.; Munuera, G. Cerium–terbium mixed oxides as potential materials for anodes in solid oxide fuel cells. J. Power Sources 2005, 151, 43–51. [Google Scholar] [CrossRef]
- van den Bossche, J.; Neyts, K.A.; de Visschere, P.; Corlatan, D.; Pauwels, H.; Vercaemst, R.; Fiermans, L.; Poelman, D.; van Meirhaeghe, R.L.; Laflére, W.H.; et al. XPS study of TbF3 and TbOF centres in ZnS. Phys. Status Solidi (a) 1994, 146, K67–K70. [Google Scholar] [CrossRef]
- Shilov, S.M.; Gavronskaya, K.A.; Pak, V.N. Distribution of luminescence excitation energy between Eu3+ and Tb3+ ions fixed in a perfluorosulfonic membrane. Russ. J. Gen. Chem. 2008, 78, 171–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).